Abstract
Background:
There is a growing body of evidence that physical activity (PA) improves symptoms of multiple sclerosis (MS). Despite the benefits of PA, people with MS are relatively inactive compared with their healthy counterparts. This study investigated associations between social cognitive theory (SCT) constructs and energy expenditure (EE) as an objective measure of PA in a sample of inactive people with MS.
Methods:
Participants (n = 65) completed several questionnaires and were assessed using standardized outcome measures as part of a cross-sectional analysis of baseline data from a randomized controlled trial (Step it Up).
Results:
The bivariate correlation analysis indicated that of all SCT constructs, only exercise self-efficacy was significantly correlated with EE (r = 0.297, P = .022). Multiple linear regression analysis found that exercise self-efficacy independently explained 9% of the variance in EE (R2 = 0.088). A model including exercise self-efficacy, exercise goal setting, exercise planning, and exercise benefits explained 17% of the variance in EE (F4,54 = 2.741, P = .038, R2 = 0.169). In this model, only exercise self-efficacy was significantly associated with EE scores (Exercise Self-Efficacy Scale β = .320, P = .016).
Conclusions:
The constructs of SCT explained little of the variance of objectively measured PA in a sample of inactive people with MS who volunteered for an exercise trial. The only significant variable was exercise self-efficacy, which confirms the importance of enhancing it through PA interventions.
Physical activity (PA) in the form of exercise training can improve many of the symptoms associated with multiple sclerosis (MS), including muscle strength,1,2 cardiorespiratory fitness,3 walking mobility,4 quality of life,5 and fatigue.6 Current evidence suggests that, although there are significant benefits of exercise interventions in the short term, the improvements are not maintained when exercise interventions are discontinued.7
Despite these benefits, people with MS are physically inactive.8 A recent study by Motl et al.9 found that people with MS were 2.3 times less likely to report sufficient PA than their healthy counterparts. There is a body of evidence that physical inactivity is associated with reduced quality of life10 and increased risk of cardiorespiratory disease.11
Therefore, it is important to develop interventions that will facilitate long-term adherence to exercise and positively influence PA behavior change. The recommendation of the Medical Research Council framework for complex interventions is that the development and evaluation of theory is essential before developing successful interventions.12 To that end, an examination of existing literature suggests that factors such as self-efficacy, outcome expectations, enjoyment, and goal setting are important constructs in facilitating positive changes in PA behavior in people with MS,13–15 and these factors form important constructs of social cognitive theory (SCT).16
Social cognitive theory is one of the most widely adopted theoretical frameworks for understanding and optimizing PA behavior. It is characterized by four constructs: self-efficacy, outcome expectations, goal setting, and barriers and facilitators. Social cognitive theory constructs have been previously studied in people with MS,17–26 with studies reporting significant correlations between SCT constructs and PA; however, most of the studies included only some constructs from the SCT framework. For example, Suh et al.,17 in their observational study of social cognitive predictors of PA in 68 people with relapsing-remitting MS, did not include measures of exercise planning and PA barriers and facilitators. Similarly, Dlugonski et al.,21 examining social cognitive correlates of PA in 54 inactive adults with MS, used the SCT constructs of self-efficacy, goal setting, and outcome expectations only. The present study aimed to examine all the SCT constructs by adding components such as PA barriers and facilitators and exercise planning.
Most work in this field has focused on self-reported measures of PA rather than on objective measures. Healthy population literature suggests using energy expenditure (EE) output27 if total PA levels and not PA intensity or duration are desired, and this article, therefore, reports objective PA and all the SCT constructs.
Because most studies have been completed in North America with general MS populations, this study adds to our understanding of PA theory by examining the associations among SCT constructs, demographic features, and objective measures of PA in a sample of ambulatory inactive people with MS in Europe.
Methods
Study Design
This was a cross-sectional study that aimed to examine the relationship between SCT constructs and EE as an objective measure of PA. Baseline data from a multicenter, double-blind randomized controlled trial (RCT) of an exercise-plus–behavior change intervention in people with MS, namely, Step it Up,28 were included. The study was performed in agreement with the Declaration of Helsinki and was approved by the Health Service Executive Mid-West Research Ethics Committee, the Galway University Hospitals Clinical Research Ethics Committee, and the University of Limerick, Faculty of Education and Health Sciences Research Ethics Committee.
Participants
Baseline data were collected for 65 people with MS who participated in the RCT. Participants were recruited using social media, e-mail, and postal communications of the MS Society of Ireland. Participants were also recruited via neurology clinics in three cities in the west and south of Ireland. People who were interested in participating in the RCT were invited to contact the research team at the University of Limerick via telephone or e-mail, wherein they were given an opportunity to ask questions about Step it Up. Potential participants were screened over the telephone, and participant information leaflets and informed consent forms were sent to each potential participant via post or e-mail. The inclusion criteria were 1) a physician-confirmed formal diagnosis of MS, 2) age 18 years or older, 3) a Patient-Determined Disease Steps score of 0 to 3, 4) a sedentary lifestyle (<30 minutes of moderate to strenuous exercise ≥1 days per week during the past 6 months), and 5) willing to give written informed consent. The exclusion criteria were 1) pregnancy, 2) MS relapse in the past 12 weeks, and 3) changes to MS medication or corticosteroid treatment in the past 12 weeks.
Procedure
After initial telephone contact and screening, potential participants were sent all the self-report outcome measures via post to complete before their objective assessment meeting with the blinded assessor (S.H.). Participants received instruction on how to complete each self-report measure according to standardized instructions for each measure of outcome. The postdoctoral researcher (S.H.) was blinded to allocation and conducted all the objective assessments the week preceding the start of the Step it Up intervention.
PA Measure
The SenseWear Armband (BodyMedia, Pittsburgh, PA) was used as an objective measure of PA. The armband integrates a biaxial accelerometer (longitudinal and transverse axes), a heat flux sensor, a galvanic skin response sensor, a skin temperature sensor, and a near-body ambient temperature sensor in addition to demographic data (sex, age, height, and weight). The armband was attached to the upper arm (overlying the triceps muscle). The armband data were downloaded using SenseWear Innerview Professional software, version 6.1 (BodyMedia). Proprietary algorithms were used to estimate EE in kilocalories. For the present analyses we used only participants who provided 4 days of valid daily EE data (n = 62), because preliminary analysis using the generalizability theory indicates that a minimum of any 4 days of the week provides a reliable estimate of average daily EE.29 The SenseWear Armband has demonstrated acceptable criterion validity of mean daily EE (kilocalorie estimates) in people with MS with mild disability,30 and EE was the PA estimate used in this study.
SCT Constructs
Exercise Self-Efficacy Scale
The Exercise Self-efficacy (EXSE)31 Scale was used to assess self-efficacy. This scale has six items that assess an individual's beliefs in his or her ability to engage in 20 minutes or more of moderate PA three times per week, in 1-month increments. The items were rated on a scale from 0 (not at all confident) to 100 (completely confident) and were averaged into a composite score ranging from 0 to 100. Higher scores reflect greater confidence in one's ability to regularly engage in PA.
Social Provisions Scale
The Social Provisions Scale32 was used to measure perceptions of social support. This scale contains six items that were rated on a 4-point scale ranging from 1 (strongly disagree) to 4 (strongly agree). Item scores were summed into an overall score ranging from 6 to 24. Higher scores reflect the greater provision of social support for PA.
Multidimensional Outcome Expectations for Exercise Scale
The Multidimensional Outcome Expectations for Exercise Scale (MOEES)33 was used to measure outcome expectations for exercises. This scale contains 15 items that reflect three subdomains of outcome expectations. Six items reflect physical outcome expectations, four items assess social outcome expectations, and five items measure self-evaluative outcome expectations. The 15 items were rated on a 5-point scale from 1 (strongly disagree) to 5 (strongly agree) and were summed to form three subscales of outcome expectations. Higher scores per subscale reflect greater physical, social, or self-evaluative outcome expectations.
Exercise Goal-Setting Questionnaire
The exercise goal-setting questionnaire34 was used to measure exercise goals. The questionnaire contains ten items that reflect goal setting for exercise behavior rated on a 5-point scale from 1 (does not describe) to 5 (describes completely). The item scores were summed into an overall score ranging from 10 to 50. Higher scores reflect a stronger tendency for setting goals for PA.
Exercise Planning Questionnaire
The exercise planning questionnaire34 was used to measure plans for exercise. The questionnaire contains ten items relating to exercise planning rated on a 5-point scale ranging from 1 (does not describe) to 5 (describes completely). Item scores were summed into an overall score ranging from 10 to 50. Higher scores reflect increased planning for PA behavior.
Exercise Barriers and Benefits Questionnaire
The exercise barriers and benefits questionnaire35 was used to measure the possible benefits associated with exercise participation and the barriers that would prevent participants from engaging in PA. This questionnaire contains 43 items rated on a 4-point scale ranging from 1 (strongly disagree) to 4 (strongly agree). The scale produces two scores: exercise benefits (EBe) and exercise barriers (EBar). The EBar scores range from 14 to 56. Higher scores on the EBar indicate greater barriers to exercise. The EBe scores range from 29 to 116. Higher scores on the EBe indicate higher perceived benefits of PA.
These aforementioned questionnaires addressing SCT constructs have been validated and have been used in previous research on PA in MS.15,36,37
Statistical Methods
Descriptive statistics were used to report participant clinical and demographic characteristics. Continuous variables were assessed for normality using the Shapiro-Wilk test and by visual inspection of histograms. Sex differences in EE were examined using an independent-samples t test. Bivariate relationships between the SCT variables and EE were examined using the Pearson correlation coefficient for approximately normally distributed data. Correlation coefficients were interpreted using the guidelines proposed by Cohen (1988): 0.1 is small, 0.3 is moderate, and 0.5 is large. Multiple linear regression was used to assess the independent multivariate associations between SCT variables and EE. First, exercise self-efficacy was entered as an independent predictor of EE. We further applied a theory-based analysis consistent with those of Pallant38 and Dlugonski et al.21 in which other SCT variables were added to find the optimal combination to explain the most variance. The assumptions of normality, linearity, and homoscedasticity of the residuals, which underlie regression analysis, were checked using normal probability plots and scatterplots. The variance inflation factor was used to test for multicollinearity. Multicollinearity was deemed to be a concern if the variance inflation factor coefficient was greater than 10. All the tests were completed with a 0.05 level of significance. The data were analyzed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp, Armonk, NY).
Results
Sample Characteristics
Most of the sample was female (n = 55 [84.6%]), had completed tertiary education (n = 37 [56.9%]), and was married (n = 43 [66.2%]). Most of this sample had relapsing-remitting MS (n = 54 [83.1%]). The sample median (25th-75th percentile) time since diagnosis was 5.0 (1.62–11.08) years, time since experiencing MS symptoms was 8.41 (4.50–14.0) years, and level of disability (Expanded Disability Status Scale) was 3.50 (3.00–3.50). The mean (SD) age of this sample was 41.98 (9.76) years. Table 1 presents the participant demographic and clinical characteristics.
Table 1.
Participant demographic and clinical characteristics

Descriptive Statistics
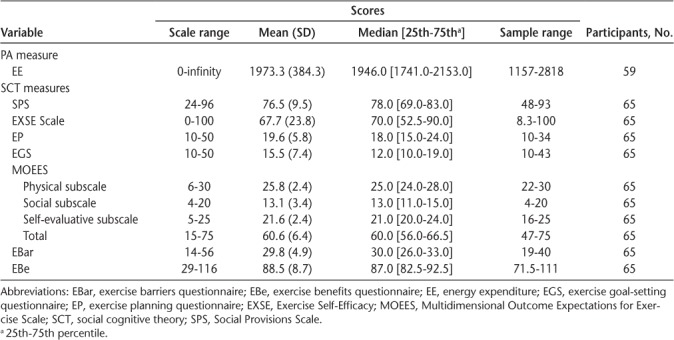
Table 2 presents baseline descriptive statistics for EE and the SCT variables. The mean score for the EXSE Scale was considerably lower than in earlier research in people with MS,20,21 whereas the mean value for the MOEES was generally comparable with that in the previously mentioned studies.
Table 2.
Baseline descriptive statistics of EE and SCT constructs
Bivariate Analysis
The bivariate correlation analysis is reported in Table 3. The correlation analysis indicated that only exercise self-efficacy was significantly correlated with EE (r = 0.297, P = .022).
Table 3.
Pearson correlation analysis between EE and social cognitive theory–based variables

Multiple Linear Regression Analyses
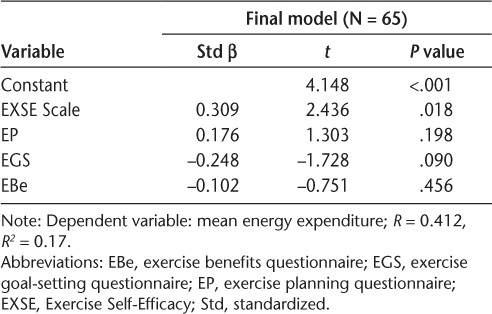
Multiple linear regression was used to assess the ability of the SCT constructs (Social Provisions Scale, EXSE Scale, exercise planning questionnaire, exercise goal-setting questionnaire, EBar and EBe questionnaire, and MOEES) to predict levels of objectively measured PA (EE). The first regression model including only exercise self-efficacy was significant and explained only 9% (R2 = 0.088) of the variance in EE (F1,57 = 5.516, P = .022, EXSE Scale β = .297). The final regression model was significant and included the EXSE Scale, exercise planning questionnaire, exercise goal-setting scale, and EBe questionnaire. The model fit was assessed using the R2 value and found that 17% of the variance in EE was explained by the model (F4,54 = 2.741, P = .038, R2 = 0.169). Residual diagnostics did not identify any outliers (all standardized residuals were between −3 and +3), and the assumptions of linearity and normality of residuals were satisfied. In this model, only exercise self-efficacy was significantly associated with EE estimates (EXSE Scale β = .320, P = .016). The final regression model is presented in Table 4.
Table 4.
Multiple linear regression examining independent associations between social cognitive theory variables and energy expenditure
Discussion
The aim of this study was to examine the relationships between all the SCT constructs and an objective measure of PA (EE) in inactive people with MS in Europe.
In this sample, only exercise self-efficacy was significantly correlated with EE. These results are consistent with previously reported associations between self-efficacy and self-reported or objectively measured PA in people with MS.17,18,20,25,26 Self-efficacy is the belief that one can successfully cope with challenging conditions,39 and many studies indicate that self-efficacy is a primary correlate of PA in general39–41 and in MS.23,24 Furthermore, a recent systematic review of correlates and determinants of PA in people with MS concluded that self-efficacy is a potential target for PA interventions.42 This study replicates the findings of previous studies and confirms that self-efficacy is an important factor in understanding PA behavior in inactive people with MS.
Importantly, this study investigated PA correlates in an inactive sample that, as volunteers for an exercise RCT, is the target of our interventions. We hypothesized that by adding SCT constructs beyond self-efficacy to the regression model we can explain more variance in EE in this group than with self-efficacy alone. Exercise goal setting had the strongest association with PA in a recent meta-analysis43 and was a moderator of the improvement in PA. In addition, people with MS have reported in qualitative studies that they would like information on the benefits of PA.44 The addition of exercise goal setting, exercise planning, and exercise benefits increased R2 in the present data. However, only exercise self-efficacy remained significant in the model. This confirms the importance of exercise self-efficacy and suggests that exercise goal setting, exercise planning, and exercise benefits should be further investigated.
Although contributing to the theory to inform the development of PA interventions, this study explained only 17% of the variance in EE and, therefore, suggests that there are other, uninvestigated constructs that may contribute to PA behavior in this population.
One limitation of this study is that we did not consider other MS symptoms or functional ability. Kayes et al.45 explored the facilitators and barriers to engagement in PA for people with MS (n = 282) and found that variables such as fatigue and perceived barriers to PA are potentially modifiable variables that could form part of the interventions to increase PA in people with MS. Also, previously published data from Suh et al.17 confirmed that more variance in PA (28%) was explained when the SCT constructs were entered into a model with functional limitations. The addition of fatigue and function are supported by a recent review of correlates and determinants of PA in persons with MS42 suggesting that fatigue, general disability level, and walking limitations are consistent correlates of PA.
A positive aspect of this study is that we used EE as an objective measure of PA, and a previous study by Coote and O'Dwyer30 found EE to be a more valid PA estimate than steps. However, the finding that EE is higher in males compared with females may be due to body composition and not to EE due to PA. This is a limitation of using EE as a proxy for PA that warrants further investigation, particularly because people with MS may expend more energy per step than healthy individuals.46
The strengths of this study are that we evaluated all the SCT constructs in a European sample of inactive, relatively newly diagnosed people with MS—the population that is the target of PA interventions. The cross-sectional nature of the data inhibits examination of the relationship between SCT variables and PA over time. The findings presented herein are from the first time point of an RCT, and the authors will collect follow-up data 10 weeks after the initial assessment and at 24- and 36-week follow-up. Although the variance in PA was explained by SCT variables, the inclusion of measures of MS symptoms and their consequences, such as cognitive function, fatigue, walking impairments, and strength limitations, and other psychosocial factors may add further clarification. This inactive sample had a relatively recent diagnosis of MS, and most participants were women with relapsing-remitting MS, had a low disability level, were well educated, and were married; therefore, the results may not be generalizable to the MS population as a whole.
In conclusion, the SCT constructs explained only 17% of the variance in PA in these inactive people with MS who volunteered for an exercise RCT. The only significant variable was exercise self-efficacy, which confirms the importance of addressing self-efficacy for exercise through PA interventions. In addition, goal setting and exercise planning may be important, as may knowledge of exercise benefits. Future studies should consider adding other behavior change constructs and MS symptoms to their investigations so that more of the variance in PA can be explained.
PRACTICE POINTS
To improve physical activity (PA), we need to understand the correlates of PA, particularly in inactive people, who are the target of our interventions.
Exercise self-efficacy was significantly correlated with PA, confirming that it is an important factor to address in interventions.
Social cognitive theory constructs alone explained only 17% of the variance in PA in this inactive sample; therefore, it is important to investigate other factors that explain more variance so that these can also be targeted in interventions.
Acknowledgments:
The authors thank Aidan Larkin, Multiple Sclerosis Society of Ireland, for his assistance with recruitment.
Financial Disclosures:
The authors have no conflicts of interest to disclose.
Funding/Support:
This work was supported by the Irish Health Research Board, Health Research Award grant HRA_PHR/2013-264.
References
- 1. Kjolhede T, Vissing K, Dalgas U.. Multiple sclerosis and progressive resistance training: a systematic review. Mult Scler. 2012; 18: 1215– 1228. [DOI] [PubMed] [Google Scholar]
- 2. Rietberg MB, Brooks D, Uitdehaag BMJ, Kwakkel G.. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev. 2005; 1: CD003980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Platta ME, Ensari I, Motl RW, Pilutti LA.. Effect of exercise training on fitness in multiple sclerosis: a meta-analysis. Arch Phys Med Rehabil. 2016; 97: 1564– 1572. [DOI] [PubMed] [Google Scholar]
- 4. Snook EM, Motl RW.. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil Neural Repair. 2009; 23: 108– 116. [DOI] [PubMed] [Google Scholar]
- 5. Motl RW, Gosney JL.. Effect of exercise training on quality of life in multiple sclerosis: a meta-analysis. Mult Scler. 2008; 14: 129– 135. [DOI] [PubMed] [Google Scholar]
- 6. Pilutti LA, Greenlee TA, Motl RW, Nickrent MS, Petruzzello SJ.. Effects of exercise training on fatigue in multiple sclerosis: a meta-analysis. Psychosom Med. 2013; 75: 575– 580. [DOI] [PubMed] [Google Scholar]
- 7. Garrett M, Hogan N, Larkin A, Saunders J, Jakeman P, Coote S.. Exercise in the community for people with multiple sclerosis: a follow-up of people with minimal gait impairment. Mult Scler. 2013; 19: 790– 798. [DOI] [PubMed] [Google Scholar]
- 8. Motl RW, McAuley E, Snook EM.. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005; 11: 459– 463. [DOI] [PubMed] [Google Scholar]
- 9. Motl RW, McAuley E, Sandroff BM, Hubbard EA.. Descriptive epidemiology of physical activity rates in multiple sclerosis. Acta Neurol Scand. 2015; 131: 422– 425. [DOI] [PubMed] [Google Scholar]
- 10. Motl RW, McAuley E, Snook EM, Gliottoni RC.. Physical activity and quality of life in multiple sclerosis: intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med. 2009; 14: 111– 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slawta JN, McCubbin JA, Wilcox AR, Fox SD, Nalle DJ, Anderson G.. Coronary heart disease risk between active and inactive women with multiple sclerosis. Med Sci Sports Exerc. 2002; 34: 905– 912. [DOI] [PubMed] [Google Scholar]
- 12. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M.. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008; 337: a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carter A, Daley A, Humphreys L, . et al. Pragmatic intervention for increasing self-directed exercise behavior and improving important health outcomes in people with multiple sclerosis: a randomized controlled trial. Mult Scler. 2014; 20: 1112– 1122. [DOI] [PubMed] [Google Scholar]
- 14. Pilutti LA, Dlugonski D, Sandroff BM, Klaren R, Motl RW.. Randomized controlled trial of a behavioral intervention targeting symptoms and physical activity in multiple sclerosis. Mult Scler. 2014; 20: 594– 601. [DOI] [PubMed] [Google Scholar]
- 15. Motl RW, Dlugonski D, Wojcicki TR, McAuley E, Mohr DC.. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult Scler. 2011; 17: 116– 128. [DOI] [PubMed] [Google Scholar]
- 16. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977; 84: 191– 215. [DOI] [PubMed] [Google Scholar]
- 17. Suh Y, Joshi I, Olsen C, Motl RW.. Social cognitive predictors of physical activity in relapsing-remitting multiple sclerosis. Int J Behav Med. 2014; 21: 891– 898. [DOI] [PubMed] [Google Scholar]
- 18. Motl RW, McAuley E, Sandroff BM.. Longitudinal change in physical activity and its correlates in relapsing-remitting multiple sclerosis. Phys Ther. 2013; 93: 1037– 1048. [DOI] [PubMed] [Google Scholar]
- 19. Motl RW, McAuley E, Wynn D, Sandroff B, Suh Y.. Physical activity, self-efficacy, and health-related quality of life in persons with multiple sclerosis: analysis of associations between individual-level changes over one year. Qual Life Res. 2013; 22: 253– 261. [DOI] [PubMed] [Google Scholar]
- 20. Suh Y, Weikert M, Dlugonski D, Sandroff B, Motl RW.. Social cognitive correlates of physical activity: findings from a cross-sectional study of adults with relapsing-remitting multiple sclerosis. J Phys Act Health. 2011; 8: 626– 635. [DOI] [PubMed] [Google Scholar]
- 21. Dlugonski D, Wojcicki TR, McAuley E, Motl RW.. Social cognitive correlates of physical activity in inactive adults with multiple sclerosis. Int J Rehabil Res. 2011; 34: 115– 120. [DOI] [PubMed] [Google Scholar]
- 22. Motl RW, Snook EM.. Physical activity, self-efficacy, and quality of life in multiple sclerosis. Ann Behav Med. 2008; 35: 111– 115. [DOI] [PubMed] [Google Scholar]
- 23. Morris KS, McAuley E, Motl RW.. Self-efficacy and environmental correlates of physical activity among older women and women with multiple sclerosis. Health Educ Res. 2008; 23: 744– 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Motl RW, Snook EM, Wynn D.. Physical activity behavior in individuals with secondary progressive multiple sclerosis. Int J MS Care. 2007; 9: 139– 142. [Google Scholar]
- 25. Motl RW, Snook EM, McAuley E, Gliottoni RC.. Symptoms, self-efficacy, and physical activity among individuals with multiple sclerosis. Res Nurs Health. 2006; 29: 597– 606. [DOI] [PubMed] [Google Scholar]
- 26. Motl RW, Snook EM, McAuley E, Scott JA, Douglass ML.. Correlates of physical activity among individuals with multiple sclerosis. Ann Behav Med. 2006; 32: 154– 161. [DOI] [PubMed] [Google Scholar]
- 27. Bassett DR, Troiano RP, McClain JJ, Wolff DL.. Accelerometer-based physical activity: total volume per day and standardized measures. Med Sci Sports Exerc. 2015; 47: 833– 838. [DOI] [PubMed] [Google Scholar]
- 28. Coote S, Gallagher S, Msetfi R, . et al. A randomised controlled trial of an exercise plus behaviour change intervention in people with multiple sclerosis: the Step it Up study protocol. BMC Neurol. 2014; 14: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norris M, Anderson R, Motl RW, Hayes S, Coote S.. Minimum number of days required for a reliable estimate of daily step count and energy expenditure, in people with MS who walk unaided. Gait Posture. 2017; 53: 201– 206. [DOI] [PubMed] [Google Scholar]
- 30. Coote S, O'Dwyer C.. Comparative validity of accelerometer-based measures of physical activity for people with multiple sclerosis. Arch Phys Med Rehabil. 2012; 93: 2022– 2028. [DOI] [PubMed] [Google Scholar]
- 31. McAuley E. Self-efficacy and the maintenance of exercise participation in older adults. J Behav Med. 1993; 16: 103– 113. [DOI] [PubMed] [Google Scholar]
- 32. Cutrona CE, Russell DW.. The provisions of social relationships and adaptation to stress. : Jones WH, Perlman D, . Advances in Personal Relationships. Vol 1. Greenwich, CT: JAI Press; 1987: 37– 67. [Google Scholar]
- 33. McAuley E, Motl RW, White SM, Wojcicki TR.. Validation of the multidimensional outcome expectations for exercise scale in ambulatory, symptom-free persons with multiple sclerosis. Arch Phys Med Rehabil. 2010; 91: 100– 105. [DOI] [PubMed] [Google Scholar]
- 34. Rovniak LS, Anderson ES, Winett RA, Stephens RS.. Social cognitive determinants of physical activity in young adults: a prospective structural equation analysis. Ann Behav Med. 2002; 24: 149– 156. [DOI] [PubMed] [Google Scholar]
- 35. Stroud N, Minahan C, Sabapathy S.. The perceived benefits and barriers to exercise participation in persons with multiple sclerosis. Disabil Rehabil. 2009; 31: 2216– 2222. [DOI] [PubMed] [Google Scholar]
- 36. Plow M, Finlayson M, Motl RW, Bethoux F.. Randomized controlled trial of a teleconference fatigue management plus physical activity intervention in adults with multiple sclerosis: rationale and research protocol. BMC Neurol. 2012; 12: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Plow MA, Resnik L, Allen SM.. Exploring physical activity behaviour of persons with multiple sclerosis: a qualitative pilot study. Disabil Rehabil. 2009; 31: 1652– 1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pallant J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using SPSS. Crows Nest, Australia: Allen & Unwin; 2010. [Google Scholar]
- 39. Bandura A.M. Self-Efficacy: The Exercise of Control. Duffield, UK: Worth Publishers; 1997. [Google Scholar]
- 40. McAuley E, Blissmer B.. Self-efficacy determinants and consequences of physical activity. Exerc Sport Sci Rev. 2000; 28: 85– 88. [PubMed] [Google Scholar]
- 41. Trost SG, Owen N, Bauman AE, Sallis JF, Brown W.. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002; 34: 1996– 2001. [DOI] [PubMed] [Google Scholar]
- 42. Streber R, Peters S, Pfeifer K.. Systematic review of correlates and determinants of physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil. 2016; 97: 633– 645.e29. [DOI] [PubMed] [Google Scholar]
- 43. Casey B, Coote S, Shirazipour C, . et al. Modifiable psychosocial constructs associated with physical activity behavior in people with multiple sclerosis: a systematic review and meta-analysis. Mult Scler. 2016; 22 suppl 3: 29– 30. [DOI] [PubMed] [Google Scholar]
- 44. Casey B, Hayes S, Browne C, Coote S.. What do people with MS want from a web-based resource to encourage increased physical activity behaviour? Disabil Rehabil. 2016; 38: 1557– 1566. [DOI] [PubMed] [Google Scholar]
- 45. Kayes NM, McPherson KM, Schluter P, Taylor D, Leete M, Kolt GS.. Exploring the facilitators and barriers to engagement in physical activity for people with multiple sclerosis. Disabil Rehabil. 2011; 33: 1043– 1053. [DOI] [PubMed] [Google Scholar]
- 46. Coote S, O'Dwyer C.. Energy expenditure during everyday activities: a study comparing people with varying mobility limitations due to multiple sclerosis and healthy controls. Disabil Rehabil. 2014; 36: 2059– 2064. [DOI] [PubMed] [Google Scholar]


