Abstract
Study Design
Secondary analysis, cross-sectional study
Background
Chronic hip joint pain (CHJP) can lead to limitations in activity participation, but the musculoskeletal factors associated with the condition are relatively unknown. Understanding the factors associated with CHJP may help develop rehabilitation strategies to improve quality of life of individuals with long-term hip pain.
Objectives
To compare measures of hip abductor muscle volume and hip abductor muscle strength between women with CHJP and asymptomatic controls.
Methods
Thirty women, 15 with CHJP and 15 matched asymptomatic controls (18–40 years of age), participated in this study. Magnetic resonance imaging was used to determine the volume of the primary hip abductor muscles, consisting of gluteus medius (GMed), gluteus minimus (GMin), a small portion of gluteus maximus (GMax), and tensor fascia latae (TFL), within a defined region of interest. Break tests were performed using a handheld dynamometer to assess hip abductor strength. During the strength test, the participant was positioned in sidelying with the involved hip in 15° abduction. Independent-sample t tests were used to compare muscle volume and strength values between those with CHJP and asymptomatic controls.
Results
Compared to asymptomatic controls, women with CHJP demonstrated significantly increased gluteal muscle volume (228±40cm3 versus 199±29cm3; p=.032), but decreased hip abductor strength (74.6±16.8Nm versus 93.6±20.2Nm; p=.009). There were no significant differences in TFL muscle volume between the two groups (p=.640).
Conclusions
Women with CHJP appear to have larger gluteal muscles, but decreased hip abductor strength compared to asymptomatic controls.
Keywords: dynamometry, femoroacetabular impingement, gluteals, magnetic resonance imaging, movement system
Introduction
Chronic hip joint pain (CHJP), often associated with labral tears,26 femoroacetabular impingement (FAI),13, 39 chondral lesions,29 and structural instability,34 can lead to significant activity limitations in young to middle-aged adults.4, 7 Individuals with CHJP often have difficulty with activities such as walking, stairs, and sitting,4, 7 leading to inability to participate in work, school, or fitness activities. CHJP is also believed to be a precursor to hip osteoarthritis (OA),6, 11, 13, 32, 39 a leading cause of reduced quality of life and restriction of activity participation for patients worldwide.8 Rehabilitation to target modifiable factors associated with CHJP may reduce pain, improve the person’s ability to participate in activity, and potentially delay or prevent hip OA. However, the evidence for rehabilitation to target these factors is limited.36 To develop effective treatment strategies, a better understanding of the factors associated with CHJP is needed. Hip muscle structure and function are two inter-related factors that may provide insight into CHJP and the associated limitations in daily activities.
The primary hip abductor muscles, gluteus medius (GMed), gluteus minimus (GMin) and tensor fascia latae (TFL), are important for hip joint function and stability.30, 37 Previous studies have shown that people with CHJP demonstrate hip abductor muscle weakness in their symptomatic hip compared to asymptomatic individuals.5, 9, 17 This weakness may be due to a number of factors including pain experienced during strength testing, muscle atrophy due to disuse, reduced neuromuscular activation, or muscle inhibition due to increased intra-articular fluid induced by injury.12 Of these, we were curious if muscle atrophy would be present in people with CHJP.
The evidence related to hip muscle volume is limited, especially in young adults with CHJP. Only one study specific to this population is available.23 Liu et al23 found that people with painful developmental dysplasia of the hip (DDH) have decreased GMed cross-sectional area (CSA) of the involved limb compared to uninvolved limb. However, a comparison to asymptomatic participants was not reported. In studies of older individuals, findings are mixed when comparing those with hip OA to asymptomatic participants. One study found no differences in gluteal CSA between those with hip OA and asymptomatic participants.3 Grimaldi et al14 reported no differences in GMed muscle volume between those with advanced hip OA, defined by Kellgren-Lawrence global scoring system (K/L)20 as grades 3–4, and matched participants without hip OA, however, those with mild OA, K/L 1–2, had increased GMed volume compared to their matched controls. Given these conflicting results and the importance of hip abductor function, more investigation is needed to better understand hip muscle structure among those with and without CHJP.
In this study, we performed a secondary analysis to compare measures of hip abductor muscle volume and hip abductor strength between women with CHJP and matched asymptomatic participants. We focused our analysis on women participants due to sample characteristics of the parent study.17 We hypothesized the involved limb in women with CHJP would have decreased hip abductor muscle volume and decreased hip abductor strength compared to asymptomatic participants.
METHODS
Participants
The 30 women in this study were a subset of a larger cohort parent study designed to assess potential risk factors for CHJP. Participants, aged 18–40 years, were recruited from Washington University School of Medicine’s orthopaedic, physical medicine and rehabilitation, and physical therapy clinics; research participant registry; and through public announcements. To be enrolled in the parent study17, a participant with CHJP had to report presence of anterior groin pain or deep hip joint pain for greater than three months. This pain needed to be reproducible with the anterior impingement test, also known as FADIR, consisting of overpressure into hip flexion, internal rotation, and adduction.27, 28 Control participants reported no current hip or other lower extremity pain. For both groups, exclusion criteria included (1) previous hip surgery or fracture, (2) contraindication to magnetic resonance imaging (MRI), (3) pregnancy, (4) self-report of neurological involvement that influenced coordination or balance and (5) BMI greater than 30 kg/m2. The BMI restriction was necessary for other procedures in the parent study. Screening tests were also performed on all participants and participants were excluded if the results indicated possible lumbar spine radiculopathy.
Control participants were matched with participants with CHJP by age (within five years), sex, BMI (within five kg/m2), and limb side. The involved hip in those with CHJP and the corresponding hip for the matched control were selected. If a participant with CHJP had bilateral hip pain, the more symptomatic hip, identified by the participant, was selected. All participants signed an informed consent statement prior to participation in the study, which was approved by Washington University School of Medicine’s Human Research Protection Office.
Instrumentation
Handheld Dynamometer
To assess hip strength, the microFET3 (Hoggan Health Industries, Salt Lake City, UT) handheld dynamometer was used. The dynamometer, reported to be accurate within 1%, was factory calibrated before the study. Handheld dynamometry is an inexpensive tool that may be used in the clinical setting. It is a reliable and valid method to measure hip muscle strength compared to isokinetic devices.2, 18
MR Images
Methods used to obtain MRIs have been previously reported.15 A 1.5T magnetic resonance system (Avanto; Siemens, Erlangen, Germany) was used to obtain a series of 3D fat suppressed gradient echo images centered at the pelvis.
Examination
A licensed physical therapist with 16 years of clinical and research experience, performed examination procedures and data collection. A research assistant assisted with examination and documenting strength data. After consent, the examiner obtained subjective data and performed tests to confirm the presence or absence of CHJP.
Prior to strength testing, participants completed self-report questionnaires including the University of California Los Angeles activity score (UCLA)1 to estimate activity levels and the Hip disability and Osteoarthritis Outcome Score (HOOS)31 to quantify hip-specific, patient-reported ability to participate in daily activities. Prior to testing, participants performed a five minute warm-up on a stationary bike or walking on a treadmill. Then a mark was placed four centimeters (cm) proximal to the lateral malleolus to provide a consistent landmark for dynamometer placement for strength testing.
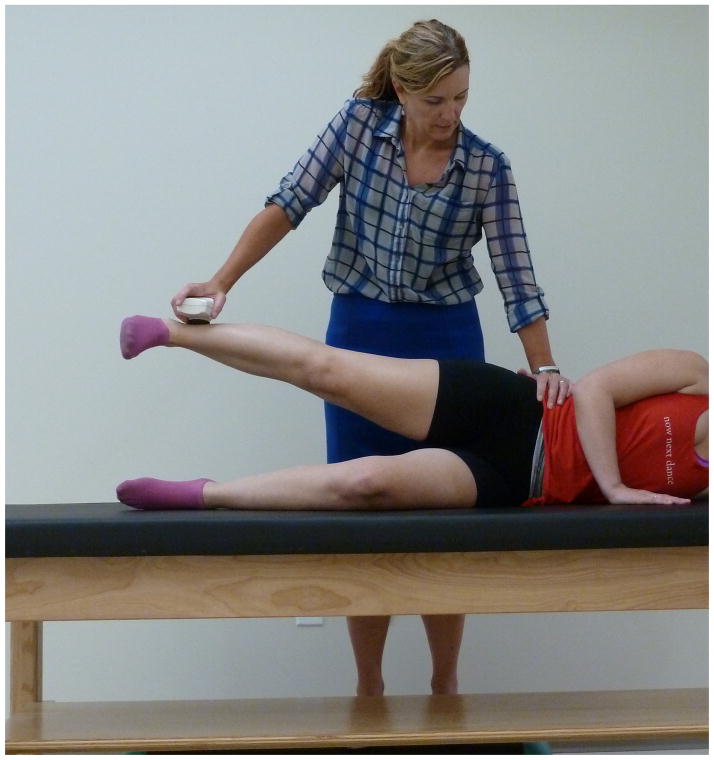
Previously reported methods were used to assess hip abductor strength.17 Briefly, patients were placed in sidelying on the side of their non-painful hip with the hip flexed to 45º and knee flexed to 90º.21 The painful hip was placed in 15º abduction, 0º flexion/extension and 0º rotation (FIGURE 1).
Figure 1.
The dynamometer was used to measure force in Newtons by performing a break test.22 To familiarize the patient to testing procedures, a submaximal test was performed. Then, three maximal tests were performed with 15 seconds rest between each trial.19, 35 To perform the break test, the participant’s limb was placed in the appropriate position. The dynamometer was placed at the previously placed mark, proximal to the lateral malleolus and counterstabilization was provided at the pelvis to prevent any extra motion at the trunk/pelvis (FIGURE 1). The examiner then placed a small amount of force through the dynamometer before slowly increasing resistance over 2–3 seconds until the participant could no longer hold the limb in 15º abduction. Verbal encouragement was provided during the tests. If there was any compensatory movement noted, the examiner would correct it and perform an additional trial. Additionally, if the value of a trial was greater than 10% different from the other trials, it was considered invalid and an additional trial was performed. During each test, the participant’s pain level was recorded on a 0–10 scale with 0 being no pain and 10 being the worst pain imaginable. The moment arm for the external resistance was the distance between the superior greater trochanter to four cm proximal to the lateral malleolus. The three valid tests were averaged to obtain average force, then multiplied by the moment arm to determine torque (Newton meters [Nm]). Test-retest reliability and standard errors of measurement can be found in TABLE 1.
TABLE 1.
Intrarater reliability for hip abductor strength and muscle size measurements.
| ICC3,3† | Hip Abductor Torque SEM | |
|---|---|---|
| Hip Abductor Strength* | 0.94 (0.67, 0.99) | 6.3Nm |
|
| ||
| ICC3,1† | Muscle Size SEM | |
|
| ||
| Gluteal Compartment Volume‡ | 0.99 (0.98–0.99) | 3.90cm3 |
| TFL Compartment Volume‡ | 0.99 (0.99–1.00) | 0.47cm3 |
Abbreviations: ICC, intraclass correlation coefficient; SEM, standard error of measurement; TFL, tensor fascia latae.
Hip abducted to 15º in sidelying. Strength tests were completed on 8 asymptomatic participants on 2 separate testing sessions at least 1 week, but no more than 2 weeks, apart.
Values in parentheses are 95% confidence interval.
MRI measurements taken using the rotation-corrected images of 20 participants, 10 participants with CHJP and 10 without, on separate occasions at least 2 weeks apart.
MRI Acquisition and Image Processing
Participants were positioned on the MRI table with their lower extremities in neutral (0º hip flexion/abduction/hip rotation). Before images were taken, a standardized method was used to optimize participant position. From a supine, knees-flexed position, the participants were asked to perform a bridge and return to supine with legs extended. Then a brief traction force was provided by the examiner, grasping both ankles and pulling in an inferior direction. Palpation and visual appraisal were used to assess positioning. Spacers were placed around the lower extremities to help maintain a neutral position. A spine coil, body matrix coil overlying the pelvis, and a peripheral angiography coil overlying the lower extremities were used during imaging. Straps were used to minimize participant movement and to keep coils in place. Scout images were obtained of the pelvis to identify capture volume. Specific parameters were as follows: slice thickness 0.82mm, Repetition time (TR) 15.96ms, Echo time (TE) 6.2ms, Field of view (FOV) 400mm at the pelvis, 512×512 matrix, no gap between slice thickness with total acquisition time being approximately 14 minutes.15
Using an independent workstation (LEONARDO; Siemens), each pelvic image was reconstructed to correct for pelvic rotation in the order of coronal, transverse and sagittal planes. Rotation correction in the coronal plane was made by aligning the inferior margins of the ischial tuberosities. In the transverse plane, this was done by aligning the bilateral posterior acetabular walls. Finally, correction in the sagittal plane was made by aligning the anterior superior iliac spine and ipsilateral anterior pubic symphysis. After post-processing, images were saved into a secure server.
The MR images were then downloaded from the server to a desktop computer and imported into Mayo Clinic Biomedical Image Resource Analyze 11.0 software (AnalyzeDirect, Overland Park, KS) to assess for study inclusion and to measure muscle volume.
MRI Participant Inclusion
The MR images from the parent study were acquired to optimally capture bony structure, therefore the images were reviewed to determine inclusion in the analysis for muscle volume.15 Imaging sequences for each participant were visually assessed to determine if the defined region of interest (ROI) for the gluteals, consisting of GMed, GMin, and a small portion of gluteus maximus (GMax), and TFL was available. To be included, images had to contain the most superior aspect of the iliac crests to the ischial tuberosities in the coronal plane and the most anterior point of the anterior superior iliac spine to the most posterior aspect of the acetabular wall in the transverse plane. Adequate visualization of each muscle group’s ROI had to be present for both participants of the matched pairs. Of the original 36 matched pairs from the parent study, 19 pairs were excluded because the MR images of at least one of the matched participants did not include the specific ROIs. Of the remaining 17 pairs, only two of the matched pairs were men. Given previous report of between-sex differences in hip muscle volume,33 we limited our analysis to the remaining 15 matched pairs of women.
Muscle Volume Measurement
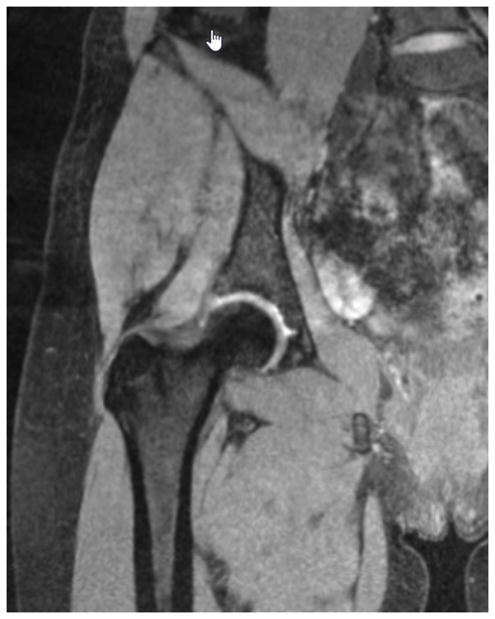
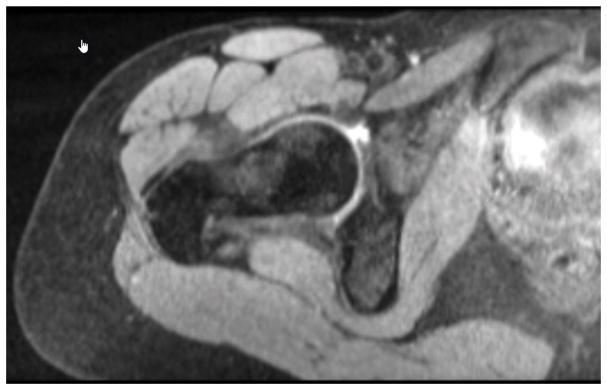
A research assistant, blinded to group status, used the rotation-corrected MR images to select slices to be measured and to complete the measurements. The ROI for each muscle was defined a priori to allow for consistency of measurement across subjects and all slices within the ROI was measured. The gluteal muscle group was measured in the coronal plane from the slice showing the first appearance of the femoral head posteriorly to the slice showing the last appearance of the femoral head anteriorly (FIGURE 2). TFL was measured in the transverse plane from the slice showing the first appearance of the ischial tuberosity distally to the slice showing the center of the femoral head (FIGURE 3). Muscle boundaries were semi-automatically outlined using the autotrace feature within the ROI for consecutive slices. The number of slices measured for each muscle group’s ROI was recorded to determine if a similar number of slices were measured between the matched pairs.
Figure 2.
Figure 3.
Intrarater reliability of the research assistant was assessed using the rotation-corrected images from 20 participants, 10 asymptomatic and 10 with CHJP. Slice selection and volume measurements were completed on two separate testing sessions, with 2 weeks between sessions. Intrarater reliability was found to be excellent (TABLE 1).
Statistics
The Shapiro-Wilk test was used to confirm normal distribution of data and Levene’s test was used to confirm equality of variance. For between-group comparisons, independent t-tests were used for continuous data and Mann-Whitney U test was used for ordinal data. A p-value less than .05 was considered significant.
RESULTS
Demographic characteristics of the participants can be found in TABLE 2. Because the two groups were matched, there were no significant differences between participants with CHJP and control participants in sex, limb side, age, and BMI. Based on the UCLA,1 both groups reported participating in high level activities like jogging, tennis, skiing, and running at least once a week. Six participants with CHJP reported bilateral pain. Participants with CHJP reported a median pain duration of 3.5 years (range of 0.4–13) and demonstrated moderate limitations in daily activities based on the HOOS subscales (TABLE 2).16, 31 Six participants reported pain duration less than one year, five between one and five years, three between six to ten years, and one greater than ten years. To assess potential bias due to excluding participants from the parent study with insufficient images, we compared those women with CHJP included in the current analysis to those who were excluded. There were no differences in age, BMI, pain duration, activity level, or HOOS subscales.
TABLE 2.
Demographics for all enrolled participants.
| CHJP (N=15) | Control (N=15) | P-Value | |
|---|---|---|---|
| Age (years)† | 28.3±4.1 | 28.3±4.4 | .97* |
| BMI (kg/m2)† | 24.0±3.3 | 24.5±3.2 | .63* |
| Limb Side | 9 right 6 left | 9 right 6 left | - |
| Average Pain Over Past Week‡ | 3/10 (1–8) | - | - |
| Worst Pain in Past Week‡ | 6/10 (3–10) | - | - |
| Average Pain Duration (Years) ‡ | 3.5 (0.4–13) | - | - |
| UCLA‡ | 10(4–10) | 9 (5–10) | .94§ |
| HOOSPain† | 72.7±14.4 | - | - |
| HOOSSymp† | 73.7±14.9 | - | - |
| HOOSADL† | 88.4±12.6 | - | - |
| HOOSSport† | 71.3±20.8 | - | - |
| HOOSQOL† | 56.8±21.0 | - | - |
Abbreviations: ADL, activities of daily living; BMI, body mass index; CHJP chronic hip joint pain; HOOS, Hip Disability and Osteoarthritis Outcome Score; QOL, quality of life; UCLA, University of California Los Angeles activity score.
Independent t-tests were used.
Values are mean±SD.
Values are median (range).
Mann-Whitney U test was performed.
Muscle Volume Differences Between Groups
Compared to the controls, participants with CHJP had significantly greater gluteal muscle volume on their involved side. There was no significant difference found in TFL muscle volume between the two groups (p=.640) (TABLE 3). There were no between group differences in the total number of slices measured for gluteals or TFL (TABLE 3).
TABLE 3.
Muscle volume, number of MRI slices per region of interest, and muscle strength values for all participants
| Muscle Volume* | CHJP (N=15) | Control (N=15) | Percent Difference | P-Value† |
|---|---|---|---|---|
| Gluteals (cm3) | 228±40 | 199±29 | 12.72 | .032 |
| TFL (cm3) | 33±11 | 31±9 | 6.06 | .640 |
|
| ||||
| Slice Number‡ | CHJP (N=15) | Control (N=15) | P-Value† | |
|
| ||||
| Gluteal Slices | 52±5 | 51±4 | .788 | |
| TFL Slices | 78±8 | 78±6 | .576 | |
|
| ||||
| Muscle Strength* | CHJP (N=15) | Control (N=15) | Percent Difference | P-Value† |
|
| ||||
| Hip Abductor Strength§ (Nm) | 74.6±16.8 | 93.6±20.2 | −20.30 | .009 |
Abbreviations: CHJP, chronic hip joint pain; TFL, tensor fascia latae.
Values are mean±SD.
Independent t-tests were used.
Hip abducted to 15º in sidelying.
Number of slices per participant.
Muscle Strength Differences Between Groups
Compared to the controls, women with CHJP had significantly decreased hip abductor muscle strength (p=.009) (TABLE 3). The majority of women with CHJP (10/15) reported no hip joint pain during strength testing of their involved side.
DISCUSSION
Our goal was to compare hip abductor muscle volume and hip abductor strength in women with CHJP and matched, asymptomatic women. Our hypotheses were partially supported. As expected, women with CHJP demonstrated decreased strength compared to controls. Surprisingly, we found that those with CHJP had larger gluteal muscle volume compared to controls. There were no differences in TFL muscle volume.
Our findings raise questions about the structure and function of hip abductors in people with CHJP. We hypothesized that pain experienced over a long period of time would result in disuse of the surrounding hip muscles, leading to muscle atrophy and eventual weakness. Instead we found larger muscle volume, which might indicate muscle hypertrophy. However other factors must be considered, including impaired neuromuscular activation during strength testing, altered architectural muscle properties, and potential presence of noncontractile tissues within the muscle in those with CHJP. Understanding the relationship among muscle structure, muscle function, and CHJP better will guide our future treatment strategies by identifying specific muscle impairments upon which to focus.
We were not surprised to find muscle weakness in women with CHJP. The participants in this study represent a subset of people from our previously published work,17 who were selected based on availability of MRI data. Additionally, previous studies have reported hip abductor weakness in similar patients compared to asymptomatic participants.5, 9 These findings suggest that rehabilitation to address muscle weakness may be an appropriate treatment approach, however we do not know the underlying mechanism of this weakness. Weakness may be due to a number of reasons, including pain experienced during testing.9, 12 In the current study, only five participants with CHJP reported experiencing pain during strength testing procedures. Other investigators have suggested weakness may be present due to impaired neural activation related to arthrogenic neuromuscular inhibition9, 12 or structural changes such as the presence of non-contractile tissues within the muscle and architectural differences.
Our findings related to muscle volume were unexpected. Because decreased muscle strength is thought to be associated with muscle atrophy, and the participants with CHJP had pain longer than three months, we expected those with CHJP to demonstrate smaller muscle volume compared to controls. Surprisingly, gluteal muscle volume was greater in those with long standing hip joint pain compared to those without pain. To our knowledge, this is the first study to report a comparison of hip abductor muscle volume in young to middle aged adults with and without CHJP. Related to our patient sample, we found one study that reported muscle CSA among patients with painful DDH.23 Liu et al23 reported the symptomatic hip in those with DDH had smaller muscle CSA compared to their contralateral, asymptomatic side, however they did not report a comparison to an asymptomatic control. We did not assess side-to-side differences in our patient sample. Six participants with CHJP demonstrated bilateral hip pain resulting in a sample of nine participants, thus limiting the power to detect differences between painful and non-painful limbs in those with unilateral pain.
Similar to our study, others have reported the comparison of hip abductor muscle size between people with hip OA and asymptomatic controls. In their systematic review, Marshall et al25 concluded that hip abductor muscle size is similar between those with hip OA and asymptomatic participants. With close assessment of the individual studies cited in their review and another recently published study, there is some evidence to suggest that findings may be affected by the participant’s age, stage of disease, or symptom duration.14, 25, 38 When comparing symptomatic limbs in those with hip OA to asymptomatic participants, Zacharias et al38 found that individuals with advanced OA (K/L 3), had significantly smaller GMax, GMed, and GMin. However, those with mild hip OA (K/L 2), had similar muscle volume to that of asymptomatic people. Grimaldi et al14 reported GMed muscle volume was 15% larger in people with mild hip OA (K/L 1–2), compared to asymptomatic participants. This is similar to the 13% greater gluteal muscle volume we found in women with CHJP compared to asymptomatic controls. This might suggest the musculature in patients with CHJP and those with early hip OA demonstrate similar characteristics.
Participants in previous studies of hip OA were older than participants in our study. It is possible the older participants in previous studies may have some age-related muscle atrophy resulting in smaller muscle volume, however, muscle atrophy was reported in only those older individuals with advanced hip OA.38 Older individuals with mild disease demonstrated no difference38 or greater muscle volume14 compared to controls. Visual assessment of our data suggests no relationship between age and gluteal muscle volume in our sample, however our sample is limited to those between 18 and 40 years old. Muscle volume may be associated with disease severity or symptom duration, with muscle atrophy occurring with more advanced OA or longer duration of symptoms.38 We do not know if OA existed in the women in our study, however, given their relatively young age the presence of OA is unlikely. Given the preliminary nature and small samples sizes of the previous studies and our current study, caution should be taken when interpreting these findings. Further investigation is needed to better understand the relationship of hip joint disease, muscle structure, and muscle function.
There are a number of possible explanations why the gluteal muscle volume in individuals with CHJP was larger than asymptomatic people. One likely explanation may be the potential presence of non-contractile tissue, such as intramuscular fat or swelling, in the gluteal ROI. The presence of intramuscular fat would result in greater muscle volume measurements, but would not contribute to the gluteals’ force-producing capabilities. In a recent study of women at risk for knee OA, knee extension strength was not associated with quadriceps muscle volume, however was associated with intramuscular fat volume, with greater intramuscular fat being associated with lower knee extensor strength.24 It is possible the women with CHJP had intramuscular fat in their gluteal muscles that we were unable to detect with our images. The images used in the current analysis were not optimized to differentiate muscle from intramuscular fat, therefore we cannot make a definitive conclusion. Our future work will include MRI sequences optimized to quantify lean muscle and intramuscular fat volume.
Muscle architectural differences between groups, such as muscle moment arm length, muscle fiber length, or pennation angle, may also explain our findings. We performed an a posteriori analysis to estimate potential differences between group gluteal moment arm lengths by measuring the perpendicular distance from the femoral head center to the GMed line of force. No differences were found indicating muscle moment arm lengths were similar between the two groups. We were unable to assess muscle fiber length or pennation angle with our data. Finally, in their article related to early hip OA, Grimaldi et al14 speculate the increased muscle volume may, in fact, be due to actual muscle hypertrophy. They reported hip abductors, GMed in particular, may be more likely to hypertrophy than to atrophy in the early phases of joint pathology. They also theorize this muscle hypertrophy may be related to changes in walking patterns, such as increased pelvic tilt or lateral shift. These are all factors that warrant further consideration for future studies.
Limitations
This study is not without limitations. Intrarater reliability did not include repeat scanning or repeat post-processing to correct for pelvic rotation, therefore, the reliability could have been less these steps were included. Additionally, this was a secondary analysis using MR images from the parent study.17 The images were optimized to measure bony morphology, and therefore not optimized to capture full hip abductor muscle volume or differentiate intramuscular fat from lean muscle. We chose to focus on the primary hip abductor muscles, therefore the contributions of muscles such as the sartorius were not included. Our 3D images, however, were acquired with small slice thickness and no gaps between slices, allowing for accurate representation of the primary hip abductors morphology within our defined ROI. For strength testing, we used 15 second rests between trials. This time may be considered short and possibly contribute to muscle fatigue with repeat testing. Upon review of the raw data, force values were not consistently highest in the first repetition or lowest in the final repetition, suggesting that fatigue was not a factor. Because the participants in this study were female and had a BMI less than 30kg/m2, the generalizability of our results is limited. BMI has been shown to be positively associated with muscle volume.10 The BMI threshold used for matching, 5kg/m2, may be considered wide, however we believe our methods are appropriate to control for the effect of BMI on our findings. We used a strict 1:1 matching in our study instead of the commonly used group matching approach. We also completed an a posteriori analysis and found the between-subject differences in BMI to be small (mean±SD: 1.2±0.8 kg/m2), with the largest between-participant difference being 3.3 kg/m2. Finally, participants with CHJP represent a heterogeneous population. To be included in the study, the participants in the CHJP had to report deep hip joint or anterior groin pain that was reproduced with the FADIR test. The FADIR test is sensitive in identifying the hip joint as the pain location, however it is not specific to a particular tissue source.27, 28 The results of this study may apply best to women with long standing hip joint pain seeking treatment.
CONCLUSION
Our study shows that women with CHJP exhibit decreased hip abductor strength, yet increased gluteal muscle volume compared to asymptomatic women. Muscle function is important and should be addressed in rehabilitation, however our study raises questions about the relationship of muscle volume and function in people with CHJP. Future research using imaging techniques to obtain full muscle volume and determine specific architectural features, such as the presence of non-contractile tissue, fat deposition, muscle fiber length, and pennation, would be useful. Studies to assess the neuromuscular activation of the hip muscles during daily tasks would also provide additional insight to our understanding and development of future treatment approaches.
KEY POINTS.
Findings
Women with CHJP were found to have decreased hip abductor strength, yet increased muscle volume of GMed, GMin, and a small portion of GMax.
Implications
Hip abductor function is important in women with CHJP, however our findings raise questions about the relationship of muscle strength and muscle volume among this population. A better understanding of this relationship will inform future treatment decisions.
Caution
Our study represents a secondary analysis of data collected during a larger cohort study. The sample size is small and includes only women with CHJP and a BMI less than 30kg/m2, thus limiting the generalizability of our results.
Acknowledgments
Funding
This work was supported by the following grants: Harris-Hayes was supported by grant K23 HD067343 and K12 HD055931 from the National Center for Medical Rehabilitation Research, National Institute of Child Health and Human Development, and National Institute of Neurological Disorders and Stroke and grant UL1 RR 024992-01 from the National Center for Research Resources, components of the National Institutes of Health and NIH Roadmap for Medical Research. Additional support was provided by Program in Physical Therapy at Washington University School of Medicine, Clinical and Translational Science Award (CTSA) Grant [UL1 TR000448] and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842
The authors would like to acknowledge Darrah Snozek for her assistance with data collection.
Footnotes
IRB Approval
This study was approved by the Human Research Protection Office of Washington University School of Medicine.
Statement of Financial Disclosure and Conflict of Interest
I affirm that I have no financial affiliation (including research funding) or involvement with any commercial organization that has a direct financial interest in any matter included in this manuscript, except as disclosed in an attachment and cited in the manuscript. Any other conflict of interest (ie, personal associations or involvement as a director, officer, or expert witness) is also disclosed in an attachment.
References
- 1.Amstutz HC, Thomas BJ, Jinnah R, Kim W, Grogan T, Yale C. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am. 1984;66(2):228–241. [PubMed] [Google Scholar]
- 2.Arnold CM, Warkentin KD, Chilibeck PD, Magnus CR. The reliability and validity of handheld dynamometry for the measurement of lower-extremity muscle strength in older adults. J Strength Cond Res. 2010;24(3):815–824. doi: 10.1519/JSC.0b013e3181aa36b8. doi:810.1519/JSC.1510b1013e3181aa1536b1518. [DOI] [PubMed] [Google Scholar]
- 3.Arokoski MH, Arokoski JP, Haara M, et al. Hip muscle strength and muscle cross sectional area in men with and without hip osteoarthritis. J Rheumatol. 2002;29(10):2185–2195. [PubMed] [Google Scholar]
- 4.Burnett RS, Della Rocca GJ, Prather H, Curry M, Maloney WJ, Clohisy JC. Clinical presentation of patients with tears of the acetabular labrum. J Bone Joint Surg Am. 2006;88(7):1448–1457. doi: 10.2106/JBJS.D.02806. [DOI] [PubMed] [Google Scholar]
- 5.Casartelli NC, Maffiuletti NA, Item-Glatthorn JF, et al. Hip muscle weakness in patients with symptomatic femoroacetabular impingement. Osteoarthr Cartil. 2011;19(7):816–821. doi: 10.1016/j.joca.2011.04.001. doi:810.1016/j.joca.2011.1004.1001. [DOI] [PubMed] [Google Scholar]
- 6.Chinzei N, Hashimoto S, Fujishiro T, et al. Inflammation and Degeneration in Cartilage Samples from Patients with Femoroacetabular Impingement. J Bone Joint Surg Am. 2016;98(2):135–141. doi: 10.2106/JBJS.O.00443. doi:110.2106/JBJS.O.00443. [DOI] [PubMed] [Google Scholar]
- 7.Clohisy JC, Baca G, Beaule PE, et al. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348–1356. doi: 10.1177/0363546513488861. doi:1310.1177/0363546513488861. [DOI] [PubMed] [Google Scholar]
- 8.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763. doi:1310.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 9.Diamond LE, Wrigley TV, Hinman RS, et al. Isometric and isokinetic hip strength and agonist/antagonist ratios in symptomatic femoroacetabular impingement. J Sci Med Sport. 2015;22(15):00202–00209. doi: 10.1016/j.jsams.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Erskine RM, Tomlinson DJ, Morse CI, et al. The individual and combined effects of obesity- and ageing-induced systemic inflammation on human skeletal muscle properties. Int J Obes (Lond) 2017;41(1):102–111. doi: 10.1038/ijo.2016.151. doi:110.1038/ijo.2016.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez M, Wall P, O’Donnell J, Griffin D. Hip pain in young adults. Aust Fam Physician. 2014;43(4):205–209. [PubMed] [Google Scholar]
- 12.Freeman S, Mascia A, McGill S. Arthrogenic neuromusculature inhibition: a foundational investigation of existence in the hip joint. Clin Biomech (Bristol, Avon) 2013;28(2):171–177. doi: 10.1016/j.clinbiomech.2012.11.014. doi:110.1016/j.clinbiomech.2012.1011.1014. [DOI] [PubMed] [Google Scholar]
- 13.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 14.Grimaldi A, Richardson C, Stanton W, Durbridge G, Donnelly W, Hides J. The association between degenerative hip joint pathology and size of the gluteus medius, gluteus minimus and piriformis muscles. Man Ther. 2009;14(6):605–610. doi: 10.1016/j.math.2009.07.004. doi:610.1016/j.math.2009.1007.1004. [DOI] [PubMed] [Google Scholar]
- 15.Harris-Hayes M, Commean PK, Patterson JD, Clohisy JC, Hillen TJ. Bony abnormalities of the hip joint: a new comprehensive, reliable and radiation-free measurement method using magnetic resonance imaging. J Hip Preserv Surg. 2014;1(2):62–70. doi: 10.1093/jhps/hnu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris-Hayes M, McDonough CM, Leunig M, Lee CB, Callaghan JJ, Roos EM. Clinical outcomes assessment in clinical trials to assess treatment of femoroacetabular impingement: use of patient-reported outcome measures. J Am Acad Orthop Surg. 2013;21(Suppl 1):S39–46. doi: 10.5435/JAAOS-5421-5407-S5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris-Hayes M, Mueller MJ, Sahrmann SA, et al. Persons with chronic hip joint pain exhibit reduced hip muscle strength. J Orthop Sports Phys Ther. 2014;44(11):890–898. doi: 10.2519/jospt.2014.5268. doi:810.2519/jospt.2014.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebert LJ, Maltais DB, Lepage C, Saulnier J, Crete M, Perron M. Isometric muscle strength in youth assessed by hand-held dynamometry: a feasibility, reliability, and validity study. Pediatr Phys Ther. 2011;23(3):289–299. doi: 10.1097/PEP.0b013e318227ccff. doi:210.1097/PEP.1090b1013e318227ccff. [DOI] [PubMed] [Google Scholar]
- 19.Ireland ML, Willson JD, Ballantyne BT, Davis IM. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003;33(11):671–676. doi: 10.2519/jospt.2003.33.11.671. [DOI] [PubMed] [Google Scholar]
- 20.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kendall F, McCreary E, Provance P, Rodgers M, Romani W. Muscles: Testing and Function With Posture and Pain. 5. Baltimore, MD: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 22.Krause DA, Schlagel SJ, Stember BM, Zoetewey JE, Hollman JH. Influence of lever arm and stabilization on measures of hip abduction and adduction torque obtained by hand-held dynamometry. Arch Phys Med Rehabil. 2007;88(1):37–42. doi: 10.1016/j.apmr.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Liu R, Wen X, Tong Z, Wang K, Wang C. Changes of gluteus medius muscle in the adult patients with unilateral developmental dysplasia of the hip. BMC Musculoskelet Disord. 2012;13:101. doi: 10.1186/1471-2474-1113-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maly MR, Calder KM, Macintyre NJ, Beattie KA. Relationship of intermuscular fat volume in the thigh with knee extensor strength and physical performance in women at risk of or with knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65(1):44–52. doi: 10.1002/acr.21868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall AR, Noronha M, Zacharias A, Kapakoulakis T, Green R. Structure and function of the abductors in patients with hip osteoarthritis: Systematic review and meta-analysis. J Back Musculoskelet Rehabil. 2016;29(2):191–204. doi: 10.3233/BMR-150614. [DOI] [PubMed] [Google Scholar]
- 26.Martin RL, Enseki KR, Draovitch P, Trapuzzano T, Philippon MJ. Acetabular labral tears of the hip: examination and diagnostic challenges. J Orthop Sports Phys Ther. 2006;36(7):503–515. doi: 10.2519/jospt.2006.2135. [DOI] [PubMed] [Google Scholar]
- 27.Martin RL, Irrgang JJ, Sekiya JK. The diagnostic accuracy of a clinical examination in determining intra-articular hip pain for potential hip arthroscopy candidates. Arthroscopy. 2008;24(9):1013–1018. doi: 10.1016/j.arthro.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 28.Maslowski E, Sullivan W, Forster Harwood J, et al. The diagnostic validity of hip provocation maneuvers to detect intra-articular hip pathology. Pm r. 2010;2(3):174–181. doi: 10.1016/j.pmrj.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy. 2005;21(4):385–393. doi: 10.1016/j.arthro.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Neumann DA. Kinesiology of the hip: a focus on muscular actions. J Orthop Sports Phys Ther. 2010;40(2):82–94. doi: 10.2519/jospt.2010.3025. [DOI] [PubMed] [Google Scholar]
- 31.Nilsdotter AK, Lohmander LS, Klassbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4:10. doi: 10.1186/1471-2474-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oner A, Koksal A, Sofu H, Aykut US, Yildirim T, Kaygusuz MA. The prevalence of femoroacetabular impingement as an aetiologic factor for end-stage degenerative osteoarthritis of the hip joint: analysis of 1,000 cases. Hip Int. 2016;26(2):164–168. doi: 10.5301/hipint.5000323. doi:110.5301/hipint.5000323. [DOI] [PubMed] [Google Scholar]
- 33.Preininger B, Schmorl K, von Roth P, et al. The sex specificity of hip-joint muscles offers an explanation for better results in men after total hip arthroplasty. Int Orthop. 2012;36(6):1143–1148. doi: 10.1007/s00264-011-1411-7. doi:1110.1007/s00264-00011-01411-00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shindle MK, Ranawat AS, Kelly BT. Diagnosis and management of traumatic and atraumatic hip instability in the athletic patient. Clin Sports Med. 2006;25(2):309–326. doi: 10.1016/j.csm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Snyder KR, Earl JE, O’Connor KM, Ebersole KT. Resistance training is accompanied by increases in hip strength and changes in lower extremity biomechanics during running. Clin Biomech (Bristol, Avon) 2009;24(1):26–34. doi: 10.1016/j.clinbiomech.2008.1009.1009. [DOI] [PubMed] [Google Scholar]
- 36.Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418–426. doi: 10.1016/j.pmrj.2013.02.005. doi:410.1016/j.pmrj.2013.1002.1005. [DOI] [PubMed] [Google Scholar]
- 37.Ward SR, Winters TM, Blemker SS. The architectural design of the gluteal muscle group: implications for movement and rehabilitation. J Orthop Sports Phys Ther. 2010;40(2):95–102. doi: 10.2519/jospt.2010.3302. doi:110.2519/jospt.2010.3302. [DOI] [PubMed] [Google Scholar]
- 38.Zacharias A, Pizzari T, English D, Kapakoulakis T, Green RA. Hip abductor muscle volume in hip osteoarthritis and matched controls. Osteoarthr Cartil. 2016;6(16):30064–30064. doi: 10.1016/j.joca.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhang C, Li L, Forster BB, et al. Femoroacetabular impingement and osteoarthritis of the hip. Can Fam Physician. 2015;61(12):1055–1060. [PMC free article] [PubMed] [Google Scholar]