Abstract
Different sampling strategies, analytic alternatives, and estimators have been proposed to better assess the characteristics of different hard-to-reach populations and their respective infection rates (as well as their sociodemographic characteristics, associated harms, and needs) in the context of studies based on respondent-driven sampling (RDS). Despite several methodological advances and hundreds of empirical studies implemented worldwide, some inchoate findings and methodological challenges remain. The in-depth assessment of the local structure of networks and the performance of the available estimators are particularly relevant when the target populations are sparse and highly stigmatized. In such populations, bottlenecks as well as other sources of biases (for instance, due to homophily and/or too sparse or fragmented groups of individuals) may be frequent, affecting the estimates.
In the present study, data were derived from a cross-sectional, multicity RDS study, carried out in 12 Brazilian cities with transgender women (TGW). Overall, infection rates for HIV and syphilis were very high, with some variation between different cities. Notwithstanding, findings are of great concern, considering the fact that female TGW are not only very hard-to-reach but also face deeply-entrenched prejudice and have been out of the reach of most therapeutic and preventive programs and projects.
We cross-compared findings adjusted using 2 estimators (the classic estimator usually known as estimator II, originally proposed by Volz and Heckathorn) and a brand new strategy to adjust data generated by RDS, partially based on Bayesian statistics, called for the sake of this paper, the RDS-B estimator. Adjusted prevalence was cross-compared with estimates generated by non-weighted analyses, using what has been called by us a naïve estimator or rough estimates.
Keywords: Brazil, HIV/AIDS, respondent-driven sampling, sexually transmitted infections, statistical methods, transgender women
1. Introduction
1.1. Major infectious diseases among transgender women
HIV/AIDS is one of the largest pandemic ever faced by humankind, with over 36 million people living with HIV/AIDS worldwide and about 5000 new HIV infections every single day. Since 2003, over 95% of those new infections have been reported in low- and middle-income countries, and around 70% of those countries have low-level or concentrated HIV/AIDS epidemics.[1]
In a concentrated epidemic, HIV spreads rapidly in one or more specific subpopulations, but its spread is relatively modest among the general population. In these contexts the individual characteristics and the structure of the networks of most-at-risk populations (MARPs), both within their own communities and respecting their putative bridges with other MARPs, as well as the population at large, have a key role in the epidemic dynamics. The current and future course of each local epidemic is usually determined by the nature and intensity of the interactions between one or more than one subpopulations with high background infection rates and the general population, since closed, small populations tend to face saturation over time. Saturation depends on the balance of susceptible and infected people vis-a-vis the homogeneity versus heterogeneity of the networks,[2] as well as the role of new birth cohorts and pathogen diversity and the immune response to it. In this sense, infections driven by multiple pathogens/strains may never saturate given segments, as have been described for dengue fever and malaria, which tend to rather become hyperendemic in some contexts and populations.[3]
Among all populations affected by HIV, evidence suggests that transgender women (TGW) carry the heaviest HIV burden worldwide.[4,5] A meta-analysis indicate that worldwide TGW are 49 times more likely to acquire HIV than adults of the same reproductive age, belonging to the general population.[6] Country reports suggest that HIV prevalence for transgender sex workers is on average 9 times higher than that for female sex workers and 3 times higher than that for male sex workers.[7] A second meta-analysis reported an overall crude HIV prevalence of 27.3% among TGW who engage in sex work, compared to 14.7% among TGW who did not report participating in sex work.[8] According to a broad review conducted in Latin America, TGW population is the most vulnerable to HIV in the region, with prevalence above 30%.[9] In Brazil, only a few local studies have been conducted so far with TGW, identifying HIV prevalence above 25%.[10,11]
Over more than 3 decades of the HIV epidemic and in varying extent respecting other sexually transmitted infections (STIs), such as HBV, syphilis, and gonorrhea, gay men have been disproportionately affected. The infection rates of HCV are not as closely associated as the classic STIs with being a member of the gay community and/or to having had sex with other men. This is probably a consequence of the major influence of parenteral risks.
A recent review conducted by the same group from Johns Hopkins University and their colleagues[12] has highlighted the increased vulnerability of gay men and other men who have sex with other men. Their infection rates are several times higher than those observed among the general population, but pale in comparison with TGW, much probably in consequence of their strong activism since the early 1980s; the successful partnership between civil society and some public and private institutions and governmental bodies; the long-term engagement in innovative interventions both in the field of prevention and treatment and, more recently, in the continuum of treatment and prevention usually called “Treatment as Prevention” (TasP), as documented by successful interventions such as iPrEx.[13]
Very little is known about the infection rates of different viral hepatitis among TGW. A more complex, nuanced dynamics should be highlighted here, with pronounced differences between hepatitis B, which is classically defined as an STI, and hepatitis C, where sexual transmission is much less frequent and parenteral transmission is pivotal. Beyond such differences between the transmission dynamics of different modalities of viral hepatitis, profound differences between successive age cohorts of people who did and did not benefit from hepatitis B vaccination as part of the regular vaccination against hepatitis B among children have been observed. The vaccination calendar that includes hepatitis B for children was fully implemented in Brazil as of the 1990s. The birth cohort effect has been documented by different Brazilian studies, carried out among several populations, for instance, among gay men.[14]
To the best of our knowledge, no comprehensive review has addressed the issue of viral hepatitis, among TGW worldwide, as has been the case in the field of HIV/AIDS. However, some studies have addressed the multiple burdens associated with female TGW who use multiple substances, among them injectable illicit substances as well as silicone. So far, the focus has been on different associated harms and HIV infection[15] and very scarce information is available about HCV infection.
Syphilis infection may potentiate HIV transmission, and high prevalence of syphilis has also been reported among TGW in Latin America.[16,17] HIV coinfection with viral hepatitis has also been reported among TGW, and those co-occurring, frequently synergic conditions have affected this population's morbidity and mortality.[18]
The increased HIV-vulnerability among TGW seems to be influenced by a variety of problems that reinforce their structural, social, and individual vulnerability.[4,5] Those aspects include family rejection, violation of the right to education and employment[19,20]; gender-based violence (GBV), including interpersonal, structural, and institutional violence[21]; lack of gender identity recognition[22]; discrimination faced in health systems/by health professionals[23,24]; and limited access to HIV prevention services, which presents a key limitation for reaching prevention goals and incorporating new prevention interventions.[9]
Although highly effective evidence-based HIV prevention strategies do exist, such as pre-exposure prophylaxis—PrEP, TGW seems to benefit less from those initiatives than other MARPs, such as men who have sex with men.[25,26] Despite much interest in and willingness to use PrEP among TGW worldwide, unfortunately the overall uptake remains low.[27,28] Frequent experiences of stigma and discrimination within health services may exert an untoward influence on this scenario, jeopardizing TGW access to and their putative benefit from PrEP.
Despite the high priority for HIV prevention among TGW, broader and specific epidemiological data on the prevalence and incidence of HIV among this population is needed to determine whether prevention strategies have worked or not, as well as to plan future efforts at the local and national level. TGW is considered a “hidden population,” as well as sex workers, men who have sex with men, and people who use drugs. These groups are often stigmatized, difficult to reach, often criminalized and cannot be accessed with traditional sampling methods.[29] Respondent-driven sampling (RDS) is been increasingly used in HIV-related studies among these populations.[30]
1.2. Methodological challenges toward a proper and accurate assessment
Several estimators have been proposed to produce theoretically unbiased population-based estimates for data collected within RDS studies.[31,32] However, despite on-going improvements in the available RDS estimators, debates continue, and there is no consensus on which estimator is more accurate.[33] As far as we are concerned, the RDS estimators available cannot directly be extended to a statistical regression model needed to measure associations and quantify risk factors for the infections. Hence, we propose a model-based estimator for RDS data which can easily be extended to a logistic regression.
In this study, we evaluate the performance of different RDS estimators in TGW inference of HIV, syphilis, HCV, and HBV prevalence, based on RDS surveys conducted in 12 Brazilian cities.
2. Methods
Between October 2016 and July 2017, an RDS study (“Divas Research”) was conducted in 12 Brazilian cities, namely Belém, Belo Horizonte, Brasília, Campo Grande, Curitiba, Fortaleza, Manaus, Porto Alegre, Recife, Rio de Janeiro, Salvador, and São Paulo. These sites were purposively selected by the Department of Surveillance, Prevention and Control of STIs, HIV/AIDS and Viral Hepatitis, Brazilian Ministry of Health. The study was sponsored by UNESCO (Project 914BRZ1138 BRAZIL AIDS-SUS).
2.1. Study population
TGW were recruited using RDS as a method to obtain a more robust and diverse sample of a hard-to-reach populations, which tends to be particularly sparse and marginalized.[29,30] TGW were eligible for the study if they self-identified themselves as transwomen, women, or other category different from the male sex designated on their birth certificate; were 18 years or older; reported spending most of their time at the selected city (living, studying, and/or working there); and received a valid study coupon. Each study participant was screened for study eligibility prior to enrollment.
Between 5 and 10 seeds diverse with respect to race/ethnicity, income, education, and age were selected as the initial recruits in each site. All participants received as a compensation $10 for recruiting peers who were eligible and enrolled into the study. For those who agreed to participate in the testing phase, Anti-HIV, Anti-Syphilis, Anti-HCV and HBsAg was offered through rapid testing.
As a rule, all patients were asked to perform rapid tests for the 4 infectious/diseases after reading and signing the Informed Consent Form (ICF), where the participant's right to refuse to take any test was guaranteed by the abovementioned ICF, in agreement with the protocol's evaluation by the FIOCRUZ IRB.
All patients received pre-test counseling, as mandated by the Brazilian legislation, describing the benefits of better knowing their health status and their right to referral to treatment for any condition made evident by the tests. On the other hand, also in agreement with the Brazilian legislation and the IRB recommendations, the putative risks associated with the testing procedures, such as the anxiety and distress that may be associated with the diagnosis of, say, to be infected by HIV or HCV were also highlighted, as well as their right to remain in the project irrespective of being tested for all diseases/infections.
2.2. Specimen collection and screening testing strategy
In order to obtain better safety and comfort for the study participant, as well as technical precision in performing the tests, we chose to collect peripheral blood in detriment of the digital puncture. The strategy also offers the advantage of avoiding a new collection procedure in case of invalid test and for the reactive samples that were sent for the respective confirmatory tests.
Peripheral blood was collected in tube with anticoagulant additive (BD Vacutainer PPT Plasma Preparation Tube, ref. 362788). The screening tests were performed from whole blood, following the procedures indicated by the manufacturers: Anti-HIV (BioEasy HIV test, ref. 03FK10), Anti-Syphilis (Alere Syphilis, ref. 06FK10), Anti-HCV (Alere HCV, ref. 02FK10) and HBsAg (VIKIA HBs Ag-Biomérieux). According to the Brazilian guidelines, the reactive samples in the first Anti-HIV test were submitted to a second rapid test (ABON HIV 1/2/ O Tri-Line). For each of the participating centers, a new, certified, and calibrated micropipette was provided, aiming for greater accuracy in the sample volume added.
2.3. Prevalence estimates
Prevalence for HIV, syphilis, HBV, and HCV was estimated using 3 different estimators. The first one is called here a “naïve estimator,” that is, a simple mean that assumes that all TGW are equally likely to participate in the study. The second estimator is the traditional RDS-II estimator.[34] The third estimator is a brand new model-based approach derived from a Bayesian logistic model with intercept only, which incorporates the sampling weights, provisionally named for the sake of the present study RDS-B. Weakly informative priors[35] were used and with given model output, we could obtain outcome prevalence by inverting the link function.
Since the data is an RDS sample,[34] each TGW has a sampling weight inversely proportional to her degree. For this purpose, we included normalized weights, such as the sum of them is equivalent to the sample size in the logistic model using the pseudo-posterior approach.[36]
There are 2 apparent advantages of using our proposed model-based Bayesian approach: priors—the weakly informative priors are recommended as the default choice of priors.[35] However, if the one which was chosen may have prior information about one outcome (prevalence), it is straightforward to add the informative prior into our model; modeling—the next step of analyzing RDS data is the search for risk factors that may be associated with the outcomes, and in a model-based approach, it is simple to add covariates and estimate odds ratios, taking into account the RDS weights.
All statistical analysis were implemented using R version 3.4.2 (R Core Team, 2017), comprising the libraries RDS[37] for the RDS-II estimates, and arm[38] for the model-based approach estimates.
2.4. Ethical considerations
The study protocol was submitted for review and approved by the Sergio Arouca National School of Public Health (ENSP/FIOCRUZ) Research Ethics Board (CAAE-49359415.9.0000.5240). Written informed consent was obtained from all participants, who could withdraw consent at any stage of the process or skip any questions perceived as too sensitive, personal, or distressing.
3. Results
3.1. Recruitment
RDS recruitment included between 5 and 10 seeds (Table 1) and all sites have chains that reached more than 4 waves (Fig. 1).
Table 1.
RDS recruitment, seeds, waves, and sample sizes by site, Brazil, 2016−2017.
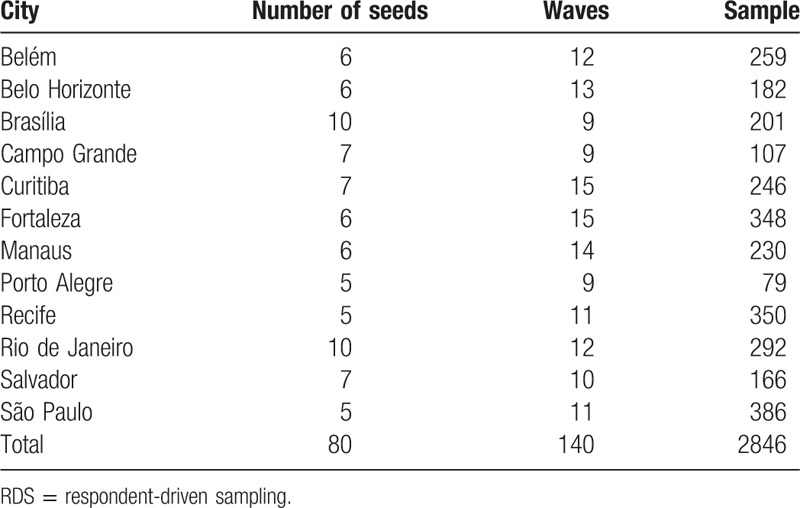
Figure 1.
Distribution of recruitment chains according to the number of recruitment waves.
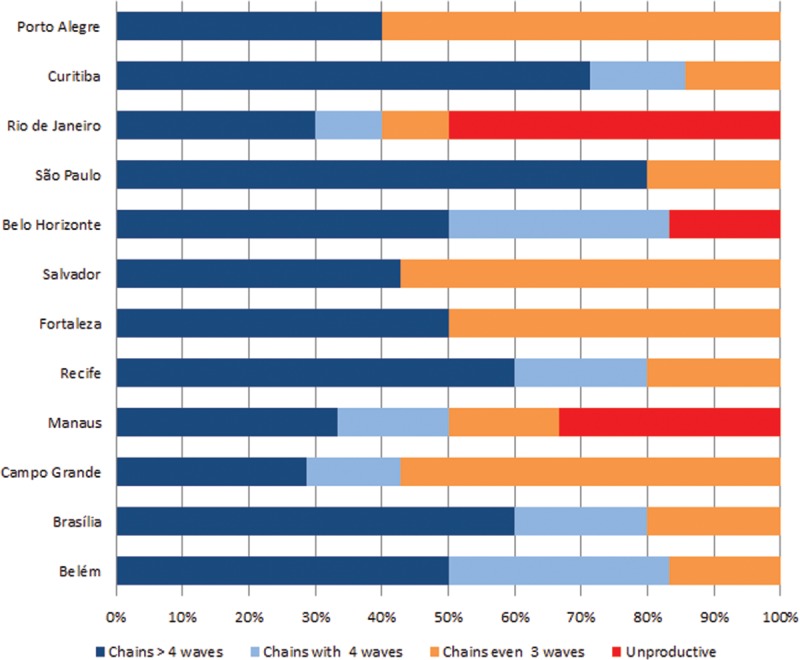
Recruitment network diagrams are shown in Figure 2, which depicts a great variability across sites. However, the majority of sites have successfully reached long or medium recruitment chains, comprising several waves. Seeds were successful at recruiting participants and generating chains with at least 3 waves in the majority of participants sites (n = 9), while in 3 cities, there were at least 1 “non-generative” (sterile) seed that recruited no participants. In Belo Horizonte, 1 seed was “non-generative,” whereas in Manaus 2 seeds and in Rio de Janeiro 5 seeds were unable to recruit participants.
Figure 2.
Recruitment networks according to research site.
São Paulo, Recife, and Curitiba had longer chains than the other sites, whereas Curitiba presented a chain with structural deformation (imposed by an external constraint, to be fully discerned by future studies). Rio de Janeiro and Manaus presented the higher proportion of very small chains, with <2 waves (Fig. 2), suggesting an underlying fragmented structure.
3.2. Social network characteristics
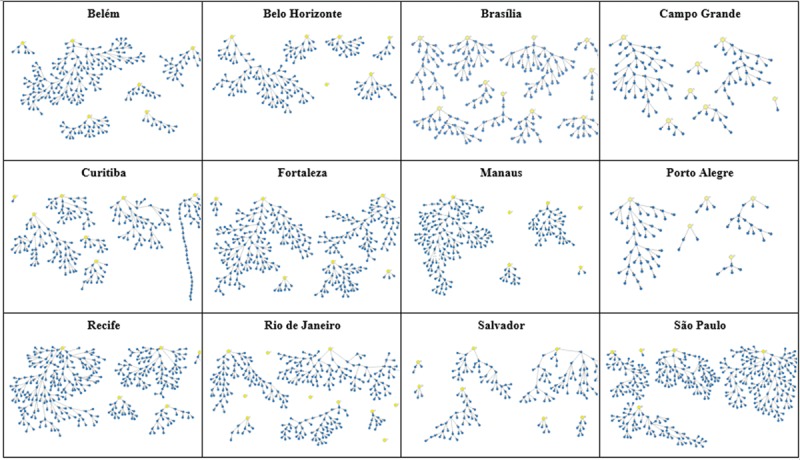
Two questions were used to assess network size: “How many transwomen do you know by name/nickname, who also know you by name/nickname and live/work/study at this city?” and “Of these, how many have you seen or spoken over phone, Facebook, or WhatsApp in the last 30 days?” There was a great variation for both outcomes within and across sites. Some participants answered that they did not know any other transwomen, while others reported knowing up to 999 transwomen by name/nickname—the average number of eligible members of individuals’ networks ranged from 54.9 to 121.9 in Recife and Rio de Janeiro, respectively (Fig. 3A). When we evaluated the network accessed within the last 30 days, the variability persisted, within and across sites, however, with lower averages, ranging from 17.7 in Curitiba to 40.0 in Rio de Janeiro (Fig. 3B).
Figure 3.
Network size per site, according to different questions. Top: using the question “How many transwomen do you know by name/nickname, who also know you by name/nickname and live/work/study at this city?”. Botton: using the question “Of these, how many have you seen or spoken over phone, Facebook, or WhatsApp in the last 30 days?”. This was the network size used in estimates.
3.3. Assessing RDS estimators
The point estimation and confidence/credibility intervals for HBV, HCV, HIV, and syphilis, according to different estimators (Naïve, RDS-II and RDS-B), are presented for each site in Figure 4.
Figure 4.
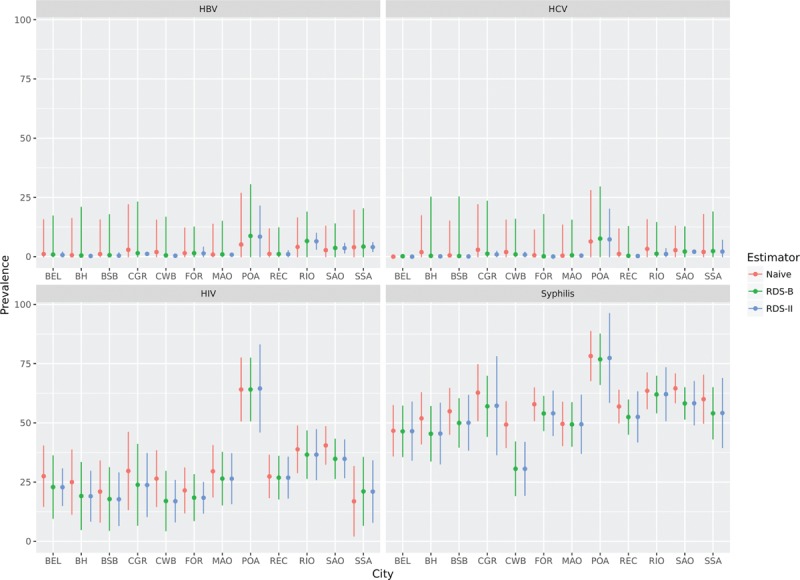
Point estimates and 95% confidence intervals for the three estimators according to research site (city) and outcome. BEL = Belm, BH = Belo Horizonte, BSB = Brasília, CGR = Campo Grande, CWB = Curitiba, FOR = Fortaleza, MAO = Manaus, POA = Porto Alegre, REC = Recife, RIO = Rio de Janeiro, SAO = São Paulo, SSA = Salvador.
HBV and HCV had very low prevalence across sites, whereas syphilis and HIV presented a relatively high prevalence. We identified higher prevalence for all the infections in the city of Porto Alegre (POA), especially syphilis and HIV. The underlying reasons that may explain such discrepancies need further investigation and will be discussed in future studies. By now, we should emphasize that Porto Alegre has the smallest number of recruitees, and statistical fluctuation is inherent in any statistical inference based on small numbers.
No matter the infection under analysis, the results from RDS-II and RDS-B tend to closely match each other, compared to their findings and those eventuating from Naïve estimates.
The congruence between results generated by RDS-II and RDS-B estimators is depicted in Figure 5. This figure presents Bland-Altman plots, comparing every single pair of estimates generated by different estimators. In addition to the mean differences when comparing the RDS-II and naïve estimators, the limits of agreement (LA) in this case are much wider than when comparing RDS-II and RDS-B. This means that not only the mean difference is higher but also the putative influence of random differences is more pronounced in the latter, which makes the results more difficult to predict. The right column of the Figure 5 makes evident the similarity of estimates based on RDS-II and RDS-B.
Figure 5.
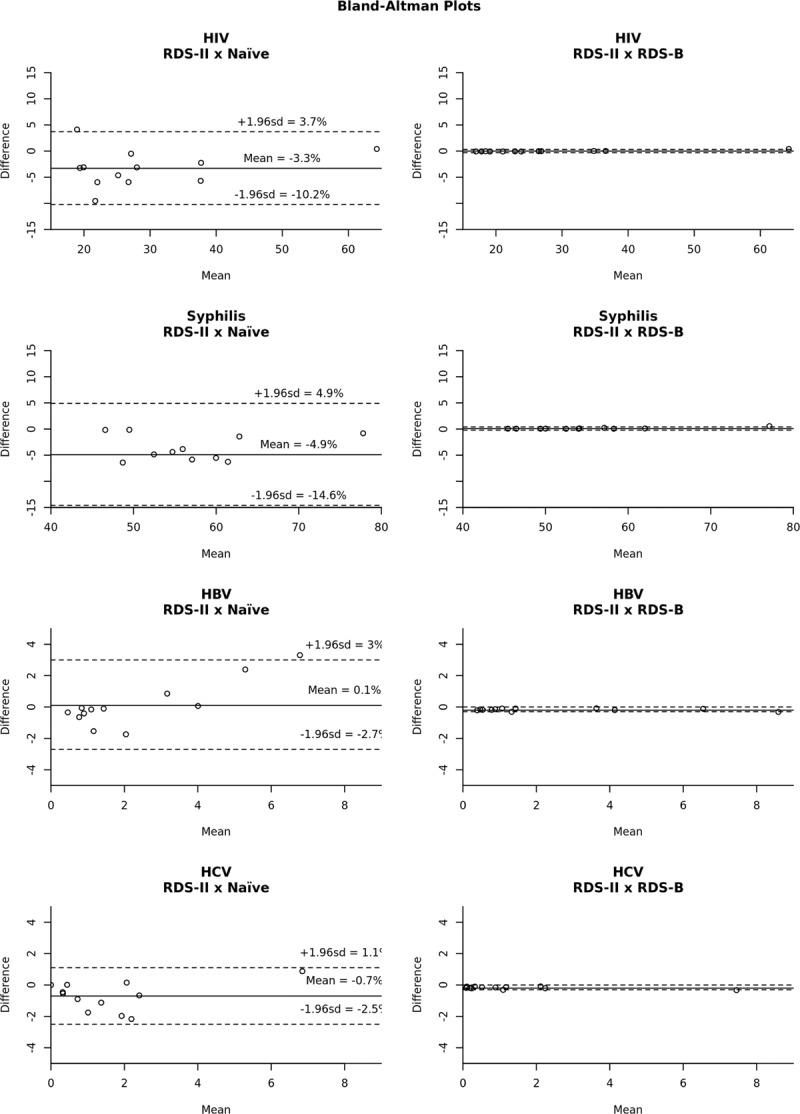
Bland-Altman plots comparing the assessment of differences between estimators. The differences of estimates for each pair of estimators is ploted against the mean of the same estimates. Solid lines are the mean differences, while traced lines represent the 95% confidence interval for the differences (limits of agreement). It is clear the concordance between RDS-II and RDS-B estimators, regardless of the outcome assessed.
4. Discussion
The present study focuses on the comparative performance of 3 different RDS estimators, which comprise an intuitive benchmark (the so-called naïve estimator), which does not take into account the underlying structure of networks, as well as some of the standard estimators used by the vast majority of RDS-based studies. Besides the cross-comparison of their performances in context (ie, taking in consideration real world findings), targeting a specific sparse and deeply stigmatized population (TGW), the study has proposed a brand new strategy based on Bayesian statistics as a new alternative to be putatively used in future studies targeting hard-to-reach populations.
In a simulation study carried out by our group,[39] we have found that the performance of RDS-II and naïve estimators tend to be similar when the underlying structure of networks approaches the random structure of networks emulating Erdös-Renyi's seminal description of random networks.
However, for different underlying network structures the naïve estimator tends to be biased.
Actual societies as well as social networks observed at the meso- and micro-levels are highly structured instead of sets defined by random interactions. In this sense, both simulation and empirical studies should take into consideration such underlying structures. Such structures are by no means negligible, respecting the performance of estimators, and in a broader sense, the accuracy of estimates of any hard-to-reach populations.
Real societies are clearly structured, making the initial assumptions of RDS as advanced by its originator, Douglas Heckathorn (such as the putative existence of a so-called “single-component,” putatively uniting all subsets of specific/local networks)[30,40] naïve versions of a much more complex and challenging reality. Whether first-degree Markov chains may or may not explain the observed findings. Classic adjustments (eg, homophily) might be complemented by innovative insights about the nature and the ways RDS-based studies could be better understood and interpreted.
The understanding of RDS as a Monte-Carlo Markov Chain,[41] as well as the proposition of new estimators, incorporating insights from Bayesian statistics seem to be useful alternatives to properly handle the current challenges facing the method and empirical challenges faced by several studies, such as those formerly carried out in Brazil.[42]
In the absence of any gold standard and the brand new findings about the complex and pervasive role of bottlenecks in any chain-referral study, diversity and flexibility of estimators are much needed. Brand new sampling and analytic strategies have been proposed to address the complex influence of such bottlenecks[43] or the entirely new methodological approach advanced.[44]
So far these approaches remain insightful theoretical approaches, but have not been implemented and/or used in the analyses of empirical studies, to the best of our knowledge. In the meantime, statisticians, computer scientists, and mathematicians, as well as those engaged in the practical aspects of study implementation, and analysis of empirical studies should do their best to gather as many evidences as possible to advance culturally sensitive public policies and to improve the consistence and validity of their assessments.
In this sense, the very high infection rates among TGW made evident by the present study, as well as by studies carried out all over the world (including Brazil) are of major concern. Evidently, sound and humane policies to ameliorate the life conditions and the health status of TGW are urgent, since current findings are simply unacceptable on ethical and humane grounds.
They seem to be the consequence of entrenched prejudice and dire conditions of life of this marginalized population. Notwithstanding, despite the evident fact that advocacy is the key and urgently needed, it is not enough. Sound empirical evidence and social mobilization should advance side-by-side and should mutually reinforce each other. The current article emphasizes methodological challenges and ways to ameliorate their untoward consequences. They must be complemented by future publications, targeting the full public health dimension of the findings, as well as the specificities of each one of the contexts under analysis.
Author contributions
Conceptualization: C.A. Velasco-de-Castro, C.F. Coutinho, J.C. Mota, L.S. Bastos, L.G. Toledo, M.S. Malta.
Data curation: D.R. Group.
Formal analysis: C.M. Santos, C.A. Velasco-de-Castro, J.C. Mota, L.S. Bastos, S.M. Brignol, S.L. Sperandei, T.S. Travassos.
Funding acquisition: C.A. Velasco-de-Castro, C.F. Coutinho, J.C. Mota, L.G. Toledo, M.S. Malta.
Investigation: L.G. Toledo, M.S. Malta.
Methodology: C.M. Santos, L.S. Bastos, S.M. Brignol, S.L. Sperandei, T.S. Travassos.
Project administration: C.F. Coutinho, D.R. Group, L.G. Toledo, M.S. Malta.
Software: C.M. Santos, L.S. Bastos, S.L. Sperandei, T.S. Travassos.
Supervision: C.A. Velasco-de-Castro, C.F. Coutinho, D.R. Group, J.C. Mota, L.S. Bastos, L.G. Toledo.
Validation: D.R. Group.
Writing – original draft: C.A. Velasco-de-Castro, L.S. Bastos, S.M. Brignol, S.L. Sperandei.
Writing – review & editing: C.F. Coutinho, D.R. Group, J.C. Mota, L.S. Bastos, L.G. Toledo, M.S. Malta.
Footnotes
Abbreviations: MARPs = most-at-risk populations, RDS = respondent-driven sampling, STI = sexually transmitted infection, TGW = transgender women.
Divas Research Group: The full list of the PIs of each one of the 12 sites, as well as the scientific coordinators of each subarea, is available at the end of the manuscript (It can be displayed as a web appendix, at the editors’ discretion).
Funding financial support for this study was provided by Brazilian Ministry of Health, through its Secretariat for Health Surveillance and its Department of Prevention, Surveillance and Control of Sexually Transmitted Infections, HIV/AIDS and Viral Hepatitis.
This study was carried out with data provided by the Department of Surveillance, Prevention and Control of Sexually Transmitted Infections, HIV/AIDS and Viral Hepatitis, of the Secretariat for Health Surveillance of the Ministry of Health.
The authors report no conflicts of interest.
References
- [1].Joint United Nations Programme on HIV/AIDS (UNAIDS), 2017. Available at: http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf Accessed October 19, 2017. [Google Scholar]
- [2].Joo J, Lebowitz JL. Behavior of susceptible-infected-susceptible epidemics on heterogeneous networks with saturation. Phys Rev E Stat Nonlin Soft Matter Phys 2004;69(part 2):066105. [DOI] [PubMed] [Google Scholar]
- [3].Das MK, Prajapati BK, Tiendrebeogo RW, et al. Malaria epidemiology in an area of stable transmission in tribal population of Jharkhand, India. Malar J 2017;16:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Poteat TC, Keatley J, Wilcher R, et al. Evidence for action: a call for the global HIV response to address the needs of transgender populations. J Int AIDS Soc 2016;19(3 suppl 2):21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reisner SL, Poteat T, Keatley J, et al. Global health burden and needs of transgender populations: a review. Lancet 2016;388:412–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baral SD, Poteat T, Strömdahl S, et al. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:214–22. [DOI] [PubMed] [Google Scholar]
- [7].Joint Programme on HIV/AIDS (UNAIDS). The Gap Report 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf Accessed October 19, 2017. [Google Scholar]
- [8].Operario D, Soma T, Underhill K. Sex work and HIV status among transgender women: systematic review and meta-analysis. J Acquir Immune Defic Syndr 2008;48:97–103. [DOI] [PubMed] [Google Scholar]
- [9].Silva-Santisteban A, Eng S, de la Iglesia G, et al. HIV prevention among transgender women in Latin America: implementation, gaps and challenges. J Int AIDS Soc 2016;19(suppl 2):20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grinsztejn B, Jalil EM, Monteiro L, et al. Transgender Study Team. Unveiling of HIV dynamics among transgender women: a respondent-driven sampling study in Rio de Janeiro, Brazil. Lancet HIV 2017;4:e169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Costa AB, Fontanari AM, Jacinto MM, et al. Population-based HIV prevalence and associated factors in male-to-female transsexuals from Southern Brazil. Arch Sex Behav 2015;44:521–4. [DOI] [PubMed] [Google Scholar]
- [12].Beyrer C, Baral SD, Collins C, et al. The global response to HIV in men who have sex with men. Lancet 2016;388:198–206. [DOI] [PubMed] [Google Scholar]
- [13].Anderson PL, Glidden DV, Liu A, et al. iPrEx Study Team. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012;4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oliveira MP, Matos MA, Silva ÁM, et al. Prevalence, risk behaviors, and virological characteristics of hepatitis B virus infection in a group of men who have sex with men in Brazil: results from a respondent-driven sampling survey. PLoS One 2016;11:e0160916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Benotsch EG, Zimmerman RS, Cathers L, et al. Non-medical use of prescription drugs and HIV risk behaviour in transgender women in the Mid-Atlantic region of the United States. Int J STD AIDS 2016;27:776–82. [DOI] [PubMed] [Google Scholar]
- [16].Solomon MM, Mayer KH, Glidden DV, et al. iPrEx Study Team. Syphilis predicts HIV incidence among men and transgender women who have sex with men in a preexposure prophylaxis trial. Clin Infect Dis 2014;59:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fernandes FR, Zanini PB, Rezende GR, et al. Syphilis infection, sexual practices and bisexual behaviour among men who have sex with men and transgender women: a cross-sectional study. Sex Transm Infect 2015;91:142–9. [DOI] [PubMed] [Google Scholar]
- [18].Poteat T, Scheim A, Xavier J, et al. Global epidemiology of HIV infection and related syndemics affecting transgender people. J Acquir Immune Defic Syndr 2016;72(suppl 3):S210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wilson EC, Garofalo R, Harris RD, et al. Transgender Advisory Committee and the Adolescent Medicine Trials Network for HIV/AIDS Interventions. Transgender female youth and sex work: HIV risk and a comparison of life factors related to engagement in sex work. AIDS Behav 2009;13:902–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sterzing PR, Ratliff GA, Gartner RE, et al. Social ecological correlates of polyvictimization among a national sample of transgender, genderqueer, and cisgender sexual minority adolescents. Child Abuse Negl 2017;67:1–2. [DOI] [PubMed] [Google Scholar]
- [21].Lyons T, Krüsi A, Pierre L, et al. Negotiating violence in the context of transphobia and criminalization: the experiences of trans sex workers in Vancouver, Canada. Qual Health Res 2017;27:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Khan SI, Hussain MI, Parveen S, et al. Living on the extreme margin: social exclusion of the transgender population (hijra) in Bangladesh. J Health Popul Nutr 2009;27:441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Poteat T, German D, Kerrigan D. Managing uncertainty: a grounded theory of stigma in transgender health care encounters. Soc Sci Med 2013;84:22–9. [DOI] [PubMed] [Google Scholar]
- [24].Collier R. Addressing transgender discrimination in health. CMAJ 2015;187:E493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Deutsch MB, Glidden DV, Sevelius J, et al. iPrEx investigators. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV 2015;2:e512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Grant RM, Anderson PL, McMahan V, et al. iPrEx Study Team. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014;14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marshall BD, Mimiaga MJ. Uptake and effectiveness of PrEP for transgender women. Lancet HIV 2015;2:e502–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wilson EC, Jin H, Liu A, et al. Knowledge, indications and willingness to take pre-exposure prophylaxis among transwomen in San Francisco, 2013. PLoS One 2015;10:e0128971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Magnani R, Sabin K, Saidel T, et al. Review of sampling hard-to-reach and hidden populations for HIV surveillance. AIDS 2005;19(suppl 2):S67–72. [DOI] [PubMed] [Google Scholar]
- [30].Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl 1997;44:174–99. [Google Scholar]
- [31].Salganik MJ, Heckathorn D. Sampling and estimation in hidden populations using respondent-driven sampling. Sociol Methodol 2004;34:193–240. [Google Scholar]
- [32].Gile KJ. Improved inference for respondent-driven sampling data with application to HIV prevalence estimation. J Am Stat Assoc 2011;106:135–46. [Google Scholar]
- [33].Salganik MJ. Commentary: respondent-driven sampling in the real world. Epidemiology 2012;23:148–50. [DOI] [PubMed] [Google Scholar]
- [34].Volz E, Heckathorn DD. Probability based estimation theory for respondent driven sampling. J Off Stat 2008;24:79–97. [Google Scholar]
- [35].Gelman A, Jakulin A, Pittau MA, et al. A weakly informative default prior distribution for logistic and other regression models. Ann Appl Stat 2008;2:1360–83. [Google Scholar]
- [36].Savitsky TD, Toth D. Bayesian estimation under informative sampling. Electron J Statist 2016;10:1677–708. [Google Scholar]
- [37].Handcock MS, Fellows IE, Gile KJ. (2012) RDS: Respondent-Driven Sampling, Version 0.8-1. Project home page at http://hpmrg.org, URL https://CRAN.R-project.org/package=RDS. Accessed December 10, 2017. [Google Scholar]
- [38].Gelman A, Su Y-S. arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. R package version 1.8-5, 2015. [Google Scholar]
- [39].Sperandei S, Bastos LS, Ribeiro-Alves M, et al. Assessing respondent-driven sampling: a simulation study across different networks. Social Networks 2018;52:48–55. [Google Scholar]
- [40].Heckathorn DD. Snowball versus respondent-driven sampling. Sociol Methodol 2011;41:355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Khabbazian M, Hanlon B, Russek Z, et al. Novel sampling design for respondent-driven sampling. Available at: https://arxiv.org/abs/1606.00387 [accessed on October 24, 2017]. [Google Scholar]
- [42].Toledo L, Codeço CT, Bertoni N, et al. Brazilian Multicity Study Group on Drug Misuse. Putting respondent-driven sampling on the map: insights from Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr 2011;57(suppl 3):S136–43. [DOI] [PubMed] [Google Scholar]
- [43].Goel S, Salganik MJ. Respondent-driven sampling as Markov chain Monte Carlo. Stat Med 2009;28:2202–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Berchenko Y, Rosenblatt J, Frost, SDW. Modeling and analysing respondent driven sampling as a counting process. Available at: https://arxiv.org/abs/1304.3505 [accessed on October 24, 2017]. [Google Scholar]


