The American Society for Gastrointestinal Endoscopy (ASGE) Technology Committee provides reviews of existing, new, or emerging endoscopic technologies that have an impact on the practice of GI endoscopy. Evidence-based methodology is used, with a MEDLINE literature search to identify pertinent clinical studies on the topic and a MAUDE (U.S. Food and Drug Administration Center for Devices and Radiological Health) database search to identify the reported adverse events of a given technology. Both are supplemented by accessing the “related articles” feature of PubMed and by scrutinizing pertinent references cited by the identified studies. Controlled clinical trials are emphasized, but in many cases data from randomized controlled trials are lacking. In such situations, large case series, preliminary clinical studies, and expert opinions are used. Technical data are gathered from traditional and Web-based publications, proprietary publications, and informal communications with pertinent vendors.
Technology Status Evaluation Reports are drafted by 1 or 2 members of the ASGE Technology Committee, reviewed and edited by the committee as a whole, and approved by the Governing Board of the ASGE. When financial guidance is indicated, the most recent coding data and list prices at the time of publication are provided. For this review, the MEDLINE database was searched through August 2016 for articles related to cryotherapy, using the words cryotherapy, gastrointestinal tract, cryoablation, cryospray, cryosurgery, liquid nitrogen, liquid carbon dioxide, cryoballoon, and Barrett’s esophagus. Technology Status Evaluation Reports are scientific reviews provided solely for educational and informational purposes. Technology Status Evaluation Reports are not rules and should not be construed as establishing a legal standard of care or as encouraging, advocating, requiring, or discouraging any particular treatment or payment for such treatment.
Background
Cryotherapy uses extremely cold temperatures for tissue destruction. In the GI tract, cryotherapy can be performed using devices designed for use with endoscopes. During cryotherapy, a cryogen (a substance used to produce very low temperatures) is used to freeze the target tissue, and repeated freeze/thaw cycles result in the destruction of abnormal tissue. In endoscopic cryotherapy, the cryogen is typically a liquefied gas, such as nitrogen or carbon dioxide, that may be either directly applied to tissue or used within a balloon device. Endoscopic cryotherapy was first used for the treatment of Barrett’s esophagus (BE), and the indications for its use in other GI disorders are expanding.
Technology under review
Mechanism of action
Cryotherapy induces cell necrosis for therapeutic purposes through cycles of controlled local freezing and thawing of the tissue. Application of a cryogen to tissue is believed to result in necrosis of mucosal and submucosal lesions by several mechanisms. Freezing of tissue results in the formation of ice crystals within the intracellular and extracellular spaces, leading to cell membrane disruption, protein denaturation, and osmotic gradients that lead to cell dehydration.1, 2, 3, 4, 5, 6 Cells in the periphery of ablation zones that are not immediately destroyed by direct cryoablation-induced injury may subsequently die by upregulation of apoptosis, thought to be mediated by cytochrome C release.1, 7, 8
The thawing component of cryotherapy also appears to be an important mechanism for cell death.9 During thawing, ice crystals fuse and further damage cell membranes. In addition, vascular stasis due to endothelial damage, platelet aggregation, and formation of microthrombi results in ischemic necrosis.10, 11, 12
For cell destruction by cryotherapy, the tissue temperature must reach a critical threshold that is unique to the cell type and the environment of the targeted tissue, but typically ranges between −20°C and −50°C.13 Because collagen and elastin fibers are less sensitive to the effects of cryotherapy than are epithelial cells, the tissue structure remains intact, reducing the risk of perforation.13, 14, 15, 16 The extent of tissue destruction is also dependent on the number of freeze/thaw cycles applied.12, 13, 17, 18
Types of endoscopic cryotherapy and equipment
Three cryotherapy systems developed for use in GI endoscopy have been cleared by the U.S. Food and Drug Administration and are marketed in the United States. Two of the systems use a pressurized liquefied gas spray as the cryogen (truFreeze, CSA Medical, Lexington, Mass, and Polar Wand, GI Supply, Camp Hill, Pa), whereas the third uses a cryogenic balloon that requires direct contact with the target tissue (Coldplay CryoBalloon Focal Ablation System, C2 Therapeutics, Redwood City, Calif). Of note, production and sale of the Polar Wand system was suspended by the manufacturer in March 2016.
Cryotherapy using liquid nitrogen
The first cryotherapy system developed for endoscopic use (truFreeze) uses liquid nitrogen delivered through a spray catheter. Its first endoscopic use was reported in 2005.19 This system has gone through several iterations, and currently the third-generation device is available.
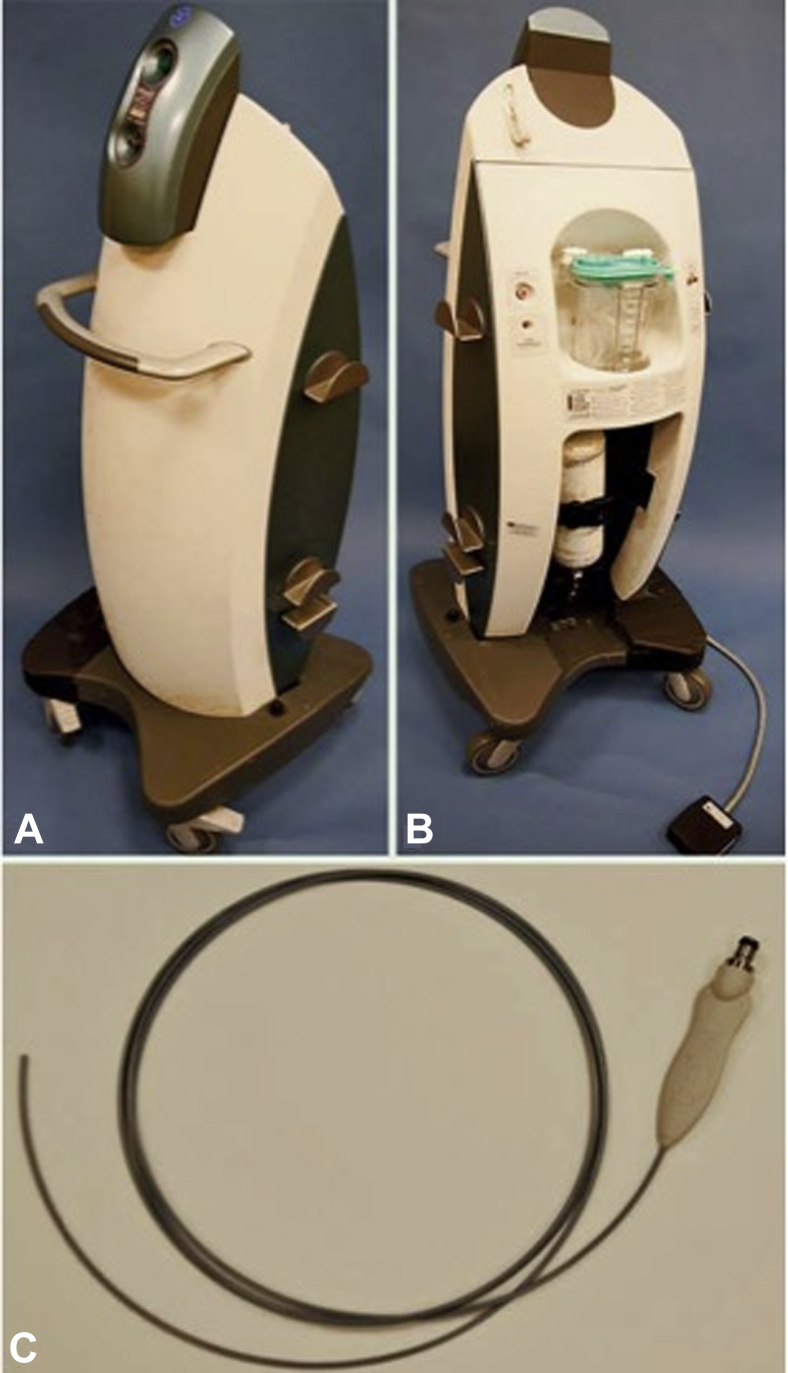
The truFreeze system allows for freezing of GI mucosal tissue to −196°C with the use of a low-pressure, non-contact spray and comprises a console unit, 2 foot pedal controls, a spray catheter, and a decompression tube. The console houses a liquid nitrogen tank and compressor and a suction canister, and it features a touch screen control (Fig. 1). The single-use spray catheter is 213 cm long, is 7F in diameter, and is constructed of polyimide reinforced with a stainless steel mesh. The catheter connects to a liquid nitrogen compressor within the console and can be inserted through any endoscope with a ≥2.8-mm instrument channel. The foot pedals initiate the flow of liquid nitrogen from the compressor to the catheter and suction through a 20F dual-channel decompression tube that is positioned coaxially with the endoscope for active venting. Liquid nitrogen is dispersed from the catheter in a low-pressure (<5 psi) spraylike fashion, resulting in flash freezing (Fig. 2). The noncontact delivery allows for ablation of topographically variable lesions; flat, nodular, and masslike tissue can be ablated.
Figure 1.

Liquid nitrogen spray cryotherapy console.
(Image used with permission from CSA Medical, Inc)
Figure 2.
Liquid nitrogen spray cryotherapy catheter.
(Image used with permission from CSA Medical, Inc)
Nitrogen rapidly expands from liquid to gas, so a dual-channel decompression tube is placed before treatment to allow active and passive gas venting to prevent GI luminal perforation. The suction tubing connects to the console and allows for continuous suction throughout the entire procedure.20 The current system also has a pressure-sensing capability that audibly alerts the endoscopist when luminal pressures are elevated above a set threshold.
After endoscopic visualization of the area of interest, the spray catheter is advanced through the instrument channel to the target ablation site. The tip of the catheter is positioned 0.5 cm to 1 cm away from the tissue to be treated. The area of interest is then treated according to the dosimetry protocol for the tissue being ablated.21, 22, 23, 24 The timer is usually started once a white frost is formed. After each application of cryogen, the frozen tissue is allowed to thaw before beginning the next cycle (Video 1, available online at www.VideoGIE.org). Dosimetry protocols for liquid nitrogen cryotherapy that have been reported for common disease indications are described in Table 1.
Table 1.
Reported dosimetry for endoscopic GI cryotherapy
| Indication | Number of cycles | Time per cycle (seconds) | Study reference |
|---|---|---|---|
| Liquid nitrogen cryotherapy | |||
| Barrett’s esophagus with dysplasia/IMCA | 2 | 20 | 22, 23 |
| Palliation of esophageal cancer | 3 | 20 | 33 |
| GAVE | 1 | HFA | 36 |
| Radiation proctopathy | 4 | 10 | 38 |
| Carbon dioxide cryotherapy | |||
| Barrett’s esophagus with dysplasia/IMCA | 5-7 | 15-30 | 25, 26 |
| Palliation of esophageal cancer | NDA | NDA | NDA |
| GAVE | 1 | HFA | 37 |
| Radiation proctopathy | NDA | NDA | NDA |
| Nitrous oxide cryotherapy | |||
| Barrett’s esophagus with dysplasia | 1 | 6-10 | 31 |
| Palliation of esophageal cancer | NDA | NDA | NDA |
| GAVE | NDA | NDA | NDA |
| Radiation proctopathy | NDA | NDA | NDA |
GAVE, Gastric antral vascular ectasia; HFA, cryogen applied until a hard freeze was achieved; IMCA, intramucosal carcinoma; NDA, no data available.
Cryotherapy using carbon dioxide
The Polar Wand is a noncontact spray cryotherapy system that uses liquid carbon dioxide (CO2) as the cryogen, allowing freezing of GI mucosal tissue to −78°C.25 This system includes a console unit, 2 foot pedal controls, a spray catheter, and a decompression tube (Fig. 3). The console houses a liquid CO2 compressor and tank and a suction canister. The CO2 evacuation tube for this system has a cap on the distal end of the tube that will fit on endoscopes with an outer diameter of 9 to 10 mm (Fig. 4). This allows the gas evacuation tube to be advanced in tandem with the endoscope.25, 26 A 7F 200-cm-long single-use polyethylene catheter attaches to the console and is advanced through the instrument channel of the endoscope for spray application of the cryogen. A dual foot pedal system is used; depression of the blue pedal activates the flow of CO2 and simultaneous suction at an adjustable level for CO2 evacuation, whereas depression of the yellow pedal activates full suction only. The system delivers cryogen at a rate of 6 to 8 L/min at a pressure of 450 to 750 psi.26
Figure 3.
Carbon dioxide spray cryotherapy system. A and B, Carbon dioxide spray cryotherapy console. C, Carbon dioxide spray cryotherapy catheter.
(Image used with permission from Thieme Medical Publishers.)
Figure 4.
Carbon dioxide spray cryotherapy distal attachment cap with the spray catheter.
(Image used with permission from GI Supply.)
The manufacturer recommends priming the catheter before introduction into the endoscope. Otherwise, the use of the spray catheter is similar to the truFreeze system. The timing for the dosimetry is started when ice formation begins. Reported dosimetry protocols for liquid CO2 cryotherapy for common disease indications are listed in Table 1.
Cryotherapy using nitrous oxide–inflated balloon
The Coldplay CryoBalloon Focal Ablation System is the most recently developed endoscopic cryotherapy system. The device comprises a 175-cm-long, 3.6-mm diameter, balloon-tipped catheter that attaches to a battery-powered handle with a trigger (Fig. 5). The handle contains a 23.5-g cartridge of liquid nitrous oxide. The balloon catheter is compatible with endoscopes with a working channel diameter of ≥3.7 mm. Both the catheter and the handle are single-use items.
Figure 5.
Nitrous oxide cryotherapy system.
(Image courtesy of C2 Therapeutics.)
After endoscopic visualization of the area of interest, the 3-cm-long balloon is inflated by pressing the trigger once and releasing it. The balloon is designed to self-inflate to the size of the esophagus (approximately 20 to 35 mm) at a low pressure generated by the nitrous oxide system. Continued activation of the trigger delivers the cryogen from the cartridge to the balloon. The cryogen emerges from a 1-mm opening in the side of the catheter, delivering a focal spray within the balloon that is perpendicular to the axis of the catheter. The liquid nitrous oxide focally cools the area of the balloon exposed to the cryogen to −85 °C. As such, the tissue directly apposed to the area of the balloon receiving the stream of cryogen from the catheter will be ablated (focal areas of treatment). A brief test spray helps confirm proper catheter position. Prolonged trigger activation delivers a preset amount of cryogen that may be modified by adjusting the program on the handle. The site of ablation can be changed by rotating the catheter (to change the ablation site at the same level) or by inserting or retracting the balloon catheter to ablate a site at a different level. When the balloon is deflated, the remaining gas is vented back to the handle (Video 2, available online at www.VideoGIE.org).
Each cartridge allows for ablation of 2 to 3 sites. The cartridges can be replaced without removing the catheter from the endoscope. The current balloon catheter is designed for treating focal areas, and a pear-shaped balloon intended to facilitate focal ablation at the gastroesophageal junction is also available. A 360° ablation device that works in a similar fashion is under development.
Outcomes data
Endoscopic cryotherapy in the esophagus
Cryotherapy for Barrett’s esophagus using liquid nitrogen
In a prospective multicenter study, 80 patients with dysplastic BE completed endoscopic cryotherapy every 2 to 3 months until there was no endoscopic evidence of BE and no histologic evidence of dysplasia, followed by surveillance endoscopies up to 2 years.27 Complete eradication of dysplasia (CE-D) was achieved in 67 out of 80 patients (84%) and complete eradication of intestinal metaplasia (CE-IM) in 51 out of 80 patients (64%). Prior smaller studies reported similar outcomes with liquid nitrogen cryoablation for BE.22, 23
Two retrospective studies evaluated the incidence of recurrent IM in patients with BE with high-grade dysplasia (HGD) who initially attained CE-IM with liquid nitrogen cryoablation.21, 28 In 1 study, recurrent IM was seen in 11 out of 36 patients (30%), including 6 out of 36 (17%) with dysplasia over 3 years of follow-up.28 Another study reported recurrent IM in 13 out of 32 patients (41%), including recurrent HGD in 6 out of 32 patients (19%) over a 2-year follow-up period.21
The efficacy of liquid nitrogen spray cryotherapy for the treatment of refractory or recurrent dysplasia after radiofrequency ablation (RFA) failure was assessed in a retrospective study of 16 patients.29 CE-D was achieved in 12 patients (75%) and CE-IM in 5 patients (31%). Three patients developed esophageal strictures that responded to endoscopic dilation.29
Cryotherapy for Barrett’s esophagus using liquid CO2
The efficacy of endoscopic cryoablation of BE using liquid CO2 spray was evaluated in a prospective pilot study of 22 patients.26 Twenty patients (90.9 %) had CE-IM after a median of 2 sessions (range, 1 - 3 sessions). At 6 months follow-up, recurrence was detected in 3 out of 20 (15 %) of the patients who had initially achieved CE-IM.26 A retrospective study reported the outcomes in 64 patients who underwent CO2 cryotherapy for the treatment of BE-HGD or intramucosal cancer, with a median follow-up time of 4.2 years.25 At 1 year, the overall complete response rates were 94% for HGD and 55% for IM. Recurrent or new IM was detected during surveillance in 31% of the patients who had initially attained CE-IM.25 Although most studies assessing the use of cryotherapy for the treatment of BE have reported benefit, 1 study in which CO2 spray was used for ablation of Barrett’s neoplasia was terminated early because of lack of efficacy.30
Cryotherapy for Barrett’s esophagus using nitrous oxide–inflated balloon
In a multicenter pilot study, 39 BE patients underwent 56 focal ablations of 6, 8, or 10 seconds’ duration. Conversion of BE to neosquamous epithelium was observed at follow-up endoscopy 6 to 8 weeks later in 6 out of 10 (60%) areas treated for 6 seconds, 23 out of 28 (82%) areas treated for 8 seconds, and 18 out of 18 (100%) areas treated for 10 seconds.31
Cryotherapy for esophageal cancer
The ability of cryotherapy to treat into the deep submucosa makes it a potential treatment option for esophageal cancer.32 In a multicenter retrospective study, 79 patients with esophageal cancer who either failed or were ineligible for conventional therapy underwent endoscopic cryotherapy with liquid nitrogen as a salvage therapy for local disease control.33 Fifty-three patients (67%) had received previous therapies including EMR, photodynamic therapy, chemoradiation, argon plasma coagulation, RFA, or a combination of these therapies. The tumor stages in the 49 patients who completed cryotherapy treatment were T1 in 36 patients and T2 to T4 in 13 patients. Complete endoscopic remission was observed in 26 out of 36 (72.2%) patients with T1 stage tumors compared with 4 out of 12 patients (33%) with stage T2 to stage T4 disease.32 Other smaller case series have reported similar outcomes with the adjunctive use of cryotherapy for early esophageal cancer.34, 35
Endoscopic cryotherapy in the stomach and duodenum
Studies using endoscopic cryotherapy in the stomach and duodenum have primarily assessed its utility for control of active bleeding or prevention of future bleeding. An initial pilot study evaluated 7 patients with gastric or duodenal arteriovenous malformations (AVMs), 7 patients with gastric antral vascular ectasia (GAVE), and 5 patients with radiation gastropathy.36 In all patients, previous endoscopic therapy including laser, thermal, and electrosurgical coagulation had failed. The patients underwent a mean of 3.4 cryotherapy sessions using liquid nitrogen. The best response to cryotherapy was seen in patients with AVMs and GAVE, with cessation of bleeding in 6 out of 7 patients and 5 out of 7 patients, respectively.36 Cryotherapy was less effective in patients with radiation gastropathy, with cessation of bleeding noted in only 2 out of 5 patients.36
Another study evaluated 12 transfusion-dependent GAVE patients, 8 of whom had refractory or recurrent disease after thermal therapy. All patients underwent 3 sessions of CO2 spray cryotherapy.37 Six patients experienced a complete response, defined as a significant improvement in endoscopic appearance, increasing hemoglobin level, and no transfusion requirements. Six patients had a partial response, defined as incomplete ablation on endoscopy and reduced transfusion requirements.37
Endoscopic cryotherapy in the rectum
The application of cryotherapy in the rectum has focused primarily on the treatment of radiation proctopathy (RP), although data are limited. A case series of 26 patients with recurrent GI bleeding treated with liquid nitrogen cryoablation included 7 patients with RP; this study reported cessation of bleeding in all 7 patients.36 In a prospective case series of 10 patients with RP treated with liquid nitrogen cryoablation, 6 out of 7 patients (86%) experienced a significant decrease in rectal bleeding after treatment.38
Safety
Adverse Events
Overall, cryotherapy in the GI tract appears to be well-tolerated and safe. The majority of reported adverse events are from studies in which spray cryotherapy was used in the esophagus. Adverse events are mostly self-limited and include chest pain, esophagitis, sore throat, lip ulcer, esophageal ulcers, and dysphagia.19, 25, 26, 34, 39 Strictures requiring endoscopic dilation have been reported in 3% to 13% of treated patients, with a favorable response to endoscopic dilation in all cases.21, 23, 33, 34 Gastric perforation has been reported with the use of both liquid nitrogen and liquid CO2 systems.30, 34 No mortality has been reported with the use of endoscopic cryotherapy. There are limited data on adverse events associated with the use of the Coldplay CryoBalloon; as yet, only minor pain has been reported.31
Contraindications
Ulcerations, mucosal breaks, and eosinophilic esophagitis are considered to be relative contraindications to cryotherapy.40, 41 Patients with surgically altered anatomy, including those who have undergone gastric bypass surgery, may be at increased risk for perforation, and thus spray cryotherapy is not recommended for these patients.41, 42
Ease of use
The technical complexity of cryotherapy is similar to that of other ablative techniques such as RFA and argon plasma coagulation. Cryotherapy can be safely performed with a variety of sedation approaches, including moderate sedation, deep sedation, or general anesthesia. The sedation approach should be individually tailored for each patient based on comorbidities, body habitus, and anticipated procedure duration. Some physicians administer small doses of glycopyrrolate to reduce secretions.
The use of a transparent distal cap that attaches to the endoscope may help with visualization during spray cryotherapy. During use of the truFreeze system, the decompression tube may hinder visualization of the target site. In these situations, the endoscope tip can be used to reposition the decompression tube. The presence of the decompression tube may increase the difficulty of treatment when luminal stenosis is present, such as in patients with esophageal cancer.
The CryoBalloon currently is available only as a focal treatment device; as such, long segments of mucosal pathology may be cumbersome to treat. The CryoBalloon also requires the use of a therapeutic endoscope with a ≥3.7-mm instrument channel.
Financial considerations
The spray cryotherapy systems require a capital investment for purchase of a console. This cost may be potentially defrayed if the system is shared with other providers who use cryotherapy in their practice (eg, pulmonology, dermatology, gynecology, and surgery). The liquefied gas tanks must be periodically filled, adding limited expense. Because the CryoBalloon system consists of disposable components, it does not require a capital investment. List prices for endoscopic cryotherapy systems available in the United States are depicted in Table 2.
Table 2.
List prices for endoscopic cryotherapy systems approved for use in the United States
| Platform | Equipment | Single vs multiuse | Cost |
|---|---|---|---|
| Liquid nitrogen cryotherapy | Console | Multiuse | $105,000 |
| Spray catheter with decompression tube | Single use | $1995 | |
| Visualization cap | Single use | $50 | |
| Nitrous oxide cryotherapy | CryoBalloon focal ablation catheter | Single use | $1200 |
| CryoBalloon focal controller | Single use | $750 | |
| Nitrous oxide cartridge | Single use | $60 | |
| Carbon dioxide cryotherapy | Currently not available |
There are no Current Procedural Terminology (CPT) codes specific to endoscopic cryotherapy, but existing codes for ablative procedures may be used. The CPT code 43229 is used for esophagoscopy with ablation of tumor(s), polyp(s), or other lesion(s), whereas 43270 is used to report EGD with ablation. Both codes include dilation (if needed), which is thus not separately reportable. Flexible sigmoidoscopy with ablation may be reported using 45346, whereas 45388 may be used to report colonoscopy with ablation.
As an alternative, endoscopists may consider reporting an unlisted service code, (ie, unlisted procedure, esophagus – 43499; in stomach – 43999; in rectum – 45999; in colon 45399). However, users should review the work relative value units (RVUs) and practice expenses associated with cryotherapy before deciding whether to report with existing ablation codes or whether to use an unlisted service code. When unlisted codes are reported, additional supporting documentation should be included with each claim. The information should detail the nature, extent, and need for the procedure and the time, effort, and equipment necessary to provide the procedure. Additional items to include are the complexity of symptoms, final diagnosis, pertinent patient findings, diagnostic and therapeutic procedures, concurrent problems, and follow-up care. It is helpful in such a cover letter to compare the work RVU or total RVU for the procedure with an existing code of similar intraservice time and intensity (eg, 43229 or 43270).
Areas for future research
The majority of studies assessing endoscopic cryotherapy are small and retrospective. Larger studies, preferably prospective in design, would help to better determine the efficacy and durability of cryotherapy for the treatment of various GI disorders. The available cryotherapy systems vary significantly, both in the temperature attained by the cryogen and in the cost of the system. However, any difference in clinical efficacy or cost effectiveness between the different systems remains unknown at this time.
Efficacy of cryotherapy has not been compared to other ablative modalities such as RFA. Head-to-head comparative studies will help determine the best use of each modality and allow practitioners to tailor treatment to the needs of each patient.
Outcomes in a very small number of patients with non-T1 esophageal cancers receiving salvage cryotherapy have been intriguing. Also, a possible synergistic effect between cryotherapy and chemotherapy has been suggested in a murine model.43 Further studies evaluating cryotherapy for luminal cancers in a palliative, salvage, or adjunctive approach are needed to define potential roles for cryotherapy in the treatment of GI malignancies.
Summary
Endoscopic cryotherapy has shown safety and efficacy in the ablation of mucosal lesions such as dysplastic BE and for hemostasis of bleeding lesions such as GAVE and radiation proctopathy. The ease of use and the relatively low cost of cryotherapy make it a potentially attractive treatment modality for these indications.
Disclosure
Dr Navaneethan is a consultant for AbbVie and Janssen and is a member of the speakers’ bureau for Takeda. Dr Parsi is a consultant for Boston Scientific. Dr Thosani is a consultant for Boston Scientific and Mederi. All other authors disclosed no financial relationships relevant to this publication.
Footnotes
This document was reviewed and approved by the governing board of the American Society for Gastrointestinal Endoscopy (ASGE).
Supplementary data
Liquid nitrogen cryotherapy in a patient with dysplastic Barrett's esophagus.
Nitrous oxide cryotherapy. (Video courtesy of C2 Therapeutics.)
References
- 1.Erinjeri J.P., Clark T.W. Cryoablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21:S187–S191. doi: 10.1016/j.jvir.2009.12.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gage A.A., Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 3.Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology. 1977;14:251–272. doi: 10.1016/0011-2240(77)90175-4. [DOI] [PubMed] [Google Scholar]
- 4.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984;247:C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 5.Privalov P.L. Cold denaturation of proteins. Crit Rev Biochem Mol Biol. 1990;25:281–305. doi: 10.3109/10409239009090612. [DOI] [PubMed] [Google Scholar]
- 6.Whittaker D.K. Mechanisms of tissue destruction following cryosurgery. Ann R Coll Surg Engl. 1984;66:313–318. [PMC free article] [PubMed] [Google Scholar]
- 7.Hanai A., Yang W.L., Ravikumar T.S. Induction of apoptosis in human colon carcinoma cells HT29 by sublethal cryo-injury: mediation by cytochrome c release. Int J Cancer. 2001;93:526–533. doi: 10.1002/ijc.1359. [DOI] [PubMed] [Google Scholar]
- 8.Clarke D.M., Robilotto A.T., Rhee E. Cryoablation of renal cancer: variables involved in freezing-induced cell death. Technol Cancer Res Treat. 2007;6:69–79. doi: 10.1177/153303460700600203. [DOI] [PubMed] [Google Scholar]
- 9.Baust J.G., Gage A.A. The molecular basis of cryosurgery. BJU Int. 2005;95:1187–1191. doi: 10.1111/j.1464-410X.2005.05502.x. [DOI] [PubMed] [Google Scholar]
- 10.Kahlenberg M.S., Volpe C., Klippenstein D.L. Clinicopathologic effects of cryotherapy on hepatic vessels and bile ducts in a porcine model. Ann Surg Oncol. 1998;5:713–718. doi: 10.1007/BF02303482. [DOI] [PubMed] [Google Scholar]
- 11.Weber S.M., Lee F.T., Jr., Chinn D.O. Perivascular and intralesional tissue necrosis after hepatic cryoablation: results in a porcine model. Surgery. 1997;122:742–747. doi: 10.1016/s0039-6060(97)90082-9. [DOI] [PubMed] [Google Scholar]
- 12.Muguruma N., Marcon N.E. Technique and emerging role of cryotherapy. Tech Gastrointest Endosc. 2010;12:44–48. [Google Scholar]
- 13.Weber S.M., Lee F.T. Cryoablation: history, mechanism of action, and guidance modalitites. In: Sonnenberg E., McMullen W., Solbiati L., editors. Tumor ablation. Springer; New York: 2010. [Google Scholar]
- 14.Yeh C.J. Cryosurgical treatment of melanin-pigmented gingiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:660–663. doi: 10.1016/s1079-2104(98)90199-8. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd J.P., Dawber R.P. Wound healing and scarring after cryosurgery. Cryobiology. 1984;21:157–169. doi: 10.1016/0011-2240(84)90207-4. [DOI] [PubMed] [Google Scholar]
- 16.Gage A.A., Baust J.M., Baust J.G. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59:229–243. doi: 10.1016/j.cryobiol.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatsutani K., Rubinsky B., Onik G. Effect of thermal variables on frozen human primary prostatic adenocarcinoma cells. Urology. 1996;48:441–447. doi: 10.1016/S0090-4295(96)00199-9. [DOI] [PubMed] [Google Scholar]
- 18.Ravikumar T.S., Steele G., Jr., Kane R. Experimental and clinical observations on hepatic cryosurgery for colorectal metastases. Cancer Res. 1991;51:6323–6327. [PubMed] [Google Scholar]
- 19.Johnston M.H., Eastone J.A., Horwhat J.D. Cryoablation of Barrett's esophagus: a pilot study. Gastrointest Endosc. 2005;62:842–848. doi: 10.1016/j.gie.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Dumot J.A., Greenwald B.D. Cryotherapy for Barrett's esophagus: does the gas really matter? Endoscopy. 2011;43:432–433. doi: 10.1055/s-0030-1256332. [DOI] [PubMed] [Google Scholar]
- 21.Gosain S., Mercer K., Twaddell W.S. Liquid nitrogen spray cryotherapy in Barrett's esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc. 2013;78:260–265. doi: 10.1016/j.gie.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Greenwald B.D., Dumot J.A., Horwhat J.D. Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esophagus. 2010;23:13–19. doi: 10.1111/j.1442-2050.2009.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaheen N.J., Greenwald B.D., Peery A.F. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71:680–685. doi: 10.1016/j.gie.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro A., Bejarano P., Livingstone A. Depth of injury caused by liquid nitrogen cryospray: study of human patients undergoing planned esophagectomy. Dig Dis Sci. 2014;59:1296–1301. doi: 10.1007/s10620-013-2991-4. [DOI] [PubMed] [Google Scholar]
- 25.Canto M.I., Shin E.J., Khashab M.A. Safety and efficacy of carbon dioxide cryotherapy for treatment of neoplastic Barrett's esophagus. Endoscopy. 2015;47:582–591. doi: 10.1055/s-0034-1391734. [DOI] [PubMed] [Google Scholar]
- 26.Xue H.B., Tan H.H., Liu W.Z. A pilot study of endoscopic spray cryotherapy by pressurized carbon dioxide gas for Barrett's esophagus. Endoscopy. 2011;43:379–385. doi: 10.1055/s-0030-1256334. [DOI] [PubMed] [Google Scholar]
- 27.Ghorbani S., Tsai F.C., Greenwald B.D. Safety and efficacy of endoscopic spray cryotherapy for Barrett's dysplasia: results of the National Cryospray Registry. Dis Esophagus. 2016;29:241–247. doi: 10.1111/dote.12330. [DOI] [PubMed] [Google Scholar]
- 28.Halsey K.D., Chang J.W., Waldt A. Recurrent disease following endoscopic ablation of Barrett's high-grade dysplasia with spray cryotherapy. Endoscopy. 2011;43:844–848. doi: 10.1055/s-0030-1256649. [DOI] [PubMed] [Google Scholar]
- 29.Sengupta N., Ketwaroo G.A., Bak D.M. Salvage cryotherapy after failed radiofrequency ablation for Barrett's esophagus-related dysplasia is safe and effective. Gastrointest Endosc. 2015;82:443–448. doi: 10.1016/j.gie.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Verbeek R.E., Vleggaar F.P., Ten Kate F.J. Cryospray ablation using pressurized CO2 for ablation of Barrett's esophagus with early neoplasia: early termination of a prospective series. Endosc Int Open. 2015;3:E107–E112. doi: 10.1055/s-0034-1390759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholvinck D.W., Kunzli H.T., Kestens C. Treatment of Barrett's esophagus with a novel focal cryoablation device: a safety and feasibility study. Endoscopy. 2015;47:1106–1112. doi: 10.1055/s-0034-1392417. [DOI] [PubMed] [Google Scholar]
- 32.Dumot J.A., Greenwald B.D. Argon plasma coagulation, bipolar cautery, and cryotherapy: ABC's of ablative techniques. Endoscopy. 2008;40:1026–1032. doi: 10.1055/s-0028-1103414. [DOI] [PubMed] [Google Scholar]
- 33.Greenwald B.D., Dumot J.A., Abrams J.A. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686–693. doi: 10.1016/j.gie.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dumot J.A., Vargo J.J., 2nd, Falk G.W. An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635–644. doi: 10.1016/j.gie.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Saligram S., Chennat J., Hu H. Endotherapy for superficial adenocarcinoma of the esophagus: an American experience. Gastrointest Endosc. 2013;77:872–876. doi: 10.1016/j.gie.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Kantsevoy S.V., Cruz-Correa M.R., Vaughn C.A. Endoscopic cryotherapy for the treatment of bleeding mucosal vascular lesions of the GI tract: a pilot study. Gastrointest Endosc. 2003;57:403–406. doi: 10.1067/mge.2003.115. [DOI] [PubMed] [Google Scholar]
- 37.Cho S., Zanati S., Yong E. Endoscopic cryotherapy for the management of gastric antral vascular ectasia. Gastrointest Endosc. 2008;68:895–902. doi: 10.1016/j.gie.2008.03.1109. [DOI] [PubMed] [Google Scholar]
- 38.Moawad F.J., Maydonovitch C.L., Horwhat J.D. Efficacy of cryospray ablation for the treatment of chronic radiation proctitis in a pilot study. Dig Endosc. 2013;25:174–179. doi: 10.1111/j.1443-1661.2012.01355.x. [DOI] [PubMed] [Google Scholar]
- 39.Barthel J.S., Kucera S., Harris C. Cryoablation of persistent Barrett's epithelium after definitive chemoradiation therapy for esophageal adenocarcinoma. Gastrointest Endosc. 2011;74:51–57. doi: 10.1016/j.gie.2011.03.1121. [DOI] [PubMed] [Google Scholar]
- 40.Halsey K.D., Greenwald B.D. Cryotherapy in the management of esophageal dysplasia and malignancy. Gastrointest Endosc Clin N Am. 2010;20:75–87. doi: 10.1016/j.giec.2009.07.009. vi-vii. [DOI] [PubMed] [Google Scholar]
- 41.Greenwald B.D., Lightdale C.J., Abrams J.A. Barrett's esophagus: endoscopic treatments II. Ann N Y Acad Sci. 2011;1232:156–174. doi: 10.1111/j.1749-6632.2011.06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen A.M., Pasricha P.J. Cryotherapy for Barrett's esophagus: Who, how, and why? Gastrointest Endosc Clin N Am. 2011;21:111–118. doi: 10.1016/j.giec.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Forest V., Peoc'h M., Campos L. Effects of cryotherapy or chemotherapy on apoptosis in a non-small-cell lung cancer xenografted into SCID mice. Cryobiology. 2005;50:29–37. doi: 10.1016/j.cryobiol.2004.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Liquid nitrogen cryotherapy in a patient with dysplastic Barrett's esophagus.
Nitrous oxide cryotherapy. (Video courtesy of C2 Therapeutics.)