Abstract
Physical activity and stress are both environmental modifiers of Alzheimer's disease (AD) risk. Animal studies of physical activity in AD models have largely reported positive results, however benefits are not always observed in either cognitive or pathological outcomes and inconsistencies among findings remain. Studies using forced exercise may increase stress and mitigate some of the benefit of physical activity in AD models, while voluntary exercise regimens may not achieve optimal intensity to provide robust benefit. We evaluated the findings of studies of voluntary and forced exercise regimens in AD mouse models to determine the influence of stress, or the intensity of exercise needed to outweigh the negative effects of stress on AD measures. In addition, we show that chronic physical activity in a mouse model of AD can prevent the effects of acute restraint stress on Aβ levels in the hippocampus. Stress and physical activity have many overlapping and divergent effects on the body and some of the possible mechanisms through which physical activity may protect against stress-induced risk factors for AD are discussed. While the physiological effects of acute stress and acute exercise overlap, chronic effects of physical activity appear to directly oppose the effects of chronic stress on risk factors for AD. Further study is needed to identify optimal parameters for intensity, duration and frequency of physical activity to counterbalance effects of stress on the development and progression of AD.
Keywords: Alzheimer's disease, Amyloid, Stress, Exercise, Physical activity
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder for which there is currently no effective treatment. The increasing social, physical, and economic burden of AD for both patients and caregivers is becoming a major public health concern in many areas of the world. The complexity of the disease, involving multiple pathological changes, interactions with risk-associated genes, and effects of behavioral or environmental modifiers have made finding a successful treatment difficult. Multifactorial treatment strategies will likely be necessary to provide a significant benefit to both patients and caregivers.
It is generally accepted that physical activity decreases the risk of developing AD and many other disorders that occur with age (Adlard and Cotman, 2004; Kennedy et al., 2017; Paillard et al., 2015). Higher rates of reported exercise are correlated with improved AD biomarkers in cognitively normal older adults (Liang et al., 2010). However, some forms of physical activity can induce stress and the effects of intensity, duration, frequency, and mode of activity needed to maximize health benefits are not fully understood. Psychological stress is associated with increased risk of many disorders and has been repeatedly shown to exacerbate symptoms and accelerate disease onset in AD (Baglietto-Vargas et al., 2015; Csernansky et al., 2006; Dong et al., 2004; Selye, 1955). Conversely, mild stress can be beneficial, particularly for cognitive function (Bos et al., 2014). The circumstances under which stress or physical activity occur may determine its effects on AD pathology.
Acute stress activates the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS), which increases the release of glucocorticoids (GCs) and catecholamines (Smith and Vale, 2006). These molecules then initiate a neuroendocrine response, mobilizing lipids, glucose, and other resources to facilitate cognitive and physical demands of a “fight or flight” challenge. In conditions of acute psychological stress, these neuroendocrine responses are not tied to an increased metabolic demand. In chronic stress, this prolonged activation of the stress system has been linked to a large number of comorbidities ranging from metabolic dysfunction and cardiovascular disorders, to cognitive dysfunction and psychological disorders, such as depression (McEwen, 2017). The effects of psychological stress are likely to be exaggerated in the presence of physical inactivity due to lack of use of the physiological and metabolic products produced in preparation for the body's anticipated physical response to a threat. For example, increasing pro-inflammatory cytokines to help repair tissue damage due to physical exertion is counterproductive in the absence of the tissue damage and may lead to aberrant responses in the system (Fleshner and Crane, 2017). Several of these physiological changes associated with chronic stress are also risk factors for AD (Mayeux and Stern, 2012).
Chronic physical activity may play a protective role in stress system dysregulation and increase resistance to stress-related disorders (Tsatsoulis and Fountoulakis, 2006). A recent review by Nation et al. (2011) carefully outlines the effects of stress and exercise specifically on the neurovascular system in AD, and highlights the importance of uncovering the mechanisms and interactions involved in the effects of exercise and stress on AD pathophysiology so that new behavioral and/or pharmacological strategies can be developed for effective treatment of the disease. Many studies have examined the effects of stress or exercise on AD neuropathology or cognitive function independently, but few have analyzed the interactions between stress and exercise on the pathophysiology and cognitive symptoms of the disease. The goal of this review is to evaluate the interaction between physical activity and stress on AD pathophysiology in the context of forced versus voluntary physical activity. Does physical activity promote stress resistance or resilience (or both) to AD pathology? Do parameters of physical activity such as intensity or duration matter such that there is a threshold to counteract the negative effects of psychological stress on AD pathology?
2. Physical activity terminology and characteristics
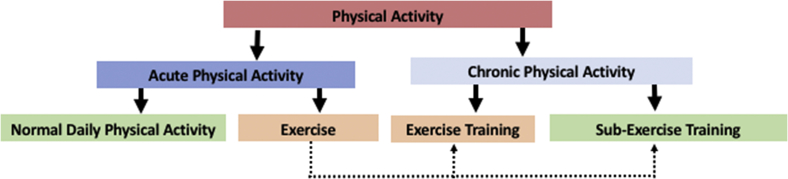
Physical activity refers to any movement of the body resulting from skeletal muscle contraction that elevates total body energy expenditure above that at rest (Caspersen et al., 1985). Physical activity encompasses a wide variety of endeavors, including walking around the block, gardening, mowing the lawn, or running several miles. Caspersen et al. (1985) suggest that physical activity can be categorized in multiple ways, for the purposes of this review we categorized physical activity as acute or chronic (Fig. 1). Acute physical activity refers to one bout or session, such as walking to the store or a long bicycle ride, and can be subdivided into normal daily physical activity and exercise. Chronic physical activity refers to a structured, repetitive regimen carried out over weeks, months, or years intended to improve psychological or physical health, and can be subdivided into exercise training and sub-exercise training. Exercise training refers to chronic physical activity with a goal of increasing physical fitness above that of a sedentary individual (e.g. improved cardiovascular or muscle endurance, muscle strength, or flexibility). Exercise training regimens must be of sufficient intensity (physically taxing), duration (length of time exercise is performed), and frequency (number of exercise bouts performed) to overload the body and increase function. For a chronic physical activity regimen to meet the criteria of exercise training an increase in a physiological function must be demonstrated (referred to as an exercise training effect). Examples of an exercise training effect would be an increase in maximum oxygen consumption, an increase in the activity of a skeletal muscle mitochondrial enzyme associated with the Krebs cycle or electron transport chain (e.g. citrate synthase), or an increase in muscle strength. Sub-exercise training chronic physical activity refers to a structured regimen where the intensity, duration, and frequency are not at a high enough level to change physical fitness above that of a sedentary individual, such as a casual daily walk.
Fig. 1.
Classification of Physical Activity. Acute physical activity refers to an individual bout of activity that can be part of the normal process of conducting daily business (normal daily physical activity) or it can be exercise which refers to a planned session of physical activity such as walking, jogging, bicycling, swimming, weight lifting or stretching intended to be part of a chronic physical activity program with the goal of improving health. Chronic physical activity is a regimen of exercise carried out over time with the goal of improving physical or psychological health. Exercise training is a chronic physical activity regimen of sufficient intensity, duration, and frequency to increases physical fitness. Sub-exercise training is a chronic physical activity regimen that is not of sufficient intensity, duration, or frequency to improve physical fitness, but may reduce psychological stress and improve psychological health.
3. Studies of the effects of physical activity in mouse models of AD: can we determine the effects of stress?
Environmental factors influence disease onset and progression in AD, and being able to uncover the experience-dependent mechanisms for modifying the course of the disease is important to provide treatment guidelines and predict the success of therapeutic interventions. Studies of the effects of physical activity in animal models of AD have primarily used two methods: voluntary running on a wheel (Table 1) or running at a fixed speed on a treadmill (Table 2). Forced exercise, typically in the form of treadmill running, has been shown to increase biomarkers of the stress response in rodents (Moraska et al., 2000; Svensson et al., 2016; Yanagita et al., 2007). Due to the findings by Dong et al. (2008, 2004) and others (Baglietto-Vargas et al., 2015; Carroll et al., 2011) that chronic stress exacerbates AD pathology in mouse models, it is important to determine the influence of stress in forced exercise studies. Treadmill running provides a measure of control over exercise intensity and duration not available in voluntary running conditions; however, the psychological stress component could possibly counteract some of the positive benefits of chronic physical activity. Presumably voluntary exercise results in less stress on the mouse than forced exercise and comparison of voluntary and forced exercise in exercise training and sub-exercise training regimens can provide insight into the opposing relationship that psychological stress and physical activity have on biomarkers of AD.
Table 1.
Summary of studies investigating chronic voluntary physical activity in transgenic mouse models of Alzheimer's disease.
| Model (Strain and Gender) | Physical Activity Type | Intensity (estimated) and Duration | Frequency | Major AD Outcomes | Reference |
|---|---|---|---|---|---|
| TgCRND8 Female |
Wheel running | 3.7–6.0 m/min 12 h/day |
7 days/week 5 months From 1 to 6 months of age |
↓ amyloid plaque ↑MWM |
Adlard et al., 2005 |
| APP23 Female |
Wheel running | Multiple animals per cage w/single running wheel Distance run not reported |
Beginning at 10 weeks of age Ending at 11 months of age |
MWM (ns) Amyloid plaque (ns) |
Wolf et al., 2006 |
| APPsw Male or Female not specified |
Wheel running | Multiple animals per cage w/single running wheel Distance run not reported |
Beginning at 6 weeks of age Ending at 9 months of age |
MWM (ns) RAWM (ns) Cortisol (ns) Cytokines (ns) Amyloid plaque (ns) Synaptophysin (ns) |
Cracchiolo et al., 2007 |
| Tg2576 Male or Female not specified |
Wheel running | 2.4 m/min 12 h/day |
7 day/week 3 weeks Age ranged from 16 to 18 months at beginning of treatment |
↓ soluble Aβ40 Aβ42 (ns) Aβ insoluble fraction (ns) ↓TNF-α and IL-1β |
Nichol et al., 2008 |
| Tg2576 Male and Female |
Wheel running | 12 h/day Distance run not reported |
3 weeks in 16–18 month old mice | ↑RAWM although performance similar to group with locked wheel |
Nichol et al., 2007 |
| Tg2576 Male or Female not specified |
Wheel running | 12 h/day Distance run not reported |
7 days/week 3 weeks Age ranged from 15 to 19 months at beginning of treatment |
Insoluble Aβ40 and Aβ42 (ns) ↑RAWM both reference and working memory |
Parachikova et al., 2008 |
| TgCRND8 Male |
Wheel running Animals housed individually |
2.0 m/min 12 h/day |
7 days/week Treatment from 80 to 150 days of age (10 weeks) |
NOR (ns) Barnes maze (ns) Spontaneous behavior (ns) Corticosterone (ns) Amyloid plaque (ns) |
Richter et al., 2008 |
| Tg2576 Male and Female |
Wheel running | 10.9 m/min 60 min/day |
5 day/week 16 weeks From 5 to 9 months of age |
↓amyloid plaque Soluble Aβ (ns) ↑NOR ↑hippocampal volume |
Yuede et al., 2009 |
| APP23 Female |
Wheel Running | 12 h/day | 7 days/week 2 groups: Animals had access to running for 10 days one group was 6 months of age the other was 18 months of age |
6 month group: No amyloid plaque present Adult hippocampal neurogenesis (ns) 18 month group: Amyloid plaque (ns) ↑ Adult hippocampal neurogenesis |
Mirochnic et al., 2009 |
| THY-Tau22 Male |
Wheel Running | 12 h/day Distance run not reported |
7 days/week 9 months from 3 to 12 months of age |
↑Y-Maze ↓pathological tau species Astrogliosis (ns) Inflammation (ns) Cholinergic neurons (ns) BDNF (ns) |
Belarbi et al., 2011 |
| 3xTg-AD Male and Female |
Wheel running | 12 h/day Distance run not reported |
7 days/week 2 groups: One month beginning at 3 months of age 6 months beginning at 1 month of age |
1 month group: ↑MWM Corner test (ns) Open field (ns) Dark-light box test (ns) Boissier's 4 hole board test (ns) Soluble Aβ (ns) Amyloid plaque (ns) 6 month group: ↑MWM Corner test (ns) Open field (ns) Dark-light box test (ns) Boissier's 4 hole board test (ns) Soluble Aβ (ns) Amyloid plaque (ns) ↓oxidative stress |
García-Mesa et al., 2011 |
| 3xTg-AD male |
Wheel running | 4.0 m/min 12 h/day |
7 days/week 6 months from 6 to 12 months of age |
↑MWM ↑sensorimotor function ↑dark-light box test ↑Boissier's hole board test ↓oxidative stress ↓amyloid plaque phosphorylated tau (ns) |
García-Mesa et al., 2012 |
| SAMP8 Female |
Wheel running | 0.55 m/min 12 h/day |
7 days/week 8 weeks from 6 months of age to 8 months of age |
↑growth factors in plasma and cortex Plasma triglycerides (ns) |
Cosín-Tomás et al., 2014 |
| 3xTg-AD Male or Female Not specified |
Wheel running | 12 h/day distance not reported |
7 days/week for 6 months from 1 to 7 months of age |
↑Neuroprotective factors ↑synaptic proteins APP (ns) Tau (ns) |
Revilla et al., 2014 |
| 3xTg-AD female |
Wheel running | 3.3 m/min 12 h/day |
7 days/week 3 months from 12 to 15 months of age |
↑MWM ↑locomotion ↑dark-light box test ↑Boissier's hole board test ↓oxidative stress ↓amyloid plaque ↓phosphorylated tau |
García-Mesa et al., 2016 |
| APPswe/PS1ΔE9 Male or Female not specified |
Wheel Running | 12 h/day Distance run not reported |
7 days/week 10 weeks beginning at 5 months of age |
↑MWM ↓Aβ oligomers ↓amyloid plaque ↓phosporylated tau ↓inflammation ↓neuronal cell loss ↑neurogenesis |
Tapia-Rojas et al., 2016 |
| TgCRND8 Male or Female not specified |
Wheel Running | 13.75 m/min 12 h/day |
7 days/week Two groups: 1 month or running from 3 to 4 months of age 2 months of running from 3 to 5 months of age |
1 month group: Open field (ns) ↑Y-Maze ↑ neurogenesis amyloid plaque area, size, and number (ns) 2 month group: ↑ Y-Maze ↓amyloid plaque area, size, and number ↑ neurogenesis |
Maliszewska-Cyna et al., 2016 |
Abbreviations: MWM = Morris Water Maze, NOR = Novel Object Recognition, RAWM = Radial Arm Water Maze, BDNF = Brain Derived Neurotrophic Factor, APP = Amyloid Precursor Protein.
Clarifications:
1. In our experience mice exposed to running wheels 24 h per day, 7 days per week run in the wheel almost constantly during the 12 h dark cycle and almost not at all during the light cycle. Therefore, in this summary we report exercise duration as 12 h/day.
2. We estimated daily exercise intensity (speed) in studies that reported average distance run by dividing distance (meters) by 720 min (minutes in 12 h). This likely underestimates intensity slightly as the mice do not run constantly for the full 12 h dark cycle.
3. Several studies reported in this summary employed multiple groups as part of larger enrichment studies. For these studies, we only report findings comparing the group that only had access to a running wheel to the group receiving no enrichment.
Table 2.
Summary of studies investigating chronic forced physical activity in transgenic mouse models of Alzheimer's disease.
| Model (Strain and Gender) | Physical Activity Type |
Intensity and Duration | Frequency | Major AD Outcomes | Reference |
|---|---|---|---|---|---|
| NSE/APPsw Male or Female not specified |
Treadmill running | 13.2 m/min, 0% grade 1 h/day |
5 days/week 16 weeks Beginning at 13 months of age |
↑MWM ↓ Aβ42 deposition ↑BDNF and GLUT-1 ↑HSP70 ↑SOD and catalase ↓pro-apoptotic proteins |
Um et al., 2008 |
| NSE/APPsw Male or Female not specified |
Treadmill running | 13.2 m/min, 0% grade 1 h/day |
5 days/week 16 weeks Beginning at 13 months of age |
↑MWM ↓ Aβ42 deposition ↑BDNF and GLUT-1 ↓pro-apoptotic proteins ↑HSP70 ↑SOD and catalase ↓Brain Total cholesterol, glucose, and insulin |
Cho et al., 2010 |
| NSE/hPS2m Male or Female not specified |
Treadmill running | 12 m/min, 0% grade 1 h/day 5 days/week |
5 days/week 3 months Beginning at 24 months of age |
↑MWM ↓ Aβ42 deposition ↓tau phosporylation ↑BDNF, NGF, CREB ↑HSP70 ↑MAPK signaling ↓neuronal apoptosis |
Um et al., 2011 |
| NSE/hPS2m Male or Female not specified |
Treadmill running | 12 m/min, 0% grade 1 h/day |
5 days/week 12 weeks Beginning at 24 months of age |
↑MWM ↓Aβ42 deposition ↓BACE-1 activity (β-secretase) ↓ER stress |
Kang et al., 2013 |
| 3xTg-AD Male and Female |
Treadmill running | 4.2 m/min, 0% grade 30 min/day |
5 days/week 5 weeks beginning at 6 months of age |
Corner test (ns) Dark-Light box test (ns) MWM (ns) T-Maze (ns) ∼ Aβ40; Aβ42 (ns in females, improved ratio in males) Tau (ns) |
Giménez-Llort et al., 2010 |
| APP/PS1 Male or Female not specified |
Treadmill running No electric shock motivation |
5-11 m/min, 0% grade 30 min/day |
5 days/week 5 months from 3 to 8 months of age |
↑MWM ↑synaptic plasticity, LTP ↓BDNF mRNA |
Liu et al., 2011 |
| APP/PS1 Female |
Treadmill running No electric shock motivation |
5-11 m/min, 0% grade 30 min/day |
5 days/week 5 months from 3 to 8 months of age |
↓amyloid plaque ↓soluble Aβ40 APP (ns) ↓sAPPβ, sAPPα (ns) ↓CTFα and CTFβ ↓hyperphosphorylated tau |
Liu et al., 2013 |
| APPswe/PS1dE9 Male |
Treadmill running Gentle tail touching motivation no electric shock |
Gradual distance increase 70–300 m/day with gradual speed increase from 5 to 8 to 10–15 m/min | 6 days/week 5 months beginning at 4 months of age |
↑MWM Amyloid plaque (ns) ↓microglial activation |
Xiong et al., 2015 |
| APP/PS1 | Treadmill running No electric shock motivation |
5-11 m/min, 0% grade 30 min/day |
5 days/week 5 months 2 groups; one beginning at 3 months of age the other beginning at 12 months of age |
Young group: ↑MWM ↓amyloid plaque ↓guanidine extracted soluble Aβ40 and Aβ42 ↑LTP Old group: ↑MWM Amyloid plaque in old mice (ns) ↓guanidine extracted soluble Aβ40 and Aβ42 ↑LTP |
Zhao et al., 2015 |
| APP/PS1 Male |
Treadmill running Gentle tail touching motivation no electric shock |
Week 1: 5 m/min, 0% grade 10 min/day Week 2: 10 m/min, 0% grade Time increased by 10 min each day Week 3–5: 10 m/min, 0% grade 60 min/day |
5 days/week 5 weeks 2 groups; one beginning at 7 months of age the other at 24 months of age |
Adult group: Passive avoidance (ns) ↓ anxiety MWM (ns) ↓guanidine extracted soluble Aβ40 and Aβ42 Few amyloid plaques detected in SED or EX BDNF (ns) Aged group: Passive avoidance (ns) Anxiety (ns) ↑MWM soluble Aβ40 and Aβ42 not assessed ↓ amyloid plaque |
Ke et al., 2011 |
| APP/PS1 Male |
Treadmill running | 11 m/min 30 min/day 0% grade |
5 days/week 20 weeks; from 3 months of age to 8 months of age |
↑ Y maze ↑ Passive Avoidance ↑ Mitochondrial function and antioxidant enzymes ↓ Mitochondrial ROS ↓ Aβ42 |
Bo et al., 2014 |
| APP/PS1 Male |
Treadmill running | 10 m/min 20 min/day 0% grade |
5 days/week 4 months; from 6 months of age to 10 months of age |
↑ MWM ↑ White matter capillary length and volume White matter capillary surface area (ns) |
Zhang et al., 2017 |
| 3xTg-AD Male or Female not specified |
Treadmill running Gentle tail touching motivation no electric shock |
10 m/min 20 min/day 0% grade |
5 days/week 12 weeks 2 groups; one beginning at 4 months of age the other beginning at 24 months of age |
Young group: ↑MWM Aβ40 and Aβ42 (ns) Amyloid plaque (ns) APP (ns) Presenilin 1 (ns) CFTβ (ns) ADAM10&17 (ns) BACE1 (ns) IDE (ns) Neprilysin (ns) ↓phosporylated tau ↑PSD-95 and synaptophysin BDNF and NGF (ns) Old group: ↑MWM ↓ Aβ40 and Aβ42 ↓Amyloid plaque ↓APP ↓Presenilin 1 ↓BASE1 ↓ CFTβ ADAM10&17 (ns) BACE1 (ns) IDE (ns) Neprilysin (ns) ↓phosporylated tau ↑PSD-95 and synaptophysin ↑BDNF NGF (ns) |
Cho et al., 2015 |
| APPswe/PS1dE9 Male and Female |
Treadmill running | Acclimation at 10 m/min 20–60 min/day first 4 weeks gradually increasing to 12 m/min 1hr/day 0% grade |
5 days/week for 9 weeks beginning at 6 weeks of age | ↑Conditioned fear memory ↑dendritic complexity ↑BDNF signaling pathways ↑LRP1 ↓Aβ40 and Aβ42 |
Lin et al., 2015 |
| NSE/htau23 Male or Female not specified |
Treadmill running | Acclimation at 9 m/min for 20 min/day Gradual increase from 12 m/min for 30 min/day to 14.4 m/min for 50 min/day 0% grade |
5 days/week for 12 weeks | ↑improved autophagy ↑GSK-3β ↓tau and phospho-tau ↑MWM |
Kang and Cho, 2015 |
| Tg-NSE/hPS2m Male or Female not specified |
Treadmill running | 12 m/min 1 h/day 0% grade |
5 days/week for 3 months from 24 months of age | ↑MWM ↑anti-apoptotic factors ↑insulin signaling pathways ↓COX-2 ↑BDNF ↑HSP70 |
Koo et al., 2013 |
| 3xTg-AD Male |
Forced Wheel running | 8 m/min 1 h/day 0% grade |
2 groups: 1x/week or 3x/week for 12 weeks from 2.5 to 6 months of age |
Improvement in blood and brain inflammatory chemokines in the 3x/week group, but not the 1x/week group | Haskins et al., 2016 |
| Tg2576 Male and Female |
Treadmill running | 10.9 m/min, 0% grade 1 h/day |
5 day/week 16 weeks From 5 to 9 months of age |
↓amyloid plaque Soluble Aβ (ns) NOR (ns) ↑hippocampal volume |
Yuede et al., 2009 |
| Tg2576 Male |
Treadmill running | Low Intensity group: 15 m/min 0% grade Hi Intensity group: 32 m/min 10% grade 1 h/day |
5 days/week 3 months From 3 to 6 months of age |
↓soluble Aβ40 and Aβ42 HI > LOW > SED ↑numerous Aβ clearance proteins HI > LOW > SED |
Moore et al., 2016 |
Abbreviations: MWM = Morris Water Maze, APP = Amyloid Precursor Protein, BDNF = Brain Derived Neurotrophic Factor, HSP = Heat Shock Protein, CTF = C Terminal Fragment, SOD = Superoxide Dismutase, LTP = Long Term Potentiation, NGF = Nerve Growth Factor, ROS = reactive oxygen species.
We directly evaluated the effects of intensity-matched forced and voluntary chronic physical activity regimens on the cognitive and pathological changes in a transgenic AD mouse model and found that both forms of chronic physical activity decreased plaque count and increased hippocampal volume, but only mice in the voluntary group performed better on a cognitive task (Yuede et al., 2009). Psychological stress in forced exercise bouts can be due to the physical discomfort of the shock stimulus at the back of the treadmill belt designed to motivate the mice to run, or by the emotional distress associated with having to run at a constant predetermined speed. To differentiate between those two forms of stress we included a group that received the foot shock only, but did not run. Interestingly, we did not find a significant impairment in our shock-only control group suggesting that the shock exposure the forced exercise group received during treadmill running was not a strong enough stressor to have an effect on AD pathology. However, the animals in the forced exercise group did not achieve all of the same benefits as the animals in the voluntary exercise group indicating a qualitative difference between the physical activity regimens. We concluded that it was more likely that the psychological stress associated with the forced exercise mitigated some of the beneficial effects, particularly on cognition, realized by the voluntary exercise group.
4. Forced versus voluntary chronic physical activity: does intensity matter?
The results of studies employing physical activity in mouse models of AD are largely positive (Table 1, Table 2), however there are studies reporting little to no improvement in AD pathology or cognitive benefit of the physical activity regimen (Cracchiolo et al., 2007; Giménez-Llort et al., 2010; Richter et al., 2008; Wolf et al., 2006). Mice in all of these studies have increased physical activity, though it is difficult to determine exactly how much exercise training the animals received. To date, only two studies using mouse models of AD have assessed an exercise training effect (Lin et al., 2015; Moore et al., 2016). It is conceivable that the stress involved in treadmill running in animal models is enough to counteract the beneficial effects of chronic physical activity if the intensity, duration, and frequency are below a critical threshold. Currently, it is not possible to differentiate the studies listed in Table 1, Table 2 as to whether they represent significant exercise training or sub-exercise training chronic physical activity regimens. Since voluntary exercise is often studied by giving mice unrestricted access to a running wheel in their cage and reporting total distance, it is difficult to precisely determine exercise intensity. We attempted to estimate intensity based on the parameters reported in the studies of voluntary exercise in animal models of AD in Table 1. We calculated speed by dividing the total distance reported by the amount of time the animal likely ran on the running wheel each day. In the studies where distance was reported, most exercise intensities ranged from roughly 0.55–6 m/min each day, however we note that these are likely an underestimation of the actual intensity. If a mouse utilizes the running wheel during the 12 h dark cycle but only runs for a total of 4 h, their intensity would be higher than we estimate. From our own experience, we found that when given access to a running wheel for only 1 h a day, Tg2576 mice voluntarily ran at an average speed of 10.9 m/min over the four month course of the study. Several studies employing voluntary physical activity show improvements in Aβ or tau pathology, cognitive measures, and secondary physiological measures that could lead to improvement in relation to AD pathology, such as increased neurogenesis (Adlard et al., 2005; Belarbi et al., 2011; García-Mesa et al., 2016; Maliszewska-Cyna et al., 2016; Tapia-Rojas et al., 2016; Yuede et al., 2009). While others report effects in only cognition or other factors related to AD pathogenesis (Cosín-Tomás et al., 2014; García-Mesa et al., 2012, 2011; Mirochnic et al., 2009; Nichol et al., 2008, 2007; Parachikova et al., 2008; Revilla et al., 2014). Since intensity is a controllable parameter in forced exercise regimens, we can directly compare results of different intensities in the studies listed in Table 2. All studies using forced exercise regimens of at least 10 m/min/day report improvement in pathophysiological or cognitive effects of AD in the mouse model (Bo et al., 2014; Cho et al., 2015, 2010; Kang et al., 2013; Kang and Cho, 2015; Ke et al., 2011; Koo et al., 2013; Lin et al., 2015; Liu et al., 2013, 2011; Moore et al., 2016; Um et al., 2011, 2008; Xiong et al., 2015; Yuede et al., 2009; Zhang et al., 2017; Zhao et al., 2015). A study of lower intensity (8 m/min) forced wheel running in 3xTG-AD male mice found that physical activity 3 times per week for 12 weeks improved inflammatory markers, while once a week did not have an effect indicating that frequency of physical activity plays a role (Haskins et al., 2016). Additionally, lower intensity treadmill running, 4.2 m/min, did not result in significant changes in pathology female 3xTG mice, although subtle benefits on Aβ40/42 ratio were found in male mice (Giménez-Llort et al., 2010) suggesting exercise intensity may play a role in the magnitude of the benefits on AD pathology.
What level of chronic physical activity is necessary to reduce AD pathology, and is there an exercise intensity/duration/frequency threshold where the benefits outweigh the effects of psychological stress? In our initial study, exercise intensity, duration, and frequency of the forced treadmill group was matched to that of the wheel running group as our focus was to evaluate the potential negative effects of psychological stress associated with forced exercise. We subsequently evaluated the effects of exercise intensity (keeping duration and frequency constant) on AD pathology. In pilot studies, we discovered that Tg2576 mice running at 15 m/min on a level treadmill for 60 min/day, 5 days/week, for 8 weeks had an increased citrate synthase activity, whereas mice running at 10 m/min for the same duration and frequency only had a trend towards an increase in citrate synthase activity. We further discovered that most Tg2576 mice were capable of running at a speed of 32 m/min on a 10% incline for 1 h. These investigations indicated that the running speeds previously utilized by ourselves and others to investigate the effects of chronic physical activity on AD pathology were relatively low, and that running speeds of around 10 m/min are near the threshold separating sub-exercise training from exercise training in transgenic model of AD.
We then evaluated the effect of exercise training intensity on soluble Aβ concentrations by comparing Tg2576 mice running at 32 m/min on a 10% incline for 60 min/day, 5 days/week, for 12 weeks (from 3 to 6 months of age) to a sedentary group, and a group running at 15 m/min on a level treadmill for the same duration and frequency. The exercise training effect (citrate synthase activity) was significantly greater in the high intensity group compared to the low intensity group, and the low intensity group was greater than the sedentary group demonstrating a dose-dependent exercise training effect. In both the cortex and hippocampus, soluble Aβ40 and Aβ42 were decreased in an exercise training dose-dependent manner, and proteins involved in Aβ clearance (neprilysin, insulin degrading enzyme, MMP9, LRP1, and HSP70) were all increased in a dose-dependent manner. We have followed up using the same groups, but continuing the exercise regimen for 12 months ending at 15 months of age. Improvements in pathological markers of AD also translated into robust improvements in cognitive function in the high intensity group, whereas cognitive improvements in the low intensity group were subtle compared to the sedentary group (manuscript in preparation).
Previous studies of the effects of chronic physical activity on AD pathology and cognition, summarized in Table 1, Table 2 suggest an effect of exercise intensity, particularly for forced exercise regimens, which was confirmed by our studies. One complicating factor when comparing exercise intensities among these studies is the differences in mouse models as it is likely that some models are capable of greater running speeds than others. We have recently begun studying the effects of exercise in APP/PS1 mice and have discovered that the majority of these mice are only capable of running at a speed of 24 m/min on a 10% incline for 1 h compared to 32 m/min for Tg2576 mice. While it is clear that exercise intensity does matter for beneficial impact on AD pathology, one question that remains is where the intensity threshold exists for which the beneficial effects of physical activity do not outweigh the negative effects of stress.
5. Does chronic physical activity protect against the effects of acute psychological stress in AD?
Acute stress has rapid effects on the levels of Aβ in the mouse brain. Kang et al. (2007) tested the effects of acute restraint stress on Aβ in Tg2576 mice. Using a microdialysis technique to measure soluble Aβ in the brain (Cirrito et al., 2003), they found that acute restraint stress significantly increased Aβ levels in the hippocampus, and levels remained high for at least 13 h following the stressful experience. Blocking action potentials during restraint stress blocked the increase in Aβ suggesting that increased neuronal activity in the hippocampus during restraint stress was driving the increase in extracellular Aβ levels. Administration of corticotrophin releasing factor (CRF), but not corticosterone, mimicked the effects of restraint stress on extracellular Aβ indicating that this effect occurred through the actions of CRF in the brain.
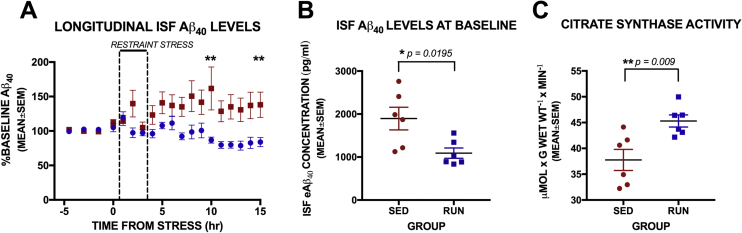
To determine if exercise training could protect against this effect of acute psychological stress on Aβ levels in the brain, we measured Aβ before, during, and after acute restraint stress in 4 month old female APP/PS1 mice following 8 weeks of treadmill running at an intensity of 15 m/min, 60 min/day, 5 days a week. We used the same techniques for stress, microdialysis, and Aβ quantification as reported in (Kang et al., 2007) and all methods and procedures were conducted in accordance with the guidelines established by the IACUC and Animal Studies Committee at Washington University School of Medicine. Interestingly, the stress-induced effect on extracellular Aβ levels in the hippocampus was prevented, not just diminished, in the exercise trained female mice compared to sedentary female mice (Fig. 2A). Aβ levels fluctuate around baseline for the entire 15 h period following stress, while Aβ levels in the sedentary mice continued to rise significantly above baseline following acute restraint stress. In addition, baseline Aβ levels in the hippocampus of exercise trained mice were significantly lower, by 42%, than those in sedentary mice (Fig. 2B). To establish a significant physiological effect of exercise training, we also measured citrate synthase activity (procedures as reported in Moore et al., 2016) in the soleus muscle and found significantly higher activity following 8 weeks of exercise training compared to sedentary mice (Fig. 2C).
Fig. 2.
Effects of chronic exercise on acute restraint stress in female APP/PS1 mice.
(A) Aβ levels in the hippocampus were measured every hour and change from baseline values were calculated in response to acute restraint stress in 4 month old female APP/PS1 mice. Total number of mice was 12, with n = 6 per group, however the microdialysis probe clogged in one mouse in the RUN group during restraint stress resulting in n = 5 for longitudinal analysis. Two way Repeated Measures ANOVA show significant interaction between group and time [F(21,189) = 3.663, p < 0.0001]. Simple main effects with Bonferroni correction for multiple comparisons indicate a significant increase from baseline in the SED group at hours 2, 5, 8, 9, 10 and 15 h from stress, while no significant change from baseline was seen in the RUN group. Between subjects comparisons show differences between groups at hour 10 (**p = 0.0095) and 15 (**p = 0.0051) following stress. (B) Mice in the RUN group had significantly lower concentrations in ISF Aβ in the hippocampus at baseline compared to SED group [unpaired t-test: t = 2.778, df = 10, p = 0.0195]. (C) Measures of citrate synthase activity in the soleus muscle were significantly higher in the RUN group compared to SED animals [unpaired t-test: t = 3.204, df = 10, p = 0.0094] indicating a significant exercise training effect following the 8-week exercise regimen.
Exercise training increases aspects of several Aβ clearance pathways, and this effect is intensity-dependent (Moore et al., 2016). In our current experiments, we again observed a significant difference in baseline Aβ levels in the hippocampus of exercise trained APP/PS1 mice compared to sedentary controls (Fig. 2B). The net effect of more effective clearance pathways in place during acute psychological stress could lead to a much lower increase in Aβ in response to stress such that we could not detect an increase and rapid recovery in Aβ levels. It is also possible that exercise training prevents the increased neuronal activity in response to psychological stress which is underlying the protective effect of exercise training on Aβ presented here. Further studies measuring activity in the hippocampus are planned to determine the mechanism. Because exercise training could be a form of repeated, controllable stress, it is also conceivable that treadmill running in mice makes the animals resistant to the HPA axis effects of acute psychological stress, and subsequently to the physiological changes that increase AD pathology following acute psychological stress. The dual effects of psychological stress resistance and improved Aβ clearance most likely have combined positive effects on the development and progression of AD pathology. These results indicate that exercise training in female mice provides resistance to the effects of acute psychological stress on extracellular Aβ levels. We are currently analyzing differences in gene expression in the hippocampus of exercise trained and untrained mice to determine the mechanisms underlying this effect.
6. Does chronic physical activity provide resistance or resilience to stress in AD?
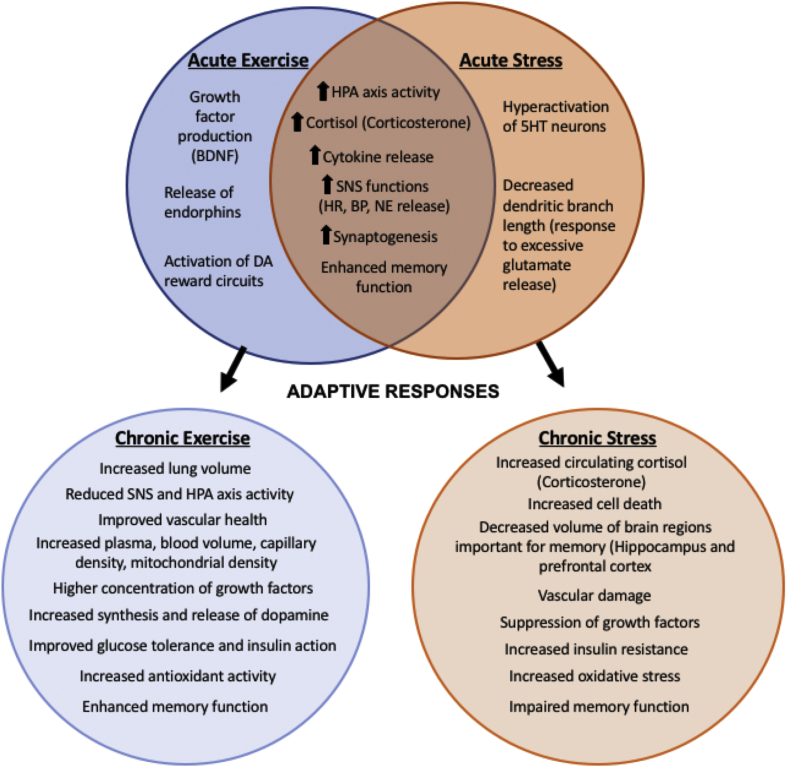
Physical activity has shown to be protective against several stress-related psychiatric disorders (King et al., 1993; Taylor et al., 1985) even though acute exercise can be similar to acute stress in that it stimulates the SNS and HPA axis. The stress response is a physiological reaction to environmental changes that can be positive and proadaptive or negative and maladaptive (Musazzi et al., 2017). Chronic exercise training can have adaptive effects on the homeostatic regulation of the system that may improve resistance and/or resilience to psychological and physical stress and therefore be protective or preventative against disease. In contrast, chronic stress results in several maladaptive changes that can accelerate or potentiate disease (Fig. 3). Among other things, acute exercise increases pro-inflammatory cytokines, increases oxidative stress, and increases cortisol/corticosterone. When combined with physical activity in a chronic manner, this response can train the body to adapt or be more resilient to psychological and physical stress, or counteract inflammatory or other negative physiological effects of AD, leading ultimately to protection or slowing of the disease progression (Cavalcante et al., 2017; Pedersen and Saltin, 2006).
Fig. 3.
Acute and Chronic Effects of Stress and Exercise. Acute effects of exercise and stress overlap, but lead to divergent chronic adaptations that change risk for developing AD. The acute physiological effects of stress and exercise have several similar aspects that could lead to improvement or impairment in AD. Adaptive responses to chronic stress increase risk for AD, while the adaptive responses to chronic exercise decrease risk for AD.
As defined in Fleshner et al. (2011) stress resistance refers to an increase in the duration and/or intensity of a stressor needed to cross the threshold from adaptive to maladaptive responses to psychological stress. Stress resilience, in contrast, refers to facilitated recovery after stressor exposure. Individuals who are stress resistant are able to endure higher levels of psychological or physical stress before experiencing negative consequences, while individuals who are more resilient are able to bounce back more quickly following a stressful experience. Monika Fleshner's laboratory has done many elegant studies defining the impact of physical activity on psychological stress in rats. Six weeks of physical activity in the form of voluntary wheel running has stress-buffering effects on the immune system of male rats (reviewed in Fleshner, 2005), inflammatory cytokines (Speaker et al., 2014), anxiety-related behaviors and social avoidance (Greenwood et al., 2003). Studies by Greenwood et al. (2013) evaluated the importance of the controllability of chronic physical activity on neural circuits involved in stress resistance. While voluntary exercise is a natural reward and is a motivator for some people, the amount of control a person has over their physical activity may have similar aspects to forced exercise. For example, if a person does not enjoy running, do they experience the same benefits as someone who does? Greenwood and colleagues have attempted to dissect this by making the degree of controllability over wheel running a variable in their studies. Both voluntary and forced wheel running protected against stress-induced behavioral changes in rats. They also found dopamine reward-related plasticity enhanced in both voluntary and forced running conditions, and these changes in plasticity contribute to stress resistance independent of the controllability of the chronic physical activity (Herrera et al., 2016). In fact, despite the apparent markers of chronic stress in the forced wheel running rats, benefits from the physical activity were still evident in behavioral responses and neurochemical pathways involved in stress resistance. The differences in intensity and duration of exercise as well as the amount of psychological stress perceived with either type of exercise, forced or voluntary, may play a role in which functional or physiological domain positive effects are conferred. So, thresholds for the intensity of physical activity to provide psychological stress resistance or resilience may differ depending on the physiological or behavioral outcome of interest.
Environmental enrichment has been shown to more quickly reduce plasma corticosterone levels in mice following acute restraint stress, suggestive of a stress resilience effect (Meijer et al., 2007). It appears that some aspects of acute or subthreshold voluntary physical activity promotes stress resiliency, while chronic physical activity may promote resistance. Stress resistance and resilience are dependent on multiple genetic and environmental factors, and the mechanisms of stress resiliency are important to uncover for identifying new approaches for the treatment of stress-related disorders, including AD.
7. What are the possible mechanisms for the stress-buffering effects of exercise training on AD pathology?
7.1. Improved Aβ clearance
A variety of stressors (both physical and psychological) can induce the upregulation of heat shock proteins (HSPs), which are designed to protect the cell against stress-induced oxidative, heat, and cytokine insult (Kiang and Tsokos, 1998). HSPs have also been reported to prevent protein aggregation and refold damaged proteins (Moseley, 1997). Voluntary physical activity increases levels of HSPs in the rat brain, and particularly in response to acute psychological or physical stress (Campisi et al., 2003). Since exercise training increases levels of HSP70 in a dose-dependent manner, this is one possible mechanism for physical activity to confer stress resilience or resistance to AD by promoting clearance of Aβ or other proteins/debris from the brain. Exercise intensity dependent increases in mRNA levels in the hippocampus and cortex for Aβ clearance factors such as; neprilysin, HSP70, LRP1, and LRP2 have also been shown (Moore et al., 2016) as well as, increases in protein levels in the cortex and hippocampus for neprilysin, insulin degrading enzyme (IDE), MMP9, LRP1, and HSP70. In addition to changes in receptors and enzymes, another factor that may contribute to clearance efficiency is cerebral blood flow. Chronic stress decreases local cerebral blood flow in the rat hippocampus (Endo et al., 1999), and region-specific increases in cerebral blood flow have been reported following physical activity of moderate intensity (approximately 60% maximal oxygen uptake) in humans (Ide and Secher, 2000; Ogoh and Ainslie, 2009). An overall improvement in global or regional cerebral blood flow could minimize the effects of hypoperfusion in AD, which may also promote clearance of Aβ and other AD related proteins.
7.2. Improved neuroprotection
Brain-derived neurotrophic factor (BDNF), which is highly expressed in brain regions involved in AD, has many beneficial effects on cognition and synaptic plasticity (Fumagalli et al., 2006). Chronic stress has been shown to reduce BDNF levels, particularly in the hippocampus, in several animal models (Aleisa et al., 2006; Schaaf et al., 2000; Smith et al., 1995). Higher serum levels of BDNF are correlated with lower risk for developing dementia (Aisen, 2014; Laske et al., 2011). In humans, acute exercise causes an increase in BDNF in peripheral blood that is directly correlated with exercise intensity (Dinoff et al., 2017), and chronic exercise training results in higher resting levels of BDNF in circulation in both men and women (Dinoff et al., 2016). Stem cell production is modulated by homeostasis to keep pace with physiological demands such as exercise (Nakada et al., 2011), so physical activity and exercise training may prevent the age-related decline in neurogenesis and impaired HPA axis homeostasis. Keeping these homeostatic mechanisms intact for a longer time would slow aspects of disease progression driven by disrupting metabolism and HPA axis function. Adlard and Cotman (2004) reported that voluntary physical activity protected against a stress-induced decrease in hippocampal BDNF, and that corticosterone directly modulates alterations in hippocampal BDNF. Short term (5 days of 30 min/day at 15 m/min) treadmill exercise in rats also increased BDNF, which was suppressed by chronic (2hr/day) immobilization stress (Fang et al., 2013). Immobilization stress reduced synaptic markers, which was reversed by treadmill exercise. Voluntary physical activity (wheel running) did not have a significant effect on neurogenesis and LTP until 56 days of running (Patten et al., 2013), suggesting it may take longer to reach a physiological benefit with voluntary physical activity compared to exercise training. It is well established that physical activity and exercise training increase hippocampal BDNF (Cotman and Berchtold, 2002; van Praag et al., 2005, 1999) and given the correlations with BNDF and dementia risk in humans, increasing hippocampal BDNF through exercise is another pathway through which exercise can buffer the effects of stress in AD.
Increased levels of reactive oxygen species in the brain occur as we age and play a role in many chronic diseases, including AD. Chronic stress accelerated AD pathology and increased markers of oxidative stress in Tg2576 mice (Lee et al., 2009). While high levels of acute physical activity increase oxidative stress, chronic physical activity regimens have been shown to decrease the activity of enzymes that generate oxidation, and increase the activity of antioxidant enzymes such as SOD1 and SOD3 (reviewed in Kojda and Hambrecht, 2005). In AD transgenic mouse models this has been demonstrated to occur with both voluntary (García-Mesa et al., 2016, 2012, 2011) and forced (Bo et al., 2014; Cho et al., 2010; Um et al., 2008) chronic physical activity. While it remains to be determined if exercise training after damage from oxidative stress has occurred can successfully repair damage, it appears that exercise training prior to the development of AD pathology may provide resistance to the negative effects of oxidative stress in AD, as well as protect against stress-induced increases in oxidative stress in the brain.
7.3. Improved homeostatic functions
Peripheral insulin resistance is associated with impaired cognition and lower hippocampal volume (Rasgon et al., 2011), and alterations in insulin sensitivity and insulin signaling occur in individuals with AD (reviewed in Stanley et al., 2016). Increasing glucose in the blood and brain increases extracellular Aβ in the brains of APP/PS1 mice (Macauley et al., 2015). Acute stress increases glucose in the blood/brain to mobilize resources for the fight or flight response, while chronic stress leads to insulin resistance and impaired glucose metabolism. Exercise training results in multiple beneficial adaptations in skeletal muscles to increase glucose transporters and improve overall glucose control. The effects of exercise on glucose regulation have been shown to be intensity dependent with higher intensity exercise resulting in more significant changes in glucose regulation or insulin sensitivity (Malin et al., 2016). Improved regulation of glucose metabolism improves whole body homeostasis which ultimately protects against the effects of stress and the development/progression of AD through multiple pathways.
A review by Nation et al. (2011) lays out the involvement of both stress and exercise on the neurovascular system in AD. The well documented effects of exercise and physical fitness on the cardiovascular system have important indications for exercise training in the prevention and treatment of AD. The more common sporadic form of AD is closely associated with vascular disease, with many major risk factors for cardiovascular disease also being recognized as risk factors for AD (Martins et al., 2006). Many of the physiological processes shown to be impaired in AD are improved with increased physical activity. Furthermore, many of the physiological processes shown to be altered with chronic stress are factors that worsen cardiovascular disease and AD. Therefore, there is a close relationship between the cardiovascular and stress systems and both play a role in the development and progression of AD. Physical fitness to improve the cardiovascular system appears to directly counteract the effects of stress on AD pathology.
Exercise has been shown to provide resistance to stress-induced immune impairment, particularly in response to a challenge (Fleshner, 2005). The immune response regulation is impaired in AD (Marx et al., 1999) and exercise may benefit AD in this regard by strengthening the immune system, as well as suppressing the immune response to stress (stress resistance) in the first place. Studies in aged animals show that areas of the brain, particularly the hippocampus, have an exaggerated immune response with prolonged elevation of pro-inflammatory cytokines following an immune challenge (Barrientos et al., 2015, 2012), suggesting regulation of the immune system in the brain decreases with age. Physical activity has become increasingly recognized as a potential intervention to reduce chronic inflammation (Arikawa et al., 2011; Di Raimondo et al., 2013; Gleeson et al., 2011). Resistance to stress-induced suppression of immune function was not observed in conditions of forced exercise, however (Fleshner, 2005), suggesting that the negative effects of stress occurring at the same time as physical activity may suppress benefits on immune system regulation. Future studies to determine if the benefits in immune function are exercise intensity dependent or if duration of physical activity is a more critical factor for modulating immune responses to stress in AD will be important.
Sleep disturbances and circadian rhythm dysfunction are tightly linked with the development and progression of AD (reviewed in Musiek et al., 2015). Many studies have found impairments in the sleep-wake cycle increase cognitive and pathophysiological changes in AD mouse models (Holth et al., 2017; Kang et al., 2009; Roh et al., 2014). Studies of the effects of exercise in animals and humans have shown increased sleep quality (Lancel et al., 2003; Netzer et al., 2001; Torsvall et al., 1984), while chronic stress shortens duration of sleep and disrupts sleep maintenance resulting in overall reduced sleep quality (Cheeta et al., 1997). Long term physical activity changes sleep physiology (Lancel et al., 2003), with wheel running mice showing fewer, but longer sleep episodes compared to sedentary controls indicating better sleep consolidation. Voluntary physical activity in rats also prevented stress-induced disruptions in sleep quality (Thompson et al., 2016). McCurry et al. (2011) studied the effects of 30 min of continuous walking/day for at least 4 days/week on the effect of sleep in patients with AD. They found that physical activity resulted in significantly less total wake time and better sleep efficiency after six months of intervention. The type of exercise or the stress involved may play a role in the degree of influence exercise has on sleep. Improving sleep quality is beneficial in AD and determining mechanisms to protect the brain from stress-induced sleep dysfunction could be an important intervention strategy.
7.4. Improved regulation in neurotransmitter systems
Stress and exercise also affect several neurotransmitter systems. Glucocorticoids influence synaptic plasticity by altering glutamate receptor density and function in the hippocampus, amygdala and prefrontal cortex (de Kloet et al., 2002; Yuen et al., 2011). GCs enhance calcium influx through NMDA receptors, which underlies both LTP and LTD (Tse et al., 2011). This has an acute effect of memory enhancement (Yuen et al., 2011), however chronic over-excitation or excessively high corticosterone concentrations lead to reduced LTP and memory impairment (Zhang et al., 2012). Exercise can induce changes in regulation of the glutamatergic system that together with upregulation of neurotrophic factors may be protective against excitotoxicity associated with over-activation of the glutamatergic system caused by stress (Dietrich et al., 2005). Exercise, and mild stress, increase norepinephrine release which stimulates phosphorylation of the AMPA receptor subtype GluR1 and mobilizes GluR1-containing receptors to the synapse, therefore enhancing LTP (Hu et al., 2007). Repeatedly activating the NE system through physical activity may protect against psychological stress by producing a habituation-like effect on the system. For example, chronic physical activity induced an adaptation in the locus coeruleus that helped constrain activity in this brain region during exposure to non-exercise stressors such as shock (Dishman et al., 1997; Greenwood et al., 2003).
There is also evidence that the serotonergic system is severely affected in AD and that increasing serotonergic signaling can reduce Aβ levels in the brain (Cirrito et al., 2011; Fisher et al., 2016; Li et al., 2017; Sheline et al., 2014). Stress affects many aspects of the serotonergic system with regulation by GCs depending on the brain region and type of stressor involved. Greenwood et al. (2005) reported hyperactivation of serotonergic neurons in response to acute stress, which could lead to depletion of serotonin under chronic conditions. Regular aerobic physical activity has been shown to increase serum levels of serotonin in humans (Fumoto et al., 2010). Increasing serotonin in the brains of APP/PS1 mice, either directly or through receptor signaling mechanisms, resulted in a significant decrease in Aβ levels (Cirrito et al., 2011). Taken together, increasing serotonin regulation in the brain through physical activity may protect against stress-induced depletion of serotonin and improve cognitive and pathophysiological features in AD.
8. Summary and conclusions
Humans are constantly bombarded by physicians and the media with challenges to reduce psychological stress and increase physical activity to improve health and reduce risk of chronic disease, including AD. For some individuals, however, both the thought and act of daily exercise imposes psychological stress. It is clear that stress increases the risk and exacerbates the symptoms of AD (Dong and Csernansky, 2009). It is equally clear that chronic physical activity decreases AD risk (Intlekofer and Cotman, 2013). Given that acute exercise can both cause stress and be protective against AD when incorporated into a chronic exercise training regimen, this interaction has been investigated in an attempt to elucidate the net effect of these two opposing forces on the disease process.
Voluntary wheel running has been utilized in transgenic mouse models of AD to investigate the effects of chronic physical activity on AD related outcomes in the absence of psychological stress that can accompany an exercise bout. Taken collectively, the results of these studies (summarized in Table 1) suggest that chronic voluntary physical activity is beneficial in enhancing physiological parameters and cognitive function associated with reduced AD risk. However, not all studies confirm this, and the effects are subtle in most of the studies that do support this concept. One likely factor causing the discrepancy in these studies is that exercise intensity is directly related to the magnitude of the effect of physical activity on improving AD-related outcomes, and there is an intensity threshold below which the effects are not realized. While mice run great distances in wheel running paradigms, the average intensity at which they run is typically very low compared to treadmill running regimens. Establishing physiological training effects in these regimens will be important for determining where this threshold lies.
Treadmill running introduces the element of psychological stress into the paradigm as the mouse must keep up with the set speed of the treadmill and, in most cases, must avoid a small electrical shock if it does not maintain the treadmill speed. Our study (Yuede et al., 2009) directly comparing wheel running vs. treadmill running of the same intensity, duration, and frequency demonstrated that when psychological stress is removed from the chronic physical activity condition, AD-related outcomes are improved to a greater degree than when psychological stress is present. However, treadmill running did result in improved AD outcomes over sedentary mice indicating that the benefits of forced exercise outweighed the negative effects of the psychological stress associated with the activity. When taken collectively, studies that employed treadmill running as the sole chronic physical activity treatment (summarized in Table 2) suggest that the benefits of physical activity outweigh the detrimental effects of psychological stress on AD outcomes. As with wheel running, this conclusion is not universally supported by all studies and, of course, the results are more subtle than robust. Treadmill running intensity overall was greater than that estimated in the wheel running studies (4.2–32.0 m/min), but training effects with both types of running might actually be relatively low as a significant increase in citrate synthase levels as an objective marker of exercise training was found at higher intensities (Lin et al., 2015; Moore et al., 2016). Tg2576 mice running at 15 m/min had modest improvement in AD outcomes similar to those of previous studies employing treadmill running. However, mice running at 32 m/min on a 10% grade, a much greater intensity than other treadmill running studies, showed robust improvement in several pathophysiological markers of AD (Moore et al., 2016). Both groups had elevated soleus muscle citrate synthase activity demonstrating a greater exercise training effect in the high intensity group compared to the low intensity group. These results provide strong evidence for a dose-response of exercise training on AD risk outweighing the negative impact of the stress involved in forced treadmill exercise. Differences in intensity, which is rarely measured directly by something such as citrate synthase activity, between exercise studies relating to AD may also account for apparent inconsistent findings in the literature.
Future studies are needed into the effects of the quantity (intensity, duration, and frequency) of chronic physical activity on AD outcomes as well as the thresholds needed to induced stress resistance or resilience and the mechanisms associated with those outcomes. Health benefits from chronic physical activity regimens could result directly from physical activity enhancing cell, tissue, and organ function or it could come indirectly from the acute bouts of physical activity within the chronic regimen resulting in reducing psychological stress, i.e., taking a walk on a nature trail is a good way to “blow off steam.” Theoretically, exercise training would primarily provide health benefits by directly enhancing health associated with enhanced physiological function, while sub-exercise training physical activity regimens would primarily act by relieving psychological stress.
Many epidemiological studies support the idea that increased physical activity can slow the progression of AD and other neurodegenerative disorders (Beckett et al., 2015; Lautenschlager et al., 2008; Reynolds et al., 2016). A report by Norton et al. (2014) found that 21% of AD cases in the US could be attributed to physical inactivity, making chronic physical activity a powerful tool for decreasing AD risk. While psychological stress is clearly associated with declines in AD, the benefits from chronic physical activity may outweigh the amount of stress associated with the regimen. A study by Ridgel et al. (2009) evaluated the effects of forced and voluntary exercise on motor function in individuals with Parkinson's disease and found that only the individuals who were forced to achieve an exercise intensity that was higher than their voluntary exercise rate showed significant disease symptom-modifying benefits from the intervention. Although the ideal physical activity regimen for individuals with AD has yet to be clearly described, there is increasing evidence that improving muscle strength, balance, and aerobic capacity will provide valuable health benefits to counteract the pathophysiology of the disease.
The effects of acute exercise and stress have many overlapping components that allow for dissecting out positive stress and negative stress on the system. The opposing effects of stress and exercise allow us to identify mechanisms that can determine vulnerability or resistance to AD. Of course, these mechanisms do not occur in isolation and many interactions could either enhance or diminish the positive effects of exercise on stress in AD. The effects of stress on the system and the circuitry involved in the stress response differ between males and females, and is more recently becoming the focus of many important studies determining the risk of stress on the development of AD (Bangasser et al., 2017). It is also possible that the effects of exercise on the neuroendocrine system are different for males and females. The effects of physical activity in AD can be viewed as a multi-target drug, especially in counteracting the well-documented negative effects of stress on the disease. These interactions need to be studied further to fully understand the frequency, intensity, and duration of physical activity required as well as the timing performed in relation to stress to determine the optimal guidelines to target different aspects of the disease.
Acknowledgements
Support for this work provided in part by an award from the Alzheimer's Association SAGA-17-419511 (JRC), and NIH grants P50 AG005681 and P01 NS074969.
References
- Adlard P.A., Cotman C.W. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–992. doi: 10.1016/j.neuroscience.2003.12.039. https://doi.org/10.1016/j.neuroscience.2003.12.039 [DOI] [PubMed] [Google Scholar]
- Adlard P.A., Perreau V.M., Pop V., Cotman C.W. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J. Neurosci. Off. J. Soc. Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. https://doi.org/10.1523/JNEUROSCI.0496-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen P.S. Serum brain-derived neurotrophic factor and the risk for dementia. J. Am. Med. Assoc. 2014;311:1684–1685. doi: 10.1001/jama.2014.3120. https://doi.org/10.1001/jama.2014.3120 [DOI] [PubMed] [Google Scholar]
- Aleisa A.M., Alzoubi K.H., Gerges N.Z., Alkadhi K.A. Chronic psychosocial stress-induced impairment of hippocampal LTP: possible role of BDNF. Neurobiol. Dis. 2006;22:453–462. doi: 10.1016/j.nbd.2005.12.005. https://doi.org/10.1016/j.nbd.2005.12.005 [DOI] [PubMed] [Google Scholar]
- Arikawa A.Y., Thomas W., Schmitz K.H., Kurzer M.S. Sixteen weeks of exercise reduces C-reactive protein levels in young women. Med. Sci. Sports Exerc. 2011;43:1002–1009. doi: 10.1249/MSS.0b013e3182059eda. https://doi.org/10.1249/MSS.0b013e3182059eda [DOI] [PubMed] [Google Scholar]
- Baglietto-Vargas D., Chen Y., Suh D., Ager R.R., Rodriguez-Ortiz C.J., Medeiros R., Myczek K., Green K.N., Baram T.Z., LaFerla F.M. Short-term modern life-like stress exacerbates Aβ-pathology and synapse loss in 3xTg-AD mice. J. Neurochem. 2015;134:915–926. doi: 10.1111/jnc.13195. https://doi.org/10.1111/jnc.13195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser D.A., Dong H., Carroll J., Plona Z., Ding H., Rodriguez L., McKennan C., Csernansky J.G., Seeholzer S.H., Valentino R.J. Corticotropin-releasing factor overexpression gives rise to sex differences in Alzheimer's disease-related signaling. Mol. Psychiatr. 2017;22(1126) doi: 10.1038/mp.2016.185. https://doi.org/10.1038/mp.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R.M., Frank M.G., Watkins L.R., Maier S.F. Aging-related changes in neuroimmune-endocrine function: implications for hippocampal-dependent cognition. Horm. Behav. 2012;62:219–227. doi: 10.1016/j.yhbeh.2012.02.010. https://doi.org/10.1016/j.yhbeh.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R.M., Thompson V.M., Kitt M.M., Amat J., Hale M.W., Frank M.G., Crysdale N.Y., Stamper C.E., Hennessey P.A., Watkins L.R., Spencer R.L., Lowry C.A., Maier S.F. Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol. Aging. 2015;36:1483–1495. doi: 10.1016/j.neurobiolaging.2014.12.003. https://doi.org/10.1016/j.neurobiolaging.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett M.W., Ardern C.I., Rotondi M.A. A meta-analysis of prospective studies on the role of physical activity and the prevention of Alzheimer's disease in older adults. BMC Geriatr. 2015;15 doi: 10.1186/s12877-015-0007-2. https://doi.org/10.1186/s12877-015-0007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belarbi K., Burnouf S., Fernandez-Gomez F.-J., Laurent C., Lestavel S., Figeac M., Sultan A., Troquier L., Leboucher A., Caillierez R., Grosjean M.-E., Demeyer D., Obriot H., Brion I., Barbot B., Galas M.-C., Staels B., Humez S., Sergeant N., Schraen-Maschke S., Muhr-Tailleux A., Hamdane M., Buée L., Blum D. Beneficial effects of exercise in a transgenic mouse model of Alzheimer's disease-like Tau pathology. Neurobiol. Dis. 2011;43:486–494. doi: 10.1016/j.nbd.2011.04.022. https://doi.org/10.1016/j.nbd.2011.04.022 [DOI] [PubMed] [Google Scholar]
- Bo H., Kang W., Jiang N., Wang X., Zhang Y., Ji L.L. Exercise-induced neuroprotection of Hippocampus in APP/PS1 transgenic mice via upregulation of mitochondrial 8-Oxoguanine DNA glycosylase. Oxid. Med. Cell. Longev. 2014;2014 doi: 10.1155/2014/834502. https://doi.org/10.1155/2014/834502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos M.G.N., Schuijer J., Lodestijn F., Beckers T., Kindt M. Stress enhances reconsolidation of declarative memory. Psychoneuroendocrinology. 2014;46:102–113. doi: 10.1016/j.psyneuen.2014.04.011. https://doi.org/10.1016/j.psyneuen.2014.04.011 [DOI] [PubMed] [Google Scholar]
- Campisi J., Leem T.H., Greenwood B.N., Hansen M.K., Moraska A., Higgins K., Smith T.P., Fleshner M. Habitual physical activity facilitates stress-induced HSP72 induction in brain, peripheral, and immune tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R520–R530. doi: 10.1152/ajpregu.00513.2002. https://doi.org/10.1152/ajpregu.00513.2002 [DOI] [PubMed] [Google Scholar]
- Carroll J.C., Iba M., Bangasser D.A., Valentino R.J., James M.J., Brunden K.R., Lee V.M.-Y., Trojanowski J.Q. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. https://doi.org/10.1523/JNEUROSCI.3836-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Publ. Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- Cavalcante P.A.M., Gregnani M.F., Henrique J.S., Ornellas F.H., Araújo R.C. Aerobic but not resistance exercise can induce inflammatory pathways via toll-like 2 and 4: a systematic review. Sports Med. - Open. 2017;3(42) doi: 10.1186/s40798-017-0111-2. https://doi.org/10.1186/s40798-017-0111-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeta S., Ruigt G., van Proosdij J., Willner P. Changes in sleep architecture following chronic mild stress. Biol. Psychiatr. 1997;41:419–427. doi: 10.1016/S0006-3223(96)00058-3. https://doi.org/10.1016/S0006-3223(96)00058-3 [DOI] [PubMed] [Google Scholar]
- Cho J., Shin M.-K., Kim D., Lee I., Kim S., Kang H. Treadmill running reverses cognitive declines due to Alzheimer disease. Med. Sci. Sports Exerc. 2015;47:1814–1824. doi: 10.1249/MSS.0000000000000612. https://doi.org/10.1249/MSS.0000000000000612 [DOI] [PubMed] [Google Scholar]
- Cho J.Y., Um H.S., Kang E.B., Cho I.H., Kim C.H., Cho J.S., Hwang D.Y. The combination of exercise training and alpha-lipoic acid treatment has therapeutic effects on the pathogenic phenotypes of Alzheimer's disease in NSE/APPsw-transgenic mice. Int. J. Mol. Med. 2010;25:337–346. doi: 10.3892/ijmm_00000350. [DOI] [PubMed] [Google Scholar]
- Cirrito J.R., Disabato B.M., Restivo J.L., Verges D.K., Goebel W.D., Sathyan A., Hayreh D., D'Angelo G., Benzinger T., Yoon H., Kim J., Morris J.C., Mintun M.A., Sheline Y.I. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14968–14973. doi: 10.1073/pnas.1107411108. https://doi.org/10.1073/pnas.1107411108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito J.R., May P.C., O'Dell M.A., Taylor J.W., Parsadanian M., Cramer J.W., Audia J.E., Nissen J.S., Bales K.R., Paul S.M., DeMattos R.B., Holtzman D.M. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J. Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosín-Tomás M., Alvarez-López M.J., Sanchez-Roige S., Lalanza J.F., Bayod S., Sanfeliu C., Pallàs M., Escorihuela R.M., Kaliman P. Epigenetic alterations in hippocampus of SAMP8 senescent mice and modulation by voluntary physical exercise. Front. Aging Neurosci. 2014;6(51) doi: 10.3389/fnagi.2014.00051. https://doi.org/10.3389/fnagi.2014.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C.W., Berchtold N.C. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cracchiolo J.R., Mori T., Nazian S.J., Tan J., Potter H., Arendash G.W. Enhanced cognitive activity – over and above social or physical activity – is required to protect Alzheimer's mice against cognitive impairment, reduce Aβ deposition, and increase synaptic immunoreactivity. Neurobiol. Learn. Mem. 2007;88:277–294. doi: 10.1016/j.nlm.2007.07.007. https://doi.org/10.1016/j.nlm.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky J.G., Dong H., Fagan A.M., Wang L., Xiong C., Holtzman D.M., Morris J.C. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am. J. Psychiatr. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. https://doi.org/10.1176/ajp.2006.163.12.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet E.R., Grootendorst J., Karssen A.M., Oitzl M.S. Gene x environment interaction and cognitive performance: animal studies on the role of corticosterone. Neurobiol. Learn. Mem. 2002;78:570–577. doi: 10.1006/nlme.2002.4079. [DOI] [PubMed] [Google Scholar]
- Di Raimondo D., Tuttolomondo A., Buttà C., Casuccio A., Giarrusso L., Miceli G., Licata G., Pinto A. Metabolic and anti-inflammatory effects of a home-based programme of aerobic physical exercise. Int. J. Clin. Pract. 2013;67:1247–1253. doi: 10.1111/ijcp.12269. https://doi.org/10.1111/ijcp.12269 [DOI] [PubMed] [Google Scholar]
- Dietrich M.O., Mantese C.E., Porciuncula L.O., Ghisleni G., Vinade L., Souza D.O., Portela L.V. Exercise affects glutamate receptors in postsynaptic densities from cortical mice brain. Brain Res. 2005;1065:20–25. doi: 10.1016/j.brainres.2005.09.038. https://doi.org/10.1016/j.brainres.2005.09.038 [DOI] [PubMed] [Google Scholar]
- Dinoff A., Herrmann N., Swardfager W., Lanctôt K.L. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur. J. Neurosci. 2017;46:1635–1646. doi: 10.1111/ejn.13603. https://doi.org/10.1111/ejn.13603 [DOI] [PubMed] [Google Scholar]
- Dinoff A., Herrmann N., Swardfager W., Liu C.S., Sherman C., Chan S., Lanctôt K.L. The effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): a meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163037. https://doi.org/10.1371/journal.pone.0163037 e0163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman R.K., Renner K.J., Youngstedt S.D., Reigle T.G., Bunnell B.N., Burke K.A., Yoo H.S., Mougey E.H., Meyerhoff J.L. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res. Bull. 1997;42:399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- Dong H., Csernansky J.G. Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. J. Alzheimers Dis. JAD. 2009;18:459–469. doi: 10.3233/JAD-2009-1152. https://doi.org/10.3233/JAD-2009-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Goico B., Martin M., Csernansky C.A., Bertchume A., Csernansky J.G. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. https://doi.org/10.1016/j.neuroscience.2004.05.040 [DOI] [PubMed] [Google Scholar]
- Dong H., Yuede C.M., Yoo H.-S., Martin M.V., Deal C., Mace A.G., Csernansky J.G. Corticosterone and related receptor expression are associated with increased β-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155:154–163. doi: 10.1016/j.neuroscience.2008.05.017. https://doi.org/10.1016/j.neuroscience.2008.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y., Nishimura J.I., Kobayashi S., Kimura F. Chronic stress exposure influences local cerebral blood flow in the rat hippocampus. Neuroscience. 1999;93:551–555. doi: 10.1016/s0306-4522(99)00176-1. [DOI] [PubMed] [Google Scholar]
- Fang Z.H., Lee C.H., Seo M.K., Cho H., Lee J.G., Lee B.J., Park S.W., Kim Y.H. Effect of treadmill exercise on the BDNF-mediated pathway in the hippocampus of stressed rats. Neurosci. Res. 2013;76:187–194. doi: 10.1016/j.neures.2013.04.005. https://doi.org/10.1016/j.neures.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Fisher J.R., Wallace C.E., Tripoli D.L., Sheline Y.I., Cirrito J.R. Redundant Gs-coupled serotonin receptors regulate amyloid-β metabolism in vivo. Mol. Neurodegener. 2016;11(45) doi: 10.1186/s13024-016-0112-5. https://doi.org/10.1186/s13024-016-0112-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M. Physical activity and stress resistance: sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc. Sport Sci. Rev. 2005;33:120–126. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- Fleshner M., Crane C.R. Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol. 2017;38:768–776. doi: 10.1016/j.it.2017.08.002. https://doi.org/10.1016/j.it.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M., Maier S.F., Lyons D.M., Raskind M.A. The neurobiology of the stress-resistant brain. Stress. 2011;14:498–502. doi: 10.3109/10253890.2011.596865. https://doi.org/10.3109/10253890.2011.596865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F., Racagni G., Riva M.A. The expanding role of BDNF: a therapeutic target for Alzheimer's disease? Pharmacogenomics J. 2006;6:8–15. doi: 10.1038/sj.tpj.6500337. https://doi.org/10.1038/sj.tpj.6500337 [DOI] [PubMed] [Google Scholar]
- Fumoto M., Oshima T., Kamiya K., Kikuchi H., Seki Y., Nakatani Y., Yu X., Sekiyama T., Sato-Suzuki I., Arita H. Ventral prefrontal cortex and serotonergic system activation during pedaling exercise induces negative mood improvement and increased alpha band in EEG. Behav. Brain Res. 2010;213:1–9. doi: 10.1016/j.bbr.2010.04.017. https://doi.org/10.1016/j.bbr.2010.04.017 [DOI] [PubMed] [Google Scholar]
- García-Mesa Y., Colie S., Corpas R., Cristòfol R., Comellas F., Nebreda A.R., Giménez-Llort L., Sanfeliu C. Oxidative stress is a central target for physical exercise neuroprotection against pathological brain aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2016;71:40–49. doi: 10.1093/gerona/glv005. https://doi.org/10.1093/gerona/glv005 [DOI] [PubMed] [Google Scholar]
- García-Mesa Y., Giménez-Llort L., López L.C., Venegas C., Cristòfol R., Escames G., Acuña-Castroviejo D., Sanfeliu C. Melatonin plus physical exercise are highly neuroprotective in the 3xTg-AD mouse. Neurobiol. Aging. 2012;33:1124. doi: 10.1016/j.neurobiolaging.2011.11.016. https://doi.org/10.1016/j.neurobiolaging.2011.11.016 e13-29. [DOI] [PubMed] [Google Scholar]
- García-Mesa Y., López-Ramos J.C., Giménez-Llort L., Revilla S., Guerra R., Gruart A., Laferla F.M., Cristòfol R., Delgado-García J.M., Sanfeliu C. Physical exercise protects against Alzheimer's disease in 3xTg-AD mice. J. Alzheimers Dis. JAD. 2011;24:421–454. doi: 10.3233/JAD-2011-101635. https://doi.org/10.3233/JAD-2011-101635 [DOI] [PubMed] [Google Scholar]
- Giménez-Llort L., García Y., Buccieri K., Revilla S., Suñol C., Cristofol R., Sanfeliu C. Gender-specific neuroimmunoendocrine response to treadmill exercise in 3xtg-ad mice. Int. J. Alzheimer's Dis. 2010;2010:128354. doi: 10.4061/2010/128354. https://doi.org/10.4061/2010/128354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11:607–615. doi: 10.1038/nri3041. https://doi.org/10.1038/nri3041 [DOI] [PubMed] [Google Scholar]
- Greenwood B.N., Foley T.E., Burhans D., Maier S.F., Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033:164–178. doi: 10.1016/j.brainres.2004.11.037. https://doi.org/10.1016/j.brainres.2004.11.037 [DOI] [PubMed] [Google Scholar]
- Greenwood B.N., Kennedy S., Smith T.P., Campeau S., Day H.E.W., Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003;120:269–281. doi: 10.1016/s0306-4522(03)00047-2. https://doi.org/10.1016/S0306-4522(03)00047-2 [DOI] [PubMed] [Google Scholar]
- Greenwood B.N., Spence K.G., Crevling D.M., Clark P.J., Craig W.C., Fleshner M. Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. Eur. J. Neurosci. 2013;37:469–478. doi: 10.1111/ejn.12044. https://doi.org/10.1111/ejn.12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins M., Jones T.E., Lu Q., Bareiss S.K. Early alterations in blood and brain RANTES and MCP-1 expression and the effect of exercise frequency in the 3xTg-AD mouse model of Alzheimer's disease. Neurosci. Lett. 2016;610:165–170. doi: 10.1016/j.neulet.2015.11.002. https://doi.org/10.1016/j.neulet.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Herrera J.J., Fedynska S., Ghasem P.R., Wieman T., Clark P.J., Gray N., Loetz E., Campeau S., Fleshner M., Greenwood B.N. Neurochemical and behavioral indices of exercise reward are independent of exercise controllability. Eur. J. Neurosci. 2016;43:1190–1202. doi: 10.1111/ejn.13193. https://doi.org/10.1111/ejn.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holth J.K., Mahan T.E., Robinson G.O., Rocha A., Holtzman D.M. Altered sleep and EEG power in the P301S Tau transgenic mouse model. Ann. Clin. Transl. Neurol. 2017;4:180–190. doi: 10.1002/acn3.390. https://doi.org/10.1002/acn3.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Real E., Takamiya K., Kang M.-G., Ledoux J., Huganir R.L., Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. https://doi.org/10.1016/j.cell.2007.09.017 [DOI] [PubMed] [Google Scholar]
- Ide K., Secher N.H. Cerebral blood flow and metabolism during exercise. Prog. Neurobiol. 2000;61:397–414. doi: 10.1016/s0301-0082(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Intlekofer K.A., Cotman C.W. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol. Dis. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. https://doi.org/10.1016/j.nbd.2012.06.011 Special Issue: Gene-environment interactions in neuropsychiatric disorders. [DOI] [PubMed] [Google Scholar]
- Kang E.-B., Cho J.-Y. Effect of treadmill exercise on PI3K/AKT/mTOR, autophagy, and Tau hyperphosphorylation in the cerebral cortex of NSE/htau23 transgenic mice. J. Exerc. Nutr. Biochem. 2015;19:199–209. doi: 10.5717/jenb.2015.15090806. https://doi.org/10.5717/jenb.2015.15090806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E.-B., Kwon I.-S., Koo J.-H., Kim E.-J., Kim C.-H., Lee J., Yang C.-H., Lee Y.-I., Cho I.-H., Cho J.-Y. Treadmill exercise represses neuronal cell death and inflammation during Aβ-induced ER stress by regulating unfolded protein response in aged presenilin 2 mutant mice. Apoptosis. Int. J. Program. Cell Death. 2013;18:1332–1347. doi: 10.1007/s10495-013-0884-9. https://doi.org/10.1007/s10495-013-0884-9 [DOI] [PubMed] [Google Scholar]
- Kang J.-E., Cirrito J.R., Dong H., Csernansky J.G., Holtzman D.M. Acute stress increases interstitial fluid amyloid-β via corticotropin-releasing factor and neuronal activity. Proc. Natl. Acad. Sci. Unit. States Am. 2007;104:10673–10678. doi: 10.1073/pnas.0700148104. https://doi.org/10.1073/pnas.0700148104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.-E., Lim M.M., Bateman R.J., Lee J.J., Smyth L.P., Cirrito J.R., Fujiki N., Nishino S., Holtzman D.M. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. https://doi.org/10.1126/science.1180962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H.-C., Huang H.-J., Liang K.-C., Hsieh-Li H.M. Selective improvement of cognitive function in adult and aged APP/PS1 transgenic mice by continuous non-shock treadmill exercise. Brain Res. 2011;1403:1–11. doi: 10.1016/j.brainres.2011.05.056. https://doi.org/10.1016/j.brainres.2011.05.056 [DOI] [PubMed] [Google Scholar]
- Kennedy G., Hardman R.J., Macpherson H., Scholey A.B., Pipingas A. How does exercise reduce the rate of age-associated cognitive Decline? A review of potential mechanisms. J. Alzheimers Dis. 2017;55:1–18. doi: 10.3233/JAD-160665. https://doi.org/10.3233/JAD-160665 [DOI] [PubMed] [Google Scholar]
- Kiang J.G., Tsokos G.C. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol. Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- King A.C., Taylor C.B., Haskell W.L. Effects of differing intensities and formats of 12 months of exercise training on psychological outcomes in older adults. Health Psychol. 1993;12:292–300. doi: 10.1037//0278-6133.12.4.292. https://doi.org/10.1037/0278-6133.12.4.292 [DOI] [PubMed] [Google Scholar]
- Kojda G., Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc. Res. 2005;67:187–197. doi: 10.1016/j.cardiores.2005.04.032. https://doi.org/10.1016/j.cardiores.2005.04.032 [DOI] [PubMed] [Google Scholar]
- Koo J.H., Kwon I.S., Kang E.B., Lee C.K., Lee N.H., Kwon M.G., Cho I.H., Cho J., yong Neuroprotective effects of treadmill exercise on BDNF and PI3-K/Akt signaling pathway in the cortex of transgenic mice model of Alzheimer's disease. J. Exerc. Nutr. Biochem. 2013;17:151–160. doi: 10.5717/jenb.2013.17.4.151. https://doi.org/10.5717/jenb.2013.17.4.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancel M., Droste S.K., Sommer S., Reul J.M.H.M. Influence of regular voluntary exercise on spontaneous and social stress-affected sleep in mice. Eur. J. Neurosci. 2003;17:2171–2179. doi: 10.1046/j.1460-9568.2003.02658.x. [DOI] [PubMed] [Google Scholar]
- Laske C., Stellos K., Hoffmann N., Stransky E., Straten G., Eschweiler G.W., Leyhe T. Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. Int. J. Neuropsychopharmacol. 2011;14:399–404. doi: 10.1017/S1461145710001008. https://doi.org/10.1017/S1461145710001008 [DOI] [PubMed] [Google Scholar]
- Lautenschlager N.T., Cox K.L., Flicker L., Foster J.K., Bockxmeer F.M., van Xiao J., Greenop K.R., Almeida O.P. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. J. Am. Med. Assoc. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. https://doi.org/10.1001/jama.300.9.1027 [DOI] [PubMed] [Google Scholar]
- Lee K.-W., Kim J.-B., Seo J.-S., Kim T.-K., Im J.-Y., Baek I.-S., Kim K.-S., Lee J.-K., Han P.-L. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. J. Neurochem. 2009;108:165–175. doi: 10.1111/j.1471-4159.2008.05769.x. https://doi.org/10.1111/j.1471-4159.2008.05769.x [DOI] [PubMed] [Google Scholar]
- Li X., Wang Q., Hu T., Wang Y., Zhao J., Lu J., Pei G. A tricyclic antidepressant, amoxapine, reduces amyloid-β generation through multiple serotonin receptor 6-mediated targets. Sci. Rep. 2017;7:4983. doi: 10.1038/s41598-017-04144-3. https://doi.org/10.1038/s41598-017-04144-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K.Y., Mintun M.A., Fagan A.M., Goate A.M., Bugg J.M., Holtzman D.M., Morris J.C., Head D. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann. Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. https://doi.org/10.1002/ana.22096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-W., Shih Y.-H., Chen S.-J., Lien C.-H., Chang C.-Y., Huang T.-Y., Chen S.-H., Jen C.J., Kuo Y.-M. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer's disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 2015;118:189–197. doi: 10.1016/j.nlm.2014.12.005. https://doi.org/10.1016/j.nlm.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Liu H., Zhao G., Cai K., Zhao H., Shi L. Treadmill exercise prevents decline in spatial learning and memory in APP/PS1 transgenic mice through improvement of hippocampal long-term potentiation. Behav. Brain Res. 2011;218:308–314. doi: 10.1016/j.bbr.2010.12.030. https://doi.org/10.1016/j.bbr.2010.12.030 [DOI] [PubMed] [Google Scholar]
- Liu H., Zhao G., Zhang H., Shi L. Long-term treadmill exercise inhibits the progression of Alzheimer's disease-like neuropathology in the hippocampus of APP/PS1 transgenic mice. Behav. Brain Res. 2013;256:261–272. doi: 10.1016/j.bbr.2013.08.008. https://doi.org/10.1016/j.bbr.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Meijer M.K., Sommer R., Spruijt B.M., van Zutphen L.F.M., Baumans V. Influence of environmental enrichment and handling on the acute stress response in individually housed mice. Lab. Anim. 2007;41:161–173. doi: 10.1258/002367707780378168. https://doi.org/10.1258/002367707780378168 [DOI] [PubMed] [Google Scholar]
- Macauley S.L., Stanley M., Caesar E.E., Yamada S.A., Raichle M.E., Perez R., Mahan T.E., Sutphen C.L., Holtzman D.M. Hyperglycemia modulates extracellular amyloid-β concentrations and neuronal activity in vivo. J. Clin. Invest. 2015;125:2463–2467. doi: 10.1172/JCI79742. https://doi.org/10.1172/JCI79742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin S.K., Rynders C.A., Weltman J.Y., Barrett E.J., Weltman A. Exercise intensity modulates glucose-stimulated insulin secretion when adjusted for adipose, liver and skeletal muscle insulin resistance. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154063. https://doi.org/10.1371/journal.pone.0154063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliszewska-Cyna E., Xhima K., Aubert I. A comparative study evaluating the impact of physical exercise on disease progression in a mouse model of Alzheimer's disease. J. Alzheimers Dis. JAD. 2016;53:243–257. doi: 10.3233/JAD-150660. https://doi.org/10.3233/JAD-150660 [DOI] [PubMed] [Google Scholar]
- Martins I.J., Hone E., Foster J.K., Sünram-Lea S.I., Gnjec A., Fuller S.J., Nolan D., Gandy S.E., Martins R.N. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Mol. Psychiatr. 2006;11(721) doi: 10.1038/sj.mp.4001854. https://doi.org/10.1038/sj.mp.4001854 [DOI] [PubMed] [Google Scholar]
- Marx F., Blasko I., Grubeck-Loebenstein B. Mechanisms of immune regulation in Alzheimer's disease: a viewpoint. Arch. Immunol. Ther. Exp. 1999;47:205–209. [PubMed] [Google Scholar]
- Mayeux R., Stern Y. Epidemiology of alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a006239. https://doi.org/10.1101/cshperspect.a006239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry S.M., Pike K.C., Vitiello M.V., Logsdon R.G., Larson E.B., Teri L. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer's disease: results of a randomized, controlled trial. J. Am. Geriatr. Soc. 2011;59:1393–1402. doi: 10.1111/j.1532-5415.2011.03519.x. https://doi.org/10.1111/j.1532-5415.2011.03519.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Neurobiological and systemic effects of chronic stress. Chronic Stress Thousand Oaks Calif. 2017;1 doi: 10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirochnic S., Wolf S., Staufenbiel M., Kempermann G. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus. 2009;19:1008–1018. doi: 10.1002/hipo.20560. https://doi.org/10.1002/hipo.20560 [DOI] [PubMed] [Google Scholar]
- Moore K.M., Girens R.E., Larson S.K., Jones M.R., Restivo J.L., Holtzman D.M., Cirrito J.R., Yuede C.M., Zimmerman S.D., Timson B.F. A spectrum of exercise training reduces soluble Aβ in a dose-dependent manner in a mouse model of Alzheimer's disease. Neurobiol. Dis. 2016;85:218–224. doi: 10.1016/j.nbd.2015.11.004. https://doi.org/10.1016/j.nbd.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Moraska A., Deak T., Spencer R.L., Roth D., Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1321–R1329. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- Moseley P.L. Heat shock proteins and heat adaptation of the whole organism. J. Appl. Physiol. Bethesda Md. 1997;1985(83):1413–1417. doi: 10.1152/jappl.1997.83.5.1413. https://doi.org/10.1152/jappl.1997.83.5.1413 [DOI] [PubMed] [Google Scholar]
- Musazzi L., Tornese P., Sala N., Popoli M. Acute or Chronic? A stressful question. Trends Neurosci. 2017;40:525–535. doi: 10.1016/j.tins.2017.07.002. https://doi.org/10.1016/j.tins.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Musiek E.S., Xiong D.D., Holtzman D.M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med. 2015;47:e148. doi: 10.1038/emm.2014.121. https://doi.org/10.1038/emm.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D., Levi B.P., Morrison S.J. Integrating physiological regulation with stem cell and tissue homeostasis. Neuron. 2011;70:703–718. doi: 10.1016/j.neuron.2011.05.011. https://doi.org/10.1016/j.neuron.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation D.A., Hong S., Jak A.J., Delano-Wood L., Mills P.J., Bondi M.W., Dimsdale J.E. Stress, exercise, and Alzheimer's disease: a neurovascular pathway. Med. Hypotheses. 2011;76:847–854. doi: 10.1016/j.mehy.2011.02.034. https://doi.org/10.1016/j.mehy.2011.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer N.C., Kristo D., Steinle H., Lehmann M., Strohl K.P. REM sleep and catecholamine excretion: a study in elite athletes. Eur. J. Appl. Physiol. 2001;84:521–526. doi: 10.1007/s004210100383. https://doi.org/10.1007/s004210100383 [DOI] [PubMed] [Google Scholar]
- Nichol K.E., Parachikova A.I., Cotman C.W. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav. Brain Res. 2007;184:124–132. doi: 10.1016/j.bbr.2007.06.027. https://doi.org/10.1016/j.bbr.2007.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol K.E., Poon W.W., Parachikova A.I., Cribbs D.H., Glabe C.G., Cotman C.W. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J. Neuroinflammation. 2008;5(13) doi: 10.1186/1742-2094-5-13. https://doi.org/10.1186/1742-2094-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S., Matthews F.E., Barnes D.E., Yaffe K., Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. https://doi.org/10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- Ogoh S., Ainslie P.N. Cerebral blood flow during exercise: mechanisms of regulation. J. Appl. Physiol. Bethesda Md. 2009;1985(107):1370–1380. doi: 10.1152/japplphysiol.00573.2009. https://doi.org/10.1152/japplphysiol.00573.2009 [DOI] [PubMed] [Google Scholar]
- Paillard T., Rolland Y., de Souto Barreto P. Protective effects of physical exercise in Alzheimer's disease and Parkinson's disease: a narrative review. J. Clin. Neurol. 2015;11:212–219. doi: 10.3988/jcn.2015.11.3.212. https://doi.org/10.3988/jcn.2015.11.3.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parachikova A., Nichol K.E., Cotman C.W. Short-term exercise in aged Tg2576 mice alters neuroinflammation and improves cognition. Neurobiol. Dis. 2008;30:121–129. doi: 10.1016/j.nbd.2007.12.008. https://doi.org/10.1016/j.nbd.2007.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten A.R., Sickmann H., Hryciw B.N., Kucharsky T., Parton R., Kernick A., Christie B.R. Long-term exercise is needed to enhance synaptic plasticity in the hippocampus. Learn. Mem. Cold Spring Harb. N. 2013;20:642–647. doi: 10.1101/lm.030635.113. https://doi.org/10.1101/lm.030635.113 [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sports. 2006;16(Suppl 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. https://doi.org/10.1111/j.1600-0838.2006.00520.x [DOI] [PubMed] [Google Scholar]
- Rasgon N.L., Kenna H.A., Wroolie T.E., Kelley R., Silverman D., Brooks J., Williams K.E., Powers B.N., Hallmayer J., Reiss A. Insulin resistance and hippocampal volume in women at risk for Alzheimer's disease. Neurobiol. Aging. 2011;32:1942–1948. doi: 10.1016/j.neurobiolaging.2009.12.005. https://doi.org/10.1016/j.neurobiolaging.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla S., Suñol C., García-Mesa Y., Giménez-Llort L., Sanfeliu C., Cristòfol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology. 2014;81:55–63. doi: 10.1016/j.neuropharm.2014.01.037. https://doi.org/10.1016/j.neuropharm.2014.01.037 [DOI] [PubMed] [Google Scholar]
- Reynolds G.O., Otto M.W., Ellis T.D., Cronin-Golomb A. The therapeutic potential of exercise to improve mood, cognition, and sleep in Parkinson's disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2016;31:23–38. doi: 10.1002/mds.26484. https://doi.org/10.1002/mds.26484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H., Ambrée O., Lewejohann L., Herring A., Keyvani K., Paulus W., Palme R., Touma C., Schäbitz W.-R., Sachser N. Wheel-running in a transgenic mouse model of Alzheimer's disease: protection or symptom? Behav. Brain Res. 2008;190:74–84. doi: 10.1016/j.bbr.2008.02.005. https://doi.org/10.1016/j.bbr.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Ridgel A.L., Vitek J.L., Alberts J.L. Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabil. Neural Repair. 2009;23:600–608. doi: 10.1177/1545968308328726. https://doi.org/10.1177/1545968308328726 [DOI] [PubMed] [Google Scholar]
- Roh J.H., Jiang H., Finn M.B., Stewart F.R., Mahan T.E., Cirrito J.R., Heda A., Snider B.J., Li M., Yanagisawa M., de Lecea L., Holtzman D.M. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer's disease. J. Exp. Med. 2014;211:2487–2496. doi: 10.1084/jem.20141788. https://doi.org/10.1084/jem.20141788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf M.J., De Kloet E.R., Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress Amst. Neth. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress and disease. Science. 1955;122:625–631. doi: 10.1126/science.122.3171.625. https://doi.org/10.1126/science.122.3171.625 [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., West T., Yarasheski K., Swarm R., Jasielec M.S., Fisher J.R., Ficker W.D., Yan P., Xiong C., Frederiksen C., Grzelak M.V., Chott R., Bateman R.J., Morris J.C., Mintun M.A., Lee J.-M., Cirrito J.R. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3008169. https://doi.org/10.1126/scitranslmed.3008169 236re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.A., Makino S., Kvetnansky R., Post R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speaker K.J., Cox S.S., Paton M.M., Serebrakian A., Maslanik T., Greenwood B.N., Fleshner M. Six weeks of voluntary wheel running modulates inflammatory protein (MCP-1, IL-6, and IL-10) and DAMP (Hsp72) responses to acute stress in white adipose tissue of lean rats. Brain. Behav. Immun., Exercise Immunology in Health and Disease. 2014;39:87–98. doi: 10.1016/j.bbi.2013.10.028. https://doi.org/10.1016/j.bbi.2013.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M., Macauley S.L., Holtzman D.M. Changes in insulin and insulin signaling in Alzheimer's disease: cause or consequence? J. Exp. Med. 2016;213:1375–1385. doi: 10.1084/jem.20160493. https://doi.org/10.1084/jem.20160493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M., Rosvall P., Boza-Serrano A., Andersson E., Lexell J., Deierborg T. Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiol. Stress. 2016;5:8–18. doi: 10.1016/j.ynstr.2016.09.002. https://doi.org/10.1016/j.ynstr.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Rojas C., Aranguiz F., Varela-Nallar L., Inestrosa N.C. Voluntary running attenuates memory loss, decreases neuropathological changes and induces neurogenesis in a mouse model of Alzheimer's disease. Brain Pathol. Zurich Switz. 2016;26:62–74. doi: 10.1111/bpa.12255. https://doi.org/10.1111/bpa.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.B., Sallis J.F., Needle R. The relation of physical activity and exercise to mental health. Publ. Health Rep. 1985;100:195–202. [PMC free article] [PubMed] [Google Scholar]
- Thompson R.S., Roller R., Greenwood B.N., Fleshner M. Wheel running improves REM sleep and attenuates stress-induced flattening of diurnal rhythms in F344 rats. Stress Amst. Neth. 2016;19:312–324. doi: 10.1080/10253890.2016.1174852. https://doi.org/10.1080/10253890.2016.1174852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsvall L., Akerstedt T., Lindbeck G. Effects on sleep stages and EEG power density of different degrees of exercise in fit subjects. Electroencephalogr. Clin. Neurophysiol. 1984;57:347–353. doi: 10.1016/0013-4694(84)90158-5. [DOI] [PubMed] [Google Scholar]
- Tsatsoulis A., Fountoulakis S. The protective role of exercise on stress system dysregulation and comorbidities. Ann. N. Y. Acad. Sci. 2006;1083:196–213. doi: 10.1196/annals.1367.020. https://doi.org/10.1196/annals.1367.020 [DOI] [PubMed] [Google Scholar]
- Tse Y.C., Bagot R.C., Hutter J.A., Wong A.S., Wong T.P. Modulation of synaptic plasticity by stress hormone associates with plastic alteration of synaptic NMDA receptor in the adult hippocampus. PLoS One. 2011;6:e27215. doi: 10.1371/journal.pone.0027215. https://doi.org/10.1371/journal.pone.0027215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um H.-S., Kang E.-B., Koo J.-H., Kim H.-T., Jin-Lee, Kim E.-J., Yang C.-H., An G.-Y., Cho I.-H., Cho J.-Y. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer's disease. Neurosci. Res. 2011;69:161–173. doi: 10.1016/j.neures.2010.10.004. https://doi.org/10.1016/j.neures.2010.10.004 [DOI] [PubMed] [Google Scholar]
- Um H.S., Kang E.B., Leem Y.H., Cho I.H., Yang C.H., Chae K.R., Hwang D.Y., Cho J.Y. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer's disease in an NSE/APPsw-transgenic model. Int. J. Mol. Med. 2008;22:529–539. [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. https://doi.org/10.1038/6368 [DOI] [PubMed] [Google Scholar]
- van Praag H., Shubert T., Zhao C., Gage F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. Off. J. Soc. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. https://doi.org/10.1523/JNEUROSCI.1731-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S.A., Kronenberg G., Lehmann K., Blankenship A., Overall R., Staufenbiel M., Kempermann G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol. Psychiatr. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. https://doi.org/10.1016/j.biopsych.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Xiong J.Y., Li S.C., Sun Y.X., Zhang X.S., Dong Z.Z., Zhong P., Sun X.R. Long-term treadmill exercise improves spatial memory of male APPswe/PS1dE9 mice by regulation of BDNF expression and microglia activation. Biol. Sport. 2015;32:295–300. doi: 10.5604/20831862.1163692. https://doi.org/10.5604/20831862.1163692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita S., Amemiya S., Suzuki S., Kita I. Effects of spontaneous and forced running on activation of hypothalamic corticotropin-releasing hormone neurons in rats. Life Sci. 2007;80:356–363. doi: 10.1016/j.lfs.2006.09.027. https://doi.org/10.1016/j.lfs.2006.09.027 [DOI] [PubMed] [Google Scholar]
- Yuede C.M., Zimmerman S.D., Dong H., Kling M.J., Bero A.W., Holtzman D.M., Timson B.F., Csernansky J.G. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer's disease. Neurobiol. Dis. 2009;35:426–432. doi: 10.1016/j.nbd.2009.06.002. https://doi.org/10.1016/j.nbd.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E.Y., Liu W., Karatsoreos I.N., Ren Y., Feng J., McEwen B.S., Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol. Psychiatr. 2011;16:156–170. doi: 10.1038/mp.2010.50. https://doi.org/10.1038/mp.2010.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chao F.-L., Zhou C.-N., Jiang L., Zhang L., Chen L.-M., Luo Y.-M., Xiao Q., Tang Y. Effects of exercise on capillaries in the white matter of transgenic AD mice. Oncotarget. 2017;8:65860–65875. doi: 10.18632/oncotarget.19505. https://doi.org/10.18632/oncotarget.19505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sheng H., Qi J., Ma B., Sun J., Li S., Ni X. Glucocorticoid acts on a putative G protein-coupled receptor to rapidly regulate the activity of NMDA receptors in hippocampal neurons. Am. J. Physiol. Endocrinol. Metab. 2012;302:E747–E758. doi: 10.1152/ajpendo.00302.2011. https://doi.org/10.1152/ajpendo.00302.2011 [DOI] [PubMed] [Google Scholar]
- Zhao G., Liu H.L., Zhang H., Tong X.J. Treadmill exercise enhances synaptic plasticity, but does not alter β-amyloid deposition in hippocampi of aged APP/PS1 transgenic mice. Neuroscience. 2015;298:357–366. doi: 10.1016/j.neuroscience.2015.04.038. https://doi.org/10.1016/j.neuroscience.2015.04.038 [DOI] [PubMed] [Google Scholar]