Abstract
The aim of this study is to investigate in vitro the anticancer, antioxidant, and antibacterial activities of three low molecular weight subfractions I, II and III isolated from secondary metabolites produced by the wood degrading fungus Cerrena unicolor. The present study demonstrated that the low molecular weight subfractions III exhibited the strongest inhibitory activity towards breast carcinoma cells MDA-MB-231, prostatic carcinoma cells PC3, and breast cancer cells MCF7 with the half-maximal inhibitory concentration (IC50) value of 52,25 μg/mL, 60,66 μg/mL, and 54,92 μg/mL, respectively. The highest percentage of inhibition was noted at a concentration of 300 μg/mL in all the examined tumor lines. A significant percentage (59.08%) of ex-LMSIII inhibition of the MDA-MB-231 tumor line was reached at a concentration of 15 μg/ml, while the concentration applied did not affect normal human fibroblast cells. The low molecular weight subfraction III was the most effective and additionally showed the highest free radical 1,1-diphenyl-2-picryl-hydrazyl scavenging activity (IC50 20.39 μg/mL) followed by the low molecular weight subfraction I (IC50 64.14 μg/mL) and II (IC50 49.22 μg/mL). The antibacterial activity of the tested preparations was evaluated against three microorganisms: Bacillus subtilis, Staphylococcus aureus, and Escherichia coli. The MIC minimal inhibitory concentration (MIC) values for the low molecular weight subfraction I, II, and III showed a stronger inhibition effect on S. aureus than on B. subtilis and E. coli cells. The MIC values for the low molecular weight subfraction II against S. aureus, B. subtilis, and E. coli were 6.25, 12.5, and 100 mg/mL, respectively.
Introduction
Medicinal mushrooms have long been used in Asian countries due to their bioactivities such as anticancer, antioxidant, antimicrobial, hepatoprotective, antineurodegenerative, antidiabetic, antiangiogenic, and hypoglycemic effects in animals and in humans [1–4]. Of all known species of mushrooms, 650 have documented medicinal properties but, surprisingly, only approximately 20 are in common clinical use at present [5]. Some edible mushrooms, e.g. Grifola fron dosa, Lentinus edodes, Flammulina velutipes, Pleurotus ostreatus, Tremella mesenterica, and Hericium erinaceus, are used for medicinal applications. There are also some non-edible mushroom species used solely for medicinal purposes, for instance Ganoderma lucidum, Schizophyllum commune, or Trametes versicolor. Bioactive substances from fungi can generally be divided into two groups of high molecular weight compounds, which include primarily polysaccharides and proteins and low molecular weight compounds, such as indoles, terpenoids, or phenols. The second group comprises low-molecular-weight secondary compounds that can penetrate the cell membrane and act as effectors of specific signal transduction pathways [4–6]. Substances belonging to both groups have great medical potential. In addition to their nutritional values, they possess antitumor, antibacterial, antiviral, and immunomodulatory activities [7–9]. One of the most widely investigated groups of preparations isolated from higher fungi (Basidiomycota), especially wood degrading species, are enzymes involved in degradation of the lignocellulose complex, where oxidative and hydrolytic enzymes cooperate, including laccases, peroxidases and other oxidases, (hemi)cellulases, and different glycosidases [10,11]. Bioactive proteins constitute another important type of functional components in mushrooms with an increasing potential pharmaceutical value [12]. Hu et al. (2011) demonstrated that the fruiting bodies of the mushroom Agrocybe cylindracea produce a laccase with HIV-1 reverse transcriptase inhibitory activity and antiproliferative activity against HepG2 cells and MCF7 cells [13]. Another very well-studied group of fungal bioactive compounds comprises polysaccharides [14–16]. Natural extracts obtained from mushrooms have been used for many years for different health purposes. Aqueous extracts of Funalia trogii have been shown to have in vitro and in vivo anti-tumor efficacy [17]. Other authors reported cytotoxic and mutagenic effects of F. trogii and C. versicolor extracts on the HeLa cervical cancer cell line and human fibroblast cells [18]. It has also been reported that ethanol extracts from Ramaria flava exhibit a wide range of anticancer, antioxidant, and antibiotic activities [4]. Many substances isolated from fungi have been described as supplements to full mushroom extracts. Liu et al. (2006) isolated a xylose-specific lectin with antimitogenic and antitumor activities from fresh fruiting bodies of Xylaria hypoxylon [19]. It has been reported that lectins isolated from Pholiota adiposa and Ganoderma microsporum exhibited antiviral and antitumor activities as well [20–21]. Mushrooms are also a very efficient source of many bioactive phenolic substances, e.g. phenolic acids, flavonoids, hydroxybenzoic acids, hydroxycinnamic acids, lignans, tannins, stilbenes, oxidized polyphenols, and terpenoids [22–23]. It has been found that many phenolic compounds are very effective free-radical scavengers or metal inactivators [24]. Antioxidant compounds, i.e. phenolics, polysaccharides, tocopherols, flavonoids, carotenoids, glycosides, ergothioneine, and ascorbic acid, are found in fruiting bodies, mycelium, and cultures fluid [25]. Interestingly, the antioxidant potential in mushrooms is higher than in the most commonly used vegetables and fruits. Free radicals are known to induce oxidative damage in physiologically important biomolecules and play an important role in processes of aging, progress of cardiovascular diseases, cancer, impaired immune system, or inflammatory diseases [26]. Some authors have described isolation of various extracellular polyphenols with antioxidant properties, e.g. from the culture broth of Inonotus xeranticus, Phellinus linteus [27], and Ramaria flava [4].
For many years, C. unicolor was regarded as a nonedible fungus and was intensively studied as an efficient producer of extracellular laccase produced in noninduced conditions of growth [28–29]. The results presented in our earlier paper show that laccase and total ex-LMS (extracellular low molecular weight secondary metabolites) produced by C. unicolor species possess cytotoxic and antiproliferative activity against cervical cancer cells (SiHa and CaSki) and melanomic cells [30–31]. The cytotoxic activity of laccase preparations towards several hematological malignancies has been described as well [32]. C. unicolor cultures are a very promising source of other bioactive substances, not only laccase, whose properties have been partially determined [33].
The aim of the present report will be to evaluate the antitumor, antioxidant, and antibacterial activities of three subfractions separated from the total ex-LMS produced by C. unicolor.
Materials and methods
Mushroom growing conditions and separation of fungal samples
Cerrena unicolor (Bull. ex Fr.) Murr. was obtained from the culture collection of the Regensburg University and deposited in the fungal collection of the Department of Biochemistry (Maria Curie-Sklodowska University, Poland) under the strain number 139 (ITS sequence deposited in GenBank under accession number DQ056858) [34]. The fermentor scale cultivation was performed at 26°C in a 2.5 L Bioflo III (New Brunswick Scientific, New Brunswick, NJ, USA) fermentor containing 2 L of a sterilized Lindenberg and Holm medium optimized as described by Janusz et al. in [29]. The medium in the fermentor was inoculated with crumbled fungal mats (10% of the total medium volume), aerated, and stirred at 100 rpm. The onset of the idiophase (the stage of the production of secondary metabolites) was determined as recommended by Jennings and Lysek [35]. 10-day-old idiophasic cultures were harvested and filtered through Miracloth (Calbiochem). The biomass and culture fluid obtained were used for further assays. The culture liquid obtained after mycelium separation was centrifuged at 10.000 ×g for 15 min. The supernatant was immediately subdivided into two fractions on the ultrafiltration system Pellicon 2 Mini holder (Millipore, Bedford, MA, USA) with an Ultracel mini cartridge (10 kD cut-off). The starting fraction containing compounds with a molecular weight below 10 kDa had already been used as a source of low molecular weight metabolites (extracellular low molecular weight subfraction, ex-LMS) and had been tested as described in our previous publication [32]. The antioxidant and pro-oxidative properties, the antibacterial, antiviral, immunomodulatory, and anticancer activities, and the toxicity of the ex-LMS fraction have also been presented in previously published reports [30–33]. In this study, a fraction containing compounds below 10 kDa was fractionated on a Sephadex G-10 column (20 cm x 2 cm) into two subfractions (below and above 700 Da)–ex-LMSI and ex-LMSII. Additionally, the same fraction (below 10 kDa) was precipitated using an ammonium sulfate-saturated solution for isolation of low molecular weight proteins and dialyzed against 4 L of distilled water at 4°C–ex-LMSIII. The content of ammonium sulfate was checked with the BaCl test. The three ex-LMSI, ex-LMSII, and ex-LMSIII fractions were subsequently lyophilized and used as a source of natural low molecular weight metabolites.
Biochemical analysis
Determination of proteins, carbohydrates, and phenolic compounds
Protein concentrations were determined using the Bradford reagent and bovine serum albumin as a standard [36]. The total content of the phenolic compounds was determined with diazosulfanilamide using the DASA test [37], where the absorbance was measured at 500 nm and vanillic acid was used as a standard. The total carbohydrate content was determined by the phenol-sulfuric acid assay with D-glucose as a standard [38].
FT-IR spectroscopy analysis of ex-LMS samples
The analyses of ex-LMSI, ex-LMSII, and ex-LMSIII were performed using lyophilizates. FTIR spectroscopy was performed with a spectrometer (Thermo Scientific Nicolet 8700A with FT Ramana Nicolet NXR module) in the wavelength range 4000–400 cm−1.
Antioxidant properties
Free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH)-scavenging test
The total antioxidant capacity of the three fractions was determined using the DPPH radical as a reagent, according to the procedure described by Paduch et al. [39]. This method is based on the ability of 1,1-diphenyl-2-picrylhydrazyl (DPPH) to decolorize in the presence of antioxidants. Subsequently, 100 μL of the test compound at concentrations ranging from 6.25 to 800 μg/mL were mixed with 0.1 mL of the DPPH solution (0.2 mg/mL in ethanol) and the absorbance at 515 nm was determined after 2, 5, 10, 15, 20, and 30 min of incubation at room temperature. Trolox and ascorbic acid (Vit. C), i.e. the well-known standards with strong antioxidant activities, were used as positive controls. The percentage of inhibition of DPPH oxidation was calculated according to the following formula:
where Ac means the absorbance of the control sample andAt means the absorbance of the standard or tested compound. The antioxidant ability of the sample was expressed as IC50.
[2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] (ABTS) radical-scavenging test
The ABTS radical-scavenging activities of the fractions were determined using the method of van den Berg et al. [40], Duo-Chuan [41], and Re et al. [42] with modification. A stock solution was prepared by dissolving 7.4 mM ABTS and 2.6 mM potassium persulfate in MQ water. After 16 h, the concentrated ABTS stock solution was diluted with phosphate buffered saline (PBS) pH 7.4 to absorbance recorded at 734 nm. Subsequently, 10 μL of the analyzed compound at concentrations ranging from 6.25 to 800 μg/mL were mixed with 990 μL of the ABTS radical solution and the absorbance was measured. The percentage of inhibition of ABTS oxidation was calculated using the following formula:
where Acontrol means the absorbance of the control and Asample is the absorbance at 734 nm of the tested compound/standard. A Trolox and ascorbic acid calibration curve was prepared for a concentration range from 6.25 to 800 μg/mL and IC50 values were obtained.
Hydroxyl radical-scavenging activity assay
The OH radical-scavenging activity assay was conducted according to the Fenton method [43–45] with some modifications. 100 μL of the sample were incubated with a mixture containing 20 μL of FeSO4 · 7H2O (9 mM), 20 μL of a hydroxybenzoic acid solution with ethanol (9 mM) and 20 μL of H2O2 (8.8 mM) in a 37°C water bath for 30 minutes. The percent OH radical-scavenging effect of each sample was calculated using the following equation:
where Acontrol is the absorbance of the control reaction and the sample is replaced by 100 mL ethanol. The tests were performed in triplicate.
Analysis of the antibacterial activity
Inhibitory zone assay
Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 25922), and Bacillus subtilis (ATCC 6633) bacterial strains were used as indicator bacteria and inoculated into a commercially available Muller-Hinton Agar II medium (LabM (TM), IDG plc, UK), 38g/L, with the inoculum solution (100 μL) of ca. 1×105 CFU/mL of each kind of microorganisms smeared on the standard assay medium. 100 μL of the specimen (concentration 1 mg/mL) were added into the agar well in the center of Petri dishes and left to incubate for 2 hours at room temperature; afterwards, the plates were transferred to 37°C for 18 h. Sterilized physiological saline was used as a control. After the incubation of inoculated bacterial cultures treated with ex-LMSI, ex-LMSII, and ex-LMSIII, the inhibition zones were measured.
Minimal inhibitory concentration (MIC)
The minimal inhibitory concentration (MIC) of ex-LMSI, ex-LMSII, and ex-LMSIII was evaluated using the two-fold serial dilution method [46]. The samples were dissolved in methanol (filtered, 0.22 μm) and then diluted to obtain 200 mg/mL stock solutions. 0.5 mL of the stock solution was incorporated into 0.5 mL of sterilized nutrient broth for bacteria and serially diluted to achieve 100, 50, 25, 12.5, and 6.25 mg/mL, respectively. A 100-μL aliquot of the standardized suspension of the test bacteria (105CFU/mL) was transferred to the wells of a 96-well tissue culture plate. Then, another 100 μL of diluted samples were added to each well and the inoculated 96-well tissue culture plates were incubated at 37°C for 24 h. The MIC was defined as the lowest concentration of samples inhibiting the visible growth of the tested microorganisms.
Anticancer assay
The test sample was subjected to the MTT assay to determine the in vitro cell growth inhibitory activity against the human cancer cell lines (MCF7, MDA-MB-231, and PC3) [47]. The cells were grown in tissue culture flasks in RPMI 1640 medium at 37°C in an atmosphere of 5% CO2 and 100% relative humidity in a CO2 incubator. A 100-μL aliquot of cells (105 cells/mL) was transferred to the wells of a 96-well tissue culture plate. The cells were allowed to grow for 12 h and then they were treated with the sample. 100 μL of the test samples (300, 150, 15, 1.5, and 0.15 μg/mL) were added to the wells, and the cells were further incubated for another 48 h at 37°C in an atmosphere of 5% CO2. 20 μL of MTT (5 mg/mL in phosphate-buffered saline) were then added to each well and the cells were further cultured for 4 h. After removal of the medium, 100 μL of dimethyl sulfoxide (DMSO) was added to each well [4]. The absorbance was measured on a microplate reader (Thermo LabSystems, Grand Rapids, OH, USA) at the wavelength of 570 nm [26]. Suitable blanks and a positive control were also included, and paclitaxel was used as the positive control. The inhibition percentage was calculated using the following formula:
where Acontrol is the absorbance of the control reaction and Asample is the absorbance in the presence of the sample. Each test was done in triplicate and the concentration required for 50% inhibition of viability (IC50) was determined.
Statistical analysis
All results presented in the paper are expressed as mean and standard deviation (±SD) from three experiments (n = 3). Differences in mean values between the groups were analyzed by a one-way analysis of variance (ANOVA) with post-hoc Tukey HSD test and all tests were considered statistically significant at p ≤ 0.05.
Results and discussion
C. unicolor represents wood degrading fungi from the phylum Basidiomycota, which are exceptionally useful biotechnological tools in various industrial processes. In recent years, C. unicolor has been extensively studied as a very efficient source of extracellular laccase, which is an enzyme used on a wide scale in various industries. Recently, our results have indicated that laccase (LAC), crude endopolysaccharides (c-EPL), and the extracellular low molecular weight subfraction (ex-LMS) exhibit pro- and antioxidant properties as well as immunomodulatory, antibacterial, antiviral, and anticancer activities [30–33]. In this study, we tested the antioxidant, antibacterial, and cytotoxic activities of three subfractions of low molecular weight metabolites: ex-LMSI, ex-LMSII, and ex-LMSIII derived from the total C. unicolor ex-LMS preparation.
Characterization of the biochemical properties of ex-LMSI, ex-LMSII, and ex-LMSIII
C. unicolor is a source of natural active low molecular weight metabolites. Our analysis focused on three fractions of extracellular low molecular weight secondary metabolites from C. unicolor cultures (ex-LMSI, ex-LMSII, and ex-LMSIII). Our earlier study of the chemical composition of ex-LMS from C. unicolor showed the presence of sugars (total carbohydrates: 780.07 μg/mL, reducing sugars: 507.14 μg/mL, total polysaccharides: 272.93 μg/mL), proteins (189 μg/mL), and phenolic compounds (15 μM) [33]. The analysis of the chemical composition (concentrations of proteins, total carbohydrates, and total phenolic compounds) of idiophasic ex-LMSI, ex-LMSII, and ex-LMSIII isolated from C. unicolor revealed distinct differences between the investigated preparations (Table 1). The ex-LMSIII fraction contained an evidently higher amount of proteins (43.7 μg/mL) than ex-LMSI (2.25 μg/mL) and ex-LMSII (4.87 μg/mL). The concentration of phenolic compounds was significantly higher in ex-LMSI (20.56 μM) than in ex-LMSII (4.45 μM) and ex-LMSIII (5.8 μM). The concentration of total carbohydrates was the highest in ex-LMSI, i.e. 36.82 μg/mL. Other authors showed the presence of the analyzed groups of compounds in full mushroom extracts obtained using organic solvents [48]. The presence of phenolic compounds, such as quercetin, chrysin, and pinocembrin, in water extracts from Ramaria flava has been confirmed in other investigations [4].
Table 1. Chemical composition of ex-LMSI, ex-LMSII, and ex-LMSIII isolated from C. unicolor.
Yield of total carbohydrates, concentration of phenolic compounds, and protein content. The samples of ex-LMS were dissolved in distilled water (1 mg/mL) and used for the tests.
| Samples | Protein (μg/mL*) |
Total carbohydrate (μg/mL*) |
Total phenolic compounds (μM) |
|---|---|---|---|
| ex-LMS I | 12.25 ± 4.3a | 36.82 ± 4.2a | 20.56 ± 1.6a |
| ex-LMS II | 4.87 ± 0.6b | 29.35 ± 1.4b | 4.45 ± 0.4b |
| ex-LMS III | 43.7 ± 2.6c | 5.48 ± 0.7c | 5.8 ± 0.4b |
All results are expressed as mean ± SD from three experiments (n = 3). Values with different letters within the columns are significantly different (p ≤ 0.05).
* μg of substances for 1g of dry mass of lyophilized preparation.
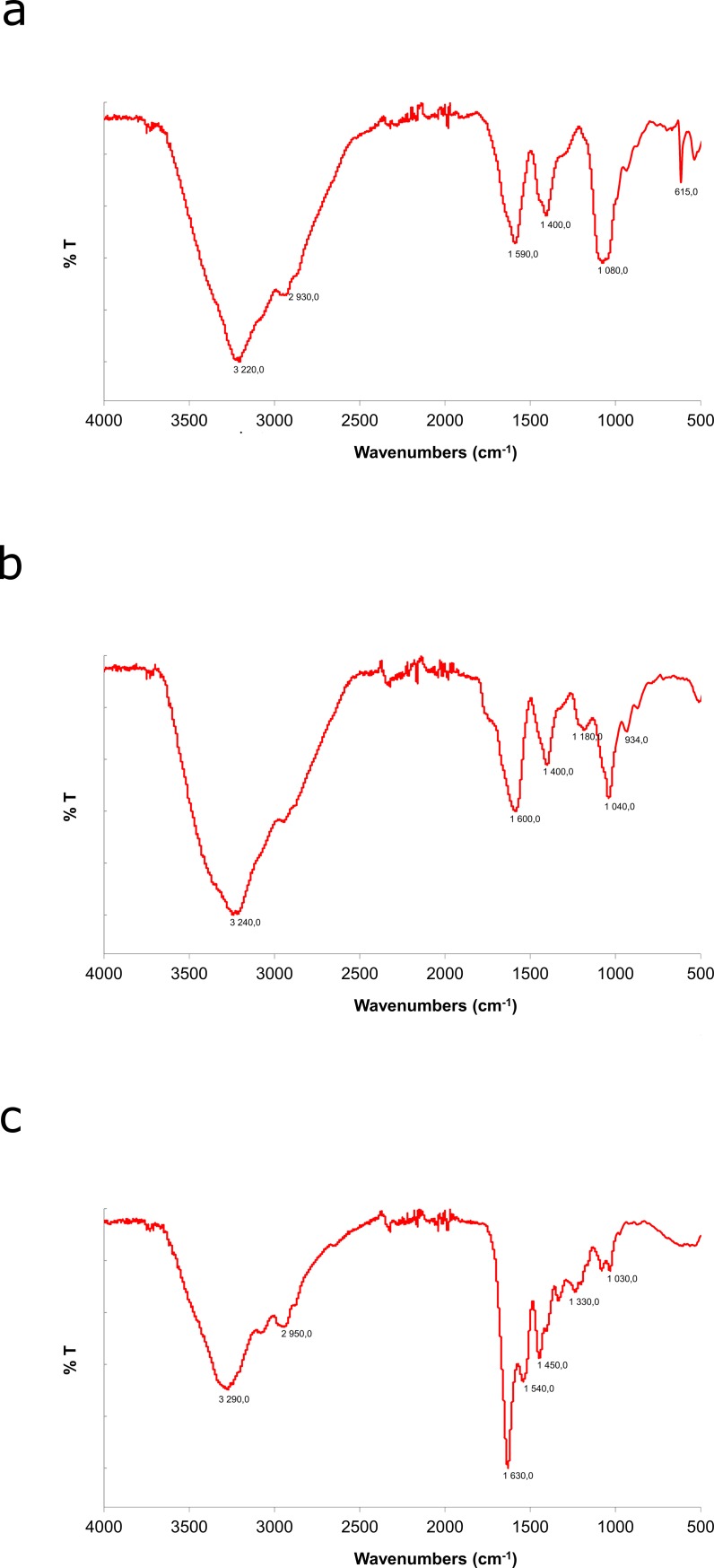
The FT-IR spectrum analysis of the ex-LMS subfractions of the C. unicolor idiophasic cultures demonstrated an aminoglycoside substance pattern (Fig 1). The characteristic strong broad band ca. 3200 cm−1 indicates the presence of OH stretching in hydrogen bonds [14]. The absorption bands between 1600 and 1400 cm−1 are attributed to the stretching vibration of the C–O bond of the carboxyl group, characteristic for proteins [49]. The band ca. 1000 cm−1 indicates the presence of O substituted glucose residues and β-linkages in the glucosidic chain [49]. The absorption band at 500–600 cm−1 suggests that the ex-LMS fraction contains pyranose rings in its structure. Analysis of the FT-IR spectrum of the ex-LMSIII fraction (c) showed higher absorption for proteins at ca. 1600 cm-1 and lower at ca. 3200 cm-1 compared to the ex-LMSI (a) and ex-LMSII (b) fractions. Similarly, the absorption at 1000 cm-1 is lower in the case of the ex-LMSIII fraction than for ex-LMSI and ex-LMSII.
Fig 1.
FT-IR analysis (FT-IR spectra of ex-LMSI (a), ex-LMSII (b), and ex-LMSIII (c) isolated from C. unicolor). FTIR spectroscopy was performed with a spectrometer (Thermo Scientific Nicolet 8700A with FT Raman Nicolet NXR module) in the wavelength range 4000–400 cm−1.
Antioxidant properties of ex-LMSI, ex-LMSII, and ex-LMSIII
Since our previous analyses showed that the scavenging ability of ex-LMS was at the same level or even higher in comparison to the model antioxidant substances, i.e. Trolox and ascorbic acid [33], we also checked the antioxidant capacity of the subfractions prepared in this study. In this research, we determined the scavenging properties of ex-LMSI, ex-LMSII, and ex-LMSIII using three methods: DPPH, ABTS, and OH radical scavenging assays. The analysis revealed strong reducing activity of these subfractions. The scavenging activity of the fractions tested was compared to those of ascorbic acid and Trolox. The IC50 values were 63.69 μg/mL for Trolox and 41.25 μg/mL for ascorbic acid in the DPPH method, 42.11 μg/mL and 28.23 μg/mL in the the ABTS method, and 54.61 μg/mL and 39.25 μg/mL in the the OH method, respectively. The DPPH radical-scavenging capacity of the analyzed samples is shown in Table 2. The activity can be evaluated by determination of the IC50 values, which correspond to the concentration of fungal samples that are able to scavenge 50% of free radicals present in the reaction mixture. High-IC50 values indicate low antioxidant activity. Ex-LMSIII shows the highest DPPH radical-scavenging activity, followed by ex-LMSI and ex-LMSII. The IC50 values of the samples were 20.39, 64.14, and 49.22 μg/mL, respectively. The measurements of the ABTS test showed slightly lower scavenging capacity of the ex-LMS fractions (in particular ex-LMSIII) in relation to the DPPH method (Table 2). The IC50 values in the ABTS scavenging method were 81.12 μg/mL (ex-LMSI), 39.78 μg/mL (ex-LMSII), and 31.49 μg/mL (ex-LMSIII). The hydroxyl radical and its subsequent radicals are the most harmful reactive oxygen species, as they are mainly responsible for the oxidative injury in many biomolecules [4,16]. In the method, hydroxyl radicals are produced via the Fenton reaction in the system. The scavenging capacity of hydroxyl radicals in the samples is compared to a positive control consisting of ascorbic acid and Trolox. The scavenging effects of the samples are shown in Table 2. The IC50 values of the samples ranged from 49.13 to 69.12 μg/mL. Based on the comparison of the IC50 values, the order of the hydroxyl radical-scavenging activity was found to be as follows: ex-LMSIII fraction > ex-LMSII fraction > ex-LMSII fraction (Table 2).
Table 2. IC50 values in the DPPH, ABTS, and OH radical-scavenging activity assay of ex-LMS I, ex-LMS II, and ex-LMS III isolated from C. unicolor submerged cultures.
| IC50 (μg/mL) | |||
|---|---|---|---|
| DPPH radical scavenging | ABTS radical scavenging | OH radical scavenging | |
| ex-LMS I | 64.14 ± 2.27a | 81.12 ± 3.29a | 69.12 ± 1.82a |
| ex-LMS II | 49.22 ± 1.83b | 39.78 ± 2.09b | 57.94 ± 1.27b |
| ex-LMS III | 20.39 ± 4.17c | 31.49 ± 4.91b | 49.13 ± 1.34c |
All results are expressed as mean ± SD from three experiments (n = 3). Values with different letters within the columns are significantly different (p ≤ 0.05).
The strong antioxidant activity of fungal extracts is most often correlated with high content of total phenols. The present results demonstrate stronger activity of ex-LMSIII than that of the standard antioxidants (ascorbic acid and Trolox). Our results indicate that the analyzed ex-LMS fractions with lower phenol content exert a stronger radical scavenging effect, suggesting that phenols are not the main factor in their antioxidant activity. In our previous study, we demonstrated the antioxidant activity of the ex-LMS starting fraction of C. unicolor. The scavenging abilities of C. unicolor ex-LMS at the concentration range of 6.25–800 μg/mL were between 20 and 90% for ABTS and between 10 and 59% for DPPH. The IC50 values in the case of the ABTS- and DPPH-scavenging tests were 25.0 μg/mL and 85.3 μg/mL, respectively [33]. To our knowledge, there is no adequate data on C. unicolor comparable to the data obtained in our work. Literature data confirm the antioxidant activity of non-phenolic substances of fungal origin. Antioxidant metabolites, i.e. 2,4,6-trimethylacetophenone imine, glutamyl tryptophan, azatadine, and lithocholic acid glycine conjugate isolated from Boletus spp. exhibited antioxidant activity [50].
Antibacterial activity of ex-LMSI, ex-LMSII, and ex-LMSIII
A wide range of components derived from mushrooms have been reported to possess antibacterial properties. Our previous studies have shown that total ex-LMS was much more effective towards E. coli and S. aureus bacterial cells than the extracellular fraction of laccase or the polysaccharide fraction from C. unicolor [33]. The activity of ex-LMSI, ex-LMSII, and ex-LMSIII was tested against bacteria, and penicillin sodium was used as a standard drug for comparison. The test microorganisms used in the present studies included E. coli (Gram-negative bacteria) as well as S. aureus and B. subtilis (Gram-positive bacteria). In our experiments, the low-molecular subfractions ex-LMSI, ex-LMSII, and ex-LMSIII showed relatively strong antimicrobial activity against all the Gram-positive and Gram-negative bacterial species used (Tables 3 and 4). The inhibition zone values obtained during the experimental cycle indicate that S. aureus was the most susceptible bacterium. In turn, B. subtilis and E. coli exhibited the lowest sensitivity. The ex-LMSII fraction was the most effective against all the microorganisms tested. These results are in agreement with the reports demonstrating that, in general, Gram-positive bacteria are considered more sensitive to different natural and synthetic compounds than Gram-negative bacteria due to the differences in the structure of their cell walls [4,51]. The cell wall of Gram-positive bacteria is composed of peptidoglucans and teichoic acids, while the cell wall of Gram-negative bacteria is composed of peptidoglucans, lipopolysaccharides, and lipoproteins [52–53]. This observation is in agreement with other studies [20,54] that have demonstrated greater sensitivity of Gram-positive bacteria than that of Gram-negative bacteria. The results described above are similar to those presented in this report.
Table 3. Antibacterial activities (inhibitory zone assay) of ex-LMSI, ex-LMSII, and ex-LMSIII (1mg/mL) isolated from C. unicolor submerged cultures.
| Diameters of the inhibition zones (mm) | |||
|---|---|---|---|
| E. coli | S. aureus | B. subtilis | |
| ex-LMS I | 1.68 ± 0.16ab | 2.38 ± 0.14a | 1.69 ± 0.11a |
| ex-LMS II | 1.96 ± 0.12a | 3.21 ± 0.23b | 2.76 ± 0.19b |
| ex-LMS III | 1.55 ± 0.14b | 2.36 ± 0.30a | 2.76 ± 0.19b |
| physiological saline | -a | -a | -a |
| penicillin sodiumb | 2.86 ± 0.17c | 3.08 ± 0.26b | 3.01 ± 0.16b |
All results are expressed as mean ± SD from three experiments (n = 3). Values with different letters within the columns are significantly different (p ≤ 0.05).
aNot detected.
bThe concentration of penicillin sodium was 0.5 mg/well for the Gram-negative microorganisms and 0.25 mg/well for the Gram-positive bacteria.
Table 4. Antibacterial activities (minimum inhibitory concentration) of ex-LMSI, ex-LMSII, and ex-LMSIII (1mg/mL) isolated from C. unicolor submerged cultures.
| Minimum inhibitory concentration (mg/mL) | |||
|---|---|---|---|
| E. coli | S. aureus | B. subtilis | |
| ex-LMS I | 50.00a | 12.50a | 25.00a |
| ex-LMS II | 100.00b | 6.25b | 12.50b |
| ex-LMS III | 100.00b | 50.00c | 25.00a |
All results are expressed as mean ± SD from three experiments (n = 3). Values within the column are significantly different (p ≤ 0.05).
The MIC values for the ex-LMS fractions against B. subtilis, S. aureus, and E. coli showed that ex-LMSI and ex-LMSII exerted a higher inhibition effect on S. aureus than on B. subtilis and E. coli (Tab. 4). The ex-LMSII fraction was the most effective as previously. The MIC values for this fraction against B. subtilis, S. aureus, and E. coli were 12.5, 6.25, and 100 mg/mL, respectively.
These results suggest that the extracellular low molecular weight secondary metabolites derived from the C. unicolor culture fluid, especially ex-LMSII, may be an interesting source of antibacterial substances.
Anticancer activity of ex-LMSI, ex-LMSII, and ex-LMSIII
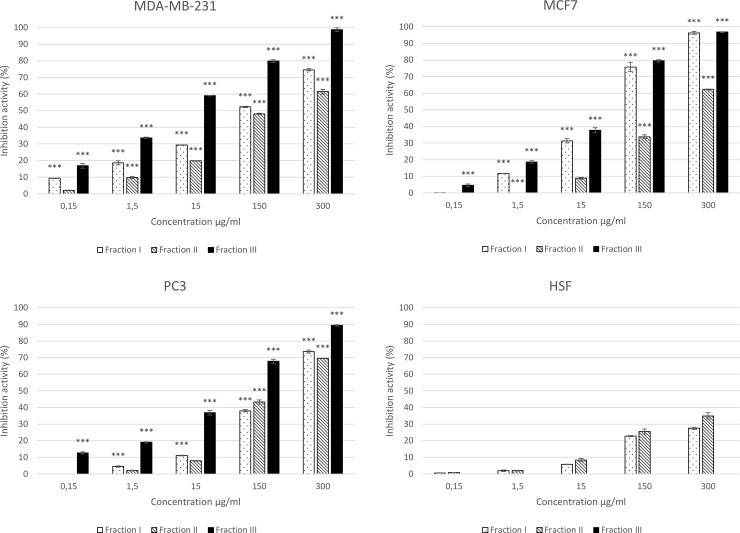
Approximately 651 species of higher basidiomycetes with anticancer activity have been described in the literature [55]. For example, an ethanol extract of Antrodia cinnamomea mycelia was found to possess high antihepatoma activity. Isolation of antiproliferative metabolites from fungal cultures and investigation of their mechanism of action may lead to the development of a new class of anticancer drugs [56]. In our previous studies, we have shown that the C. unicolor ex-LMS total fraction has antiproliferative properties toward murine melanoma B16-F10 cells [31]. To study the growth inhibitory activity of the ex-LMS fractions from C. unicolor in vitro, human cancer cell lines (MCF7, MDA-MB-231, and PC3) were incubated with various concentrations of ex-LMSI, ex-LMSII, and ex-LMSIII (Fig 2).
Fig 2. Cytotoxic effect (inhibition activity of ex-LMSI, ex-LMSII, and ex-LMSIII isolated from C. unicolor towards three cancer cell lines MCF7, MDA-MB-23, and PC3 as well as the HSF line).
The MTT assay was performed after 48 h of incubation. Values are mean ± SD (n = 3).
The potentially toxic effect of the investigated subfractions was assessed using the MTT assay (Table 5). The inhibition of the viability of all these three human cancer cells by ex-LMSI, ex-LMSII, and ex-LMSIII affected the normal human fibroblast cells in a very negligible way. The highest percentage of inhibition was obtained at a concentration of 300 μg/mL over all the examined tumor cell lines, and ex-LMSIII proved to be the most effective.
Table 5. Cytotoxic IC50 of ex-LMSI, ex-LMSII, and ex-LMSIII isolated from C. unicolor against cancer cells.
| IC50 (μg/mL) | ||||
|---|---|---|---|---|
| ex-LMSI | ex-LMSII | ex-LMSIII | Paclitaxel (positive control) |
|
| PC3 | 97.17 ± 3.23a | 103.32 ± 6.71a | 60.66 ± 4.67b | 11.73 ± 7.14c |
| MDA-MB-231 | 74.91 ± 4.51a | 89.85 ± 3.89b | 52.25 ± 6.48c | 14.38 ± 2.30d |
| MCF7 | 66.22 ± 3.85a | 109.45 ± 6.49b | 54.92 ± 2.27c | nd* |
| HSF | >100 | >100 | >100 | nd* |
All results are expressed as mean ± SD from three experiments (n = 3). Values with different letters within the rows are significantly different (p ≤ 0.05).
* Not detected.
Literature data indicating the antitumor activity of fungi are primarily focused on the use of mycelium extracts. For example, Liu et al. demonstrated the cytotoxic activity of a Ramaria flava ethanolic extract against human cancer cell lines (BGC-803, NCI-H520, and MDAMB-231) [4]. They demonstrated that the ethanol extract had the strongest growth inhibitory activity against human breast cancer cells of the MDA-MB-231 line (IC50 = 66.54 ± 4.27 μg/ml), and the inhibition rate was 71.66% at a concentration of 200 μg/ml. The in vitro anticancer activity of ethanol extracts was also confirmed by Wu et al. investigating the effects of Fomitopsis pinicola, Ganoderma sinense, Fomitopsis officinalis, and Polyporus melanopus extracts on cancer cell lines HepG2 and S-180 [57]. In our study, a significant percentage (59.08%) of ex-LMSIII inhibition of the MDA-MB-231 tumor line was observed at a concentration of 15 μg/ml, which did not affect normal human fibroblast cells. As shown by the literature data, most studies related to the antitumor properties of fungi focus on alcoholic or aqueous extracts or substances isolated from the mycelium. In our study, we used a low molecular weight fraction of the post-culture fluid obtained in large quantities during liquid mushroom culture. The fraction has not been used in investigations of fungal antitumor activity to date. The omission of organic extraction in the separation procedure is also very important especially from the biomedical point of view. All these results suggest that the ex-LMSI, ex-LMSII, and ex-LMSIII subfractions can be a rich source of highly bioactive substances, especially with cytotoxic potential.
Conclusions
It was found in the present study that the three bioactive subfractions of low molecular weight secondary metabolites: ex-LMSI, ex-LMSII, and ex-LMSIII isolated from C. unicolor culture fluid possessed anticancer, antioxidant, and antibacterial properties. Our results show that post-culture medium containing secondary metabolites of less than 10 kDa can be a rich source of natural anticancer, antioxidant, and antibiotic substances and can be important in the control of various human and animal diseases. These results suggest that C. unicolor can be an effective source of pharmaceuticals.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by statutory research BS/UMCS from the Ministry of Science and Higher Education in Poland, numbers BS-P-11-010-16-1-09 and BS-P-11-010-17-1-11.
References
- 1.Chen YJ, Jiang S, Jin YX, Yin YL, Yu GJ, Lan XQ, et al. Purification and characterization of an antitumor protein with deoxyribonuclease activity from edible mushroom Agrocybe aegerita. Mol Nutr Food Res. 2012;56: 1729–1738. doi: 10.1002/mnfr.201200316 [DOI] [PubMed] [Google Scholar]
- 2.Elsayed EA, Enshasy HE, Wadaan MAM, Ramlan A. Mushrooms: a potential natural source of anti-inflammatory compounds for medical applications. Mediat Inflamm. 2014;1: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu T, Beelman RB. The bioactive compounds in medicinal mushrooms have potential protective effects against neurodegenerative diseases. Adv Food Technol Nutr Sci Open J. 2015;1(2): 62–65. [Google Scholar]
- 4.Liu K, Wang J, Zhao L, Wang Q. Anticancer, antioxidant and antibiotic activities of mushroom Ramaria flava. Food Chem Toxicol. 2013;58: 375–380. doi: 10.1016/j.fct.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Worthington J, Rashid S. The therapeutic potential of mushroom extracts In Natural Products as Future Medicinal Agents. Old City Publishing Inc., USA, 2009. [Google Scholar]
- 6.Mahajna J, Dotan N, Zaidman BZ, Petrova RD, Wasser SP. Pharmacological values of medicinal mushrooms for prostate cancer therapy: the case of Ganoderma lucidum. Nutr Cancer. 2009;61: 16–26. doi: 10.1080/01635580802379323 [DOI] [PubMed] [Google Scholar]
- 7.Lindequist U, Niedermeyer THJ, Jülich WD. The pharmacological potential of mushrooms. Evid. Complement. Alternat. Med., 2005;2: 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren L, Perera C, Hemar Y. Antitumor activity of mushroom polysaccharides: a review. Food Funct, 2012;3: 1118–1130. doi: 10.1039/c2fo10279j [DOI] [PubMed] [Google Scholar]
- 9.Rajewska J, Bałasińska B. Związki biologicznie aktywne zawarte w grzybach jadalnych i ich korzystny wpływ na zdrowie. Postepy Hig. Med. Dosw. 2004;58: 352–357. [PubMed] [Google Scholar]
- 10.Baldrian P, Valaskova V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev. 2008;32: 501–521. doi: 10.1111/j.1574-6976.2008.00106.x [DOI] [PubMed] [Google Scholar]
- 11.Hatakka A, Hammel KE. Fungal biodegradation of lignocellulose: Mycota X Industrial Applications (2nd edn) (Hofrichter M, ed.), Springer-Verlag; 2010. [Google Scholar]
- 12.Xu X, Yan H, Chen J, Zhang H. Bioactive proteins from mushrooms. Biotechnol Adv. 2011;29: 667–674. doi: 10.1016/j.biotechadv.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 13.Hu DD, Zhang RY, Zhang GQ, Wang HX, Ng TB. A laccase with antiproliferative activity against tumor cells from an edible mushroom, white common Agrocybe cylindracea. Phytomedicine. 2011;18: 374–379. doi: 10.1016/j.phymed.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 14.Kozarski M, Klaus A, Niksic M, Jakovljevic D, Helsper JPFG, Van Griensven LJLD. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011; 129: 1667–1675. [Google Scholar]
- 15.Pan Y, Dong S, Hao Y, Zhou Y, Ren X, Wang J, et al. Ultrasonicassisted extraction process of crude polysaccharides from Yunzhi mushroom and its effect on hydroxyproline and glycosaminoglycan levels. Carbohydr Polym. 2010;81: 93–96. [Google Scholar]
- 16.Zhang BZ, Yan PS, Chen H, He J. Optimization of production conditions for mushroom polysaccharides with high yield and antitumor activity. Carbohydr Polym. 2012;87: 2569–2575. [Google Scholar]
- 17.Rashid S, Unyayar A, Mazmanci MA, McKeown SR, Banat IM, Worthington J. A study of anti-cancer effects of Funalia trogii in vitro and in vivo. Food and Chemical Toxicology. 2011;49: 1477–1483. doi: 10.1016/j.fct.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 18.Unyayar A, Demirbilek M, Turkogl M, Celik A, Mazmanci M, Erkurt A, et al. Evaluation of Cytotoxic and Mutagenic effects of Coriolus versicolor and Funalia trogii extracts on mammalian cells. Drug Chem Toxicol. 2006;1: 1–15. [DOI] [PubMed] [Google Scholar]
- 19.Liu QH, Wang HX, Ng TB. First report of a xylose-specific lectin with potent hemagglutinating, antiproliferative and anti-mitogenic activities from a wild ascomycete mushroom. Biochim Biophys Acta. 2006;1760(12): 1914–1919. doi: 10.1016/j.bbagen.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 20.Zhang GQ, Sun J, Wang HX. A novel lectin with antiproliferative activity from the medicinal mushroom Pholiota adiposa. Acta Biochim Pol. 2009;56(3): 415–421. [PubMed] [Google Scholar]
- 21.Lin CH, Sheu GT, Lin YW, Yeh CS, Huang YH, Lai YC, et al. A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochem. 2010;45(9): 1537–1542. [Google Scholar]
- 22.Cote J, Caillet S, Doyon G. Bioactive compounds in cranberries and their biological properties. Crit Rev Food Sci Nutr. 2010;50(7): 666–679. doi: 10.1080/10408390903044107 [DOI] [PubMed] [Google Scholar]
- 23.D’Archivio M, Filesi C, Vari R, Scazzocchio B, Masella R. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 2010;11: 1321–1342. doi: 10.3390/ijms11041321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dziezak JD. Antioxidants-The ultimate answer to oxidation. Food Techn. 1986;40(9): 94. [Google Scholar]
- 25.Sanchez C. Reactive oxygen species and antioxidant properties from mushrooms. Synt and Sys Biotechnol. 2017;2: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JL, Liu K, Gong WZ, Wang Q, Xu DT, Liu MF, et al. Anticancer, antioxidant, and antimicrobial activities of anemone (Anemone cathayensis). Food Sci Biotechnol. 2012;21: 551–557. [Google Scholar]
- 27.Jung J-Y, Lee L-K, Seok S-J, Lee H-J, Kim Y-H, Yun B-S. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J of Appl Microbiol. 2008;104,6: 1824–1832. [DOI] [PubMed] [Google Scholar]
- 28.Rogalski J, Dawidowicz A, Jóźwik E, Leonowicz A. Immobilization of laccase from Cerrena unicolor on controlled porosity glass. J Mol Cat B. 1999;6: 29–39. [Google Scholar]
- 29.Janusz G, Rogalski J, Szczodrak J. Increased production of laccase by Cerrena unicolor in submerged liquid cultures. World J Microbiol Biotechnol. 2007;23(10): 1459–1464. [Google Scholar]
- 30.Mizerska-Dudka M, Jaszek M, Błachowicz A, Rejczak TP, Matuszewska A, Osińska-Jaroszuk M, et al. Fungus Cerrena unicolor as an effective source of new antiviral, immunomodulatory, and anticancer compounds. Int J Biol Macromol. 2015;79: 459–68. doi: 10.1016/j.ijbiomac.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 31.Statkiewicz M, Matuszewska A, Jaszek M, Janusz G, Osinska M, Sulej J, et al. Antimelanomic effects of high–and low–molecular weight bioactive subfractions isolated from the mossy maze mushroom, Cerrena unicolor (Agaricomycetes). Int J of Med Mushrooms. 2017;19(7): 619–628. [DOI] [PubMed] [Google Scholar]
- 32.Matuszewska A, Karp M, Jaszek M, Janusz G, Osińska-Jaroszuk M, Sulej J et al. Laccase purified from Cerrena unicolor exerts antitumor activity against leukemic cells. Oncol Let. 2016;11(3): 2009–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaszek M, Osińska-Jaroszuk M, Janusz G, Matuszewska A, Stefaniuk D, Sulej J, et al. New bioactive fungal molecules with high antioxidant and antimicrobial capacity isolated from Cerrena unicolor idiophasic cultures. BioMed Res Int. 2013;2013: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janusz G, Mazur A, Checinska A, Malek W, Rogalski J, Ohga S. Cloning and characterization of a laccase gene from biotechnologically important basidiomycete Cerrerna unicolor. Journal of the Faculty of Agriculture, Kyushu University. 2012;57(1): 41–49. [Google Scholar]
- 35.Jennings D. and Lysek G. Fungal Biology: Understanding the Fungal Lifestyle, BIOS Scientific Publishers Ltd., Oxford, UK, 1999. [Google Scholar]
- 36.Bradford MM. A rapid and sensitive method for the quantitation ofmicrogramquantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72: 248–254. [DOI] [PubMed] [Google Scholar]
- 37.Malarczyk E. Transformation of phenolic acids by Nocardia. Acta Microbiol Polon. 1998; 38: 45–53. [Google Scholar]
- 38.DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem.1956;28,3: 350–356. [Google Scholar]
- 39.Paduch R, Matysik G, Wojciak-Kosior M, Kandefer-Szerszen M. Skalska-Kamińska A. Nowak-Kryska M, et al. Lamium album extracts express free radical scavenging and cytotoxic activities. Polish Journal of Environmental Studies. 2008;17,4: 569–580. [Google Scholar]
- 40.Van den Berg R, Haenen GRMM, van den Berg H, Bast A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999; 66,4: 511–517. [Google Scholar]
- 41.Duo-Chuan L. Review of fungal chitinases. Mycopathologia. 2006;161,6: 345–360. doi: 10.1007/s11046-006-0024-y [DOI] [PubMed] [Google Scholar]
- 42.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol and Med. 1999;26,9–10: 1231–1237. [DOI] [PubMed] [Google Scholar]
- 43.Grymonpré DR, Sharma AK, Finney WC, Locke BR. The role of Fenton’s reaction in aqueous phase pulsed streamer corona reactors. Chem Eng J. 2001;82: 189–207. [Google Scholar]
- 44.Wu XJ, Hansen C. Antioxidant capacity, phenolic content, and polysaccharide content of Lentinus edodes grown in whey permeate-based submerged culture J Food Sci. 2008;73: 1–8. [DOI] [PubMed] [Google Scholar]
- 45.Liu K, Wang JL, Gong WZ, Xiao X, Wang Q. Antioxidant activities in vitro of ethanol extract and fractions from mushroom Lenzites Betulina. J Food Biochem. 2012; doi: 10.1111/j.1745-4514.2012.00666.x [Google Scholar]
- 46.Siddiqi R, Naz S, Ahmad S, Sayeed SA. Antimicrobial activity of the polyphenolic fractions derived from Grewia asiatica, Eugenia jambolana and Carissa carandas. Int J Food Sci Technol. 2011;46: 250–256. [Google Scholar]
- 47.Khan N, Hadi N, Afaq F, Syed DN, Kweon MH, Mukhtar H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28: 163–173. doi: 10.1093/carcin/bgl145 [DOI] [PubMed] [Google Scholar]
- 48.Smolskait L, Venskutonis PR, Talou T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT—Food Scien Technol. 2015;60: 462–471. [Google Scholar]
- 49.Manrique GD, and Lajolo FM. FT-IR spectroscopy as a tool formeasuring degree of methyl esterification in pectins isolated fromripening papaya fruit. Postharvest Biol and Technol. 2002;25,1: 99–107. [Google Scholar]
- 50.Yuswan MHMY, Al-Obaidi JR, Rahayu A. New bioactive molecules with potential antioxidant activity from various extracts of wild edible Gelam mushroom (Boletus spp.). Adv Biosci Biotechnol. 2015;6: 320–329. [Google Scholar]
- 51.Siddiqi R, Naz S, Ahmad S, Sayeed SA. Antimicrobial activity of the polyphenolic fractions derived from Grewia asiatica, Eugenia jambolana and Carissa carandas. Int J Food Sci Technol. 2011;46: 250–256. [Google Scholar]
- 52.Heijenoort J. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology 2001;11: 25–36. [DOI] [PubMed] [Google Scholar]
- 53.Kosanić M, Ranković B, Stanojković T. Antioxidant, antimicrobial and anticancer activity of 3 Umbilicaria species. J Food Sci. 2012;77: T20–T25. doi: 10.1111/j.1750-3841.2011.02459.x [DOI] [PubMed] [Google Scholar]
- 54.Gezer K, Duru M, Kivrak I, Turkoglu A, Mercan N, Turkoglu H, et al. Free-radical scavenging capacity and antimicrobial activity of wild edible mushroom from Turkey. Afr J Biotechnol. 2006;5: 1924–1928. [Google Scholar]
- 55.Fan L, Pan H, Soccol AT, Pandey A, Soccol CR. Advances in mushroom research in the last decade. Food Technol Biotechnol. 2006;44: 303–311. [Google Scholar]
- 56.Chen YY1, Chou PY, Chien YC, Wu CH, Wu TS, Sheu MJ. Ethanol extracts of fruiting bodies of Antrodia cinnamomea exhibit anti-migration action in human adenocarcinoma CL1-0 cells through the MAPK and PI3K/AKT signaling pathways. Phytomedicine. 2012. June 15;19(8–9):768–78. doi: 10.1016/j.phymed.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 57.Wu HT, Lu FH, Su YC, Ou HY, Hung HC, Wu JS, et al. In vivo and in vitro anti-tumor effects of fungal extracts. Molecules 2014;19(2): 2546–2556. doi: 10.3390/molecules19022546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.