Abstract
Background
The major efficacy trials on diabetes prevention have used resource-intensive approaches to identify high-risk individuals and deliver lifestyle interventions. Such strategies are not feasible for wider implementation in low- and middle-income countries (LMICs). We aimed to evaluate the effectiveness of a peer-support lifestyle intervention in preventing type 2 diabetes among high-risk individuals identified on the basis of a simple diabetes risk score.
Methods and findings
The Kerala Diabetes Prevention Program was a cluster-randomized controlled trial conducted in 60 polling areas (clusters) of Neyyattinkara taluk (subdistrict) in Trivandrum district, Kerala state, India. Participants (age 30–60 years) were those with an Indian Diabetes Risk Score (IDRS) ≥60 and were free of diabetes on an oral glucose tolerance test (OGTT). A total of 1,007 participants (47.2% female) were enrolled (507 in the control group and 500 in the intervention group). Participants from intervention clusters participated in a 12-month community-based peer-support program comprising 15 group sessions (12 of which were led by trained lay peer leaders) and a range of community activities to support lifestyle change. Participants from control clusters received an education booklet with lifestyle change advice. The primary outcome was the incidence of diabetes at 24 months, diagnosed by an annual OGTT. Secondary outcomes were behavioral, clinical, and biochemical characteristics and health-related quality of life (HRQoL). A total of 964 (95.7%) participants were followed up at 24 months. Baseline characteristics of clusters and participants were similar between the study groups. After a median follow-up of 24 months, diabetes developed in 17.1% (79/463) of control participants and 14.9% (68/456) of intervention participants (relative risk [RR] 0.88, 95% CI 0.66–1.16, p = 0.36). At 24 months, compared with the control group, intervention participants had a greater reduction in IDRS score (mean difference: −1.50 points, p = 0.022) and alcohol use (RR 0.77, p = 0.018) and a greater increase in fruit and vegetable intake (≥5 servings/day) (RR 1.83, p = 0.008) and physical functioning score of the HRQoL scale (mean difference: 3.9 score, p = 0.016). The cost of delivering the peer-support intervention was US$22.5 per participant. There were no adverse events related to the intervention. We did not adjust for multiple comparisons, which may have increased the overall type I error rate.
Conclusions
A low-cost community-based peer-support lifestyle intervention resulted in a nonsignificant reduction in diabetes incidence in this high-risk population at 24 months. However, there were significant improvements in some cardiovascular risk factors and physical functioning score of the HRQoL scale.
Trial registration
Australia and New Zealand Clinical Trials Registry ACTRN12611000262909.
Thirunavukkarasu Sathish and colleagues report findings from a community, peer-supported trial to reduce diabetes onset in high-risk individuals in India.
Author summary
Why was this study done?
In low- and middle-income countries (LMICs), there is an urgent need to develop low-cost strategies for identifying high-risk individuals and delivering lifestyle interventions to prevent diabetes.
What did the researchers do and find?
We conducted a cluster-randomized controlled trial in a community-setting in India to test whether a peer-support lifestyle intervention could reduce diabetes risk at 24 months.
We identified high-risk individuals on the basis of a simple diabetes risk score.
There was a nonsignificant reduction in diabetes risk in this high-risk population at 24 months. However, there were significant improvements in some risk factors for cardiovascular disease and a measure of quality of life.
The intervention cost was low at US$22.5 per participant over 12 months.
What do these findings mean?
Risk scores for better identifying people at highest risk for diabetes are needed, particularly in resource-constrained settings.
It is important to identify ways to improve program adherence and engagement, possibly by using more flexible modes of program delivery, e.g., at worksites and by text messaging.
Introduction
Type 2 diabetes is a major public health problem worldwide [1]. Globally, an estimated 425 million people have diabetes, and the majority of those (79%) are living in low- and middle-income countries (LMICs) such as India [1]. A large proportion of people with diabetes are undiagnosed, and many present with complications at the time of diagnosis [1]. Diabetes imposes a large economic burden on individuals, their families, and national health systems [1]. Therefore, there is an urgent need to develop and implement effective and cost-effective measures to prevent diabetes.
The major efficacy trials have shown that lifestyle interventions targeting physical activity, dietary changes, and weight loss are effective [2–5] and cost-effective [6,7] in preventing type 2 diabetes among people with impaired glucose tolerance (IGT). However, while this is encouraging, the real challenge is to deliver such interventions under ‘real-world’ conditions [8]. The efficacy trials required the expensive oral glucose tolerance test (OGTT) to identify high-risk individuals and involved specialized multidisciplinary teams (e.g., physicians, nurses, dieticians, exercise physiologists) to deliver interventions. These are important factors limiting the translation of findings from the efficacy trials to real-world settings, particularly in LMICs, thereby requiring alternative strategies for identifying high-risk individuals and delivering interventions [9].
Mass screening with an OGTT to identify high-risk individuals is highly challenging in LMICs because of the cost and the limited availability of trained clinical staff and accredited laboratories [10]. Diabetes risk scores are low-cost, noninvasive, and easy-to-administer screening tools, which could reduce the number of OGTTs when used in a stepwise screening approach [11]. International guidelines and expert groups recommend using diabetes risk scores as the first screening step to identify people who may be at high risk, with blood tests undertaken to confirm high-risk status (i.e., prediabetes). These high-risk individuals can then be referred to a lifestyle intervention program [12,13]. However, even these approaches require blood testing on up to 50% of adults, posing difficulties in low-resource settings.
Lifestyle interventions evaluated in the major efficacy trials have involved resource-intensive, individualized counselling delivered on a one-to-one basis or in groups by highly trained health professionals [2–5]. In the Chinese Da Qing IGT and Diabetes Study [3], physicians delivered one individual counselling session and group counselling sessions every week for one month, followed by monthly for three months and every three months for the next 5.8 years. In the United States Diabetes Prevention Program (US DPP) [2], participants in the lifestyle intervention group received 16 individual counselling sessions from case managers within the first 24 weeks following randomization, and then, face-to-face contacts (individual or group) were made every two months for another 2.5 years. Over three years, the intervention costs were US$2,780 per participant [14]. In the Finnish Diabetes Prevention Study [5], participants received one-to-one individualized counselling sessions from nutritionists. Seven sessions were delivered in the first year and one session every three months thereafter until the end of the study at six years. Exercise physiologists guided participants to increase their physical activity through individualized resistance training sessions. In the Indian Diabetes Prevention Programme (IDPP) [4], although the intervention was less labor- and resource-intensive (US$225 per participant over three years) [6] than other efficacy trials, it was delivered on a one-to-one basis by physicians, dieticians, and social workers. These intervention strategies are not feasible for wider implementation in real-world settings in LMICs, where the burden of diabetes is substantial [1] and where the availability of highly trained health professionals for program delivery is very limited [15]. Peer support is an alternative strategy to encourage people to make and sustain healthy lifestyle changes [16]. Peer support refers to the provision of practical, social, and emotional ongoing support from nonprofessionals for complex health behaviors [17]. A recent systematic review has shown that peer support is effective in bringing behavior change in prevention and management of various health conditions, including HIV/AIDS, maternal and child health, and mental health, as well as diabetes, cardiovascular disease, and other chronic conditions [17]. Peer-support interventions are low-cost, culturally appropriate, and potentially scalable [17].
The Kerala Diabetes Prevention Program (K-DPP) was a cluster randomized controlled trial (RCT) of a peer-support lifestyle intervention implemented in a community setting in India [18]. In this paper, we aimed to examine whether the intervention could reduce diabetes incidence at 24 months among high-risk individuals identified on the basis of a diabetes risk score.
Methods
Ethics statement
The study was approved by the Health Ministry Screening Committee of the Government of India; ethics committees of the Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCT/IEC-333/May 2011), Trivandrum, India; Monash University (CF11/0457-2011000194); and The University of Melbourne (1441736) in Australia. Written informed consent was obtained from all study participants.
Study design
This study is reported in accordance with the Consolidated Standards of Reporting Trials guidelines for cluster RCTs (S1 Checklist) [19]. The details of the K-DPP study design have been described in detail elsewhere [18], and the protocol is available at https://link.springer.com/article/10.1186/1471-2458-13-1035. Briefly, K-DPP was a cluster RCT conducted in 60 polling areas (clusters) of Neyyattinkara taluk (subdistrict) in Trivandrum district, Kerala state. Polling areas are well-defined and identifiable locations demarcated with landmarks such as hills, rivers, roads, streets, etc. by the Election Commission of India [20]. A cluster design was chosen for the study, as the risk of contamination would otherwise be high among individuals from the same community.
Randomization and masking
At the time of study enrolment, Neyyattinkara taluk had 603 polling areas across four legislative assembly constituencies (LACs). Although there were maps available to locate polling areas in each of the four LACs, there was no single map connecting the four LACs, which could show the contiguous polling areas across the borders of LACs. To reduce the risk of selecting contiguous polling areas, we removed 244 polling areas that were located along the borders of the four LACs and selected at random 60 of the remaining 359 polling areas. The 60 polling areas were then randomly assigned in a 1:1 ratio to the control group (received an education booklet on general lifestyle advice) or the intervention group (peer-support lifestyle intervention) by an independent statistician using a computer-generated randomization sequence. After randomization, in both the intervention and control groups, there were two contiguous polling areas. Therefore, one polling area from each pair was replaced with a nearby polling area, which were at least two kilometers apart. Participants were masked to group assignment until the completion of the baseline assessment. Field staff members administering questionnaires (no group-specific questions were included in the questionnaires) and undertaking measurements at baseline and follow-ups, along with laboratory technicians and investigators, were masked. Field staff members administering the process evaluation questionnaire to intervention participants were not masked.
Participants
From the electoral roll of each of the 60 polling areas, 80 individuals (age 30 to 60 years) were selected randomly and were approached through home visits. Eligibility criteria included no history of diabetes or other chronic illness that might affect their participation in the trial, being literate in the local language (Malayalam), not being pregnant, and not taking medications known to affect glucose tolerance (glucocorticoids, antiretroviral drugs and antipsychotics). Those satisfying the eligibility criteria were screened using the Indian Diabetes Risk Score (IDRS), which comprises four simple parameters: age, family history of diabetes, physical activity (regular exercise or strenuous work), and waist circumference [21]. At the time of study enrolment, the IDRS was the only risk score from India that had been previously evaluated in a cohort study, with a score of ≥60 being a strong predictor of incident diabetes in Asian Indians [22]. Therefore, we chose the IDRS to screen and recruit our trial participants. Those with an IDRS score ≥60 were invited to attend a community-based clinic to undergo a 75-gm OGTT. Clinics were conducted in local neighborhoods in community buildings (e.g., schools, library halls, church halls). The OGTT was performed according to the World Health Organization (WHO) guidelines [23]. A venous blood sample was taken after an overnight fast for at least eight hours, and a second blood sample was collected two hours after oral ingestion of 75-g glucose dissolved in 250–300 ml of water. Those with fasting plasma glucose (FPG) ≥7.0 mmol/l or 2-hr plasma glucose (2-hr PG) ≥11.1 mmol/l or both were diagnosed to have diabetes based on the American Diabetes Association (ADA) criteria [12]. They were then referred to a healthcare facility and were excluded from the study. The remaining individuals were enrolled in the trial irrespective of their baseline glucose tolerance. If the participant had not fasted for the recommended time, they were asked to attend another clinic in a nearby neighborhood on a different day.
Interventions
Detailed information on the development and cultural adaptation of the intervention program have been reported elsewhere [24,25]. Briefly, the main theory underpinning the intervention program was the Health Action Process Approach model [26] with more emphasis given to collectivistic rather than individualistic strategies during the intervention design phase. The intervention program was adapted from the Finnish Good Ageing in Lahti Region (GOAL) program [27] and the Australian Greater Green Triangle (GGT) Diabetes Prevention Project [28] through situational analysis, needs assessment, and cultural translation [24,25]. This adaptation process was guided by the Intervention Mapping Approach [29]. The program utilized the core functions of peer support identified in the US Peers for Progress Program [16] and incorporated behavior change strategies that were identified from the needs assessment study [25]. The intervention model and program were tested and further refined following piloting with two groups in 2012–2013 [18].
The 12-month peer-support program consisted of 15 group sessions: an introductory session delivered by the K-DPP team; two education sessions conducted by local experts; and 12 sessions delivered by trained lay peer leaders. An introductory session was planned to introduce the group participants to the program and its mentoring style. The needs assessment study showed that at least one peer group session per month would be optimal and feasible to deliver the intervention [25], and thus 12 peer group sessions were planned. The needs assessment study also emphasized the importance of including sessions on diabetes prevention and management by local experts, as the knowledge on these among people with prediabetes in Kerala was low [25]. Furthermore, during the pilot phase [18], peer leaders were selected from within the groups, and their level of diabetes-specific knowledge was also limited. Therefore, we decided that information would be delivered by experts through two education sessions, and the peer leaders’ role would be to help participants to translate the information into their daily lives. To minimize resources, the education sessions were delivered to participants from two to three neighborhoods within close proximity. Participants were encouraged to bring family members along to further extend the reach of the education.
Group sessions and community activities
All group sessions were held in the local community in a convenient neighborhood facility (e.g., schools, community halls). Sessions were conducted during weekends at a convenient time for participants. In the introductory group session (lasted for 60–90 minutes), the K-DPP team introduced the program and its benefits to the participants and their family members. The two education sessions (each lasting for half a day) were conducted by experts in the field of diabetes, nutrition, and physical activity. These sessions focused on the etiology of diabetes, risk factors, misconceptions around diabetes, and the role of lifestyle change in preventing and managing diabetes. The experts also reinforced the role and importance of peer support in behavior change and encouraged participants to attend the peer group sessions. During the introductory group session, each group selected two peer leaders (one male, one female) from among their participants with assistance from the K-DPP team. Peer leaders were identified on the basis of their willingness to lead the group, social credibility, and acceptability by the group. Peer groups consisted of 10 to 23 participants, with approximately equal numbers of males and females. The first two peer group sessions were held fortnightly, and the subsequent 10 sessions were held every month thereafter, with each session lasting 60 to 90 minutes. Each session had specific objectives and structured content to be covered, which maps onto interventions delivered in the Finnish GOAL program [27,30] and the Australian GGT project [28] (refer to S1 Table for further details).
Based on a sociobehavioral intervention model for lifestyle change [26], the peer group sessions aimed to achieve the following key lifestyle objectives:
Increasing physical activity
Promoting healthy eating habits
Maintaining appropriate body weight by balancing calorie intake and physical activity
Tobacco cessation
Reducing alcohol consumption
Ensuring adequate sleep
Lifestyle change strategies for increasing physical activity focused on identifying enjoyable activities for individuals and groups (e.g., walking groups, yoga sessions) and building those activities into each participant’s daily routine. Advice on healthy diet included increasing the intake of fruits and vegetables and reducing the portion size of rice and intake of fried foods and refined sugars. Participants used goal setting, action planning and self-recording of activities in their program workbook as key behavioral strategies for increasing physical activity and healthy eating. Participants received a handbook containing information on peer support, its benefits, and its role in assisting with sustainable lifestyle changes related to reducing diabetes risk. Body weight of participants was measured during the peer group sessions. Peer leaders also had regular contact with their group participants between the formal group sessions in order to reinforce the program objectives, update on the content of missed sessions, and encourage goal attainment and attendance at the next peer group session. Group participants were also encouraged by their peer leaders to participate in community activities such as establishing kitchen gardens, yoga training, and walking groups to support lifestyle change. These activities were led by peer leaders with assistance from local resource persons (LRPs) (community volunteers). While groups were encouraged to keep meeting at completion of the formal program at 12 months, there was no structured support provided for this.
Training, quality assurance and support for peer leaders
Peer leaders were provided two training sessions (each of two days’ duration) by the K-DPP staff members: an intervention manager (registered nurse with a PhD in public health) and an intervention assistant (medical social worker). These sessions aimed to build peer leaders’ basic knowledge about diabetes, to emphasize the role of a peer leader, and to provide skills on group facilitation, communication, goal setting, and promoting community activities. Refer to S1 Text for the content of these training sessions. Peer leaders were provided with a workbook, which outlined peer group sessions’ objectives, along with an activity guide and exercises to prepare them for conducting the sessions. Peer leaders were also given measuring cups and spoons to assist them in educating the participants about the daily recommended food quantities such as consumption of oil, sugar, salt, rice, and vegetables. Trained LRPs were asked to attend the peer group sessions as observers and to complete a detailed checklist to ensure program fidelity.
During the program delivery, peer leaders were also supported in the following ways:
Telephone calls were made by the K-DPP team before and after each session to discuss the sessions and any challenges faced by the peer leaders.
Practical support was provided by LRPs to help organize the logistics for local program delivery.
Reimbursement of costs for attending training sessions was provided.
Control participants received an education booklet concerning information about diabetes and its risk factors, as well as standard advice about lifestyle change.
Procedures
Participants were assessed at baseline, 12 months, and 24 months. During each assessment, field staff members administered standardized and validated questionnaires to collect measures of sociodemographic characteristics, lifestyle behaviors, medical history, and health-related quality of life (HRQoL). Self-reported levels of physical activity were measured using the Global Physical Activity Questionnaire [31]. Intake of fruits and vegetables were assessed using a food frequency questionnaire [32]. HRQoL was assessed using the 36-item Short-Form (SF-36) health survey [33]. The SF-36 is divided into eight scales (physical functioning, role limitation—physical, role limitation—emotional, bodily pain, general health, mental health, social functioning, and vitality) and two domains (physical component summary and mental component summary). Scores for each of the scales and domains range from 0 to 100, with higher scores indicating better quality of life. The SF-36 data were converted into a six-dimensional health state called the Short Form 6 Dimension (SF-6D), whose score ranges between 0.29 (worse health) and 1.00 (full health). Following the 12-month intervention period, a process evaluation questionnaire was administered to intervention participants by field staff members who were different from those administering main questionnaires. During each assessment, anthropometry (height, weight, fat percent, muscle mass, waist circumference, and hip circumference) and blood pressure were measured, and blood samples were taken for the OGTT, HbA1c, and lipids according to standard protocols [34]. Individuals diagnosed with diabetes on the OGTT at 12-month follow-up were referred to healthcare facilities for treatment and care. However, they were still followed-up, although an OGTT was not performed at 24 months; instead, FPG alone was measured. Blood samples were centrifuged within 30 minutes of collection and transported in boxes with dry ice to a nationally accredited laboratory. Plasma glucose was measured using the hexokinase method on a COBAS 6000 analyzer, with kits supplied from Roche Diagnostics, Switzerland. HbA1c was measured using the high-performance liquid chromatography method on a D-10 BIORAD analyzer and lipids by enzymatic methods on a COBAS 6000 analyzer, using kits supplied by Roche Diagnostics, Switzerland. Low-density lipoprotein (LDL) cholesterol was estimated using the Friedewald equation [35] for participants with triglycerides ≤4.52 mmol/l, and for the rest, values obtained from the direct method were used in the analysis.
Outcomes
The primary outcome was the incidence of diabetes at 24 months, diagnosed by an annual OGTT, according to the ADA criteria (FPG ≥7.0 mmol/l and/or 2-hr PG ≥11.1 mmol/l) [12]. Participants who were diagnosed with diabetes by a physician and taking antidiabetic medications (‘clinical diagnosis’) subsequent to entry in the trial were also included in the primary outcome. Secondary outcomes included weight, waist circumference, waist-to-hip ratio, fat percent, muscle mass, systolic and diastolic blood pressure, FPG, 2-hr PG, HbA1c, lipid profile, IDRS score, ≥5 servings of fruit and vegetables intake per day, physical activity, tobacco use, alcohol use, and HRQoL.
Statistical analysis
Assuming an annual incidence of diabetes of 18.3% in the control group [4], an intracluster correlation coefficient (ICC) of 0.02 for plasma glucose [36], an average of 17 participants per group, at 5% significance with 80% power, allowing a loss to follow-up of 10%, the numbers of participants and polling areas per study group required were 510 and 30 respectively, to detect a relative risk reduction (RRR) of 30% at 24 months [18]. Funding dictated that the primary outcome be measured at 24 months’ follow-up. Since ICC values for diabetes incidence were not available from published studies, ICC for plasma glucose from a previous study [36] was used. However, a positive ICC for diabetes incidence was not observed in the trial. Hence, the 32% inflation of sample size for a design effect was redundant.
Baseline characteristics of clusters and participants are summarized using mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables and frequency and percentage for categorical variables. The analyses observed intention-to-treat, i.e., participants and clusters were analyzed according to the group to which they were allocated. There were a few changes to the analysis plan specified in the study protocol [18]. For the primary outcome analysis, instead of logistic regression and Cox-proportional hazards regression, to estimate the relative risk (RR) (and 95% confidence interval [CI] and P value) at 24 months, we used log binomial models estimated by generalized estimating equations (GEE) with an exchangeable working correlation structure and robust standard errors to account for clustering by polling areas. This approach gave improved interpretability of the intervention effect (as increased cumulative incidence (RR) rather than increased odds) and accorded with the use of RR in the protocol’s sample size. As diabetes was only observed systematically at 12- and 24-month time points a discrete time proportional hazards model was considered appropriate in place of the Cox model but this model provides little extra information above and beyond the log-binomial model for diabetes incidence at 12 and 24 months. We also conducted post hoc subgroup analyses by baseline glucose tolerance: normal glucose tolerance (NGT), isolated impaired fasting glucose (IFG), and IGT defined by the ADA criteria [12] and the WHO criteria [23]. To examine the heterogeneity of intervention effect by subgroup, an interaction term between the intervention assignment and subgroup was included in the GEE models, and its significance was tested using the Wald test. The subgroup analyses were done because the current literature on diabetes prevention programs has been largely limited to people with IGT [37], yet the target population in the real world is much broader.
For the analysis of continuous secondary outcomes, mixed-effects linear regression models were used and included outcomes at baseline, 12 months and 24 months, and included all participants with outcome data available at one or more of these timepoints. Skewed variables were log-transformed before analysis. Study group (intervention vs. control), timepoint (follow-up vs. baseline) and a study group-by-timepoint interaction were specified as fixed effects. Random effects were specified for polling areas, to account for the clustered study design, and for participants, to account for correlation between the repeated measurements on the same individual. The P value of the study-group-by-timepoint interaction was used to test the difference in change between study groups. For categorical secondary outcomes, the log binomial model was used.
We assessed the sensitivity of the primary outcome analysis to missing outcome data using multilevel multiple imputation (MMI), accounting for clustering [38]. We performed 10 imputations using GEE to fit log binomial imputation models for missing outcomes and with study group and the following baseline covariates included as auxiliary variables: age, sex, education, occupation, monthly household expenditure, current tobacco use, current alcohol use, fruit and vegetable intake (in servings/day), leisure time physical activity, family history of diabetes, body mass index, waist-to-hip ratio, fat percent, muscle mass, systolic blood pressure, diastolic blood pressure, FPG, 2-hr PG, HbA1c, LDL cholesterol, and triglycerides. The log RR (and its standard error) was computed on each multiply imputed dataset, and the results were combined to obtain the multiple imputation estimate using Rubin’s rule [39]. MMI was performed using the R Jomo package [40].
The costs associated with delivering the peer-support intervention over 12 months were estimated across five major categories (training sessions for peer leaders and LRPs, group sessions, resource materials, administrative costs, and community activities). The K-DPP personnel (intervention manager and intervention assistant) were interviewed to estimate the amount of time they spent on various intervention activities. Personnel costs were calculated based on the actual salary (or remuneration) paid to the intervention manager, intervention assistant, local experts, and LRPs. Nonpersonnel costs (travel, food and logistics, rent for venues, phone calls, designing and printing charges for resource materials, and administrative costs) were estimated based on the actual expenditure. The cost figures were obtained from the finance registers. The cost estimates in Indian Rupees (INR) were converted to US dollars using an exchange rate of INR58.6 = US$1 for the year 2013 [41].
A two-sided P value <0.05 was considered statistically significant for all analyses. Analyses were performed using Stata version 14.2 (StataCorp LP, College Station, Texas, USA), R 3.4.3, or Microsoft Excel 2016 (Microsoft corporation, Redmond, Washington, USA).
Results
Participant flow and characteristics
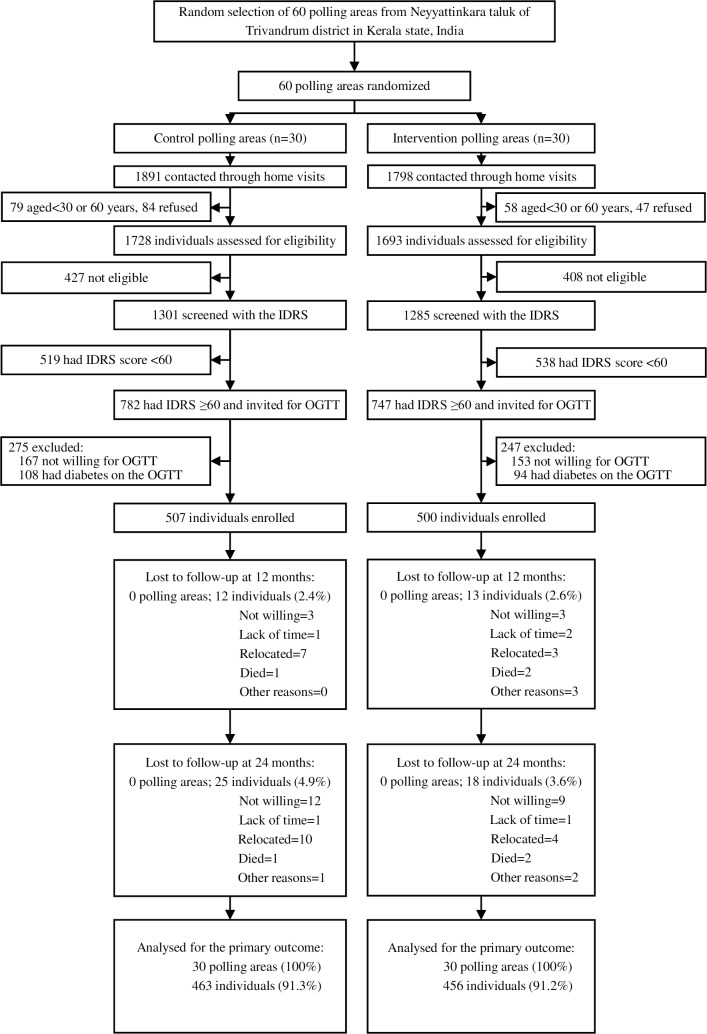
Participants from the 60 polling areas were recruited between January 20, 2013, and October 27, 2013. Fig 1 shows the trial profile. A total of 3,689 individuals were contacted through home visits, of whom 137 (3.7%) did not satisfy the age criteria and were therefore excluded. Of the remaining 3,552 individuals, 131 (3.7%) declined participation, and 3421 were assessed for eligibility, of whom 835 (24.4%) were not eligible. Of 2,586 eligible individuals screened with the IDRS, 1,529 (59.1%) had a score ≥60, of whom 1,209 (79.1%) attended community-based clinics and underwent an OGTT. After excluding 202 (16.7%) individuals with diabetes, 1,007 (507 in the control group and 500 in the intervention group) were enrolled in the trial. Baseline characteristics of clusters and participants were similar between the study groups (Table 1). Participants’ mean age was 46.0 years, and the majority were male (52.8%), educated up to secondary school (75.6%), and employed (72.3%). According to the ADA criteria [12], 11.5% had IGT, 57.5% had isolated IFG, and 31.0% had NGT. The corresponding figures for the WHO criteria [23] were 11.5%, 22.5%, and 66.0%, respectively. The prevalence of several cardiovascular risk factors was high at baseline, as reported previously [42]. All clusters and 95.7% (964/1007) of participants were followed-up at 24 months (95.1% in the control group; 96.4% in the intervention group).
Fig 1. Trial profile.
IDRS, Indian Diabetes Risk Score; OGTT, oral glucose tolerance test.
Table 1. Baseline characteristics of clusters and participants by study group.
|
Excluded = 250: Not able to speak, read and write the local language = 40 Known T2DM = 160 Other chronic illness = 39 Pregnancy = 2 Taking medications known to influence glucose tolerance = 9 |
Control group | Intervention group |
|---|---|---|
| Cluster level | ||
| No. of polling areas | 30 | 30 |
| Average no. of individuals/polling area | 1100 | 1150 |
| Individual level | ||
| No. of participants | 507 | 500 |
| Socio-demographic characteristics | ||
| Age (years), mean (SD) | 45.7 (7.4) | 46.2 (7.6) |
| Female, n (percent) | 236 (46.6) | 239 (47.8) |
| Education, n (percent) Up to primary Middle Secondary Higher secondary Vocational education College or above |
117 (23.1) 143 (28.2) 123 (23.5) 42 (8.3) 31 (6.1) 51 (10.1) |
136 (27.2) 129 (25.8) 114 (22.8) 43 (8.6) 28 (5.6) 50 (10.0) |
| Occupation, n (percent) Skilled/unskilled Homemaker Unemployed/retired |
361 (71.2) 139 (27.4) 7 (1.4) |
367 (73.4) 129 (25.8) 4 (0.8) |
| Monthly household expenditure (INR), median (IQR) | 6000 (5000 to 10000) | 7000 (5000 to 10000) |
| Marital status, n (percent) Married Separated Divorced Widowed Single |
482 (95.1) 2 (0.4) 1 (0.2) 19 (3.8) 3 (0.6) |
476 (95.2) 4 (0.8) 3 (0.6) 9 (1.8) 8 (1.6) |
| Clinical characteristics | ||
| Weight (kg), mean (SD) | 64.5 (12.1) | 62.6 (11.6) |
| Waist circumference (cm), mean (SD) | 88.7 (9.7) | 87.9 (9.7) |
| Waist-to-hip ratio, mean (SD) | 0.93 (0.06) | 0.93 (0.07) |
| Fat percent (percent), mean (SD) | 30.0 (8.7) | 29.7 (8.2) |
| Muscle mass (kg), mean (SD) | 42.4 (8.4) | 41.5 (8.3) |
| Systolic blood pressure (mmHg), mean (SD) | 123.4 (17.9) | 123.0 (17.6) |
| Diastolic blood pressure (mmHg), mean (SD) | 74.8 (12.1) | 75.0 (11.5) |
| IDRS score, mean ± SD | 67.5 (8.4) | 66.8 (8.3) |
| Bio-chemical characteristics | ||
| Fasting plasma glucose (mmol/l), mean (SD) | 5.8 (0.5) | 5.8 (0.5) |
| 2-hr plasma glucose (mmol/l), mean (SD) | 6.0 (1.5) | 5.9 (1.6) |
| HbA1c (percent), mean (SD) | 5.6 (0.5) | 5.6 (0.5) |
| Total cholesterol (mmol/l), mean (SD) | 5.7 (1.1) | 5.7 (1.0) |
| LDL cholesterol (mmol/l), mean (SD) | 3.8 (0.9) | 3.8 (0.9) |
| Triglycerides (mmol/l), median (IQR) | 1.1 (0.9 to 1.6) | 1.2 (0.9 to 1.6) |
| Behavioral characteristics | ||
| ≥5 servings of fruit and vegetables/daya, n (percent) | 84 (16.6) | 91 (18.2) |
| Physically active (leisure time)b, n (percent) | 102 (20.4) | 107 (21.1) |
| Current tobacco usec, n (percent) | 92 (18.2) | 102 (20.4) |
| Current alcohol used, n (percent) | 97 (19.1) | 114 (22.8) |
| Standard drinks of alcohol (per drinking occasion)e, mean (SD) | 0.2 (0.4) | 0.2 (0.4) |
| Medical history | ||
| Family history of diabetes, n (percent) | 260 (51.3) | 222 (44.4) |
| Anti-hypertensive drugs, n (percent) | 40 (7.9) | 35 (7.0) |
| Lipid-lowering drugs, n (percent) | 12 (2.4) | 9 (1.8) |
| Health-Related Quality of Life variables | ||
| Physical functioning, mean (SD) | 77.8 (23.4) | 75.6 (23.5) |
| Bodily pain, mean (SD) | 70.5 (29.4) | 69.9 (28.8) |
| Role limitation - physical, mean (SD) | 83.8 (21.6) | 82.2 (22.0) |
| Role limitation - emotional, mean (SD) | 84.4 (21.9) | 83.4 (23.0) |
| Social functioning, mean (SD) | 88.1 (20.3) | 87.1 (22.3) |
| Vitality, mean (SD) | 73.2 (20.8) | 74.0 (20.5) |
| General health, mean (SD) | 64.6 (24.7) | 64.9 (23.5) |
| Mental health, mean (SD) | 76.7 (19.8) | 79.4 (19.0) |
| Physical component summary, mean (SD) | 49.9 (8.5) | 49.1 (8.7) |
| Mental component summary, mean (SD) | 53.2 (9.1) | 54.0 (9.1) |
| SF-6D, mean (SD) | 0.78 (0.15) | 0.78 (0.15) |
Abbreviations: IDRS, Indian Diabetes Risk Score; INR, Indian Rupees; IQR, interquartile range; LDL, low density lipoprotein; SD, standard deviation; SF-6D, Short Form 6 Dimension. Percentages may not add up to 100% due to rounding.
a One serving of fruit equals a medium-sized fruit or two small-sized fruits or half a glass of fruit juice or a bowl of grapes. One serving of vegetables (excludes tubers) equals 80 grams.
b Self-reported history of moderate or vigorous physical activities during leisure time performed in bouts of at least 10 minutes’ duration.
c Smoking or use of smokeless tobacco products (chewing tobacco and snuff) in the past 30 days.
d Drank an alcoholic drink in the past 30 days.
e One standard drink of alcohol refers to 30 ml of spirits, 120 ml of wine, 285 ml of beer, or 285 ml of toddy (palm wine).
Process evaluation
Of the 15 total program sessions, participants attended a median of 9 (IQR 3 to 13) sessions; 10.8% attended all 15 sessions, 62.4% attended seven or more sessions, and 89.2% attended at least one of these sessions. Twenty-nine out of 30 groups delivered all of their 12 peer group sessions, according to the intervention protocol. Nearly two-thirds (61.8%, 309/500) of intervention participants reported that they had regular contact with peer leaders outside the formal group sessions during the 12-month program, with a mean number of contacts being 11.3 (SD 8.1). More than half (57.2%, 286/500) reported participation in community activities, including yoga sessions, kitchen gardening, and walking groups. Among those who did not attend formal program sessions (n = 54), one-third (33.3%) still reported that they had regular contact with their peer leaders outside the group sessions, and 16.7% also reported participating in community activities. Overall, only 27 participants (5.4%) did not have any exposure to the program.
Incidence of diabetes
After a median follow-up of 24 months, overall, diabetes developed in 147 participants (144 were diagnosed on the OGTT and 3 were clinically diagnosed): 17.1% (79/463) of participants in the control group and 14.9% (68/456) of participants in the intervention group. The RR was 0.88 (95% CI 0.66–1.16), p = 0.36. The RR did not change appreciably after excluding those with baseline HbA1c ≥6.5% (n = 52) (ADA [12] and International Expert Committee [43] cutoff value for diabetes) (RR 0.93, 95% CI 0.66–1.31, p = 0.66) and was similar to the results obtained using MMI (RR 0.86, 95% CI 0.61–1.13), p = 0.29.
The RR in the IGT subgroup (ADA and WHO criteria: 0.66, 95% CI 0.45–0.98, p = 0.038) was lower than that in the isolated IFG (ADA criteria: 0.95, 95% CI 0.68–1.33, p = 0.77; WHO criteria: 0.98, 95% CI 0.68–1.42, p = 0.92) or NGT (ADA criteria: 1.23, 95% CI 0.36–4.26; WHO criteria: 1.15, 95% CI 0.66–2.01, p = 0.63) subgroups. However, there was no evidence in favor of an interaction between baseline glucose tolerance and study group on diabetes incidence (ADA criteria: p = 0.24; WHO criteria: p = 0.11).
Secondary outcomes
Table 2 shows the changes in clinical and biochemical characteristics by study group at 24 months. The IDRS score reduced in both study groups, but the reduction was greater in the intervention group by 1.50 points (p = 0.022). Table 3 shows the changes in behavioral characteristics by study group at 24 months. Compared with the control participants, intervention participants were more likely to consume ≥5 servings of fruit and vegetables per day by 83% (p = 0.008). Intervention participants were 23% less likely to consume alcohol compared with the control participants (p = 0.018), and the amount of alcohol consumed was lower among intervention participants (p = 0.030). Table 4 shows the changes in HRQoL variables by study group at 24 months. Compared with the control group, the intervention participants had a greater increase in physical functioning score of the HRQoL scale by 3.9 points (p = 0.016). The 12-month changes in secondary outcomes by study group are given in S2, S3 and S4 Tables.
Table 2. Changes in clinical and biochemical characteristics at 24 months by study group.
| Control group | Intervention group | |||
|---|---|---|---|---|
| Mean change (SD) | Mean change (SD) | Difference* (95% CI) | P value | |
| Weight (kg) | 1.24 (2.91) | 1.22 (3.27) | 0.01 (−0.34 to 0.36) | 0.95 |
| Waist circumference (cm) | 0.63 (6.97) | −0.07 (5.95) | −0.67 (−1.56 to 0.22) | 0.14 |
| Waist-to-hip ratio | −0.011 (0.062) | −0.020 (0.078) | −0.008 (−0.018 to 0.002) | 0.12 |
| Fat percent (percent) | 0.54 (3.17) | 0.61 (2.77) | 0.10 (−0.29 to 0.48) | 0.62 |
| Muscle mass (kg) | 0.52 (3.43) | 0.42 (1.69) | −0.10 (−0.39 to 0.20) | 0.52 |
| Fasting plasma glucose (mmol/l) | 0.230 (0.988) | 0.225 (0.811) | 0.014 (−0.088 to 0.115) | 0.79 |
| Two-hour plasma glucose (mmol/l) | 0.47 (2.11) | 0.43 (1.97) | −0.07 (−0.34 to 0.20) | 0.63 |
| HbA1c (percent) | 0.056 (0.603) | −0.003 (0.430) | −0.058 (−0.122 to 0.006) | 0.08 |
| Systolic blood pressure (mmHg) | 0.28 (13.29) | −0.92 (13.44) | −1.22 (−2.80 to 0.35) | 0.13 |
| Diastolic blood pressure (mmHg) | −0.49 (9.74) | −1.59 (9.86) | −1.12 (−2.29 to 0.05) | 0.06 |
| Total cholesterol (mmol/l) | −0.11 (0.87) | −0.13 (0.80) | −0.01 (−0.11 to 0.08) | 0.79 |
| LDL cholesterol (mmol/l) | −0.09 (0.79) | −0.11 (0.73) | −0.02 (−0.11 to 0.07) | 0.73 |
| Triglycerides (mmol/l)† | 1.11 (46.22) | 1.06 (44.99) | 0.96 (0.91 to 1.00) | 0.07 |
| IDRS score | −4.36 (9.61) | −5.74 (10.86) | −1.50 (−2.78 to −0.22) | 0.022 |
Abbreviations: CI, confidence interval; IDRS, Indian Diabetes Risk Score; LDL, low density lipoprotein; SD, standard deviation.
* Mixed-effects linear regression was used to estimate the difference in change between study groups.
† Geometric mean (coefficient of variation) is presented for within-group change and geometric mean ratio for between-group change for skewed triglycerides.
Table 3. Changes in behavioral characteristics at 24 months by study group.
| Control group | Intervention group | Relative risk* (95% CI) | P value | |
|---|---|---|---|---|
| (n/N [percent]) | (n/N [percent]) | |||
| ≥5 servings of fruit and vegetables/daya | ||||
| Baseline | 77/507 (15.2) | 68/500 (13.6) | 0.90 (0.63 to 1.28) | 0.56 |
| 24 months | 41/482 (8.5) | 67/482 (13.9) | 1.83 (1.17 to 2.84) | 0.008 |
| Physically active (leisure time)b | ||||
| Baseline | 107/507 (21.1) | 102/500 (20.4) | 0.94 (0.67 to 1.33) | 0.73 |
| 24 months | 87/482 (18.1) | 101/482 (21.0) | 1.20 (0.81 to 1.79) | 0.36 |
| Current tobacco usec | ||||
| Baseline | 92/507 (18.2) | 102/500 (20.4) | 1.12 (0.81 to 1.55) | 0.50 |
| 24 months | 80/482 (16.6) | 72/482 (14.9) | 0.79 (0.60 to 1.05) | 0.11 |
| Current alcohol used | ||||
| Baseline | 97/507 (19.1) | 114/500 (22.8) | 1.19 (0.87 to 1.62) | 0.29 |
| 24 months | 88/482 (18.3) | 81/482 (16.8) | 0.77 (0.62 to 0.95) | 0.018 |
| Mean (SD) | Mean (SD) | Difference† (95% CI) | P value | |
| Standard drinks of alcohol (per drinking occasion)e | ||||
| Baseline | 0.20 (0.44) | 0.23 (0.43) | 0.028 (−0.032 to 0.088) | 0.36 |
| 24 months | 0.18 (0.39) | 0.17 (0.37) | −0.044 (−0.083 to −0.004) | 0.030 |
Abbreviations: CI, confidence interval; SD, standard deviation.
* Generalized estimating equations was used to estimate the relative risk (and 95% CI).
† Mixed-effects linear regression was used to estimate the difference in mean change between study groups.
a One serving of fruit equals a medium-sized fruit or two small-sized fruits or half a glass of fruit juice or a bowl of grapes. One serving of vegetables (excludes tubers) equals 80 grams.
b Self-reported history of moderate or vigorous physical activities during leisure time performed in bouts of at least 10 minutes’ duration.
c Smoking or use of smokeless tobacco (chewing tobacco and snuff) in the past 30 days.
d Consumed an alcoholic drink (spirits, wine, beer or toddy [palm wine]) in the past 30 days.
e One standard drink of alcohol refers to 30 ml of spirits, 120 ml of wine, 285 ml of beer, or 285 ml of toddy (palm wine).
Table 4. Changes in health-related quality of life variables at 24 months by study group.
| Control group | Intervention group | |||
|---|---|---|---|---|
| Mean change (SD) | Mean change (SD) | Difference* (95% CI) | P value | |
| Physical functioning | 1.3 (25.6) | 5.0 (25.7) | 3.9 (0.7 to 7.0) | 0.016 |
| Bodily pain | 4.5 (33.4) | 3.1 (34.3) | −1.2 (−5.3 to 2.9) | 0.55 |
| Role limitation - physical | 3.3 (25.4) | 4.7 (26.9) | 1.4 (−1.8 to 4.5) | 0.40 |
| Role limitation - emotional | 3.1 (24.2) | 5.9 (26.3) | 2.5 (−0.6 to 5.6) | 0.11 |
| Social functioning | 2.1 (25.6) | 3.1 (27.0) | 1.0 (−2.1 to 4.2) | 0.52 |
| Vitality | 6.1 (22.1) | 6.1 (21.5) | 0.3 (−2.5 to 3.0) | 0.85 |
| General health | 6.2 (26.5) | 5.5 (27.3) | −0.7 (−4.1 to 2.7) | 0.68 |
| Mental health | 5.4 (22.8) | 4.6 (21.5) | −0.9 (−3.6 to 1.8) | 0.50 |
| Physical component summary | 1.1 (9.1) | 1.5 (9.0) | 0.4 (−0.7 to 1.5) | 0.46 |
| Mental component summary | 2.4 (10.4) | 2.4 (10.0) | 0.02 (−1.21 to 1.26) | 0.97 |
| SF-6D | 0.04 (0.16) | 0.05 (0.17) | 0.01 (−0.01 to 0.03) | 0.30 |
Abbreviations: CI, confidence interval; SD, standard deviation; SF-6D, Short Form 6 Dimension.
* Mixed-effects linear regression was used to estimate the difference in mean change between study groups.
Intervention costs
Table 5 shows the costs associated with delivering the peer-support intervention over 12 months. The total intervention costs amounted to US$11,225 (US$22.5 per participant). The group sessions were the largest cost contributor (52.8% of total costs), followed by designing and printing charges for resource materials (21.7%), administrative costs (13.8%), and training of peer leaders and LRPs (11.7%). Personnel costs accounted for 26.7% of the total costs. Community activities incurred no program costs.
Table 5. Costs of the peer-support lifestyle intervention over 12 months.
| Categories | Inputs | Cost (US dollars) | Percent of total cost |
|---|---|---|---|
| Training sessions | 1308 | ||
| a. Peer leader training sessions (n = 2) | 691 | ||
| Personnel cost | 135 | ||
| Travel cost | 410 | ||
| Food and logistics | 144 | ||
| Phone calls | 2 | ||
| b. LRP training sessions (n = 5) | 617 | ||
| Personnel cost | 49 | 11.7 | |
| Travel cost | 512 | ||
| Food and logistics | 53 | ||
| Phone calls | 3 | ||
| Group sessions | 5928 | ||
| a. Introductory group sessions (n = 30) | 1636 | ||
| Personnel cost | 453 | ||
| Travel cost | 861 | ||
| Rent for venues, food, and logistics | 321 | ||
| Phone calls | 1 | ||
| b. DPES (n = 14) | 999 | ||
| Personnel cost | 198 | 52.8 | |
| Travel cost | 402 | ||
| Rent for venues, food, and logistics | 397 | ||
| Phone calls | 2 | ||
| c. Peer group sessions (n = 348) | 3293 | ||
| Personnel cost | 2233 | ||
| Rent for venues, food, and logistics | 1036 | ||
| Phone calls | 24 | ||
| Resource materialsa (n = 1560) | Designing and printing charges | 2441 | 21.7 |
| Administrative costs | 1548 | 13.8 | |
| Community activitiesb | 0 | 0 | |
| Total costs | 11225 | 100 |
Abbreviations: DPES, Diabetes Prevention Education Sessions; LRP, local resource person. Costs in Indian Rupees (INR) were converted to US$ using an exchange rate of INR58.6 = US$1 for the year 2013. Personnel costs were calculated based on the time spent by the Intervention Manager (US$2.5/hr) and Intervention Assistant (US1.1/hr) for various intervention activities, and remuneration for local experts (US$25.6/session) and LRPs (US$4.7/session).
a Includes participant handbook, peer leader handbook, peer leader workbook and health education booklet.
b Includes yoga sessions, kitchen garden training, and walking groups.
Adverse events
We recorded no adverse events related to the intervention.
Discussion
Summary of principal findings
To our knowledge, K-DPP is the first RCT from a LMIC to evaluate the effectiveness of a peer-support lifestyle intervention delivered mainly by lay people in a community setting. This study showed that the intervention resulted in a non-significant reduction in diabetes incidence at 24 months in a high-risk population identified on the basis of a diabetes risk score. However, there were significant improvements in some cardiovascular risk factors, including IDRS score, fruit and vegetable intake, and alcohol use, and physical functioning score of the HRQoL scale.
Comparison with other studies
The trial was powered for a 30% RRR for diabetes incidence at 24 months and observed a 12% RRR (nonsignificant), which was lower than that reported in other effectiveness trials. A meta-analysis by Ashra and colleagues assessing the effectiveness of 13 pragmatic lifestyle interventions implemented in routine clinical practice showed that the pooled estimate of RRR was 26% [44]. In the Study on Lifestyle intervention and Impaired glucose tolerance Maastricht (SLIM) study, the RRR was 58% at three years [45]. In the Joetsu Diabetes Prevention Trial, the RRR varied from 27% (nonhospitalization with diabetes education and support) to 42% (short-term hospitalization with diabetes education and support) at three years [46]. In the Spanish Diabetes in Europe—Prevention using Lifestyle, Physical Activity and Nutritional Intervention (DE-PLAN) project, which was implemented in primary care settings, the RRR was 36% at four years [47]. In a mobile phone effectiveness study conducted in India, the RRR was 36% at two years [48]. In the Diabetes Community Lifestyle Improvement (D-CLIP) translational trial conducted in India, the RRR was 32% at three years [49]. The lower effect in our study could be attributed to the following reasons. In previous studies, most (if not all) participants had IGT, while in K-DPP, participants were identified on the basis of a risk score, and the majority had isolated IFG or NGT, albeit with a high burden of cardiovascular risk factors [42]. So far, from the limited recent literature available [49–51], there is no proven intervention to reduce diabetes incidence among those with isolated IFG. Furthermore, 24 months’ follow-up may not have been long enough to allow for an intervention effect to be observable, and thus a longer-term follow-up has been planned.
In the control group, there was a decline in fruit and vegetable intake and the reported level of physical activity at 24 months. These are consistent with findings from other recent longitudinal studies conducted in Kerala, showing that the proportion of people meeting the recommended intake of fruits and vegetables and level of physical activity is continuing to decrease over time in the absence of any intervention [52,53]. There was a greater increase in physical functioning score of the HRQoL scale in the intervention group at 24 months. Previous studies have shown that improvement in HRQoL is likely to be mediated by improved physical activity and weight loss [54].
In our study, the cost of delivering the peer-support lifestyle intervention over 12 months was US$22.5 per participant, a large percentage (52.8%) of which was accounted for by the group sessions, the main mode of formal program delivery. This is less than one-third of the intervention costs incurred in IDPP (US$75 per participant per year) [6]. The lower cost could be mainly attributed to the fact that, in IDPP, health professionals (physicians, dieticians, and social workers) were involved in delivering the intervention, while in K-DPP, the intervention was delivered mainly by lay peer leaders. In IDPP, the personnel cost was US$36 per participant, whereas in K-DPP, this cost was only US$6. However, the effect size in IDPP (28.5%) was higher than that in K-DPP (12%). It is possible that resource-intensive lifestyle interventions are more effective than low-resource interventions in reducing diabetes progression, at least in the IGT population (S5 Table and S1 Fig). If the K-DPP intervention were implemented as a real-world program, the unit costs for each individual would be much lower. This is because the one-off costs (e.g., training of peer leaders and LRPs and printing charges of resource materials) would be distributed over a much larger number of individuals, and the relative travel and administrative costs would also be lower. In K-DPP, travel and administrative costs accounted for one-third (33.3%) of the total costs. This is because the K-DPP personnel and local experts had to travel to the field, spending around three hours for every return trip. However, these costs would be relatively lower in a program setting and if the program were delivered at scale. Moreover, the very important K-DPP community activities incurred no program costs, as they were led by peer leaders with assistance from LRPs.
Strengths and limitations
The K-DPP trial has a number of strengths. The study was conducted in the Indian state of Kerala, which has a high prevalence of diabetes (approximately 20%) and several other cardiovascular risk factors [52,55,56]. The state is in the most advanced stage of epidemiological transition compared to other Indian states [57], and it is also the harbinger of the future for the rest of India in relation to the burden of chronic diseases [55,56]. Therefore, Kerala provides an ideal setting for the implementation and evaluation of a diabetes prevention program in a community setting in India. As far as we are aware, K-DPP is the first diabetes prevention trial to deliver a peer-support lifestyle intervention program mainly by trained lay people in a low- and middle-income setting. In the D-CLIP trial from Chennai, India, although peer support was provided by community volunteers, it involved a team of health coaches and fitness trainers in the delivery of intervention. Also, metformin (500 mg twice a day) was added to those at highest risk of developing diabetes [49]. Although metformin was found to be equally effective as lifestyle intervention in previous studies [2,4] and is cheap, the current evidence base supports its use only in combination with lifestyle interventions [58]. Other strengths of our trial include a very high follow-up rate at 24 months (97.5%), use of a rigorous study design, and a much better representation of women (nearly half the participants were women) compared to previous diabetes prevention trials in India [4,48,49,59]. However, there are also some study limitations. In subgroup analyses, balance of potentially confounding characteristics between the subgroups compared is not guaranteed, and the power may have been insufficient for such analyses. Data on behavioral risk factors (tobacco use, alcohol use, physical activity, and fruit and vegetable intake) were collected using questionnaires that were not validated by objective measures and are likely to be subject to response bias. It is possible that social desirability and acquiescence biases associated with the intervention may have resulted in the small differences observed in some of the behavioral outcomes at 24 months. Furthermore, we did not adjust for multiple comparison, and given the likelihood of type 1 errors [60], changes in secondary outcomes observed should be interpreted cautiously.
Implications for policy and future research
In efficacy trials of behavioral or social interventions, recruitment of highly selected individuals, resource-intensive interventions, and close monitoring to ensure compliance will almost always overestimate the outcomes that will actually be achievable under ‘real-world’ conditions [61]. However, given that the efficacy of lifestyle interventions to prevent diabetes among high-risk individuals, particularly among those with IGT, has been quite well established, it is now important to determine their effectiveness in real-world settings, in which the target population is likely to be much broader if program participants are not recruited on the basis of clinical testing [62]. Our study findings have some important implications for policy and future research with regards to diabetes prevention in India (and perhaps also in other LMICs). First, using a risk score rather than the OGTT to identify high-risk individuals was part of our strategy to develop a low-cost diabetes prevention program. While the IDRS with a score of ≥60 identified individuals with a high burden of cardiovascular risk factors [42], the majority had isolated IFG or NGT and not IGT. The results of our subgroup analyses suggest a trend towards greater reduction in diabetes incidence among those with IGT compared to those with isolated IFG or NGT. As mentioned previously, so far, lifestyle interventions have not been shown to be effective in reducing diabetes risk among those with isolated IFG [49–51]. Further research is required to determine the optimal cutoff for the IDRS to identify those at highest risk of developing diabetes. Alternatively, risk scores that are better at picking up people with IGT could be developed. Second, given the high burden of cardiovascular risk factors in the trial population [42] and improvements observed in some of these at 24 months, it is important to evaluate the potential longer-term benefits of the intervention on both diabetes incidence and cardiovascular risk. Finally, although the K-DPP intervention was low-cost and delivered mainly by lay people in community neighborhoods with support from local self-government bodies, it is important to do more research on how to increase program adherence and engagement, possibly by using more flexible modes of program delivery, e.g., at worksites and by text messaging. This research should also consider how continued program implementation beyond the current 12-month program for group participants can be supported by developing partnerships with other kinds of community organizations or partnerships that could deliver the intervention at scale in Kerala and elsewhere in India in the future.
Conclusions
In this low- and middle-income setting, a low-cost peer-support lifestyle intervention resulted in a nonsignificant reduction in diabetes incidence at 24 months in a high-risk population identified on the basis of a risk score. However, there were significant improvements in some cardiovascular risk factors and physical functioning score of the HRQoL scale.
Supporting information
CRT, cluster randomized trial.
(DOCX)
GGT, Greater Green Triangle; GOAL, Good Ageing in Lahti Region; K-DPP, Kerala Diabetes Prevention Program.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Naanki Pasricha, Anoop Velayudhan, Uma V. Shankar, and Neena Elezebeth Phillip for their contribution to the project administration and intervention delivery. We are grateful to all the study participants, data collection staff, LRPs, and elected representatives of the local self-government bodies for their participation in the study.
Abbreviations
- 2-hr PG
2-hr plasma glucose
- ADA
American Diabetes Association
- CI
confidence interval
- D-CLIP
Diabetes Community Lifestyle Improvement Program
- DE-PLAN
Diabetes in Europe—Prevention using Lifestyle, Physical Activity and Nutritional Intervention
- FPG
fasting plasma glucose
- GEE
generalized estimating equations
- GGT
Greater Green Triangle
- GOAL
Good Ageing in Lahti Region
- HRQoL
health-related quality of life
- ICC
intracluster correlation coefficient
- IDPP
Indian Diabetes Prevention Programme
- IDRS
Indian Diabetes Risk Score
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- INR
Indian Rupees
- IQR
interquartile range
- K-DPP
Kerala Diabetes Prevention Program
- LAC
legislative assembly constituency
- LDL
low density lipoprotein
- LMIC
low- and middle-income country
- LRP
local resource person
- MMI
multilevel multiple imputation
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- RCT
randomized controlled trial
- RR
relative risk
- RRR
relative risk reduction
- SD
standard deviation
- SF-36
36-item Short-Form
- SF-6D
Short Form 6 Dimension
- SLIM
Study on Lifestyle intervention and Impaired glucose tolerance Maastricht
- US DPP
United States Diabetes Prevention Program
- WHO
World Health Organization
Data Availability
All data files are available from the figshare database, https://doi.org/10.6084/m9.figshare.5661610.
Funding Statement
The research was supported by funding from the National Health and Medical Research Council (1005324), https://www.nhmrc.gov.au/, and the Fogarty International Centre (D43TW008332), https://www.fic.nih.gov/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas Eighth Edition 2017. Available from: http://www.diabetesatlas.org/.
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346: 393–403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20: 537–44. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49: 289–97. doi: 10.1007/s00125-005-0097-z [DOI] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344: 1343–50. doi: 10.1056/NEJM200105033441801 [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran A, Snehalatha C, Yamuna A, Mary S, Ping Z. Cost-effectiveness of the interventions in the primary prevention of diabetes among Asian Indians: within-trial results of the Indian Diabetes Prevention Programme (IDPP). Diabetes Care. 2007;30: 2548–2552. doi: 10.2337/dc07-0150 [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program Research Group. Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26: 2518–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portero McLellan KC, Wyne K, Villagomez ET, Hsueh WA. Therapeutic interventions to reduce the risk of progression from prediabetes to type 2 diabetes mellitus. Ther Clin Risk Manag. 2014;10: 173–188. doi: 10.2147/TCRM.S39564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldenburg B, Absetz P, Dunbar JA, Reddy P, O'Neil A. The spread and uptake of diabetes prevention programs around the world: a case study from Finland and Australia. Transl Behav Med. 2001;1: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming KA, Naidoo M, Wilson M, Flanigan J, Horton S, Kuti M, et al. An Essential Pathology Package for Low- and Middle-Income Countries. Am J Clin Pathol. 2017;147: 15–32. doi: 10.1093/ajcp/aqw143 [DOI] [PubMed] [Google Scholar]
- 11.Noble D, Mathur R, Dent T, Meads C, Greenhalgh T. Risk models and scores for type 2 diabetes: systematic review. BMJ. 2011;343: d7163 doi: 10.1136/bmj.d7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Standards of Medical Care in Diabetes—2018. Available from: http://care.diabetesjournals.org/content/41/Supplement_1/S1.
- 13.National Institute for Health and Care Excellence. Type 2 diabetes: prevention in people at high risk. Available from: https://www.nice.org.uk/guidance/ph38/resources/type-2-diabetes-prevention-in-people-at-high-risk-pdf-1996304192197.
- 14.Herman WH, Brandle M, Zhang P, Williamson DF, Matulik MJ, Ratner RE, et al. Costs associated with the primary prevention of type 2 diabetes mellitus in the Diabetes Prevention Program. Diabetes Care. 2003;26: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao M, Rao KD, Kumar AK, Chatterjee M, Sundararaman T. Human resources for health in India. Lancet. 2011;377: 587–98. doi: 10.1016/S0140-6736(10)61888-0 [DOI] [PubMed] [Google Scholar]
- 16.Boothroyd RI, Fisher EB. Peers for progress: promoting peer support for health around the world. Fam Pract. 2010;27 Suppl 1: i62–8. [DOI] [PubMed] [Google Scholar]
- 17.Fisher EB, Boothroyd RI, Elstad EA, Hays L, Henes A, Maslow GR, et al. Peer support of complex health behaviors in prevention and disease management with special reference to diabetes: systematic reviews. Clinical Diabetes and Endocrinology. 2017;3: 4 doi: 10.1186/s40842-017-0042-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sathish T, Williams ED, Pasricha N, Absetz P, Lorgelly P, Wolfe R, et al. Cluster randomized controlled trial of a peer-led lifestyle intervention program: study protocol for the Kerala diabetes prevention program. BMC Public Health. 2013;13: 1035 doi: 10.1186/1471-2458-13-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell MK, Elbourne DR, Altman DG. CONSORT statement: extension to cluster randomized trials. BMJ 2012;345:e5661 doi: 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 20.Election Commission of India. Manual on polling stations. Available from: http://eci.nic.in/eci_main/ElectoralLaws/HandBooks/Manual_on_Polling_Station_27052016.pdf.
- 21.Mohan V, Deepa R, Deepa M, Somannavar S, Datta M. A simplified Indian Diabetes Risk Score for screening for undiagnosed diabetic subjects. J Assoc Physicians India. 2005;53: 759–63. [PubMed] [Google Scholar]
- 22.Mohan V, Deepa M, Anjana RM, Lanthorn H, Deepa R. Incidence of diabetes and pre-diabetes in a selected urban south Indian population (CUPS-19). J Assoc Physicians India. 2008;56: 152–7. [PubMed] [Google Scholar]
- 23.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Available from: http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf.
- 24.Mathews E, Thomas E, Absetz P, D'Esposito F, Aziz Z, Balachandran S, et al. Cultural adaptation of a peer-led lifestyle intervention program for diabetes prevention in India: the Kerala diabetes prevention program (K-DPP). BMC Public Health. 2018;17: 974 doi: 10.1186/s12889-017-4986-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daivadanam M, Absetz P, Sathish T, Thankappan KR, Fisher EB, Philip NE, et al. Lifestyle change in Kerala, India: needs assessment and planning for a community-based diabetes prevention trial. BMC Public Health. 2013;13:95 doi: 10.1186/1471-2458-13-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwarzer R. Modeling health behavior change: How to predict and modify the adoption and maintenance of health behaviors. Appl Psychol-Int Rev. 2008;57: 1–29. [Google Scholar]
- 27.Absetz P, Oldenburg B, Hankonen N, Valve R, Heinonen H, Nissinen A, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL lifestyle implementation trial. Diabetes Care. 2009;32: 1418–1420. doi: 10.2337/dc09-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laatikainen T, Dunbar JA, Chapman A, Kilkkinen A, Vartiainen E, Heistaro S, et al. Prevention of type 2 diabetes by lifestyle intervention in an Australian primary health care setting: Greater Green Triangle (GGT) Diabetes Prevention Project. BMC Public Health. 2007;7: 249 doi: 10.1186/1471-2458-7-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eldrigde LKB, Markham CM, Ruiter RAC, Fernàndez ME, Kok G, Parcel GS. Planning health promotion programs: An Intervention Mapping approach. 4th ed. Hoboken, NJ: Wiley; 2016. [Google Scholar]
- 30.Uutela A, Absetz P, Nissinen A, Valve R, Talja M, Fogelholm M. Health psychological theory in promoting population health in Paijat-Hame, Finland: first steps toward a type 2 diabetes prevention study. J Health Psychol. 2004;9: 73–84. doi: 10.1177/1359105304036103 [DOI] [PubMed] [Google Scholar]
- 31.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health. 2009;6: 790–804. [DOI] [PubMed] [Google Scholar]
- 32.Soman CR, Shahulhameed S, Ramankutty V, Vijayakumar K, Kunjukrishnapillai R, Ajayan K, et al. Cohort profile: the PROLIFE study in Kerala, India. Int J Epidemiol. 2011;40: 10–4. doi: 10.1093/ije/dyp275 [DOI] [PubMed] [Google Scholar]
- 33.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Med Care. 1992;30: 473–83. [PubMed] [Google Scholar]
- 34.World Health Organization. The WHO STEPwise approach to chronic disease risk factor surveillance. Available from: http://apps.who.int/iris/bitstream/10665/43376/1/9241593830_eng.pdf.
- 35.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18: 499–502. [PubMed] [Google Scholar]
- 36.Parker DR, Evangelou E, Eaton CB. Intraclass correlation coefficients for cluster randomized trials in primary care: the cholesterol education and research trial (CEART). Contemp Clin Trials. 2005;26: 260–7. doi: 10.1016/j.cct.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 37.Barry E, Roberts S, Oke J, Vijayaraghavan S, Normansell R, Greenhalgh T. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: systematic review and meta-analysis of screening tests and interventions. BMJ. 2017;356: i6538 doi: 10.1136/bmj.i6538 [DOI] [PubMed] [Google Scholar]
- 38.Hossain A, DiazOrdaz K, Bartlett JW. Missing binary outcomes under covariate-dependent missingness in cluster randomized trials. Stat Med. 2017;36: 3092–3109. doi: 10.1002/sim.7334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 40.Quartagno M, Carpenter J. jomo: a package for multilevel joint modelling multiple imputation, 2015. Available from: http://CRAN.R-project.org/package=jomo.
- 41.International Monetary Fund. Exchange rate archives by month. Available from: https://www.imf.org/external/np/fin/data/param_rms_mth.aspx.
- 42.Sathish T, Oldenburg B, Tapp RJ, Shaw JE, Wolfe R, Sajitha B, et al. Baseline characteristics of participants in the Kerala Diabetes Prevention Program: a cluster randomized controlled trial of lifestyle intervention in Asian Indians. Diabet Med. 2017;34:647–653. doi: 10.1111/dme.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.International Expert Commitee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32: 1327–34. doi: 10.2337/dc09-9033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashra NB, Spong R, Carter P, Davies MJ, Dunkley A, Gillies C, et al. A systematic review and meta-analysis assessing the effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes mellitus in routine practice. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/456147/PHE_Evidence_Review_of_diabetes_prevention_programmes-_FINAL.pdf.
- 45.Roumen C, Corpeleijn E, Feskens EJ, Mensink M, Saris WH, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabet Med. 2008;25: 597–605. doi: 10.1111/j.1464-5491.2008.02417.x [DOI] [PubMed] [Google Scholar]
- 46.Kawahara T, Takahashi K, Inazu T, Arao T, Kawahara C, Tabata T, et al. Reduced progression to type 2 diabetes from impaired glucose tolerance after a 2-day in-hospital diabetes educational program: the Joetsu Diabetes Prevention Trial. Diabetes Care. 2008;31: 1949–1954. doi: 10.2337/dc07-2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costa B, Barrio F, Cabré JJ, Piñol JL, Cos X, Solé C, et al. Delaying progression to type 2 diabetes among high-risk Spanish individuals is feasible in real-life primary healthcare settings using intensive lifestyle intervention. Diabetologia. 2012;55: 1319–1328. doi: 10.1007/s00125-012-2492-6 [DOI] [PubMed] [Google Scholar]
- 48.Ramachandran A, Snehalatha C, Ram J, Selvam S, Simon M, Nanditha A, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomized controlled trial. Lancet Diabetes Endocrinol. 2013;1: 191–8. doi: 10.1016/S2213-8587(13)70067-6 [DOI] [PubMed] [Google Scholar]
- 49.Weber MB, Ranjani H, Staimez LR, Anjana RM, Ali MK, Narayan KM, et al. The Stepwise Approach to Diabetes Prevention: Results From the D-CLIP Randomized Controlled Trial. Diabetes Care. 2016;39: 1760–7. doi: 10.2337/dc16-1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies MJ, Gray LJ, Troughton J, Gray A, Tuomilehto J, Farooqi A, et al. A community based primary prevention programme for type 2 diabetes integrating identification and lifestyle intervention for prevention: the Let's Prevent Diabetes cluster randomized controlled trial. Prev Med. 2016;84: 48–56. doi: 10.1016/j.ypmed.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 51.Saito T, Watanabe M, Nishida J, Izumi T, Omura M, Takagi T, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med. 2011;171: 1352–60. doi: 10.1001/archinternmed.2011.275 [DOI] [PubMed] [Google Scholar]
- 52.Sathish T, Kannan S, Sarma SP, Razum O, Sauzet O, Thankappan KR. Seven-year longitudinal change in risk factors for non-communicable diseases in rural Kerala, India: The WHO STEPS approach. PLoS ONE. 2017;12(6): e0178949 doi: 10.1371/journal.pone.0178949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dyson PA, Anthony D, Fenton B, Stevens DE, Champagne B, Li LM, et al. Successful up-scaled population interventions to reduce risk factors for non-communicable disease in adults: results from the International Community Interventions for Health (CIH) Project in China, India and Mexico. PLoS ONE. 2015;10(4): e0120941 doi: 10.1371/journal.pone.0120941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ackermann RT, Edelstein SL, Narayan KM, Zhang P, Engelgau MM, Herman WH, et al. Changes in health state utilities with changes in body mass in the Diabetes Prevention Program. Obesity (Silver Spring). 2009;17: 2176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sathish T, Kannan S, Sarma PS, Razum O, Thankappan KR. Incidence of hypertension and its risk factors in rural Kerala, India: A community-based cohort study. Public Health. 2012;126: 25–32. doi: 10.1016/j.puhe.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 56.Thankappan KR, Shah B, Mathur P, Sarma PS, Srinivas G, Mini GK, et al. Risk factor profile for chronic non-communicable diseases: Results of a community-based study in Kerala, India. Indian J Med Res. 2010;131: 53–63. [PubMed] [Google Scholar]
- 57.India State-level Disease Burden Initiative Collaborators. Nations within a nation: variations in epidemiological transition across the states of India, 1990–2016 in the Global Burden of Disease Study. Lancet. 2017. pii: S0140-6736(17)32804-0. doi: 10.1016/S0140-6736(17)32804-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hostalek U, Gwilt M, Hildemann S. Therapeutic Use of Metformin in Prediabetes and Diabetes Prevention. Drugs. 2015;75: 1071–1094. doi: 10.1007/s40265-015-0416-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramachandran A, Snehalatha C, Mary S, Selvam S, Kumar CK, Seeli AC, et al. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2). Diabetologia. 2009;52: 1019–26. doi: 10.1007/s00125-009-1315-x [DOI] [PubMed] [Google Scholar]
- 60.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310: 170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singal AG, Higgins PD, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5: e45 doi: 10.1038/ctg.2013.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37: 922–933. doi: 10.2337/dc13-2195 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CRT, cluster randomized trial.
(DOCX)
GGT, Greater Green Triangle; GOAL, Good Ageing in Lahti Region; K-DPP, Kerala Diabetes Prevention Program.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All data files are available from the figshare database, https://doi.org/10.6084/m9.figshare.5661610.