Abstract
Background
Size mismatch between donor lungs and a recipient thorax could affect the major determinants of maximal expiratory airflow: airway resistance, propensity of airways to collapse, and lung elastic recoil.
Methods
A retrospective review of 159 adults who received bilateral lung transplants was performed. The predicted total lung capacity (pTLC) for donors and recipients was calculated based on sex and height. Size matching was represented using the following formula: pTLC ratio = donor pTLC / recipient pTLC. Patients were grouped according to those with a pTLC ratio > 1.0 (oversized) or those with a pTLC ratio ≤ 1.0 (undersized). Allograft function was analyzed in relation to the pTLC ratio and to recipient and donor predicted function.
Results
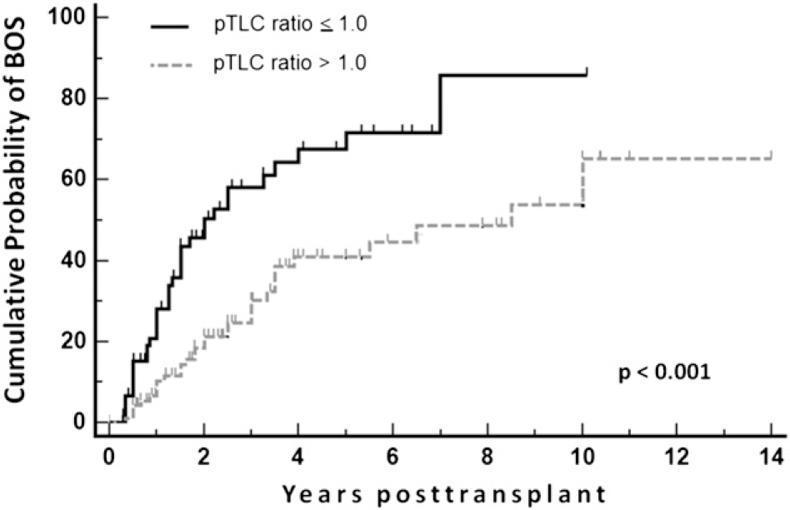
The 96 patients in the oversized cohort had a mean pTLC ratio of 1.16 ± 0.13 vs 0.89 ± 0.09 in the 63 patients of the undersized group. At 1 to 6 months posttransplant, the patients in the oversized cohort had higher FEV1/FVC ratios (0.895 ± 0.13 vs 0.821 ± 0.13, P < .01) and lower time constant estimates of lung emptying (0.38 ± 0.2 vs 0.64 ± 0.4, P < .01) than patients in the undersized cohort. Although the FVCs expressed as % predicted for the recipient were not different between cohorts, the FVCs expressed as % predicted for the donor organ were lower in the oversized cohort compared with the undersized cohort (at 1-6 months, 52.4% ± 17.1% vs 65.3% ± 18.3%, P < .001). Kaplan-Meier estimates for the occurrence of bronchiolitis obliterans syndrome (BOS) showed that patients in the oversized cohort had a lower probability of BOS (P < .001).
Conclusions
A pTLC ratio > 1.0, suggestive of an oversized allograft, is associated with higher expiratory airflow capacity and a less frequent occurrence of BOS.
Abbreviations
- BLT
bilateral lung transplant
- BOS
bronchiolitis obliterans syndrome
- CWS
chest wall strapping
- ERV
expiratory reserve volume
- FRC
functional residual capacity
- FVL
flow volume loop
- HLT
heart-lung transplant
- HR
hazard ratio
- pTLC
predicted total lung capacity
- RV
residual volume
- SUPRA pattern
supranormal expiratory airflow
- TLC
total lung capacity
- VC
vital capacity
- Ve50
expiratory airflow at 50% of the vital capacity
Size mismatch between donor lungs and a recipient thorax could affect the major determinants of maximal expiratory airflow: airway resistance, propensity of airways to collapse, and lung elastic recoil. 1 The degree of mismatch can be estimated by the ratio of predicted total lung capacities (pTLCs), using the following formula: pTLC ratio = donor pTLC / recipient pTLC.2, 3, 4 In a previous study, a flow volume loop (FVL) pattern in patients who received bilateral lung transplants (BLTs) that was characterized by supranormal expiratory airflow (SUPRA pattern) was associated with high pTLC ratios (likely the result of oversized allografts). 5 Although patients with and without the SUPRA pattern had comparable FVC and FEV1, expressed as % predicted for the recipient, there were significant differences in expiratory airflow (FEV1/FVC ratios and time constant estimates for lung emptying). A mismatch of oversized transplanted lungs that are restricted in recipients with a smaller thorax is conceptually similar to restriction of the chest wall by strapping, which causes increased elastic recoil of the lung.6, 7, 8, 9, 10 Lung size mismatch could also influence expiratory airflow through dysanapsis (interindividual differences in the relationship of lung volume and airway size). 11 In this study, we sought to evaluate the relationship of the pTLC ratio with allograft function. By convention, allograft function is related to predicted values for the recipient.3, 12, 13 However, expressing allograft function in relation to predicted values of the donor might better reflect allograft physiology. In this investigation, we characterized allograft function in relation to the predicted values of both the recipient and the donor.
Materials and Methods
This study was approved by the institutional review boards at the sites involved (e-Appendix 1A). We analyzed all BLTs for adult patients performed at Johns Hopkins Hospital from January 1, 1996, to March 1, 2010, and all BLTs performed at Inova Fairfax Hospital from January 1, 1996, to December 31, 2008. Recipients of single lung transplants were not assessed because of the potential effect of the native lung on overall lung function. All adult patients who received BLTs and were alive 3 months after transplantation with information available to calculate donor and recipient pTLC were included in this study. The pTLCs of all donors and recipients were calculated using previously described regression equations. 2
Patients were grouped according to those with a pTLC ratio > 1.0 (oversized) or ≤ 1.0 (undersized). All patients included had spirometric assessments performed, including FVLs and fiberoptic bronchoscopies, according to institutional lung transplant protocols (e-Appendix 1B). A diagnosis of bronchiolitis obliterans syndrome (BOS) and acute rejection was determined according to the standard International Society for Heart and Lung Transplantation criteria. 14 The onset of BOS was defined as an otherwise unexplained decrease of > 20% from the best posttransplant FEV1. Airway complications were defined as anastomotic or lower airway stenosis or bronchomalacia that required dilatation, stent implantation, laser therapy, or bronchoscopic follow-up.
Analysis of Allograft Function
Posttransplant pulmonary function tests were analyzed at intervals of 1 to 6 months, 6 to 12 months, 12 to 24 month, and 24 to 36 months. If multiple pulmonary function tests were available for a time interval, the one with the highest FVC was included. Two time constant estimates (τ* = FVC / [2 × Ve50], where Ve50 is expiratory airflow at 50% of the vital capacity; τ** = −1 / ln[1 − FEV1/FVC]), and a FVL slope estimate (Ve50 / [0.5 × FVC]) were calculated. 15 Allograft function was related to predicted values for both recipient and donor, derived from regression equations.16, 17 For each patient, the FVL with the highest FEV1/FVC ratio was selected (best FVL).
Statistical Analysis
Results were expressed as mean ± SD, counts, and percentages. Comparisons between groups were made using an analysis of variance one-way analysis or Fisher exact test, as appropriate. Least squares regression models were constructed using the JMP software program (www.jmpdiscovery.com) to relate the pTLC ratio to pulmonary function and to occurrence of BOS. We evaluated the impact of the pTLC ratio on the occurrence of BOS using univariable and multivariable Cox proportional hazards models and Kaplan-Meier estimates (the end of the follow-up period was diagnosis of BOS, time of death, or August 1, 2010, whatever took place first). Independent variables identified as significantly associated with the occurrence of BOS in the univariable model were included into a multivariable model. A P value < 0.05 was considered significant.
Results
Characteristics of the Stud
There were 159 adult patients who qualified for the analysis, of whom 154 received a BLT and five a heart-lung transplant (HLT). Donor and recipient characteristics are shown in Table 1. The follow-up period was completed at time of death or August 1, 2010, with a median follow-up period of 2.6 years (range, 0.3-14.2 years). The dataset included 3,783 FVL observations. The mean pTLC ratio for the study population was 1.06 ± 0.17. Patients who received transplants for COPD had the highest mean pTLC ratio (1.11 ± 0.18), and patients who received transplants for idiopathic pulmonary fibrosis had the lowest (0.98 ± 0.16) (Table 1, e-Table 1). The 96 recipients in the oversized cohort had a mean pTLC ratio of 1.16 ± 0.13. The 63 recipients in the undersized cohort had a mean pTLC ratio of 0.89 ± 0.09 (Table 2, e-Fig 1).
Table 1.
Recipient and Donor Characteristics of the Study Population
| Parameter | No. | % or SD |
|---|---|---|
| No. | 159 | 100% |
| Recipient demographics | ||
| Sex, M(F) | 77(82) | 48%(52%) |
| Age, mean, y | 44.0 | 13.9 |
| Height, mean, m | 1.68 | 0.09 |
| pTLC, mean, L | 5.81 | 1.08 |
| Donor demographics | ||
| Sex, M(F) | 92(67) | 57%(43%) |
| Age, mean, y | 38.9 | 13.4 |
| Height, mean, m | 1.70 | 0.11 |
| pTLC, mean, L | 6.08 | 1.15 |
| Donor-recipient size matching | ||
| pTLC ratio | 1.06 | 0.17 |
| pTLC absolute difference, L | 0.26 | 0.95 |
The pTLC of each donor and recipient was calculated using the regression equations recommended by the American Thoracic Society and the European Respiratory Society. 1 F = female; M = male; pTLC = predicted total lung capacity.
Table 2.
Recipient and Donor Characteristics for pTLC Ratio ≤ 1.0 (Undersized Cohort) and pTLC Ratio > 1.0 (Oversized Cohort)
| pTLC ratio ≤ 1.0 Cohort |
pTLC ratio > 1.0 Cohort |
||||
|---|---|---|---|---|---|
| Characteristics | No. | % or SD | No. | % or SD | P Value |
| No. | 63 | 40% | 96 | 60% | … |
| Recipient demographics | |||||
| Male | 39 | 62% | 38 | 39% | .008 |
| Female | 24 | 38% | 58 | 61% | .008 |
| Age, mean, y | 42.3 | 13.1 | 44.9 | 13.9 | NS |
| Height, mean, m | 1.71 | 0.10 | 1.66 | 0.09 | < .001 |
| pTLC, mean, L | 6.17 | 1.13 | 5.58 | 1.00 | < .001 |
| Donor demographics | |||||
| Male | 27 | 43% | 64 | 67% | .003 |
| Female | 36 | 57% | 32 | 33% | .003 |
| Age, mean, y | 41.2 | 13.9 | 37.4 | 12.8 | NS |
| Height, mean, m | 1.65 | 0.11 | 1.74 | 0.09 | < .001 |
| pTLC, mean, L | 5.52 | 1.09 | 6.43 | 1.03 | < .001 |
| Transplant indication | |||||
| Cystic fibrosis | 16 | 30% | 19 | 31% | NS |
| COPD | 8 | 19% | 19 | 24% | NS |
| Pulmonary hypertension | 4 | 8% | 3 | 8% | NS |
| Sarcoidosis | 10 | 19% | 11 | 15% | NS |
| Interstitial lung disease | 5 | 14% | 10 | 10% | NS |
| Congenital heart disease | 1 | 1% | 4 | 4% | NS |
| Other | 5 | 8% | 8 | 9% | NS |
| Donor-recipient size pairing | |||||
| pTLC ratio | 0.89 | 0.09 | 1.16 | 0.13 | < .001 |
| pTLC difference, L | −0.64 | 0.61 | 0.85 | 0.61 | < .001 |
| Height ratio | 0.97 | 0.04 | 1.05 | 0.04 | < .001 |
| Height difference, m | −0.05 | 0.08 | 0.07 | 0.07 | < .001 |
NS = not significant. See Table 1 legend for expansion of the other abbreviation.
Lung-Thorax Size Mismatch and Allograft Function
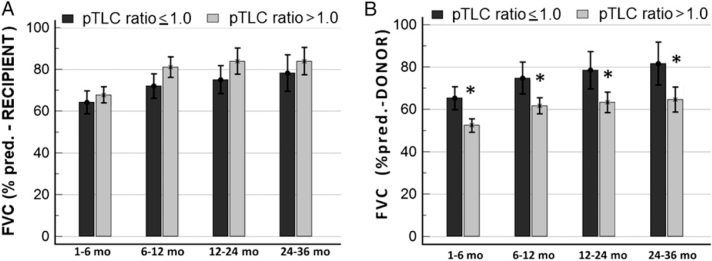
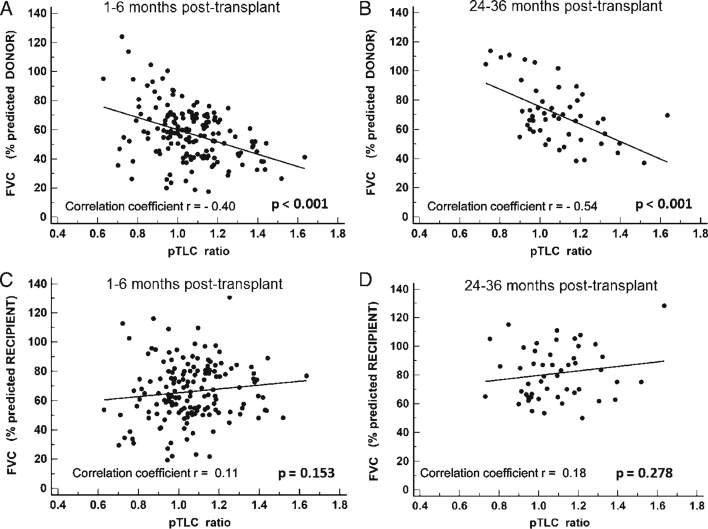
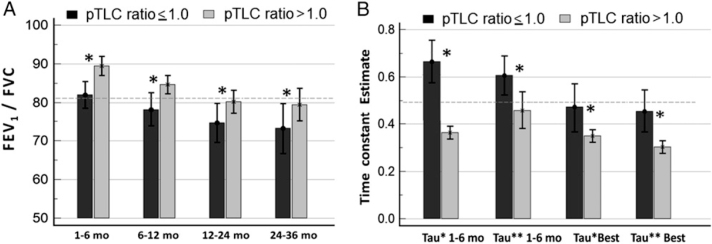
At 1 to 6 months, absolute values of FVC and FVC expressed as a percentage of recipient predicted FVC (% predicted-recipient) did not differ between groups (Fig 1A, Table 3). However, the FVCs expressed as a percentage of the donor predicted FVC (% predicted-donor) were significantly smaller in the oversized cohort at each time point (Fig 1B). At 1 to 6 months, the FVC (% predicted-donor) was 52.4% ± 17.1% in the oversized cohort and 65.3% ± 18.3% in the undersized cohort (P < .001). The FVC (% predicted-donor) was significantly related to the pTLC ratio (P < .001) (Figs 2A, 2B). The FVC (% predicted-recipient) was not significantly related to the pTLC ratio (Figs 2C, 2D). Absolute values of FEV1 were comparable between cohorts, however FEV1 (% predicted-recipient) was higher in the oversized cohort (e-Figs 2A, 2B). Ve50s, FEV1/FVC ratios, and FVL slope estimates were higher and the time constant estimates of lung emptying, τ* and τ**, were lower in the oversized cohort compared with the undersized cohort (Fig 3, Table 3). The FVL with the highest FEV1/FVC ratio in the patients in the oversized cohort demonstrated significantly higher expiratory flow capacity in all FVL parameters in comparison with patients in the undersized cohort (FEV1/FVC ratio, 0.935 ± 0.06 vs 0.871 ± 0.11; Ve50, 4.4 ± 1.7 L/s vs 3.3 ± 1.5 L/s; τ*, 0.30 ± 0.1 s vs 0.46 ± 0.4 s; τ**, 0.34 ± 0.1 s vs 0.49 ± 0.3 s; P < .01 for all).
Figure 1.
Chart shows FVC over time in cohorts with pTLC ratio ≤ 1.0 and pTLC-ratio > 1.0. A, FVC shown as % predicted for the recipient. B, FVC shown as % predicted for the donor. * = significant difference (P < .05). pred. = predicted; pTLC = predicted total lung capacity.
Table 3.
Allograft Function at the 1-Month to 6-Month Interval for pTLC Ratio ≤ 1.0 (Undersized Cohort) and pTLC Ratio > 1.0 (Oversized Cohort)
| Allograft Function at 1–6 Months | pTLC Ratio ≤ 1.0 Cohort | pTLC Ratio > 1.0 Cohort | P Value |
|---|---|---|---|
| Spirometry | |||
| FVC, L | 2.61 (1.0) | 2.54 (0.9) | .45 |
| FVC (% predicted-recipient) | 64.1 (21) | 67.7 (19) | .56 |
| FVC (% predicted-donor) | 65.3 (21.5) | 52.4 (14.7) | < .001 |
| FEV1, L | 2.06 (0.8) | 2.21 (0.8) | .33 |
| FEV1 (% predicted-recipient) | 61.4 (23.1) | 71.8 (27) | .024 |
| FEV1 (% predicted-donor) | 65.2 (18.9) | 59.6 (15.7) | .61 |
| FEV1/FVC ratio | 0.820 (0.13) | 0.895 (0.13) | < .001 |
| FVL | |||
| Ve50, L/s | 3.01 (1.5) | 4.10 (1.9) | < .001 |
| Ve50 (% predicted-recipient) | 49.9 (26) | 73.4 (31) | < .001 |
| Ve50 (% predicted-donor) | 56.5 (31) | 64.4 (28) | .1 |
| Ve50/FVC, s | 1.28 (0.8) | 1.74 (0.8) | .001 |
| Vi50, L/s | 3.87 (1.4) | 3.60 (1.4) | .31 |
| Vi50/FVC, s | 1.67 (0.6) | 1.58 (0.5) | .43 |
| Ve50/Vi50 | 0.87 (0.6) | 1.28 (0.7) | .03 |
| Time constant estimate τ* | 0.642 (0.45) | 0.379 (0.28) | < .001 |
| Time constant estimate τ** | 0.607 (0.32) | 0.427 (0.27) | < .001 |
| Slope estimate | 2.62 (1.6) | 3.54 (1.6) | < .001 |
Data shown as No. (SD). FVL = flow volume loop; Ve50 = expiratory airflow at 50% of the vital capacity; Vi50 = inspiratory airflow at 50% of the vital capacity. See Table 1 legend for expansion of other abbreviation.
Figure 2.
Charts show the relationships between lung size mismatch (pTLC ratio) and limitation of lung inflation as expressed by FVC as % predicted for donor or recipient. A, linear regression analysis between pTLC ratio and FVC (% predicted-donor) at 1-month to 6-month interval (n = 155; P < .001). B, linear regression analysis between pTLC ratio and FVC (% predicted-donor) at 24-month to 36-month interval (n = 50; P < .001). C, linear regression analysis between pTLC ratio and FVC (% predicted-recipient) at 1-month to 6-month interval (n = 155; P = .153). D, linear regression analysis between pTLC ratio and FVC (% predicted-recipient) at 24-month to 36-month interval (n = 50; P = .278). See Figure 1 legend for expansion of abbreviation.
Figure 3.
Graphs show the parameters of expiratory flow capacity according to pTLC ratio ≤ 1.0 (undersized cohort) and pTLC ratio > 1.0 (oversized cohort). A, FEV1/FVC ratio (dashed line represents the predicted FEV1/FVC ratio). B, time constant estimates (τ* = FVC / [2 × Ve50], where Ve50 is expiratory airflow at 50% of the vital capacity; τ** = −1 / ln[1 − FEV1/FVC]) (dashed line represents the predicted time constant estimate). * = significant difference (P < .05). See Figure 1 legend for expansion of abbreviation.
Determinants of Expiratory Airflow
Higher pTLC ratios were associated with higher Ve50 (% predicted-recipient; P = .001), higher FEV1/FVC ratios (P = .01), and lower time constant estimates τ* (P = .0003) and τ** (P = .001) (e-Fig 3). Expiratory flows were normalized by expressing them as a fraction of FVC (Ve50/FVC). The Ve50/FVC was higher in the oversized cohort compared with the undersized cohort (at 1-6 months, 1.74 ± 0.8/s vs 1.28 ± 0.8/s, P < .01) (e-Fig 2). The normalized mid-vital capacity inspiratory flows did not differ between the oversized and undersized cohorts (P > .1 for all comparisons) (e-Fig 2D).
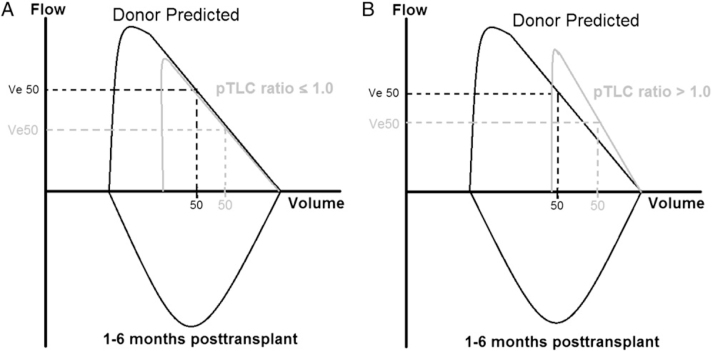
We constructed schematic FVLs for the interval of 1 to 6 months from mean values for the oversized and undersized cohorts and related these to predicted normal mean values for the donor organs (Fig 4). In the undersized cohort at any given lung volume, expiratory flows were comparable to donor predicted flows. However expiratory flows were higher than predicted for the donor organ in the oversized cohort. The slope estimates for the oversized and undersized cohorts were significantly different (3.54 ± 1.66 vs 2.62 ± 1.66; P < .001).
Figure 4.
Charts show schematic flow volume loops. A, pTLC ratio ≤ 1.0 (undersized cohort). B, pTLC ratio > 1.0 (oversized cohort). Data derived from predicted donor values and measured mean values for the 1-month to 6-month interval. Residual volume is assumed unchanged by transplantation. See Figure 1, Figure 3 legends for expansion of other abbreviations.
Lung-Thorax Size Mismatch and BOS
The median follow-up period for the study population was 2.6 years (range, 0.3-14.2 years). BOS occurred in 68 of 159 patients (42%), on average 2.1 years after transplantation (95% CI, 1.6-2.6). The patients who developed BOS had a mean pTLC ratio of 1.01 (95% CI, 0.97-1.05), whereas patients not affected by BOS had a mean pTLC ratio of 1.09 (95% CI, 1.05-1.13; P = .015). In least square regression analysis, a higher pTLC ratio predicted a lower probability for the occurrence of BOS (P = .0125) (e-Fig 4). Patients in the oversized cohort tended to have longer median follow-up periods (2.77 vs 2.34 years for those in the undersized cohort; P = .06). Thirty-four percent of patients in the oversized cohort and 56% of patients in the undersized cohort received a diagnosed of BOS (P = .006) (Table 4). The BOS-free interval prior to diagnosis tended to be longer in patients in the oversized cohort than in patients in the undersized cohort (2.51 ± 2.46 years vs 1.63 ± 1.44 years; P = .07). Patients in the oversized cohort were less likely to have BOS at any time point (P = .001) (Fig 5). The proportion of patients in the oversized and undersized cohorts who experienced acute rejection did not differ significantly (41.7% vs 39.7%; P = .69). In univariable Cox proportional hazard regression models oversizing (pTLC ratio > 1.0; hazard ratio [HR], 0.42; P = .015), the presence of acute rejection (HR, 2.9; P = .0005) and parameters of expiratory flow (FEV1/FVC-ratio, P = .01; Ve50, P = .008; Ve50 [% predicted-recipient], P = .002; time constant estimates, τ* and τ**, both P < .0001) were associated with the occurrence of BOS, (Table 5). After controlling for acute rejection, oversizing (pTLC ratio > 1.0) remained significantly associated with reduced occurrence of BOS (HR, 0.44; 95% CI, 0.25-0.79; P = .007) (Table 5).
Table 4.
Clinical Outcomes for pTLC Ratio ≤ 1.0 (Undersized Cohort) and pTLC Ratio > 1.0 Oversized Cohort
| pTLC Ratio ≤ 1.0 Cohort |
pTLC Ratio > 1.0 Cohort |
||||
|---|---|---|---|---|---|
| Clinical Outcome | n = 63 | % or SD | n = 96 | % or SD | P Value |
| Median follow-up, y | 2.34 | … | 2.77 | … | .06 |
| BOS | 35 | 56% | 33 | 34% | .006 |
| Time to BOS, y | 1.63 | 1.44 | 2.51 | 2.4 | .07 |
| Median BOS-free time, y | 2 | … | 8.2 | … | .0001 |
| Acute rejection | 25 | 39.7% | 40 | 41.7% | .8 |
| Airway complications | 19 | 30.2% | 16 | 16.8% | .05 |
BOS = bronchiolitis obliterans syndrome.
Figure 5.
Chart shows Kaplan-Meier estimates of proportion of patients with BOS, according to pTLC ratio ≤ 1.0 (undersized cohort) and pTLC-ratio > 1.0 (oversized cohort). Comparison between cohorts via log-rank test. BOS = bronchiolitis obliterans syndrome. See Figure 1 legend for expansion of other abbreviation.
Table 5.
Univariable and Multivariable Cox Proportional Hazards Models of Risk Factors for BOS
| Model | HR | 95% CI | P Value |
|---|---|---|---|
| Univariable | |||
| pTLC ratio, > 1.0 | 0.49 | 0.27–0.86 | .015 |
| Acute rejection | 2.92 | 1.59–5.34 | .0005 |
| Airway complication | 1.18 | 0.59–2.31 | .63 |
| Transplant indication: LAS group | |||
| Group A (obstructive disease) | 0.84 | 0.41–1.68 | .62 |
| Group B (pulmonary vascular disease) | 0.52 | 0.16–1.65 | .26 |
| Group C (cystic fibrosis) | 1.00 | 0.52–1.89 | .99 |
| Group D (restrictive disease) | 1.56 | 0.87–2.77 | .13 |
| FVL at 1–6 mo | |||
| FVC, L | 1.23 | 0.88–1.71 | .21 |
| FVC (% predicted-recipient), 1% | 1.01 | 0.98–1.02 | .58 |
| FVC (% predicted-donor), 1% | 1.04 | 0.71–14.62 | .13 |
| FEV1/FVC, 1% | 0.98 | 0.96–0.99 | .015 |
| Ve50, L/s | 0.78 | 0.65–0.93 | .008 |
| Ve50 (% predicted-recipient), 1% | 0.98 | 0.07–0.56 | .002 |
| Ve50/FVC, L/s | 0.55 | 0.36–0.82 | .004 |
| τ*, s | 6.66 | 2.86–15.51 | < .0001 |
| τ**, s | 5.21 | 2.30–11.78 | < .0001 |
| Multivariable | |||
| Acute rejection | 3.10 | 1.69–5.69 | .0003 |
| pTLC ratio > 1.0 | 0.44 | 0.25–0.79 | .007 |
Discussion
In this investigation of lung size mismatch and allograft function, a pTLC ratio > 1.0, suggestive of an oversized allograft, was associated with higher expiratory airflow capacity and a less frequent occurrence of BOS. A higher pTLC ratio was associated with a lower FVC, expressed as % predicted of donor lung. Expressing allograft function in relation to donor predicted lung function likely captured the actual restriction of an oversized allograft in a smaller recipient's thorax.
Prior studies concluded that wide discrepancies in lung sizing do not to influence allograft function.3, 4, 18, 19, 20 In the present study, we also found no difference in allograft function in terms of FVC, FVC (% predicted-recipient), and FEV1. However, focusing on parameters of expiratory airflow capacity, we saw the association between an oversized allograft and higher FEV1 (% predicted-recipient), higher FEV1/FVC ratios, and lower time constant estimates of lung emptying. Mason et al 3 also described in patients with emphysema and bronchiectasis the association between a higher pTLC ratio and higher FEV1 (% predicted-recipient) in the early posttransplant period, which, however, was not seen at later time points. Data expressing allograft function in relation to donor predicted function were shown in the study by Massard et al, 20 who provided posttransplant vital capacities (VCs) expressed as % predicted for the donor in 18 patients who received BLTs. Reanalyzing the published allograft function data of Massard et al showed a similar relationship between an oversized allograft and higher FEV1 (% predicted-recipient), higher FEV1/FVC ratios, and a trend correlating higher pTLC ratios with lower VCs (% predicted-donor) (e-Fig 5; e-Table 2).
Oversized Allograft and Restricted Inflation
In adults, absolute residual volume (RV) is determined by intrinsic characteristics of the lung (airway closure). 21 If the RV of the donor lung does not change after transplantation, then an oversized allograft has an RV that is large relative to the recipient's thorax. Total lung capacity (TLC) is determined by chest wall mechanics, respiratory muscles, and lung compliance. 22 Thus, oversizing (pTLC ratio > 1.0) would lead to an elevated RV/TLC ratio. An elevated RV/TLC ratio was reported in seven patients who received HLTs with larger donor lungs (mean pTLC ratio, 1.30). One year after transplant, the RV was in the predicted range for the (larger) donor lungs, whereas the TLC was in the predicted range for the recipient. 23 The functional residual capacity (FRC) of a lung transplant recipient is determined by the recipient's chest wall and donor lung mechanics. 22 A patient with an oversized allograft will likely have an FRC that is lower than the donor's FRC because of the chest wall mechanics of a relatively smaller recipient's thorax. As a consequence, expiratory reserve volume (ERV) would be reduced. Therefore, it is likely that a patient with an oversized allograft will breathe closer to the RV of the allograft. Furthermore, the increased RV/TLC ratio together with the relatively smaller TLC of the recipient will decrease the VC of the allograft out of proportion, leading to limitations to the inflation of the allograft. A prior investigation showed that an FVL pattern characterized by a SUPRA pattern was more likely to occur when the pTLC ratio was higher. 5 Patients with SUPRA patterns had an RV/TLC ratio of 54%, an RV of 112 (% predicted-recipient), and an ERV of only 0.55 L. 5 In the current study, a significant association between a higher pTLC ratio and lower FVC (% predicted-donor) was demonstrated (P < .0001, Figs 2A, 2B). The FVC (% predicted-donor) likely represents the actual limitations to the inflation of the oversized allograft.
Oversized Allografts and Determinants of Expiratory Airflow
Limitations to inflation and breathing close to RV are associated with increased elastic recoil, a key determinant of expiratory airflow.6, 7, 8, 9, 10, 24 Chest wall strapping (CWS), an experimental procedure that limits lung inflation, is conceptually similar to the transplantation of lungs that are larger than the recipient's thorax. CWS experiments performed in healthy humans caused breathing closer to RV. When FVC was limited to 50% of unstrapped VC, elastic recoil and expiratory airflows increased by approximately 60%.6, 8 The FVL pattern during CWS was altered similarly to the schematic FVL pattern shown for the oversized cohort (Fig 4B).6, 8 In patients with persistent limitations to lung inflation due to respiratory muscle weakness, increased elastic recoil persisted over time and was not related to atelectasis. 10 Lung elastic recoil was increased in 15 patients who received a BLT/HLT, who had a mean pTLC-ratio of 1.09. 25 The posttransplant TLC was not correlated with the pretransplant TLC of the donor, but fell in the expected range for the recipient. The RV remained higher than predicted for the recipient. The investigators concluded that at high lung volumes, the chest wall adapts to the transplanted lung, and at lower lung volumes, the lung adapts to the smaller chest wall. We hypothesize that the larger transplanted lungs have an RV that is large compared with the smaller chest wall. Breathing closer to the RV and limitations to inflation of the allograft could then explain the observed increase in lung elastic recoil. Dysanspsis is not a likely explanation for the differences in expiratory flows between cohorts because there was no significant difference in inspiratory airflows (e-Fig 3D). The marked difference in flows only during expiration is best explained by a difference in recoil pressures.
Lung Size Mismatch and BOS: Is the Surfactant System the Link?
Elastic recoil is determined by tissue and surface tension at the air-liquid interface. 26 Surface tension normally comprises 60% to 70% of elastic recoil and is affected by surfactant function. The surfactant system shows adaptive responses to changes in lung compliance. In a model of decreased lung compliance, increases in surfactant protein and phospholipid content mediated a compensatory reduction in surface tension. 27 Furthermore, alveolar size is a major determinant of lung distensibility. 28 With an oversized allograft, alveolar size would on average be reduced at any lung volume compared with the situation in the donor chest, leading to an increase in elastic recoil. 28 Surfactant fills in the regions adjacent to infolding of the alveoli as the lung deflates to maintain a spherical inner surface, which minimizes the increase in surface tension as the lung deflates. Thus, a chronically underinflated lung could be expected to accumulate more surfactant. On the other hand, decreased surfactant content has been described after lung transplantation, suggestive of type 2 pneumocyte dysfunction. 29 Decreased surfactant protein A levels have been linked to the onset of BOS. 30 Furthermore, risk factors for BOS can be linked to the surfactant system (Table 6).
Table 6.
The Surfactant System and Its Relationship to Risk Factors for BOS
| BOS Risk Factor | Effect on Surfactant System | Reference |
|---|---|---|
| Primary graft dysfunction | Successful treatment with surfactant | 31 |
| Acute rejection | Type 2 pneumocyte destruction and surfactant disruption | 32 |
| Rejection is associated with surfactant dysfunction | 33 | |
| Immunosuppression preserves surfactant function | 34 | |
| GERD aspiration | Inactivation of surfactant | 35 |
| Pulmonary infection | Inactivation of surfactant | 36 |
GERD = gastroesophageal reflux disease. See Table 4 legend for expansion of the other abbreviation.
Limitations
This study suggests that higher expiratory airflows in the oversized cohort (pTLC ratio > 1.0) are from increased elastic recoil. However, we have no measurements of elastic recoil to prove this hypothesis. Further prospective investigations are needed to clarify the physiologic determinants of the higher expiratory airflows in patients in the oversized cohort and the role of the surfactant system in the posttransplant period. The pTLC ratio as a marker of lung size mismatch is imprecise because it relies on regression equations based on sex and height. There is substantial overlap in the parameters of expiratory airflow between the oversized and undersized cohorts. Improved techniques like CT scan volumetry could provide better estimates of allograft and recipient thorax size. 37 This investigation does not include lung volume measurements, and our hypothesis of the effect of lung size mismatch on RV, ERV, and TLC is only supported by the literature.5, 21, 22, 23, 25 The trend toward more airway complications in the undersized cohort could have confounded the allograft function and BOS assessment. However, limiting the analysis to patients without airway complications did not change the results (e-Fig 6, e-Table 3). The assessment of risk factors for BOS is incomplete because information on primary graft dysfunction, CMV status, respiratory viral infections, or gastroesophageal reflux was not available. We cannot exclude the possibility that the results are biased by factors not accounted for, such as race, lung trimming, pleural pathology, and comorbidities.
Conclusion
An oversized allograft is associated with higher expiratory airflow and lower occurrence of BOS. The oversized cohort is conceptually similar to CWS, an experimental condition known to cause higher expiratory airflow, likely from changes in surface tension. The mechanism linking an oversized allograft to delayed occurrence of BOS deserves further investigation.
Supplementary Material
Acknowledgments
Author contributions: Dr Eberlein is the guarantor of the entire manuscript.
Dr Eberlein: contributed to the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and the revision of the article for important intellectual content.
Dr Permutt: contributed to the conception and design of the study, analysis and interpretation of data, and the revision of the article for important intellectual content.
Dr Chahla: contributed to the analysis and interpretation of data and the revision of the article for important intellectual content.
Dr Bolukbas: contributed to the analysis and interpretation of data and the revision of the article for important intellectual content.
Dr Nathan: contributed to the acquisition of data, analysis and interpretation of data, and the revision of the article for important intellectual content.
Dr Shlobin: contributed to the acquisition of data, analysis and interpretation of data, and the revision of the article for important intellectual content.
Dr Shelhamer: contributed to the analysis and interpretation of data and the revision of the article for important intellectual content.
Dr Reed: contributed to the analysis and interpretation of data and the revision of the article for important intellectual content.
Dr Pearse: contributed to the conception and design of the study, analysis and interpretation of data, and the revision of the article for important intellectual content.
Dr Orens: contributed to the analysis and interpretation of data and the revision of the article for important intellectual content.
Dr Brower: contributed to the analysis and interpretation of data and the revision of the article for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank Jenna Pearce, BA, lung transplant coordinator (Johns Hopkins Hospital), and Mary Smith, research assistant (Inova Fairfax Hospital), for administrative assistance.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/141/2/451/suppl/DC1.
Footnotes
Funding/Support: The authors have reported to CHEST that no funding was received for this study.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Pride NB, Permutt S, Riley RL, Bromberger-Barnea B. Determinants of maximal expiratory flow from the lungs. J Appl Physiol. 1967;23(5):646–662. doi: 10.1152/jappl.1967.23.5.646. [DOI] [PubMed] [Google Scholar]
- 2.Stocks J, Quanjer PH, Official statement of the European Respiratory Society Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Eur Respir J. 1995;8(3):492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 3.Mason DP, Batizy LH, Wu J. Matching donor to recipient in lung transplantation: How much does size matter? J Thorac Cardiovasc Surg. 2009;137(5):1234–1240. doi: 10.1016/j.jtcvs.2008.10.024. e1. [DOI] [PubMed] [Google Scholar]
- 4.Ouwens JP, van der Mark TW, van der Bij W, Geertsma A, de Boer WJ, Koëter GH. Size matching in lung transplantation using predicted total lung capacity. Eur Respir J. 2002;20(6):1419–1422. doi: 10.1183/09031936.02.00294402. [DOI] [PubMed] [Google Scholar]
- 5.Eberlein M, Permutt S, Brown RH. Supranormal expiratory airflow after bilateral lung transplantation is associated with improved survival. Am J Respir Crit Care Med. 2011;183(1):79–87. doi: 10.1164/rccm.201004-0593OC. [DOI] [PubMed] [Google Scholar]
- 6.Douglas NJ, Drummond GB, Sudlow MF. Breathing at low lung volumes and chest strapping: A comparison of lung mechanics. J Appl Physiol. 1981;50(3):650–657. doi: 10.1152/jappl.1981.50.3.650. [DOI] [PubMed] [Google Scholar]
- 7.Caro CG, Butler J, DuBois AB. Some effects of restriction of chest cage expansion on pulmonary function in man: an experimental study. J Clin Invest. 1960;39(4):573–583. doi: 10.1172/JCI104070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stubbs SE, Hyatt RE. Effect of increased lung recoil pressure on maximal expiratory flow in normal subjects. J Appl Physiol. 1972;32(3):325–331. doi: 10.1152/jappl.1972.32.3.325. [DOI] [PubMed] [Google Scholar]
- 9.Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol. 2010;108(1):212–218. doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estenne M, Gevenois PA, Kinnear W, Soudon P, Heilporn A, De Troyer A. Lung volume restriction in patients with chronic respiratory muscle weakness: the role of microatelectasis. Thorax. 1993;48(7):698–701. doi: 10.1136/thx.48.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis. 1980;121(2):339–342. doi: 10.1164/arrd.1980.121.2.339. [DOI] [PubMed] [Google Scholar]
- 12.Dawkins KD, Jamieson SW. Pulmonary function of the transplanted human lung. Annu Rev Med. 1986;37:263–269. doi: 10.1146/annurev.me.37.020186.001403. [DOI] [PubMed] [Google Scholar]
- 13.Mason DP, Rajeswaran J, Murthy SC. Spirometry after transplantation: how much better are two lungs than one? Ann Thorac Surg. 2008;85(4):1193–1201. doi: 10.1016/j.athoracsur.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Estenne M, Maurer JR, Boehler A. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 15.Permutt S, Menkes HA. Spirometry. Analysis of forced expiration within the time domain. In: Macklem PT, Permutt S, editors. vol 12. Dekker; New York, NY: 1979. pp. 113–152. (The Lung in the Transition between Health and Disease. Lung Biology in Health and Disease). [Google Scholar]
- 16.Gore CJ, Crockett AJ, Pederson DG, Booth ML, Bauman A, Owen N. Spirometric standards for healthy adult lifetime nonsmokers in Australia. Eur Respir J. 1995;8(5):773–782. [PubMed] [Google Scholar]
- 17.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113(5):587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 18.Otulana BA, Mist BA, Scott JP, Wallwork J, Higenbottam T. The effect of recipient lung size on lung physiology after heart-lung transplantation. Transplantation. 1989;48(4):625–629. [PubMed] [Google Scholar]
- 19.Miyoshi S, Schaefers HJ, Trulock EP. Donor selection for single and double lung transplantation. Chest size matching and other factors influencing posttransplantation vital capacity. Chest. 1990;98(2):308–313. doi: 10.1378/chest.98.2.308. [DOI] [PubMed] [Google Scholar]
- 20.Massard G, Badier M, Guillot C. Lung size matching for double lung transplantation based on the submammary thoracic perimeter. Accuracy and functional results. The Joint Marseille-Montreal Lung Transplant Program. J Thorac Cardiovasc Surg. 1993;105(1):9–14. [PubMed] [Google Scholar]
- 21.Leith DE, Mead J. Mechanisms determining residual volume of the lungs in normal subjects. J Appl Physiol. 1967;23(2):221–227. doi: 10.1152/jappl.1967.23.2.221. [DOI] [PubMed] [Google Scholar]
- 22.Rahn H, Otis AB, Chadwick LE, Fenn WO. The pressure-volume diagram of the thorax and lung. Am J Physiol. 1946;146(2):161–178. doi: 10.1152/ajplegacy.1946.146.2.161. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd KS, Barnard P, Holland VA, Noon GP, Lawrence EC. Pulmonary function after heart-lung transplantation using larger donor organs. Am Rev Respir Dis. 1990;142(5):1026–1029. doi: 10.1164/ajrccm/142.5.1026. [DOI] [PubMed] [Google Scholar]
- 24.Young SL, Tierney DF, Clements JA. Mechanism of compliance change in excised rat lungs at low transpulmonary pressure. J Appl Physiol. 1970;29(6):780–785. doi: 10.1152/jappl.1970.29.6.780. [DOI] [PubMed] [Google Scholar]
- 25.Chacon RA, Corris PA, Dark JH, Gibson GJ. Respiratory mechanics after heart-lung and bilateral lung transplantation. Thorax. 1997;52(8):718–722. doi: 10.1136/thx.52.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachofen H, Schürch S, Urbinelli M, Weibel ER. Relations among alveolar surface tension, surface area, volume, and recoil pressure. J Appl Physiol. 1987;62(5):1878–1887. doi: 10.1152/jappl.1987.62.5.1878. [DOI] [PubMed] [Google Scholar]
- 27.Dixon DL, De Pasquale CG, De Smet HR, Klebe S, Orgeig S, Bersten AD. Reduced surface tension normalizes static lung mechanics in a rodent chronic heart failure model. Am J Respir Crit Care Med. 2009;180(2):181–187. doi: 10.1164/rccm.200809-1506OC. [DOI] [PubMed] [Google Scholar]
- 28.Haber PS, Colebatch HJ, Ng CK, Greaves IA. Alveolar size as a determinant of pulmonary distensibility in mammalian lungs. J Appl Physiol. 1983;54(3):837–845. doi: 10.1152/jappl.1983.54.3.837. [DOI] [PubMed] [Google Scholar]
- 29.Hohlfeld JM, Tiryaki E, Hamm H. Pulmonary surfactant activity is impaired in lung transplant recipients. Am J Respir Crit Care Med. 1998;158(3):706–712. doi: 10.1164/ajrccm.158.3.9708063. [DOI] [PubMed] [Google Scholar]
- 30.Meloni F, Salvini R, Bardoni AM. Bronchoalveolar lavage fluid proteome in bronchiolitis obliterans syndrome: Possible role for surfactant protein A in disease onset. J Heart Lung Transplant. 2007;26(11):1135–1143. doi: 10.1016/j.healun.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 31.van der Kaaij NP, Kluin J, Haitsma JJ. Surfactant pretreatment decreases long-term damage after ischemia-reperfusion injury of the lung. Eur J Cardiothorac Surg. 2009;35(2):304–312. doi: 10.1016/j.ejcts.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 32.Semik M, Schnabel R, Bruske T. Ultrastructural studies of acute rejection following single lung transplantation in the rat—histological and immunohistological findings. Thorac Cardiovasc Surg. 1994;42(5):290–297. doi: 10.1055/s-2007-1016507. [DOI] [PubMed] [Google Scholar]
- 33.Waldhausen JA, Giammona ST, Kilman JW, Daly WJ. Effect of transplantation of canine lung on pulmonary compliance and surfactant. JAMA. 1965;191:1002–1005. doi: 10.1001/jama.1965.03080120036009. [DOI] [PubMed] [Google Scholar]
- 34.Thomas PA, Jolly PC. Preservation of pulmonary surfactant activity in canine lung allografts by immune suppressive therapy. J Thorac Cardiovasc Surg. 1968;55(3):405–410. [PubMed] [Google Scholar]
- 35.D'Ovidio F, Mura M, Ridsdale R. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6(8):1930–1938. doi: 10.1111/j.1600-6143.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 36.Ray NB, Durairaj L, Chen BB. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat Med. 2010;16(10):1120–1127. doi: 10.1038/nm.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F, Fujinaga T, Shoji T. Perioperative assessment of oversized lobar graft downsizing in living-donor lobar lung transplantation using three-dimensional computed tomographic volumetry. Transpl Int. 2010;23(9):e41–e44. doi: 10.1111/j.1432-2277.2010.01123.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.