Abstract
Surface functionalization via molecular design has been a key approach to incorporate new functionalities into existing biomaterials for biomedical application. Mussel-inspired polydopamine (PDA) has aroused great interest as a new route to the functionalization of biomaterials, due to its simplicity and material independency in deposition, favorable interactions with cells, and strong reactivity for secondary functionalization. Herein, this review attempts to highlight the recent findings and progress of PDA in bio-surface functionalization for biomedical applications. The efforts made to elucidate the polymerization mechanism, PDA structure, and the preparation parameters have been discussed. Interactions between PDA coatings and the various cell types involved in different biomedical applications including general cell adhesion, bone regeneration, blood compatibility, and antimicrobial activity have also been highlighted. A brief discussion of post-functionalization of PDA and nanostructured PDA is also provided.
Keywords: Polydopamine, Functionalization, Biomedical application, Polymerization
1. Introduction
Surface modification and functionalization play a central role in controlling surface properties and conferring new functionalities to materials, which is especially important in critical fields such as biomaterials, tissue engineering, and medical diagnostics [1–3]. In the past few decades, several key technologies have been developed to modify material surfaces, such as chemical conjugation [4], self-assembly monolayers (SAMs) [5], layer-by-layer (LBL) film deposition [6], and plasma treatment [7]. In general, however, most these surface modification techniques are time consuming and complicated processes, and, more critically, their application and quality rely largely on specific surface properties. For instance, SAMs of thiolates can only form stable layers on noble metals [5,8]; while alkylsilane SAMs can only be applied to silicon dioxide and silicon surfaces [9]. Therefore, a simple and universal surface modification approach with wide substrate applicability and easy processing is highly desirable.
In nature, mussels adhere strongly to wood or stones in wet conditions of high shear stresses from water flow. How can the mussel be an underwater specialist? The secret lies in the different mussel foot proteins secreted during adhesive formation in the adhesive plaque of mussel byssus. It was found that all these foot proteins contain 3,4-dihydroxy-L-phenylalanine (DOPA) and lysine amino acids [10,11], leading to the hypothesis that the co-existence of catechol (DOPA) and amine (lysine) groups may be crucial for achieving strong adhesion [12]. Polydopamine (PDA), containing both catechol and amine groups, was discovered to be a one-step facile surface coating method in 2007 [12]. The PDA coating has been demonstrated to functionalize a wide array of material surfaces, including superhydrophobic surfaces. Hence, PDA has opened a new route for surface modification and has garnered great interest, especially in materials science, biology and biomedical fields.
The aim of this review article is to outline the recent findings of PDA buildup mechanism and surface properties, and the development of PDA films as a smart coating material for biomedical applications. In the first part of this review, we will summarize what has been found regarding the buildup mechanisms and proposed structures of the PDA film as well as parameters that influence the film formation. In the second part, research involving the interactions of PDA coatings with mammalian cells and bacteria as well as the underlying mechanism of cell responses will be discussed. Finally, we will further present some representative applications of PDA as multifunctional coating in the biomedical field.
2. Coating mechanism, structure, and preparation
2.1. Coating mechanism and structure
The PDA is spontaneously formed by pH-induced, oxidative polymerization of dopamine-hydrochloride in alkaline solutions (pH > 7.5). To achieve PDA coatings, simple immersion of substrates in a dilute aqueous solution of dopamine (typically 2 mg/mL of dopamine in 10 mM TRIS buffer) results in spontaneous deposition of a thin PDA film [12]. Despite the ease of the PDA coating preparation, the molecular mechanism of the PDA buildup is still not fully understood, which is mainly due to the marked chemical heterogeneity and adverse physical properties of this material. Further, PDA structure can depend on the reaction conditions, e.g. buffer solution [13–15], and thus the discussion here will focus on PDA obtained by most commonly used protocol, i.e. air oxidation in TRIS or phosphate buffer.
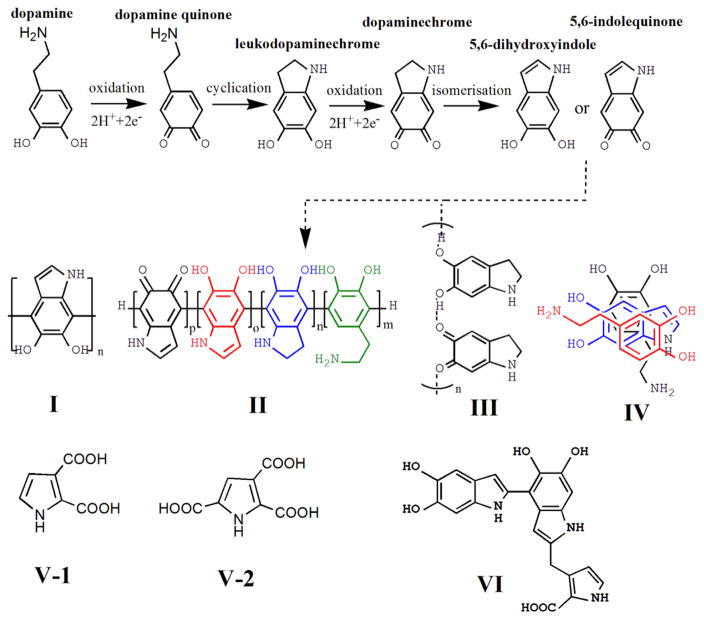
In solution, it is well known that PDA buildup shares the first steps with melanin biosynthesis: the oxidation of dopamine to dopamine-quinone, its intramolecular cyclization, oxidation to dopaminechrome, formation of 5,6-dihydroxyindole (DHI), and further oxidation to 5,6-indolequinone (IDQ) (Scheme 1) [16]. It is notable that the mixture of the dopamine, quinone and indole units may co-exist in solution after the first steps since the oxidation and cyclization may not be complete. Subsequently, the mixture of these units undergoes various pathways to form the PDA structure, which presents a puzzle: is PDA a covalent polymer or a supramolecular aggregate?
Scheme 1.
First steps of melanin formation by dopamine oxidation and proposed models of PDA structure.
In 2007, Messersmith and co-workers examined the mass spectra of PDA-coated glass using time-of-flight secondary ion mass spectrometry and found a peak at m/z 445 which was assigned to the DHI trimer [12]. Based on this observation, they suggested a polymerized structure (Scheme 1 I) wherein the DHI molecules undergo branching reactions at positions 2, 3, 4, and 7, leading to multiple isomers of dimers and later on higher oligomers, which eventually form the covalent PDA structure. However, the peak at m/z 445 was not present in the time-of-flight secondary ion mass spectrometry spectra of the PDA-coated substrates fabricated with the same protocol in other studies [17–19]. Later, Liebscher and co-workers reported investigations of the PDA structure using various spectroscopic methods, e.g. solid-state nuclear magnetic resonance, electrospray ionization high-resolution mass spectrometry, X-ray photoelectron spectroscopy [20]. Here the authors demonstrated that PDA was a covalent polymer while the buildup units consisted of mixtures of various indole units with different degrees of (un)saturation and open-chain dopamine units, rather than a single DHI unit (Scheme 1 II).
In contrast to the covalent polymer models, Bielawski and co-workers analyzed the PDA structure using a variety of solid state spectroscopic and crystallographic techniques [21]. Their data revealed the presence of hydrogen bounds to the aryl core of the PDA and stacked structures formed by monomers. Therefore, they proposed that PDA is not a covalent polymer but instead a supramolecular aggregate of monomers (primarily DHI and its dione derivative) that were held together via a combination of charge transfer, π-stacking, and hydrogen bonding interactions (Scheme 1 III). In the other study [22], Lee and co-workers monitored the PDA formation by high-performance liquid chromatography mass spectrometry and identified that a physical, self-assembled trimer of (dopamine)2/DHI (Scheme 1 IV) exists in PDA.
The proposed model was further advanced by d’Ischia and co-workers in a recent report [15]. Here the authors demonstrated that PDA consists of three main building blocks, uncyclized catecholamine/quinones, cyclized DHI unites, and pyrrolecarboxylic acids (PCA) moieties (Scheme 1 V-1 and V-2), which was the first time to be recognized. Moreover, the oligomer components only up to tetramer level were identified, thus they proposed that PDA should be represented at best as a collection of oligomeric species in which monomer units were linked through different bonding. Although they did not provide the definite evidence for a true macromolecular nature of PDA, they suggested that non-covalent interactions became significant at later stages after the oligomerization reaction has proceeded significantly.
It is notable that most previous studies on PDA structure were analyzing PDA aggregates formed in dopamine solution. However, PDA films deposited on substrates rather than PDA aggregates in solution are often utilized for biomaterial functionalization and directly involved in cell-surface interactions. The direct analysis of PDA films seems to be more meaningful for understanding basic structure-property-function relationships of the PDA. Recently, Leng and co-workers reported a structural analysis of PDA films deposited on various substrates using X-ray photoelectron spectroscopy, time-of-flight secondary ion mass spectrometry, and matrix-assisted laser desorption/ionization mass spectrometry and proposed a new PDA structure model [19]. By doing the comparative analysis of PDA films on the substrate versus PDA aggregates in solution, it was demonstrated that the dopamine monomer and TRIS molecule were present in PDA aggregates but not or limited on PDA films, although they share several common building blocks. More importantly, the (DHI)2/PCA trimer complex (Scheme 1 VI) was identified as a primary building block of PDA. Moreover, they proposed that (DHI)2/PCA trimer complex steered by the covalent interactions at the initial stages of PDA formation were further linked primarily via non-covalent interactions to build up the final PDA structure.
2.2. Coating preparation
Although preparation of PDA coatings is a rather simple one-step process, several factors during preparation, such as reaction temperature, initial dopamine concentration, solution pH, buffer and oxidant employed, have been reported to play important roles for controlling the surface characteristics of PDA coatings. Elevated temperatures can accelerate the deposition of PDA on substrates, creating a rougher and more hydrophilic PDA layer by comparison to coatings formed at lower temperature [23]. Some reports indicate that the initial reaction speed of PDA formation can reach a plateau value for dopamine concentrations higher than 1 g/L [24], while others have found a linear increase of PDA coating thickness with dopamine concentrations from 0.1 to 5.0 g/L [25]. Recently, the biphasic evolution of the PDA coating thickness as well as surface roughness with increasing initial dopamine concentrations (0.25 – 4 g/L) was found, with the initial concentration of 1.0 g/L resulting in the largest coating thickness and surface roughness [19]. This discrepancy might result from different oxygen concentration in reaction solution maintained in different studies, since the oxidant (e.g. type, concentration etc.) used in the reaction solution is another crucial factor for PDA formation. For instance, in the absence of oxygen, no visible PDA deposition was observed; while higher oxygen concentration in the dopamine solution led to not only elevated deposition rate, but also a more uniform and smooth coating [26]. Some oxidants other than oxygen such as metal ions, ammonium persulfate, have also proved to be effective in PDA formation and result in distinct deposition kinetics and optical properties [13,27,28]. Additionally, the pH and type of buffer employed also significantly affects the deposition kinetics of PDA coating formation. For example, the deposition rate reaches the highest values at pH ~8.5, and the replacement of TRIS buffer by phosphate buffer also results in higher deposition rate [13,29].
3. Cellular response to PDA coating
3.1. Cell adhesion and patterning
As a major component of naturally occurring melanin that is widely distributed in human body, PDA exhibits excellent biocompatibility. The adhesion of fibroblasts and megakaryocytes (bone marrow cells) to PDA modified surfaces has been accessed by Lee et al. in their initial report [12]. Fibroblast cell adhesion was supported on PDA coated surface as well as on unmodified controls, while limited megakaryocytic adhesion was observed on PDA coating. These observations indicated that the cytocompatibility of the PDA coatings seems cell type-dependent. The in vitro cytocompatibility of PDA coatings was further investigated by Park’s group [30], where human umbilical vein endothelial cells were cultured on PDA coated electrospun polycaprolactone nanofibers. It was found that the endothelial cells exhibited highly enhanced adhesion, viability, and stress fiber formation on PDA-coated polycaprolactone nanofibers compared to unmodified and gelatin-coated nanofibers. Moreover, the PDA coating was demonstrated to be a powerful route for converting a variety of bioinert substrates into bioactive ones, including some non-wetting surfaces [31] and 3D porous scaffolds [32], by promoting cell adhesion of several cell types, such as osteoblast, pheochromocytoma, and chondrocytes. Ku et al. [33] produced PDA coated nanofibrous scaffolds with well-aligned nanofiber and studied the effects on myoblast differentiation. They demonstrated that both myosin heavy chain expression and myoblast fusion were significantly increased on PDA-modified nanofibers.
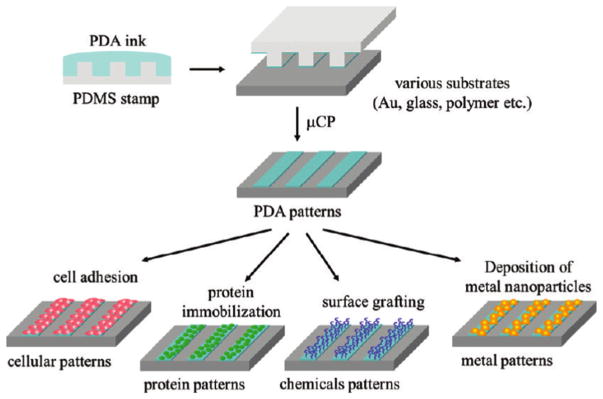
Given the strong affinity of cells to PDA coatings as well as its good stability and material independency, PDA coatings have also been utilized for cell patterning via several techniques such as photolithography [12], microfluidic technique [34], and micro-contact printing [35,36]. Compared with the former two techniques, micro-contact printing is more convenient and a cheaper way for most researchers. For instance, Sun et al.[35] showed that a PDA layer can be first coated onto the poly(dimethylsiloxane) stamps and then easily transferred onto poly(ethylene glycol) based substrates. Here the authors demonstrated better stability of printed PDA on poly(ethylene glycol) surface compared to printed proteins by culturing NIH 3T3 cells as the patterned cells retracted and began to detach within 24 h on protein-patterned substrates, whereas the PDA supported attachment and spreading of the cells. Apart from cell patterning, the patterned PDA layer can be used for protein immobilization, conjugation of thiol- or amine-containing molecules, and immobilization of metal nanoparticles to create various chemical patterns on substrates (Fig. 1) [36].
Fig. 1.
Schematic illustration of preparation of tunable micropatterned substrate based on PDA via microcontact printing and secondary reactions. Adapted and reproduced from Ref. [36].
Additionally, by taking advantage of that the conformal and uniform coating layer, as well as ease of modification of complex, 3D topographical features by PDA coating methods, the combined effects of PDA modification and topographical cues on cell behavior have been studied [37,38]. Wang et al. [37] investigated the combined effects of submicron-grooved topography and surface chemistry, such as PDA coating, on attachment, proliferation, and collagen synthesis of anterior cruciate ligament cells. They showed that the elongation and alignment of cells was mediated by the grooved topography, while cell spreading and proliferation mainly depended on surface chemistry. Further, collagen production increased both on grooved topography and PDA coatings. Zhong et al.[38] applied the PDA modification to titanium dioxide nanotubes without affecting the nanostructure morphology, and studied its combined effects on endothelial cell (EC) and smooth muscle cell (SMC) activity. Interestingly, they found that the PDA modification and nanostructures synergistically promoted EC attachment, proliferation, migration and release of nitic oxide. Meanwhile, the PDA modified nanotube surface showed inhibitory effects on SMC adhesion and proliferation.
The possible mechanism for the enhancement of cell adhesion by the PDA coatings have also been investigated [31,32]. Ku et al.[31] indicated that serum protein adsorption occurred on both unmodified and PDA-modified substrates, therefore they hypothesized that the PDA modification potentially prevented possible protein denaturation, which is responsible for enhanced cell adhesion. However, the authors did not provide data on the adsorption and conformation of adhesive serum proteins to prove their hypothesis. Tsai et al.[32] compared the cell adhesion on PDA-coated surface under serum-containing and serum-free conditions. They found that the enhancement of cell adhesion to PDA-coated surfaces was significantly higher in the presence of serum proteins than that in the absence of serum proteins. Moreover, fibronectin adsorption was higher on PDA-coated surfaces than unmodified surfaces. Therefore, they suggested that the enhancement of cell adhesion to PDA-coated surfaces is likely due to increased immobilization and/or adsorption of adhesive proteins such as fibronectin on the substrates. However, such proposed mechanism cannot explain the findings that enhancement of cell adhesion is cell type-dependent as reported by Lee et al.[12]. Interestingly, based on the new finding that the PDA coatings induced different responses of vascular ECs and SMCs, some recent studies proposed a new mechanism by which the PDA coatings modulate cell response [39–41], which will be discussed in the later Section 3.3 Blood compatibility.
3.2. Mineralization and bone regeneration
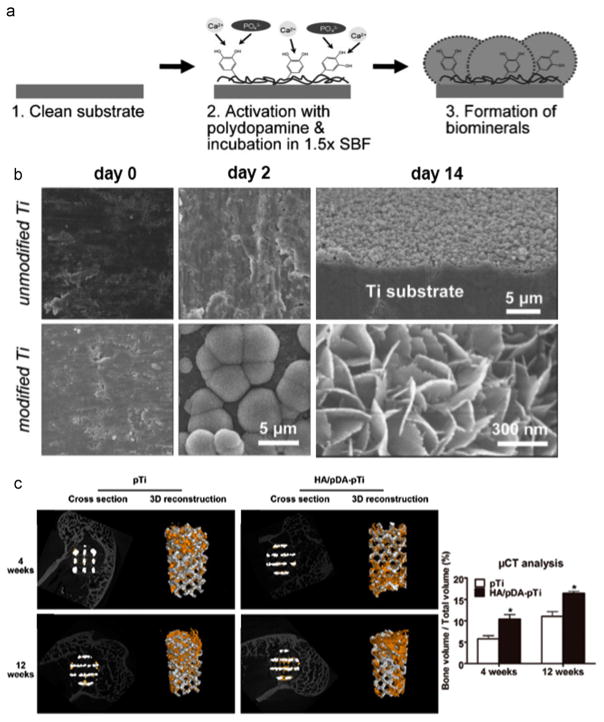
Bone injury and bone pathologies, as well as continued bone loss associated with aging, have resulted in increased research efforts focused on biomimetic materials for promoting biomineralization. However, a simple and unified way to functionalize these diverse materials to promote mineralization remains a critical challenge. In a seminal work, Ryu et al.[42] introduced a universal biomineralization route that can integrate inorganic hydroxyapatite crystals within versatile materials. They found that catecholamine moieties that are abundant in the PDA coatings are responsible not only for the chemical functionalization of a wide range of material surfaces, but also for the nucleation of hydroxyapatite by concentrating Ca2+ ions at the interface (Fig. 2a). After incubation in simulated body fluid for two weeks, the PDA coated-Titanium (Ti) was fully and uniformly covered by calcium phosphate (CaP) minerals in comparison to limited mineral deposition on the pristine Ti substrate (Fig. 2b). Another interesting study demonstrated the biomineralization of electrospun fibers by making use of PDA to bridge the minerals and polymeric fibers [43]. The obtained mineral coatings render polymeric fibers enhanced stiffness, ultimate tensile strength and toughness, which resemble closer the mechanical properties of natural bone.
Fig. 2.
The mineralization and bone regeneration facilitated by the PDA coating. a) Scheme for PDA-assisted calcium phosphate (CaP) crystal formation [42]. b) Electron microscopy images showing the effects of the PDA coating on the formation of CaP minerals after incubation the substrates in simulated body fluid (SBF) for 2 and 14 days. Adapted and reproduced from Ref. [42]. c) Microcomputed Tomography (Micro-CT) images of the porous Ti6Al4V scaffolds (pTi) and PDA-assisted hydroxyapatite coating modified scaffolds (HA/PDA-pTi) after implantation for 4 and 12 weeks (the yellow color components was newly formed bone), and the quantified percentages of regenerated bone volume/total volume in these implants. * indicate statistical significance compared to the pTi group, p < 0.05. Adapted and reproduced from Ref. [50].
Apart from facilitating the mineralization, PDA coatings also demonstrate favorable attachment and osteogenic differentiation of different cell types in vitro [44–46]. Wu et al.[44] demonstrated that PDA modified porous silicon dioxide scaffolds showed increased attachment, proliferation, and differentiation of human bone marrow stromal cells (hBMSCs), suggesting the enhanced osteogenic properties. Later, the same group developed a self-assembly method to prepare CaP/PDA nanocomposite coating on bioceramics, which stimulated the attachment, proliferation, alkaline phosphate (ALP) activity and bone-related gene expression of hBMSCs [46]. Rim et al.[45] also functionalized eletrospun fibers using PDA coatings to modulate the differentiation of human mesenchymal stem cells (hMSCs). They found that the inclusion of PDA coatings not only promoted cell attachment and proliferation, but also enhanced the osteogenic differentiation by up-regulation of osteogenic gene expression and ALP activity. In addition, PDA coatings have also been demonstrated to positively stimulate the activities of other types of bone-related cells, such as mouse osteoblastic cells and MG-63 human osteoblastic cells [47,48].
Moreover, PDA coatings have been utilized in vivo for bone regeneration. Lee et al.[49] implanted PDA modified electrospun fibers (either random or aligned topography) into a mouse calvarial defect model. The results demonstrated that regenerated bone area was maximal when mice were implanted with aligned fibers with PDA coating, indicating a positive synergistic effect on bone regeneration. It was speculated that the directed cell migration into the defect site induced by the aligned fibers and enhanced biocompatibility by the PDA coating co-contributed to the favorable bone regeneration. Another interesting study was performed by Li et al.[50] whereby Ti6Al4V scaffolds were modified by PDA-assisted hydroxyapatite coating, which enhanced the osteointegration and significantly promoted bone regeneration after implantation into rabbit femoral condylar defects at 4 and 12 weeks (Fig. 2c). Almost at the same time, Xu et al.[51] prepared a PDA-apatite hybrid nanolayer on the surfaces of the bioceramic scaffolds followed by evaluating their capability for bone regeneration in a rabbit femur defect model. It was found that the PDA/apatite nanolayer modified scaffolds induced more formation of the new bone in and around the scaffolds than unmodified scaffolds after implantation for 12 weeks.
3.3. Blood compatibility
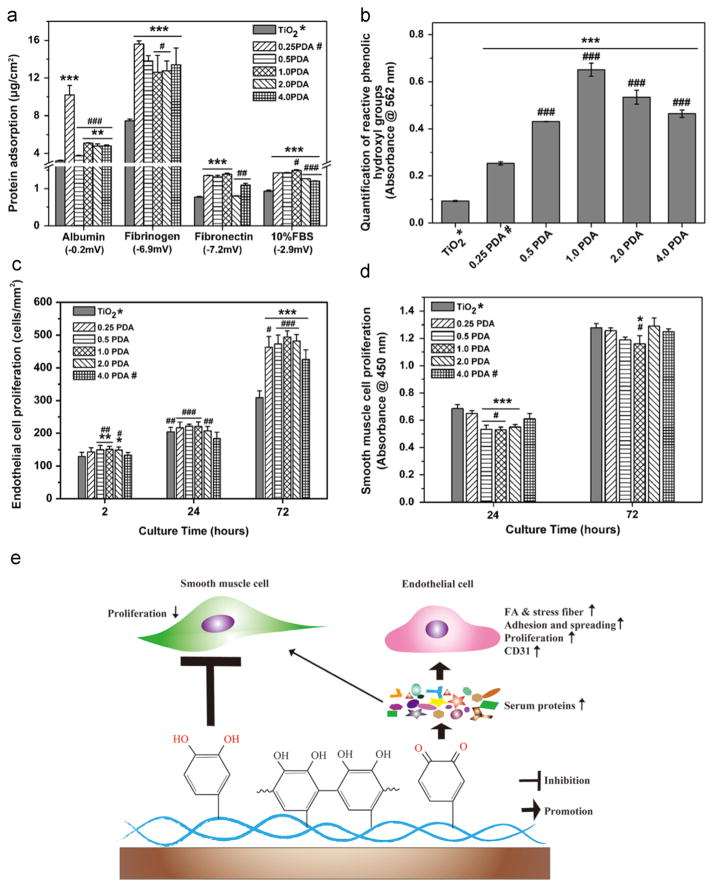
The prevalence of cardiovascular disease has led to heightened demand for vascular stents or grafts. The development of ideal vascular stents or grafts aim to favor rapid re-endothelialization by promoting vascular ECs while simultaneously inhibiting vascular SMC proliferation and improving hemocompatibility [52]. PDA coatings were initially employed to stimulate re-endothelialization of nanofibrous polycaprolactone scaffolds by Park’s group in 2010 [30]. Compared to the pristine polycaprolactone scaffolds, PDA coatings promoted EC adhesion, spreading, viability, and positive expression of EC markers, suggesting the potential application of these PDA coatings in vascular tissue engineering. More importantly, some recent studies demonstrated that PDA coatings not only significantly enhanced EC growth and functions, but also reduced SMC proliferation [39–41]. For example, Yang et al. [39] showed that PDA coatings on 316 L stainless steel stents would significantly promote endothelialization while inhibiting adhesion and proliferation of SMCs. Notably, the PDA coatings on the stents also showed good stability and resistance to the deformation behavior of compression and expansion of the stent. To further explore the mechanism by which PDA coatings modulate the behavior of ECs and SMCs in a different manner, Luo et al.[40] reported that thermal treatment of the PDA coatings could convert the primary amino and catechol groups into quinone groups by thermal oxidation in air. The authors further demonstrated that the thermal oxidation of the PDA coatings would enhance the ability to promote EC growth, but the inhibition of SMC proliferation was diminished. They concluded that the opposite effects of PDA coatings on ECs and SMCs are closely associated with phenolic/quinone groups. Interestingly, Ding et al.[41] investigated the “surface property – protein adsorption – vascular cell behavior” relationships of PDA coatings synthesized at varied initial dopamine concentrations. Their results suggested that the quinone group on PDA coatings induced a larger amount of protein adsorption, and subsequently promoted EC attachment and proliferation (Fig. 3a, c and e). Meanwhile, the reactive phenolic hydroxyl groups on PDA coatings would account for the inhibitory effects on SMCs (Fig. 3b, d and e).
Fig. 3.
The PDA coating promoted vascular EC growth while inhibited SMC proliferation. a) Quantification of the protein adsorption to PDA and pristin TiO2 sufraces. b) Quantification of reactive phenolic hydroxyl groups determined via the micro-BCA assay. c) EC proliferation determined by cell density after culturing cells for 2 h, 24 h, and 72 h. d) SMC proliferation determined by Cell Counting Kit-8 assay after culturing cells for 24 h and 72 h. e) Schematic diagram of the proposed mechanism by which PDA selectively modulating EC and SMC behavior. 0.25 PDA, 0.5 PDA, 1.0 PDA, 2.0 PDA, and 4.0 PDA represents the PDA coatings on the titanim dioxide (TiO2) surfaces prepared at various initial dopamine concentrations of 0.25, 0.5, 1.0, 2.0, 4.0 g/L in 10 mM Tris buffer, pH 8.5. Statistically significant differences are marked as follows: * vs. TiO2; # vs. 0.25 PDA for a) and b); # vs. 4.0 PDA for c) and d). Adapted and reproduced from Ref. [41].
3.4. Antimicrobial activity
Bacterial infections are prevalent in a wide variety of medical, public health, industrial and environmental settings [53]. Their increasing capability to resist multiple drugs demands development of highly potent antimicrobials. Iqbal et al.[54] found PDA has an intrinsic antimicrobial effect against E. coli by co-incubating dopamine with the bacteria solution. Their results suggested that the bacteria were encapsulated inside the PDA film by dopamine self-polymerization onto the bacteria, and the PDA shell layer prevented the bacteria multiplying. Alternatively, they hypothesized that the PDA layer may have created a barrier with reduced permeability to specific components, preventing the diffusion of nutrients necessary for bacteria survival or expulsion of metabolic waste. However, they did not assess the antimicrobial activity of PDA coatings on the material surface. In line with the strong bioactivity of PDA coatings, it was demonstrated that the deposition of PDA coatings on gold/polypyrrole bilayer actuators greatly increase the efficiency of seizing Escherichia coli (E. coli) bacteria from physiological media without compromising the actuation performance [55]. A similar conclusion was drawn by another study where the authors investigated the antibacterial performance of PDA-coated polymer/metal surfaces [56]. The results indicated that the PDA coating led to a modest increase in the attachment of E. coli and Pseudomonas aeruginosa, while no significant effects on the attachment of Staphylococcus epidermidis was observed.
In contrast, a recent study performed by Liu et al. suggested that PDA-coated zirconia significantly reduced Streptococcus gordonii and Streptococcus mutans proliferation compared to pristine zirconia surface although there was no observable effects of the PDA coating on bacterial viability [57]. More recently, an interesting study demonstrated the strong antibacterial activities of PDA coatings prepared by a facile shaking-assisted method [58]. In this study, roughened PDA (rPDA) coatings were prepared by replacing the static solution condition in the conventional synthesis procedure for smooth PDA coatings (sPDA) with a shaking solution condition. The rPDA coatings showed the same chemical features to the sPDA coatings based on the Raman spectrum, but with significantly different morphology/roughness and wettability from the sPDA coatings. Unexpectedly, the rPDA coatings were found to possess remarkably enhanced antibacterial activity against gram-positive Staphylococcus aureus, and gram-negative E. coli and Pseudomonas aeruginosa compared to the sPDA coatings as well as unmodified surfaces. The authors proposed that the contact killing resulted from the damage of the cell membranes of bacteria incubated with the rPDA coatings. This study suggested that the physical properties of PDA coatings tuned by the preparation conditions might significantly alter the anti-bacterial activities. Nevertheless, the interactions between the PDA coatings and bacteria have not been well studied. The current literature reports controversial results regarding the anti-bacterial activities of the PDA coatings; therefore the mechanism of bactericidal properties is obscure and needs further study.
4. Post-functionalization and nanostructured PDA
4.1. Ad-layer immobilization of biomolecules
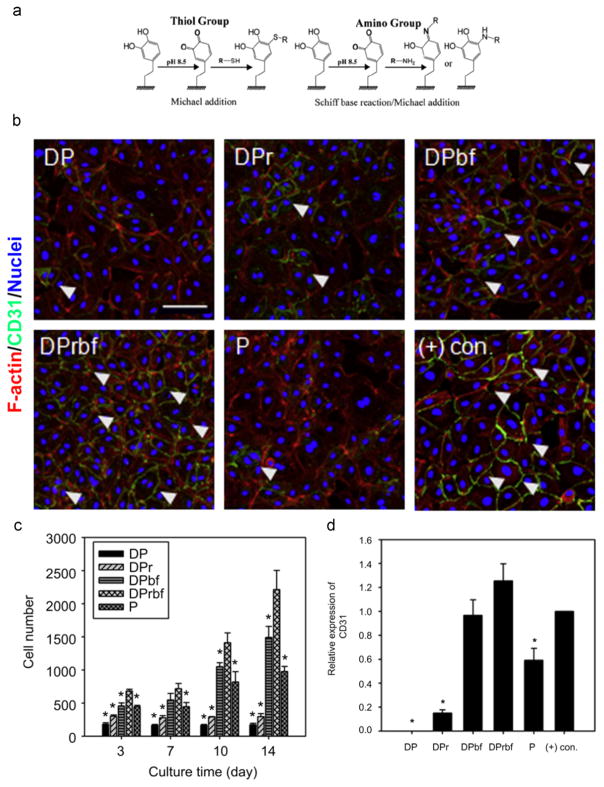
The multiple functional groups presented on PDA coatings are able to react with a wide range of molecules, providing an important platform to produce diverse biomaterials with specific functionalities. For instance, the oxidized quinone form of catechol can undergo reactions with various functional groups, including amine and thiol, via Michael addition or Schiff base reaction to form covalently grafted functional layers (Fig. 4a) [59,60]. A large number of proteins, peptides, growth factors, and other bioactive molecules have been conjugated onto substrates via the PDA coating layer, such as fibronectin [61], RGD pepetide [62], vascular endothelial growth factor [63], and selenocystamine [64]. One such example is applying PDA coatings to titanium oxide nanotubes for immobilization of the direct thrombin inhibitor – bivalirudin [65]. Interestingly, the PDA ad-layer not only increased the loading capacity of the bivalirudin, but also showed robust and controlled release of bivalirudin, which improved the hemocompatibility and competitive growth of ECs versus SMCs.
Fig. 4.
The immobilization of multiple biomelecules via the PDA coating layer. a) Reaction scheme to immobilize biomolecules with thiol or amine groups to the PDA coating layer via Michael additon and/or Schiff base reaction. Adapted and reproduced from Ref. [60]. b) Fluorescent images of human umbilical vein endothelial cells on various substrates. DP: PDA-coated poly(lactic acid-co-ε-caprolactone) film. DPr: RGD immobilized onto DP. DPbf: basic fibroblast growth factor (bFGF) immobilized onto DP. DPrbf: RGD, bFGF immobilized onto DP. P: RGD, bFGF passively adsorbed onto poly(lactic acid-co-ε-caprolactone) film. (+) con.: soluble bFGF treated group. c) Quantification of cell number after culturing human umbilical vein endothelial cells on various substrates for 3, 7, 10, and 14 days. * indicates significance as compared to the DPrbf group at each time point (p < 0.05). d) Expression of CD31 of human umbilical vein endothelial cells cultured on various substrates determined by Western blot analysis. * indicates significance as compared to the (+) con. group (p < 0.05). b), c) and d) were adapted and reproduced from Ref. [62].
Noting that the extracellular microenvironment surrounding cells in vivo is a complex array of molecular signals: (1) insoluble extracellular matrix molecules (fibrillary proteins, noncollagenous glycoproteins, and proteoglycans), (2) soluble molecules (growth factor, chemokines and cytokines), and (3) proteins on the surfaces of neighboring cells [66]; the presentation of multiple biomolecules on the surface of synthetic biomaterials is important to mimic the native cellular microenvironment in dictate of cell behavior. PDA coatings with multiple bioactive functional groups provide a simple and superior platform to immobilize multiple biomolecules onto material surfaces. For instance, Lee et al.[62] functionalized vascular graft materials with dual bioactive factors, i.e. adhesive RGD peptide as an essential extracellular matrix component and basic fibroblast growth factor, via the pre-deposited PDA layer. They confirmed that the covalently immobilized factors exhibited more stable and long-term effects towards the control of cell behavior than did passively adsorbed molecules. Moreover, it was found that the adhesion, migration, proliferation and differentiation of human umbilical vein endothelial cells were synergistically accelerated by the presence of multiple signaling factors (Fig. 4b, c, and d).
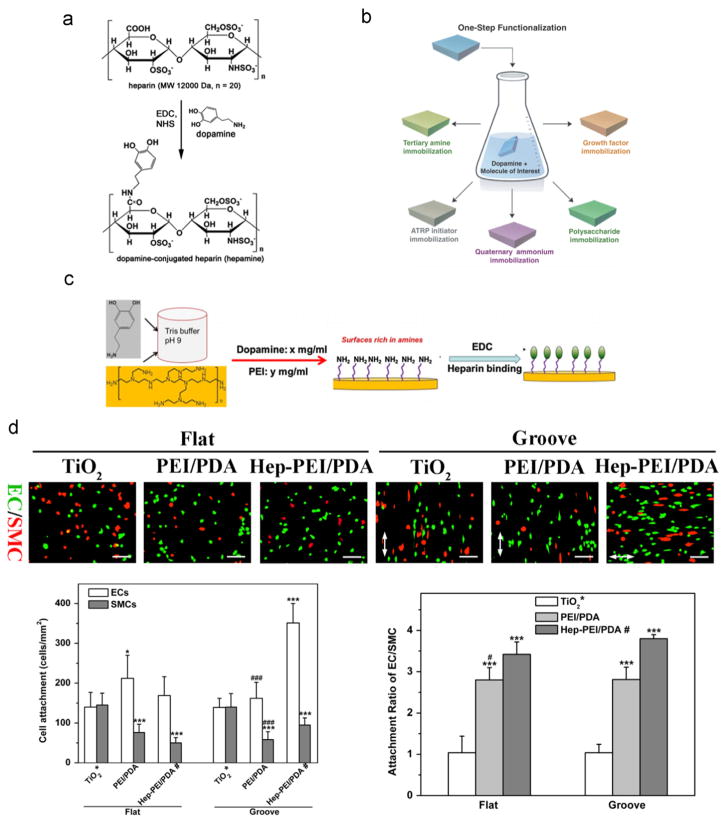
However, conjugation of biomolecules without amine and/or thiol groups via the PDA ad-layer is not very straightforward. For instance, the surface immobilization of heparin, containing sulfonic, sulfoamino, and carboxyl groups, is of great importance in the development of blood-contacting medical devices because of its ability to inactivate the blood coagulation pathway. Previous approaches for the surface immobilization of heparin, either covalent linkage or electrostatic interactions, are often time-consuming and complicated. You et al.[67] introduced a new heparin derivative by conjugation with dopamine, and the heparin-dopamine conjugate can then be further bound to various surfaces by one-step immersion of a substrate into the heparin-dopamine conjugate solution (Fig. 5a). However, the preparation of heparin-dopamine conjugation is not trivial, as it could reduce the biological activity of heparin, which presents challenges for the wide application of this method. Another interesting study developed a PDA-mediated, one-step surface immobilization strategy that is applicable not only to versatile substrates but also multiple functional compounds by simply immersing the substrates into a mixed solution of a molecule and dopamine (pH ~8.0–8.5) (Fig. 5b) [68]. This one-pot modification is excellent for molecules with amine, thiol, quaternary ammonium, and/or catechol groups, but the efficiency and biological activity of the heparin immobilization with this method is compromised because the carboxyl groups on heparin need to be activated to react with free amine on dopamine [69]. Recently, Yang et al.[70] reported a one-step process involving co-polymerization of dopamine and hexamethylendiamine, which resulted in abundant amine groups for further chemical conjugation with heparin via active ester chemistry. The immobilized heparin provided a favorable microenvironment for EC adhesion, proliferation, migration and nitric oxide release, while inhibiting SMC adhesion and proliferation. Meanwhile, Luo et al.[71] introduced an alternative approach for efficient heparinization on various substrates via the ad-layer of dopamine-assisted poly (ethylene imine) deposition (Fig. 5c). The similar amine-rich surface was created by simply immersing the substrates into the mixed solution of dopamine and poly (ethylene imine), and heparin can be effectively bound to the surface via amine/carboxyl covalent linkage. They also demonstrated high binding efficiency and enhanced biological activity of immobilized heparin by showing suppressed platelet adhesion, activation, and inhibited SMC proliferation on the heparin-binding surface. Interestingly, Ding et al.[72] utilized such method to immobilize heparin on topographically patterned substrates and studied the combined impacts of immobilized heparin and substrate topography on blood compatibility, re-endothelialization, and competitive growth of ECs over SMCs by EC/SMC co-culture. They found that combining immobilized heparin with substrate topography empowered substantially greater competitive ability of ECs over SMCs than each cue individually (Fig. 5d).
Fig. 5.
The stratgies of heparin immobilization via the PDA coating layer. a) Reaction scheme to prepare the heparin-dopamine conjugate. Adapted and reproduced from Ref. [67]. b) Schematics of the PDA-mediated, one-step surface immobilization of multiple biomolecules. Adapted and reproduced from Ref. [68]. c) Schematics of the PDA-assisted, one-step deposition of poly (ethylene imine) (PEI) for further heparin immobilization. Adapted and reproduced from Ref. [71]. d) Fluorescent images of EC/SMC co-culture, cell attachment number, and attachment ratio of EC/SMC showing the immobilized heparin via the PDA-assisted PEI deposition layer with substrate topography synergistically promoted competitive attachment of ECs over SMCs. TiO2: the pristine titanium dioxide substrate. PEI/PDA: PDA-assisted PEI coating on the TiO2 substrate. Hep-PEI/PDA: heparin immobilized onto PEI/PDA. Statistically significant differences are marked as follows: * vs. TiO2; # vs. Hep-PEI/PDA; * or # for p < 0.05; *** or ### for p < 0.001. Adapted and reproduced from Ref. [72].
4.2. Synthesis and immobilization of metal nanoparticles
The metal nanoparticles (M-NPs) have been wildly used in the biomedical field due to its excellent antimicrobial property [73]. Apart from the strong reactivity to multiple biomolecules, the diverse functional groups presented on PDA coatings also render these surfaces with strong reducing and metal binding abilities [12]. The reducing ability of PDA coatings toward metal ions makes PDA coatings a facile platform for direct synthesis of M-NPs without requiring any additional reductants or metallic seed particles. For instance, Lee’s group introduced a facile technique to form silver NPs in situ on the surface of bacterial cellulose nanofibers by first dip-coating with PDA and then immersing PDA-coated nanofibers in the silver nitrate solution [74]. Later, they combined this technique with the LBL deposition to build up multilayered M-NPs in a stepwise manner [75]. The multilayered gold and silver NPs were formed with reductive PDA coatings between layers, which served as adhesive linker between the M-NPs and the substrate, and between adjacent NP layers. Interestingly, a recent work prepared polystyrene/silver (PS/Ag) NPs via PDA coatings [76]. Briefly, PS particles were used as a template for PDA coating to form PS/PDA composite spheres. Silver precursor - [Ag(NH3)2]+ ions were added and adsorbed onto PS/PDA spheres, which was followed by their reduction into Ag NPs in situ. Antibacterial assays indicated that these PS/PDA/Ag nanocomposite particles showed enhanced antibacterial activities against E. coli and Staphylococcus aureus, while they did not show significant in vitro cytotoxicity again HEK293T human embryonic kidney cells.
Further, PDA coatings play an important role in preventing M-NPs from aggregation through quinones and unoxidized catechol groups, which better preserved antimicrobial activities of M-NPs. An interesting study performed by Lu et al.[77] introduced an effective approach for in situ growth of Ag NPs on PDA-coated silk fibers. It was demonstrated the uniform distribution of Ag NPs on PDA-coated silk. Moreover, Ag NPs decorated PDA-silk showed elevated Ag+ release, long-term release profile, and strong antibacterial ability.
4.3. PDA nanoparticles
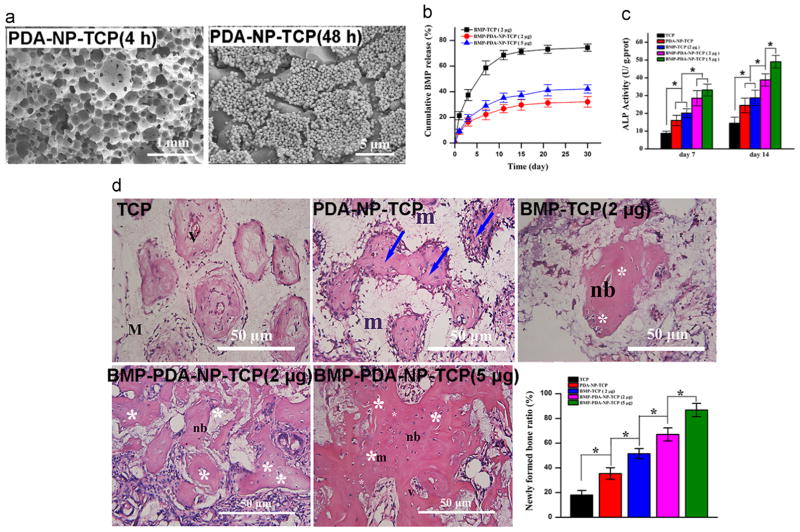
Recent advances in synthesis and functionalization of nanostructured materials, such as nanoparticles, have brought a significant increase in their biomedical applications, including imaging of cell and tissues, drug delivery, and sensing of target molecules [78]. A recent study prepared PDA nanoparticles (PDA-NPs) using oxidation and self-polymerization of dopamine in a solution containing water, ethanol, and ammonia at room temperature [79]. PDA-NPs showed good biocompatibility without long-term tissue toxicity in rats. They also underwent significant degradation. Moreover, they showed strong near-infrared adsorption and high photothermal conversion efficiency, so that after administration, they could efficiently kill cancer cells and suppress tumor growth at low laser power density and short irradiation time without damaging healthy tissues. Of great interest is the work performed by Wang et al.[80]; PDA-NPs were functionalized on various scaffolds, which not only provide favorable anchor points to capture cells and signal molecules, but also render the surface with biomimetic nanostructures (Fig. 6a). PDA-NPs help achieve well-controlled, sustained release of signal molecules, such as bone morphogenetic protein 2 (BMP-2) (Fig. 6b). It was also demonstrated that PDA-NPs functionalized scaffolds enhanced BMSC functions and bone tissue regeneration in vitro and in vivo (Fig. 6c and d).
Fig. 6.
PDA nanoparticles (PDA-NPs) for functionalization of porous scaffolds enhance tissue regeneration. a) SEM images of PDA-NPs immobilized onto β-tricalcium phosphate (TCP) scaffolds after incubation in the PDA-NP suspension for 4 h and 48 h. b) Release profiles of bone morphogenetic protein 2 (BMP-2) from various scaffolds in phosphate-buffered saline (PBS) solution. c) ALP activities of BMSCs cultured on various scaffolds for 7 and 14 days. d) Hematoxylin and eosin (H&E) staining images of various scaffolds retrieved after 12-week implantation and quantitative evaluation of newly formed bone on various scaffolds. m: material; nb: new bone; v- vessel; white asterisk: osteocyte; blue arrow: woven bone formation. Statistically significant differences are marked as follows: * for p ≤ 0.05. Adapted and reproduced from Ref. [80].
4.4. PDA capsules
The polymer capsules with well-defined structures have wide application in biomedical engineering. Over the past decades, the LBL assembly has been a prominent approach to fabricate polymer capsules with tailored properties [81]. However, the LBL process employs time-consuming assembly protocol. The simple single-step and material-independent adsorption of the PDA formation offers new opportunities in the construction of capsules, minimizing labor, cost, and assembly complexity. Caruso’s group prepared PDA capsules by the spontaneous deposition of the PDA film on to silica particles with a range of sizes (0.5–5 μm) and mesoporous structures, followed by removal of template particles to form robust capsules [82]. Meanwhile, Zhou’s group also obtained PDA capsules using polystyrene as the template, and found an interesting unidirectional loading/release behavior of PDA capsules in certain solvents when the cationic dye rhodamine 6 G was used as probing agent [83]. Later, Caruso’s group combined the advantages of one-step technique of PDA deposition with a biodegradable material poly(L-glutamic acid) to fabricate degradable capsules, allowing the tailed cargo release [84].
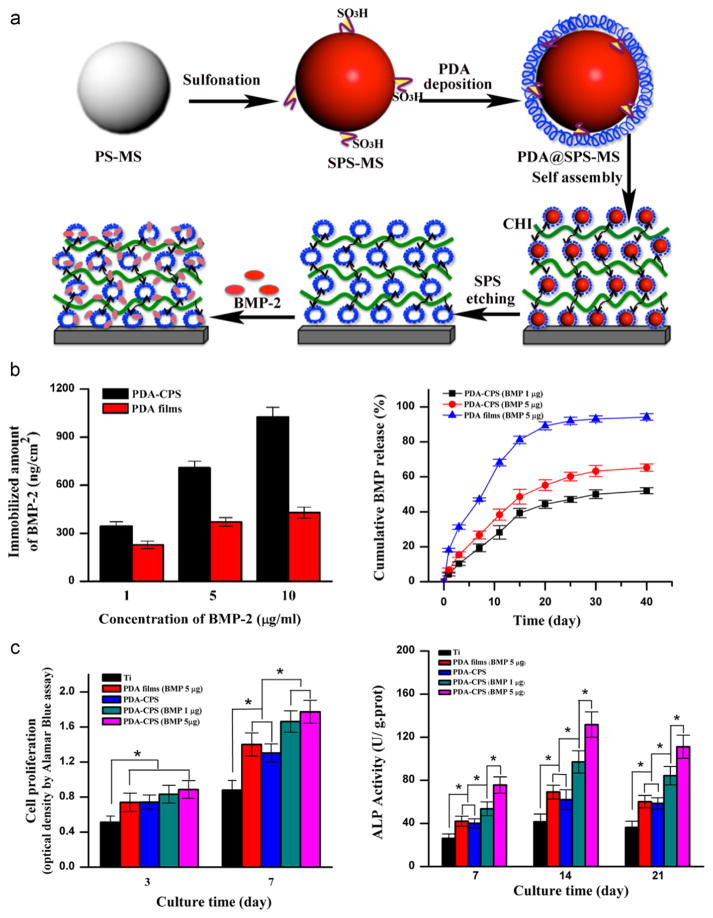
Although PDA capsules show great promises in biomedical application, their potential in control of cell responses in vitro remains largely undetermined. Biocompatibility tests demonstrated that there was negligible toxicity of PDA capsules to LIM1215 cells [82]. Recently, a facile approach has been reported to immobilize a pH-cleavable polymer-drug (anticancer drug doxorubicin) complex within PDA capsules via thiol-catechol conjugations for intracellular drug delivery [85]. The enhanced efficiency of doxorubicin-loaded PDA capsules in eradicating HeLa cancer cells compared to free doxorubicin, indicating the potential of PDA capsules as a drug delivery system. Another interesting work performed by Wang et al. fabricated microporous PDA microcapsules using LBL self-assembly (Fig. 7a) [86]. The produced microporous PDA architectures have a high capability of binding BMP-2 for sustained release of BMP-2 (Fig. 7b). More interestingly, they demonstrated that the microporous architecture and its immobilized BMP-2 synergistically enhanced the activity and osteogenetic differentiation of rat BMSCs (Fig. 7c), suggesting the utility of such microporous PDA architectures in modifying the medical devices for tissue engineering and regenerative medicine.
Fig. 7.
Self-assembling of PDA microcapsules for biomedical application. a) Schematics of the preparation of the PDA microporous architectures and subsequent loading and release of BMP-2. b) Evaluation of the BMP-2 loading ability and cumulative release from the PDA microporous architectures, i.e. PDA-CPS, and the PDA films. c) Cell proliferation and ALP activity of rat BMSCs after culturing on the pristine Ti, PDA-CPS, and PDA films. * indicates the significant difference (p < 0.05). Adapted and reproduced from Ref. [86].
5. Concluding remarks and outlook
The PDA coating employing a simple, one-step deposition protocol can be applied to virtually any substrate materials, regardless of material chemistry, geometry and dimensionality. The conformity, stability, and mechanical robustness of the PDA coating under physiological conditions make it applicable to modify biomaterials. Moreover, the presence of multiple functional motifs renders the bio-surface modified by the PDA coating with fascinating properties, not only for stimulating positive cellular responses but also for facile post-functionalization with diversity of biomolecules and metal ion/NPs, which further enable the production of diverse hybrid materials with specific functionalities. In addition, nanostructured PDA also provides biomimetic and favorable micro-environments that promote cell and tissue functions. Altogether, the PDA has emerged as a powerful and universal platform to engineer highly customized bio-surfaces for various biomedical applications.
However, there are still some open questions about PDA. For instance, as a fundamental issue, the debate regarding the PDA polymerization mechanism is still ongoing, although some monomer and oligomer units have been successfully identified as the primary building blocks of PDA. There is still no satisfactory theory describing the monomer/oligomer growth mechanism during the PDA formation. Further structural determination is important for not only PDA chemistry but also better controlled post-functionalization strategy by more in-depth understanding of the structure-property-function relationships. In addition, biodegradability is an important concern when it comes to the long-term in vivo application. The detailed evaluation of this aspect is still lacking but fairly crucial since unpolymerized dopamine was shown to be highly cytotoxic [87]. Nevertheless, we believe resolving these issues is important to further advance and expand the application of PDA in biomedical engineering.
Acknowledgments
This work was partially supported by NHLBI R01 HL119371 (W.T.).
Footnotes
Peer review under responsibility of Southwest Jiaotong University.
References
- 1.Hodneland CD, Lee YS, Min DH, Mrksich M. Selective immobilization of proteins to self-assembled monolayers presenting active site-directed capture ligands. Proc Natl Acad Sci. 2002;99:5048–5052. doi: 10.1073/pnas.072685299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 3.Wu G, Li P, Feng H, Zhang X, Chu PK. Engineering and functionalization of biomaterials via surface modification. J Mater Chem B. 2015;3:2024–2042. doi: 10.1039/c4tb01934b. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuzzo RG, Fusco FA, Allara DL. Spontaneously organized molecular assemblies. 3. Preparation and properties of solution adsorbed monolayers of organic disulfides on gold surfaces. J Am Chem Soc. 1987;109:2358–2368. [Google Scholar]
- 6.Decher G. Fuzzy nanoassemblies: toward layered polymeric multi-composites. science. 1997;277:1232–1237. [Google Scholar]
- 7.Chu PK, Chen J, Wang L, Huang N. Plasma-surface modification of biomaterials. Mater Sci Eng: R: Rep. 2002;36:143–206. [Google Scholar]
- 8.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem Rev. 2005;105:1103–1170. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhury MK, Whitesides GM. Correlation between surface free energy and surface constitution. DTIC Doc. 1992 doi: 10.1126/science.255.5049.1230. [DOI] [PubMed] [Google Scholar]
- 10.Vreeland V, Waite JH, Epstein L. Minireview—polyphenols and oxidases in substratum adhesion by marine algae and mussels. J Phycol. 1998;34:1–8. [Google Scholar]
- 11.Wiegemann M. Adhesion in blue mussels (Mytilus edulis) and barnacles (genus Balanus): mechanisms and technical applications. Aquat Sci-Res Across Boundaries. 2005;67:166–176. [Google Scholar]
- 12.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernsmann F, Ball V, Addiego F, Ponche A, Michel M, Gracio JJdA, et al. Dopamine– melanin film deposition depends on the used oxidant and buffer solution. Langmuir. 2011;27:2819–2825. doi: 10.1021/la104981s. [DOI] [PubMed] [Google Scholar]
- 14.Yue Q, Wang M, Sun Z, Wang C, Wang C, Deng Y, et al. A versatile ethanol-mediated polymerization of dopamine for efficient surface modification and the construction of functional core–shell nanostructures. J Mater Chem B. 2013;1:6085–6093. doi: 10.1039/c3tb21028f. [DOI] [PubMed] [Google Scholar]
- 15.Della Vecchia NF, Avolio R, Alfè M, Errico ME, Napolitano A, d’Ischia M. Building-Block Diversity in Polydopamine Underpins a Multifunctional Eumelanin-Type Platform Tunable Through a Quinone Control Point. Adv Funct Mater. 2013;23:1331–1340. [Google Scholar]
- 16.Li Y, Liu M, Xiang C, Xie Q, Yao S. Electrochemical quartz crystal microbalance study on growth and property of the polymer deposit at gold electrodes during oxidation of dopamine in aqueous solutions. Thin Solid Films. 2006;497:270–278. [Google Scholar]
- 17.Zeyfert CM. Surface Functionalised Emulsion-Templated Porous Polymers for in-Vitro Cell Culture. Durham University; 2010. [Google Scholar]
- 18.Schaubroeck D, Vercammen Y, Van Vaeck L, Vanderleyden E, Dubruel P, Vanfieteren J. Surface characterization and stability of an epoxy resin surface modified with polyamines grafted on polydopamine. Appl Surf Sci. 2014;303:465–472. [Google Scholar]
- 19.Ding Y, Weng L-T, Yang M, Yang Z, Lu X, Huang N, et al. Insights into the aggregation/deposition and structure of a polydopamine film. Langmuir. 2014;30:12258–12269. doi: 10.1021/la5026608. [DOI] [PubMed] [Google Scholar]
- 20.Liebscher, Mrówczyński R, Scheidt HA, Filip C, Hădade ND, Turcu R, et al. Structure of polydopamine: a never-ending story? Langmuir. 2013;29:10539–105348. doi: 10.1021/la4020288. [DOI] [PubMed] [Google Scholar]
- 21.Dreyer DR, Miller DJ, Freeman BD, Paul DR, Bielawski CW. Elucidating the structure of poly (dopamine) Langmuir. 2012;28:6428–6435. doi: 10.1021/la204831b. [DOI] [PubMed] [Google Scholar]
- 22.Hong S, Na YS, Choi S, Song IT, Kim WY, Lee H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv Funct Mater. 2012;22:4711–4717. [Google Scholar]
- 23.Jiang J, Zhu L, Zhu L, Zhu B, Xu Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir. 2011;27:14180–14187. doi: 10.1021/la202877k. [DOI] [PubMed] [Google Scholar]
- 24.Bernsmann F, Richert L, Senger B, Lavalle P, Voegel J-C, Schaaf P, et al. Use of dopamine polymerisation to produce free-standing membranes from (PLL-HA) n exponentially growing multilayer films. Soft Matter. 2008;4:1621–1624. doi: 10.1039/b806649c. [DOI] [PubMed] [Google Scholar]
- 25.Ball V, Del Frari D, Toniazzo V, Ruch D. Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: insights in the polydopamine deposition mechanism. J colloid interface Sci. 2012;386:366–372. doi: 10.1016/j.jcis.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Kim HW, McCloskey BD, Choi TH, Lee C, Kim M-J, Freeman BD, et al. Oxygen concentration control of dopamine-induced high uniformity surface coating chemistry. ACS Appl Mater interfaces. 2013;5:233–238. doi: 10.1021/am302439g. [DOI] [PubMed] [Google Scholar]
- 27.Ball V, Gracio J, Vila M, Singh MK, Metz-Boutigue M-Hln, Michel M, et al. Comparison of Synthetic Dopamine–Eumelanin Formed in the Presence of Oxygen and Cu2+ Cations as Oxidants. Langmuir. 2013;29:12754–12761. doi: 10.1021/la4029782. [DOI] [PubMed] [Google Scholar]
- 28.Wei Q, Zhang F, Li J, Li B, Zhao C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym Chem. 2010;1:1430–1433. [Google Scholar]
- 29.Dang Y, Xing C-M, Quan M, Wang Y-B, Zhang S-P, Shi S-Q, et al. Substrate independent coating formation and anti-biofouling performance improvement of mussel inspired polydopamine. J Mater Chem B. 2015;3:4181–4190. doi: 10.1039/c5tb00341e. [DOI] [PubMed] [Google Scholar]
- 30.Ku SH, Park CB. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials. 2010;31:9431–9437. doi: 10.1016/j.biomaterials.2010.08.071. [DOI] [PubMed] [Google Scholar]
- 31.Ku SH, Ryu J, Hong SK, Lee H, Park CB. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials. 2010;31:2535–2541. doi: 10.1016/j.biomaterials.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Tsai W-B, Chen W-T, Chien H-W, Kuo W-H, Wang M-J. Poly (dopamine) coating of scaffolds for articular cartilage tissue engineering. Acta Biomater. 2011;7:4187–4194. doi: 10.1016/j.actbio.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Ku SH, Park CB. Combined Effect of Mussel-Inspired Surface Modification and Topographical Cues on the Behavior of Skeletal Myoblasts. Adv Healthc Mater. 2013;2:1445–1450. doi: 10.1002/adhm.201300067. [DOI] [PubMed] [Google Scholar]
- 34.Ku SH, Lee JS, Park CB. Spatial control of cell adhesion and patterning through mussel-inspired surface modification by polydopamine. Langmuir. 2010;26:15104–15108. doi: 10.1021/la102825p. [DOI] [PubMed] [Google Scholar]
- 35.Sun K, Xie Y, Ye D, Zhao Y, Cui Y, Long F, et al. Mussel-inspired anchoring for patterning cells using polydopamine. Langmuir. 2011;28:2131–2136. doi: 10.1021/la2041967. [DOI] [PubMed] [Google Scholar]
- 36.Chien H-W, Kuo W-H, Wang M-J, Tsai S-W, Tsai W-B. Tunable micropatterned substrates based on poly (dopamine) deposition via microcontact printing. Langmuir. 2012;28:5775–5782. doi: 10.1021/la300147p. [DOI] [PubMed] [Google Scholar]
- 37.Wang PY, Wu TH, Chao PHG, Kuo WH, Wang MJ, Hsu CC, et al. Modulation of cell attachment and collagen production of anterior cruciate ligament cells via submicron grooves/ridges structures with different cell affinity. Biotechnol Bioeng. 2013;110:327–337. doi: 10.1002/bit.24615. [DOI] [PubMed] [Google Scholar]
- 38.Zhong S, Luo R, Wang X, Tang L, Wu J, Wang J, et al. Effects of polydopamine functionalized titanium dioxide nanotubes on endothelial cell and smooth muscle cell. Colloids Surfaces B: Biointerfaces. 2014;116:553–560. doi: 10.1016/j.colsurfb.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Tu Q, Zhu Y, Luo R, Li X, Xie Y, et al. Mussel-Inspired Coating of Polydopamine Directs Endothelial and Smooth Muscle Cell Fate for Re-endothelialization of Vascular Devices. Advanced healthcare materials. 2012;1:548–559. doi: 10.1002/adhm.201200073. [DOI] [PubMed] [Google Scholar]
- 40.Luo R, Tang L, Zhong S, Yang Z, Wang J, Weng Y, et al. In vitro investigation of enhanced hemocompatibility and endothelial cell proliferation associated with quinone-rich polydopamine coating. ACS Appl Mater interfaces. 2013;5:1704–1714. doi: 10.1021/am3027635. [DOI] [PubMed] [Google Scholar]
- 41.Ding Y, Yang Z, Bi CW, Yang M, Zhang J, Xu SL, et al. Modulation of protein adsorption, vascular cell selectivity and platelet adhesion by mussel-inspired surface functionalization. J Mater Chem B. 2014;2:3819–3829. doi: 10.1039/c4tb00386a. [DOI] [PubMed] [Google Scholar]
- 42.Ryu J, Ku SH, Lee H, Park CB. Mussel-Inspired Polydopamine Coating as a Universal Route to Hydroxyapatite Crystallization. Adv Funct Mater. 2010;20:2132–2139. [Google Scholar]
- 43.Xie J, Zhong S, Ma B, Shuler FD, Lim CT. Controlled biominer-alization of electrospun poly (ε-caprolactone) fibers to enhance their mechanical properties. Acta Biomater. 2013;9:5698–5707. doi: 10.1016/j.actbio.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 44.Wu C, Fan W, Chang J, Xiao Y. Mussel-inspired porous SiO 2 scaffolds with improved mineralization and cytocompatibility for drug delivery and bone tissue engineering. J Mater Chem. 2011;21:18300–18307. [Google Scholar]
- 45.Rim NG, Kim SJ, Shin YM, Jun I, Lim DW, Park JH, et al. Mussel-inspired surface modification of poly (L-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells. Colloids Surfaces B: Biointerfaces. 2012;91:189–197. doi: 10.1016/j.colsurfb.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 46.Wu C, Han P, Liu X, Xu M, Tian T, Chang J, et al. Mussel-inspired bioceramics with self-assembled Ca-P/polydopamine composite nanolayer: preparation, formation mechanism, improved cellular bioactivity and osteogenic differentiation of bone marrow stromal cells. Acta Biomater. 2014;10:428–438. doi: 10.1016/j.actbio.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Xu M, Zhang Y, Zhai D, Chang J, Wu C. Mussel-inspired bioactive ceramics with improved bioactivity, cell proliferation, differentiation and bone-related gene expression of MC3T3 cells. Biomater Sci. 2013;1:933–941. doi: 10.1039/c3bm60028a. [DOI] [PubMed] [Google Scholar]
- 48.Steeves AJ, Atwal A, Schock SC, Variola F. Evaluation of the direct effects of poly (dopamine) on the in vitro response of human osteoblastic cells. J Mater Chem B. 2016;4:3145–3156. doi: 10.1039/c5tb02510a. [DOI] [PubMed] [Google Scholar]
- 49.Lee J-h, Lee YJ, Cho H-j, Shin H. Guidance of in vitro migration of human mesenchymal stem cells and in vivo guided bone regeneration using aligned electrospun fibers. Tissue Eng Part A. 2013;20:2031–2042. doi: 10.1089/ten.tea.2013.0282. [DOI] [PubMed] [Google Scholar]
- 50.Yang Li W, Li X, Zhang X, Wang C, Meng X, Pei Y, et al. Improving Osteointegration and Osteogenesis of Three-Dimensional Porous Ti6AI4V Scaffolds by Polydopamine-Assisted Biomimetic Hydroxyapatite Coating. ACS Appl Mater interfaces. 2015;7:5715–5724. doi: 10.1021/acsami.5b00331. [DOI] [PubMed] [Google Scholar]
- 51.Xu M, Zhai D, Xia L, Li H, Chen S, Fang B, et al. Hierarchical bioceramic scaffolds with 3D-plotted macropores and mussel-inspired surface nanolayers for stimulating osteogenesis. Nanoscale. 2016;8:13790–13803. doi: 10.1039/c6nr01952h. [DOI] [PubMed] [Google Scholar]
- 52.Ding Y, Yang Z, Bi CW, Yang M, Xu SL, Lu X, et al. Directing Vascular Cell Selectivity and Hemocompatibility on Patterned Platforms Featuring Variable Topographic Geometry and Size. ACS Appl Mater interfaces. 2014;6:12062–12070. doi: 10.1021/am502692k. [DOI] [PubMed] [Google Scholar]
- 53.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 54.Iqbal Z, Lai EP, Avis TJ. Antimicrobial effect of polydopamine coating on Escherichia coli. J Mater Chem. 2012;22:21608–21612. [Google Scholar]
- 55.Liu A, Zhao L, Bai H, Zhao H, Xing X, Shi G. Polypyrrole actuator with a bioadhesive surface for accumulating bacteria from physiological media. ACS Appl Mater interfaces. 2009;1:951–955. doi: 10.1021/am9000387. [DOI] [PubMed] [Google Scholar]
- 56.Sileika TS, Kim H-D, Maniak P, Messersmith PB. Antibacterial performance of polydopamine-modified polymer surfaces containing passive and active components. ACS Appl Mater interfaces. 2011;3:4602–4610. doi: 10.1021/am200978h. [DOI] [PubMed] [Google Scholar]
- 57.Liu M, Zhou J, Yang Y, Zheng M, Yang J, Tan J. Surface modification of zirconia with polydopamine to enhance fibroblast response and decrease bacterial activity in vitro: a potential technique for soft tissue engineering applications. Colloids Surfaces B: Biointerfaces. 2015;136:74–83. doi: 10.1016/j.colsurfb.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 58.Su L, Yu Y, Zhao Y, Liang F, Zhang X. Strong Antibacterial Polydopamine Coatings Prepared by a Shaking-assisted Method. Sci Rep. 2016:6. doi: 10.1038/srep24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee H, Rho J, Messersmith PB. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv Mater. 2009;21:431–434. doi: 10.1002/adma.200801222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu LQ, Yang WJ, Neoh K-G, Kang E-T, Fu GD. Dopamine-induced reduction and functionalization of graphene oxide nanosheets. Macromolecules. 2010;43:8336–8339. [Google Scholar]
- 61.Xie J, Michael PL, Zhong S, Ma B, MacEwan MR, Lim CT. Mussel inspired protein-mediated surface modification to electrospun fibers and their potential biomedical applications. J Biomed Mater Res Part A. 2012;100:929–938. doi: 10.1002/jbm.a.34030. [DOI] [PubMed] [Google Scholar]
- 62.Lee YB, Shin YM, Lee J-h, Jun I, Kang JK, Park J-C, et al. Polydopamine-mediated immobilization of multiple bioactive molecules for the development of functional vascular graft materials. Biomaterials. 2012;33:8343–8352. doi: 10.1016/j.biomaterials.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Wang J-l, Li B-c, Li Z-j, Ren K-f, Jin L-j, Zhang S-m, et al. Electropolymerization of dopamine for surface modification of complex-shaped cardiovascular stents. Biomaterials. 2014;35:7679–7689. doi: 10.1016/j.biomaterials.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 64.Weng Y, Song Q, Zhou Y, Zhang L, Wang J, Chen J, et al. Immobilization of selenocystamine on TiO 2 surfaces for in situ catalytic generation of nitric oxide and potential application in intravascular stents. Biomaterials. 2011;32:1253–1263. doi: 10.1016/j.biomaterials.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 65.Yang Z, Zhong S, Yang Y, Maitz MF, Li X, Tu Q, et al. Polydopamine-mediated long-term elution of the direct thrombin inhibitor bivalirudin from TiO 2 nanotubes for improved vascular biocompatibility. J Mater Chem B. 2014;2:6767–6778. doi: 10.1039/c4tb01118j. [DOI] [PubMed] [Google Scholar]
- 66.Lutolf M, Hubbell J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 67.You I, Kang SM, Byun Y, Lee H. Enhancement of blood compatibility of poly (urethane) substrates by mussel-inspired adhesive heparin coating. Bioconjugate Chem. 2011;22:1264–1269. doi: 10.1021/bc2000534. [DOI] [PubMed] [Google Scholar]
- 68.Kang SM, Hwang NS, Yeom J, Park SY, Messersmith PB, Choi IS, et al. One-step multipurpose surface functionalization by adhesive catecholamine. Adv Funct Mater. 2012;22:2949–2955. doi: 10.1002/adfm.201200177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wissink M, Beernink R, Pieper J, Poot A, Engbers G, Beugeling T, et al. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials. 2001;22:151–163. doi: 10.1016/s0142-9612(00)00164-2. [DOI] [PubMed] [Google Scholar]
- 70.Yang Y, Qi P, Wen F, Li X, Xia Q, Maitz MF, et al. Mussel-inspired one-step adherent coating rich in amine groups for covalent immobilization of heparin: hemocompatibility, growth behaviors of vascular cells, and tissue response. ACS Appl Mater interfaces. 2014;6:14608–14620. doi: 10.1021/am503925r. [DOI] [PubMed] [Google Scholar]
- 71.Luo R, Wang X, Deng J, Zhang H, Maitz MF, Yang L, et al. Dopamine-assisted deposition of poly (ethylene imine) for efficient heparinization. Colloids Surfaces B: Biointerfaces. 2016;144:90–98. doi: 10.1016/j.colsurfb.2016.03.080. [DOI] [PubMed] [Google Scholar]
- 72.Ding Y, Yang M, Yang Z, Luo R, Lu X, Huang N, et al. Cooperative control of blood compatibility and re-endothelialization by immobilized heparin and substrate topography. Acta Biomater. 2015;15:150–163. doi: 10.1016/j.actbio.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 73.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 74.Sureshkumar M, Siswanto DY, Lee C-K. Magnetic antimicrobial nanocomposite based on bacterial cellulose and silver nanoparticles. J Mater Chem. 2010;20:6948–6955. [Google Scholar]
- 75.Sureshkumar M, Lee P-N, Lee C-K. Stepwise assembly of multi-metallic nanoparticles via self-polymerized polydopamine. J Mater Chem. 2011;21:12316–12320. [Google Scholar]
- 76.Cong Y, Xia T, Zou M, Li Z, Peng B, Guo D, et al. Mussel-inspired polydopamine coating as a versatile platform for synthesizing polystyrene/Ag nanocomposite particles with enhanced antibacterial activities. J Mater Chem B. 2014;2:3450–3461. doi: 10.1039/c4tb00460d. [DOI] [PubMed] [Google Scholar]
- 77.Lu Z, Xiao J, Wang Y, Meng M. In situ synthesis of silver nanoparticles uniformly distributed on polydopamine-coated silk fibers for antibacterial application. J colloid interface Sci. 2015;452:8–14. doi: 10.1016/j.jcis.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 78.Choi J, Wang NS. Nanoparticles in biomedical applications and their safety concerns. INTECH Open Access Publisher; 2011. [Google Scholar]
- 79.Liu Y, Ai K, Liu J, Deng M, He Y, Lu L. Dopamine-Melanin Colloidal Nanospheres: an Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv Mater. 2013;25:1353–1359. doi: 10.1002/adma.201204683. [DOI] [PubMed] [Google Scholar]
- 80.Wang Z, Wang K, Zhang Y, Jiang Y, Lu X, Fang L, et al. Protein-Affinitive Polydopamine Nanoparticles as an Efficient Surface Modification Strategy for Versatile Porous Scaffolds Enhancing Tissue Regeneration. Part Part Syst Charact. 2015 [Google Scholar]
- 81.Caruso F, Caruso RA, Möhwald H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science. 1998;282:1111–1114. doi: 10.1126/science.282.5391.1111. [DOI] [PubMed] [Google Scholar]
- 82.Postma A, Yan Y, Wang Y, Zelikin AN, Tjipto E, Caruso F. Self-polymerization of dopamine as a versatile and robust technique to prepare polymer capsules. Chem Mater. 2009;21:3042–3044. [Google Scholar]
- 83.Yu B, Wang DA, Ye Q, Zhou F, Liu W. Robust polydopamine nano/microcapsules and their loading and release behavior. Chem Commun. 2009:6789–6791. doi: 10.1039/b910679k. [DOI] [PubMed] [Google Scholar]
- 84.Ochs CJ, Hong T, Such GK, Cui J, Postma A, Caruso F. Dopamine-mediated continuous assembly of biodegradable capsules. Chem Mater. 2011;23:3141–3143. [Google Scholar]
- 85.Cui J, Yan Y, Such GK, Liang K, Ochs CJ, Postma A, et al. Immobilization and intracellular delivery of an anticancer drug using mussel-inspired polydopamine capsules. Biomacromolecules. 2012;13:2225–2228. doi: 10.1021/bm300835r. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z, Li C, Xu J, Wang K, Lu X, Zhang H, et al. Bioadhesive microporous architectures by self-assembling polydopamine microcapsules for biomedical applications. Chem Mater. 2015;27:848–856. [Google Scholar]
- 87.Asanuma M, Miyazaki I, Ogawa N. Dopamine-or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res. 2003;5:165–176. doi: 10.1007/BF03033137. [DOI] [PubMed] [Google Scholar]