Abstract
Purpose
In advanced Fuchs’ endothelial corneal dystrophy (FECD), central endothelial changes do not correlate with disease severity. Peripheral endothelial cell count (ECC) has not been studied as a marker for FECD severity. The goal of this study is to determine the relationship between peripheral ECC and known clinical markers of FECD in advanced cases.
Methods
Patients with FECD examined between January 1, 2013 and September 1, 2016 by one cornea specialist were identified. Electronic medical records from all prior visits were reviewed to include eyes with high-quality central and peripheral in vivo confocal microscopy (IVCM) images performed on the same day as a clinical evaluation. Endothelial photographs were used to perform manual cell counts centrally and peripherally. Clinical grading of FECD from 1 to 4 was performed at the slit lamp.
Results
We identified 154 eyes of 126 patients that met criteria for inclusion in this study. With higher disease grades, central ECC and peripheral ECC decreased, visual acuity worsened, and central corneal thickness (CCT) increased (all p < 0.05). In patients with advanced disease (defined as either grade of 3 or 4, CCT > 700, or central ECC < 350), peripheral ECC was the best predictor of disease severity and had the highest number of statistically significant correlations with other clinical markers of disease as compared to competing variables.
Conclusion
In advanced FECD, severity is best determined by peripheral ECC as compared to central ECC, visual acuity, clinical disease grade, and CCT.
Keywords: Fuchs’ endothelial corneal dystrophy, confocal microscopy, peripheral endothelial cell count
Introduction
Fuchs’ endothelial corneal dystrophy (FECD) is a genetically complex, age-related disorder that affects 4.5% of individuals over 50 years of age and 10.5% of patients over 60 years of age.1 It leads to progressive loss of corneal endothelial cells and extracellular deposits, termed guttae. These developments compromise corneal deturgescence, resulting in swelling and a loss of vision.2–4 Stromal edema may also correlate with keratocyte depletion of the anterior cornea.5
The clinical course of FECD usually spans 10–20 years.6 During this time, guttae formation and endothelial changes start in the central cornea and spread toward the periphery.7 In early stages, FECD is characterized by central guttae accompanied by a decline in central endothelial cell count (ECC) and morphological changes to the endothelial monolayer.7 In later stages, coalescence of guttae is accompanied by a low central endothelial cell density and compromise in endothelial barrier function, causing corneal edema. Correspondingly, the area of guttae is significantly larger in the central cornea compared with paracentral and peripheral regions.8
Clinical grading of FECD severity is often determined by slit lamp microscopy, central corneal thickness (CCT), and central ECC. Slit lamp grading evaluates the area of guttae coalescence and presence of edema.6 An additional method used to determine severity is retroillumination photograpy,9,10 which has been employed to detect formation of new guttae over time.11 However, manual counting can be time-consuming. Moreover, the main limitation of central ECC is that in moderate to severe FECD, guttae replace the central endothelial monolayer and render the central ECC undetectable by most imaging modalities.12 As such, central endothelial changes do not significantly correlate with disease grade in patients with grade 3 or higher (using a modified Krachmer grading scale).12 To the best of our knowledge, peripheral ECC has not been studied as a marker for FECD severity in advanced cases.
In vivo confocal microscopy (IVCM) allows for a magnified view of all layers of the cornea, including the endothelium.13,14 IVCM can be useful in the evaluation of advanced FECD before grafting as it is able to focus on the posterior cornea despite opacification and edema.14 Furthermore, confocal microscopy is able to image the peripheral corneal endothelium circumferentially.15 In this study, we utilized Heidelberg Retina Tomograph (HRT) IVCM to detect the relationship between peripheral ECC and known clinical parameters that are currently used to denote FECD severity. We determined that peripheral ECC highly correlates with disease severity in advanced FECD, thus introducing a new imaging-based method to assess the clinical progression of FECD.
Materials and Methods
Study Population and Data Collection
Approval for this retrospective chart review was obtained from the Institutional Review Board at Massachusetts Eye and Ear Infirmary (Boston, MA, USA). The investigators recorded data in such a manner that subjects could not be identified, either directly or through linked identifiers.
We identified all patients with FECD examined at any point between January 1, 2013 and September 1, 2016 by one cornea specialist (UVJ). The electronic medical records and IVCM imaging results (including dates prior to January 1, 2013) of the patients were reviewed to include only those patients with IVCM performed on the same day as a clinic visit and with IVCM studies including high quality central and peripheral endothelial imaging. Patients were excluded from this study if IVCM image quality was poor in central or peripheral endothelial photographs, or if the patient did not have a complete clinical examination on the same day as IVCM imaging that included refraction, slit lamp examination, and pachymetry. In cases where both eyes of a patient qualified for inclusion, the two were included for separate analyses. In situations where a patient had multiple clinic visits in which the required data was available, the most recent date was selected for inclusion in this study.
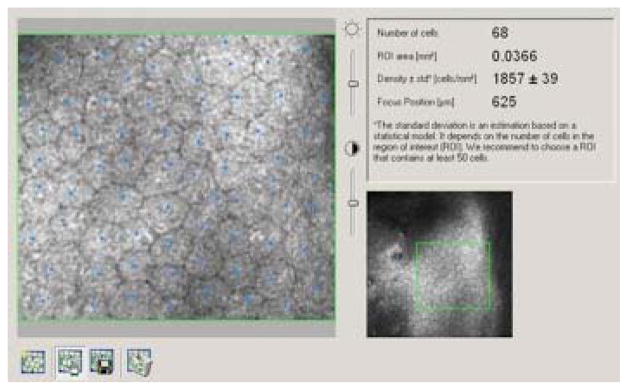
The laser scanning confocal microscope (HRT3 with Rostock Corneal Module [RCM], Heidelberg Engineering, Heidelberg, Germany) was used to obtain endothelial images and to perform manual cell counts centrally and peripherally. HRT3 images that were chosen to determine ECC for each eye had to be high resolution and have a countable mosaic of endothelial cells homogenously distributed throughout a scanned area of 400 × 400μm per image. A region of interest (ROI) with a minimum area of 0.03 mm2 was defined on the original image and the number of cells within that outline was manually counted. A sample endothelial image from a healthy eye with an ROI outlined with manual count is presented in Figure 1. In cases of advanced FECD with very few remaining endothelial cells, a representative ROI was selected to allow for at least a single countable cell, therefore allowing us to quantify a value for ECC. Subjects were instructed by the imaging technician to look in a direction off-center to obtain a peripheral endothelial photograph, which was captured 2 to 3 mm inside the limbus.
Figure 1.
Endothelial image from a healthy eye taken using Heidelberg Retina Tomograph (HRT) 3. A region of interest (ROI) with a minimum area of 0.03 mm2 was defined and the number of cells within the ROI was manually counted, resulting in a cellular density value.
Data collected on each patient included date of birth, date of visit, age at date of visit, months of follow up, gender, eye, central ECC, peripheral ECC, directional location of peripheral ECC, logarithm of the minimum angle of resolution (LogMAR) best corrected visual acuity (BCVA), clinical grade, CCT, future need for surgery, and type of surgery performed. Clinical grading of FECD was performed at the slit lamp according to the following guidelines: grade 1 – nonconfluent guttae; grade 2 – presence of any area of confluent guttae, but without edema or clinical thickening; grade 3 – confluent guttae with edema or clinical thickening; grade 4 – edema associated with whitening or haze. For eyes with visual acuity in the counting fingers range, acuity was documented as 20/400 for LogMAR conversions. To limit observer bias, the investigator who reviewed electronic medical records (JAT) was different than the researcher who performed manual cell counts using IVCM images (ZAS).
Statistical Analysis
Data were collected and tabulated into an Excel spreadsheet for analysis (Microsoft, Inc., Seattle, WA, USA). Where applicable throughout this manuscript, data is reported as mean ± standard deviation. Pearson’s correlation coefficients were used to determine relationships between variables studied. T-tests were used to compare independent means within the data. P-values less than 0.05 were considered to be statistically significant.
Results
Imaging Findings
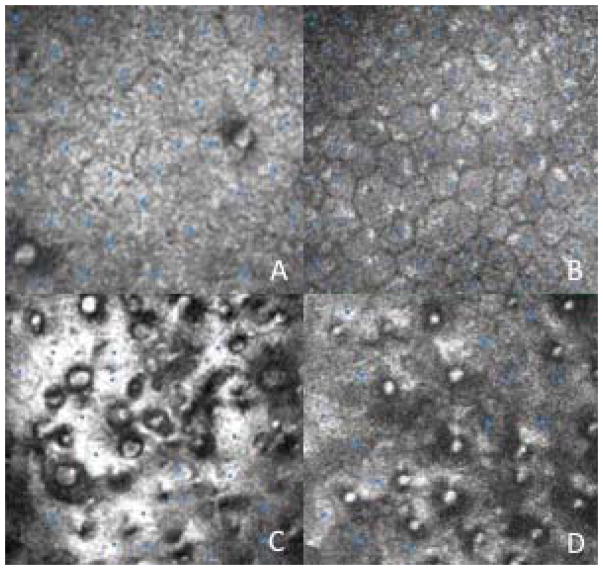
We identified 154 eyes of 126 patients with FECD that met criteria for inclusion in this study. Eyes were graded from 1 to 4, and imaging from each eye included central and peripheral endothelial photos. Sample central and peripheral endothelial photographs for grade 1 and 3 eyes after cell counts were performed are presented in Figure 2.
Figure 2.
Sample central and peripheral endothelial photographs after manual cell counts have been performed. A: Central endothelium from grade 1 eye. B: Peripheral endothelium from grade 1 eye. C: Central endothelium from grade 3 eye. D: Peripheral endothelium from grade 3 eye.
Demographic and Follow Up Data
Of the 154 eyes, 74 (48.1%) were the right eye and 80 (51.9%) were the left eye. Of the 126 patients, 82 (65.1%) were female and 44 (34.9%) were male. The mean age at the date of visit was 66.0 +/− 9.4 years (range 46 to 88 years). The mean period of follow up was 29.9 +/− 19.7 months (range 3 to 83 months) from the date of visit.
Data Stratified by Disease Grade
Table 1 presents the average age at date of visit, percent female, and clinical/imaging features after stratifying by disease grade. As shown in Table 1, with increasing disease grade, the age at visit date increased. However, this relationship was not statistically significant, although there was a trend towards statistical significance (p = 0.054). Mean disease grade among females was 1.89 +/− 0.75. Mean disease grade among males was 2.16 +/− 0.88. This difference was statistically significant (p < 0.05). With higher disease grades, central ECC and peripheral ECC decreased, visual acuity worsened, and CCT increased (Table 1). These correlations were all statistically significant (all p < 0.05).
Table 1.
Variables According to Disease Grade; ECC = endothelial cell count; LogMAR = logarithm of the minimum angle of resolution; BCVA = best corrected visual acuity; CCT = central corneal thickness
| Age at visit date | Percent female | Central ECC | Peripheral ECC | LogMAR BCVA | CCT | |
|---|---|---|---|---|---|---|
| Grade 1 | 64.8 +/− 9.8 years | 68.8% | 1392 +/− 591 | 1770 +/− 501 | 0.114 +/− 0.162 | 585 +/− 44 |
| Grade 2 | 65.6 +/− 9.1 years | 70.0% | 668 +/− 382 | 1338 +/− 352 | 0.209 +/− 0.152 | 626 +/− 43 |
| Grade 3 | 68.2 +/− 9.6 years | 46.9% | 442 +/− 257 | 831 +/− 265 | 0.432 +/− 0.303 | 673 +/− 69 |
| Grade 4 | 70.5 +/− 5.8 years | 50.0% | 205 +/− 91 | 387 +/− 202 | 0.619 +/− 0.506 | 733 +/− 116 |
Eyes Requiring Surgery During Follow Up
Forty-one eyes eventually required surgery during follow up. Of these, 26 underwent Descemet membrane endothelial keratoplasty (DMEK), 11 underwent Descemet stripping endothelial keratoplasty (DSEK), 2 underwent Descemet stripping, and 2 underwent penetrating keratoplasty. The percentage of eyes requiring surgery stratified by disease grade is presented in Table 2. As seen in Table 2, higher grades were associated with a larger proportion of eyes eventually undergoing surgery. Furthermore, the time to surgery was lower with more advanced disease grades (Table 2), and this relationship was statistically significant (p < 0.05). In addition, the time to surgery showed an inverse correlation with CCT, and this relationship was also significant (p < 0.05).
Table 2.
Requirement for Surgery According to Disease Grade
| Eyes requiring surgery | Time to surgery (months) | |
|---|---|---|
| Grade 1 | 5/48 (10.4%) | 30.8 +/− 14.6 |
| Grade 2 | 8/70 (11.4%) | 15.0 +/− 11.3 |
| Grade 3 | 24/32 (75.0%) | 9.2 +/− 10.9 |
| Grade 4 | 4/4 (100.0%) | 2.5 +/− 1.0 |
Among females, 17/99 (17.2%) eventually underwent surgery, while among males, 24/55 (43.6%) required surgery during follow up. This difference was statistically significant (p < 0.05).
Correlations Between Pairs of Clinical Variables
Table 3 presents the statistical significance of correlations between various pairs of clinical variables. When evaluating central ECC, peripheral ECC, LogMAR BCVA, disease grade, and CCT, each of the 5 variables significantly correlated with each of the other variables (Table 3).
Table 3.
Correlations Between Pairs of Clinical Variables; ECC = endothelial cell count; LogMAR = logarithm of the minimum angle of resolution; BCVA = best corrected visual acuity; CCT = central corneal thickness
| Peripheral ECC | LogMAR BCVA | Grade | CCT | |
|---|---|---|---|---|
| Central ECC | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
| Peripheral ECC | p < 0.05 | p < 0.05 | p < 0.05 | |
| LogMAR BCVA | p < 0.05 | p < 0.05 | ||
| Grade | p < 0.05 |
Correlations in Advanced Disease Grades
Among eyes with a disease grade of 3 or 4, clinical correlations are presented in Table 4. Of the 5 variables studied, peripheral ECC had the highest number of statistically significant correlations with other variables (2 statistically significant correlations). Each of the other variables had 1 or 0 statistically significant correlations. Peripheral ECC was the only variable that significantly correlated with central ECC and disease grade (Table 4).
Table 4.
Correlations Among Eyes with Grade 3 or 4 Disease; ECC = endothelial cell count; LogMAR = logarithm of the minimum angle of resolution; BCVA = best corrected visual acuity; CCT = central corneal thickness
| Central ECC | Peripheral ECC | LogMAR BCVA | Grade | CCT | |
|---|---|---|---|---|---|
| Central ECC | p < 0.05 | p > 0.10 | p = 0.090 | p > 0.10 | |
| Peripheral ECC | p < 0.05 | p > 0.10 | p < 0.05 | p > 0.10 | |
| LogMAR BCVA | p > 0.10 | p > 0.10 | p > 0.10 | p > 0.10 | |
| Grade | p = 0.089 | p < 0.05 | p > 0.10 | p > 0.10 | |
| CCT | p > 0.10 | p = 0.063 | p > 0.10 | p > 0.10 |
Correlations in Thickened Corneas
Among eyes with CCT > 700, clinical correlations are presented in Table 5. Peripheral ECC demonstrated 3 statistically significant correlations, the highest of the clinical variables studied. Peripheral ECC was the only variable studied that demonstrated a statistically significant relationship with clinical disease grade (Table 5).
Table 5.
Correlations Among Eyes with CCT > 700; ECC = endothelial cell count; LogMAR = logarithm of the minimum angle of resolution; BCVA = best corrected visual acuity; CCT = central corneal thickness
| Central ECC | Peripheral ECC | LogMAR BCVA | Grade | CCT | |
|---|---|---|---|---|---|
| Central ECC | p < 0.05 | p > 0.10 | p > 0.10 | p < 0.05 | |
| Peripheral ECC | p < 0.05 | p > 0.10 | p < 0.05 | p < 0.05 | |
| LogMAR BCVA | p > 0.10 | p > 0.10 | p > 0.10 | p > 0.10 | |
| Grade | p > 0.10 | p < 0.05 | p > 0.10 | p = 0.096 | |
| CCT | p < 0.05 | p < 0.05 | p > 0.10 | p = 0.096 |
Correlations in Cases of Low Central ECC
Among eyes with central ECC < 350, correlations are presented in Table 6. Peripheral ECC had the highest number of statistically significant correlations with other clinical variables. In addition, peripheral ECC was the only feature that significantly correlated with CCT (Table 6).
Table 6.
Correlations Among Eyes with Central ECC < 350; ECC = endothelial cell count; LogMAR = logarithm of the minimum angle of resolution; BCVA = best corrected visual acuity; CCT = central corneal thickness
| Central ECC | Peripheral ECC | LogMAR BCVA | Grade | CCT | |
|---|---|---|---|---|---|
| Central ECC | p > 0.10 | p > 0.10 | p > 0.10 | p > 0.10 | |
| Peripheral ECC | p > 0.10 | p < 0.05 | p < 0.05 | p < 0.05 | |
| LogMAR BCVA | p > 0.10 | p < 0.05 | p < 0.05 | p > 0.10 | |
| Grade | p > 0.10 | p < 0.05 | p < 0.05 | p = 0.071 | |
| CCT | p > 0.10 | p < 0.05 | p > 0.10 | p = 0.071 |
Peripheral ECC According to Location of Cell Count
Of the 154 eyes included in this study, peripheral ECC measurements were obtained inferiorly in 29 (18.8%) eyes, nasally in 62 (40.2%) eyes, superiorly in 48 (31.2%) eyes, and temporally in 15 (9.7%) eyes. There was no significant difference when comparing values from the superior and inferior peripheral cornea to the nasal and temporal peripheral cornea (p = 0.859).
Discussion
In this study, we demonstrate that peripheral ECC is a strong clinical marker of disease severity in advanced FECD, as defined by a high clinical grade, thickened central cornea, or low central ECC. In each of these scenarios, peripheral ECC was the strongest predictor of disease severity as compared to other known markers of FECD progression.
The clinical staging of FECD has been widely explored, and to this day there is no consensus on the best method to grade this condition. While slit lamp grading of the disease is common, agreement between corneal specialists on subjective and morphologic grading in FECD is only moderate.16 Perhaps the most common subjective measure of disease severity used in FECD is the Krachmer grading system, in which the disease is staged based on slit lamp findings of the confluence and area of guttae in the central corneal zone.6
Several objective methods of grading have been developed. The corneal central-to-peripheral thickness ratio is a repeatable, objective measurement that correlates with clinical severity of FECD, and also distinguishes between FECD and normal corneas.16 Alternatively, effective endothelial cell density (the product of local cell density and the fraction of a confocal image free of guttae) correlates significantly with subjective grade.17 CCT has also been shown to correlate directly with FECD severity, although the use of this measure as a substitute for disease grade is limited by the underlying effect of intraocular pressure, age, and contact lens wear on CCT.18 Finally, the peripheral guttae ratio may be useful for distinguishing advanced grades of FECD and is another potential method of grading.12 The current findings suggest that peripheral ECC is a promising imaging-based objective measure of disease severity in advanced cases.
Peripheral ECC demonstrated stronger and more correlations with disease severity compared to other disease markers (central ECC, BCVA, clinical disease grade, and CCT). The latter 4 features may be limited in their utility in advanced FECD for a number of reasons. In severe cases of FECD, the central and paracentral endothelia are nearly completely covered by guttae,12 with peripheral endothelial changes occuring later.7 As such, central ECC loses its utility as a metric for disease grade in moderate to advanced FECD, as ECC in the central and paracentral zones would not differ across grades. The earlier central degeneration and disorganization of endothelial cells may be secondary to the high density of regenerative endothelial precursor cells in the peripheral cornea.19,20 BCVA may be limited as a marker for disease severity in FECD due to the effect of coexisting cataract or retinal pathology. Clinical grading of FECD is limited by its subjectivity, with moderate agreement across specialists.16 Finally, CCT may be limited because with early central endothelial damage, the central cornea swells primarily and the peripheral cornea remains relatively unaffected until the final stages of FECD.21 Given that central corneal endothelial cells are essentially obliterated in advanced FECD, the cornea may reach its maximum point of swelling before the final stages of FECD.22
Interestingly, our findings demonstrated a higher average disease grade in males compared to females, with a higher percentage of males eventually undergoing corneal surgery. These findings parallel those reported in a study by Afshari et al., which found that men were an average of 4 years younger than women at the time of corneal transplant for FECD, and that preoperative corneal thickness was slightly greater in men.23 To the best of our knowledge, there is no known pathophysiologic mechanism for more rapid FECD progression in men compared to women. The gender disparity identified in our study may be due to the characteristic of men to often present with later ocular disease than women, and this trend has been previously demonstrated in the case of glaucoma.24
The value of IVCM in the diagnosis and evaluation of FECD has been previously described.25–27 Scanning slit IVCM has been shown to detect changes in the basal epithelium, Bowman’s layer, anterior stroma, posterior stroma, Descemet membrane, and endothelium in FECD.25 Furthermore, the ECC obtained by IVCM correlates strongly with counts obtained by standard specular microscopy.25 IVCM has been proposed as an effective means to study the corneal stroma in cases of FECD in which it may appear clinically normal but has perhaps undergone subclinical changes.27 In this regard, it may have a role in preoperative evaluation and in evaluating the response of the corneal stroma to endothelial keratoplasty.27
In advanced cases of FECD, keratoplasty remains the mainstay of treatment. While penetrating keratoplasty was once the leading surgical treatment for FECD, DSEK and DMEK have emerged as the primary methods of treatment.28–33 Descemet stripping without endothelial keratoplasty was recently identified as a potential technique in the management of FECD, although further research is needed to understand its clinical applicability.34 In our study, the percentage of eyes requiring surgical treatment for FECD increased with higher disease grades, an expected correlation given the effect of advanced FECD on visual acuity. Furthermore, the time to surgery inversely correlated with disease grade, with stage 1, stage 2, stage 3, and stage 4 disease eyes undergoing surgery an average of 30.8 months, 15.0 months, 9.2 months, and 2.5 months later, respectively. These findings may be useful in educating patients about the natural course of their disease, and may guide clinical follow up intervals.
Several weaknesses to this study have been noted. First, we did not measure the exact distance of peripheral ECC measurements from the central axis, and the location of peripheral images may have varied across eyes. However, all images were obtained by one of two technicians, both of whom practiced very similar techniques. Furthermore, the large number of eyes included in this study likely would minimize the effect of differences due to small displacements in the location of measurements. Second, we obtained a single peripheral ECC in each eye. It has been shown that the inferotemporal cornea is often the most severely damaged area in FECD, possibly due to a lack of protection by the eyelid and nose from ultraviolet light.35,36 Furthermore, we know that guttae tend to spread more horizontally than vertically.37 Thus, the location of the peripheral photograph may have affected the ECC. However, as demonstrated in our results, there was no significant difference between the superior and inferior peripheral cornea and the nasal and temporal peripheral cornea (p = 0.859). Third, LogMAR BCVA was used as a measure of disease severity in our study, but the study did not account for decreases in visual potential due to cataract or posterior pathology. For this reason, we used multiple measures of disease severity to allow for a broader picture of disease progression. Finally, although it is important to note age-related changes in central and peripheral ECC, which could have thus affected our results, we did not find a statistically significant relationship between grade and age. In addition to the large number of eyes studied in this report, strengths of this study include a single cornea specialist performing all slit lamp exams (allowing for consistent grading), and the use of two investigators for chart reviews (reducing potential observer bias).
In conclusion, in cases of advanced FECD, severity is best determined by peripheral ECC as compared to central ECC, BCVA, clinical disease grade, and CCT. Peripheral ECC may be an objective imaging-based marker to monitor FECD progression in severe cases.
Supplementary Material
Acknowledgments
Sources of public and private financial support to disclose: None
Footnotes
Authors do not have any financial or proprietary interest in products, methods, or material mentioned in this article.
This work has not been previously published or presented at a meeting.
References
- 1.Friedenwald H, Friedenwald JS. Epithelial dystrophy of the cornea. Br J Ophthalmol. 1925;9:14–20. doi: 10.1136/bjo.9.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs E. Dystrophia epithelialis corneae. Graefes Arch Clin Exp Ophthalmol. 1910;76:478–508. [Google Scholar]
- 3.Wilson SE, Bourne WM. Fuchs’ dystrophy. Cornea. 1988;7:2–18. [PubMed] [Google Scholar]
- 4.Burns RR, Bourne WM, Brubaker RF. Endothelial function in patients with cornea guttata. Invest Ophthalmol Vis Sci. 1981;20:77–85. [PubMed] [Google Scholar]
- 5.Hecker LA, McLaren JW, Bachman LA, et al. Anterior keratocyte depletion in fuchs endothelial dystrophy. Arch Ophthalmol. 2011;129:555–561. doi: 10.1001/archophthalmol.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krachmer JH, Purcell JJ, Jr, Young CW, et al. Corneal endothelial dystrophy. A study of 64 families. Arch Ophthalmol. 1978;96:2036–2039. doi: 10.1001/archopht.1978.03910060424004. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzetti DW, Uotila MH, Parikh N, et al. Central cornea guttata. Incidence in the general population. Am J Ophthalmol. 1967;64:1155–1158. [PubMed] [Google Scholar]
- 8.Giasson CJ, Solomon LD, Polse KA. Morphometry of corneal endothelium in patients with corneal guttata. Ophthalmology. 2007;114:1469–1475. doi: 10.1016/j.ophtha.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Eghrari AO, McGlumphy EJ, Iliff BW, et al. Prevalence and severity of fuchs corneal dystrophy in Tangier Island. Am J Ophthalmol. 2012;153:1067–1072. doi: 10.1016/j.ajo.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eghrari AO, Mumtaz AA, Garrett B, et al. Automated retroillumination photography analysis for objective assessment of Fuchs corneal dystrophy. Cornea. 2017;36:44–47. doi: 10.1097/ICO.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottsch JD, Sundin OH, Rencs EV, et al. Analysis and documentation of progression of Fuchs corneal dystrophy with retroillumination photography. Cornea. 2006;25:485–489. doi: 10.1097/01.ico.0000178726.11693.14. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto H, Maeda N, Soma T, et al. Quantitative regional differences in corneal endothelial abnormalities in the central and peripheral zones in Fuchs’ endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2014;55:5090–5098. doi: 10.1167/iovs.14-14249. [DOI] [PubMed] [Google Scholar]
- 13.Jonuscheit S, Doughty MJ, Ramaesh K. In vivo confocal microscopy of the corneal endothelium: comparison of three morphometry methods after corneal transplantation. Eye (Lond) 2011;25:1130–1137. doi: 10.1038/eye.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara M, Morishige N, Chikama T, et al. Comparison of confocal biomicroscopy and noncontact specular microscopy for evaluation of the corneal endothelium. Cornea. 2003;22:512–515. doi: 10.1097/00003226-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Zheng T, Le Q, Hong J, et al. Comparison of human corneal cell density by age and corneal location: an in vivo confocal microscopy study. BMC Ophthalmol. 2016;16:109. doi: 10.1186/s12886-016-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Repp DJ, Hodge DO, Baratz KH, et al. Fuchs’ endothelial corneal dystrophy: subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology. 2013;120:687–694. doi: 10.1016/j.ophtha.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaren JW, Bachman LA, Kane KM, et al. Objective assessment of the corneal endothelium in Fuchs’ endothelial dystrophy. Invest Ophthalmol Vis Sci. 2014;55:1184–1190. doi: 10.1167/iovs.13-13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopplin LJ, Przepyszny K, Schmotzer B, et al. Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Arch Ophthalmol. 2012;130:433–439. doi: 10.1001/archophthalmol.2011.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamagami S, Yokoo S, Mimura T, et al. Distribution of precursors in human corneal stromal cells and endothelial cells. Ophthalmology. 2007;114:433–439. doi: 10.1016/j.ophtha.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 20.Amann J, Holley GP, Lee SB, et al. Increased endothelial cell density in the paracentral and peripheral regions of the human cornea. Am J Ophthalmol. 2003;135:584–590. doi: 10.1016/s0002-9394(02)02237-7. [DOI] [PubMed] [Google Scholar]
- 21.Brunette I, Sherknies D, Terry MA, et al. 3-D characterization of the corneal shape in Fuchs dystrophy and pseudophakic keratopathy. Invest Ophthalmol Vis Sci. 2011;52:206–214. doi: 10.1167/iovs.09-4101. [DOI] [PubMed] [Google Scholar]
- 22.Cheng X, Petsche SJ, Pinsky PM. A structural model for the in vivo human cornea including collagen-swelling interaction. J R Soc Interface. 2015;12:20150241. doi: 10.1098/rsif.2015.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Afshari NA, Pittard AB, Siddiqui A, et al. Clinical study of Fuchs corneal endothelial dystrophy leading to penetrating keratoplasty: a 30-year experience. Arch Ophthalmol. 2006;124:777–780. doi: 10.1001/archopht.124.6.777. [DOI] [PubMed] [Google Scholar]
- 24.Crabb DP, Saunders LJ, Edwards LA. Cases of advanced visual field loss at referral to glaucoma clinics - more men than women? Ophthalmic Physiol Opt. 2017;37:82–87. doi: 10.1111/opo.12328. [DOI] [PubMed] [Google Scholar]
- 25.Mustonen RK, McDonald MB, Srivannaboon S, et al. In vivo confocal microscopy of Fuchs’ endothelial dystrophy. Cornea. 1998;17:493–503. doi: 10.1097/00003226-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Grupcheva CN, Craig JP, Sherwin T, et al. Differential diagnosis of corneal oedema assisted by in vivo confocal microscopy. Clin Exp Ophthalmol. 2001;29:133–137. doi: 10.1046/j.1442-9071.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- 27.Alomar TS, Al-Aqaba M, Gray T, et al. Histological and confocal microscopy changes in chronic corneal edema: implications for endothelial transplantation. Invest Ophthalmol Vis Sci. 2011;52:8193–8207. doi: 10.1167/iovs.11-8047. [DOI] [PubMed] [Google Scholar]
- 28.Melles GR, Eggink FA, Lander F, et al. A surgical technique for posterior lamellar keratoplasty. Cornea. 1998;17:618–626. doi: 10.1097/00003226-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Terry MA, Ousley PJ. Replacing the endothelium without corneal surface incisions or sutures: the first United States clinical series using the deep lamellar endothelial keratoplasty procedure. Ophthalmology. 2003;110:755–764. doi: 10.1016/S0161-6420(02)01939-5. [DOI] [PubMed] [Google Scholar]
- 30.Price FW, Jr, Price MO. Descemet’s stripping with endothelial keratoplasty in 200 eyes: Early challenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32:411–418. doi: 10.1016/j.jcrs.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 31.Lee WB, Jacobs DS, Musch DC, et al. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–1830. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Melles GR, Ong TS, Ververs B, et al. Descemet membrane endothelial keratoplasty (DMEK) Cornea. 2006;25:987–990. doi: 10.1097/01.ico.0000248385.16896.34. [DOI] [PubMed] [Google Scholar]
- 33.Price MO, Giebel AW, Fairchild KM, et al. Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116:2361–2368. doi: 10.1016/j.ophtha.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Borkar DS, Veldman P, Colby KA. Treatment of Fuchs endothelial dystrophy by Descemet stripping without endothelial keratoplasty. Cornea. 2016;35:1267–1273. doi: 10.1097/ICO.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 35.Adamis AP, Filatov V, Tripathi BJ, et al. Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38:149–168. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 36.Pitts DG, Cullen AP, Hacker PD. Ocular effects of ultraviolet radiation from 295 to 365 nm. Invest Ophthalmol Vis Sci. 1977;16:932–939. [PubMed] [Google Scholar]
- 37.Rosenblum P, Stark WJ, Maumenee IH, et al. Hereditary Fuchs’ dystrophy. Am J Ophthalmol. 1980;90:455–462. doi: 10.1016/s0002-9394(14)75011-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.