Abstract
Motor deficits persisting into childhood (>7 years) are associated with increased executive and cognitive dysfunction, likely due to parallel neural circuitry. This study assessed the longitudinal trajectory of motor deficits in preschool children with ADHD, compared to typically developing (TD) children, in order to identify individuals at risk for anomalous neurological development. Participants included 47 children (21 ADHD, 26 TD) ages 4–7 years who participated in three visits (V1, V2, V3), each one year apart (V1=48–71 months, V2=60–83 months, V3=72–95 months). Motor variables assessed included speed (finger tapping and sequencing), total overflow, and axial movements from the Revised Physical and Neurological Examination for Subtle Signs (PANESS). Effects for group, visit, and group-by-visit interaction were examined. There were significant effects for group (favoring TD) for finger tapping speed and total axial movements, visit (performance improving with age for all 4 variables), and a significant group-by-visit interaction for finger tapping speed. Motor speed (repetitive finger tapping) and quality of axial movements are sensitive markers of anomalous motor development associated with ADHD in children as young as 4 years. Conversely, motor overflow and finger sequencing speed may be less sensitive in preschool, due to ongoing wide variations in attainment of these milestones.
Introduction
Motor and cognitive control: shared neural circuitry
The functional maturation of human motor and executive control systems occurs in parallel—likely due to shared neural circuitry (Mahone et al., 2006; Martin, Tigera, Denckla, & Mahone, 2010). Consequently, the presence of early motor anomalies may represent a readily observable biomarker for the developmental integrity of other contiguous brain regions (Denckla, 2005; Nichols & Chen, 1981). For example, research indicates motor overflow, a potential indicator of central nervous system (CNS) anomalies, is associated more with mental/developmental age, rather than chronological age (Cohen, Taft, Mahadeviah, & Birch, 1967). Brain regions supporting motor control undergo rapid development in the early years of life and continue to mature into young adulthood (Mahone et al., 2006; Roeder et al., 2008). While more basic (repetitive) motor skills are well developed by age 6–7 years, efficiency of more complex (sequenced) movements (Martin et al., 2010) and disappearance of nonessential associated movements (e.g., overflow) occur much later in development, due in part to the more protracted period of cortical and transcallosal myelination supporting motor inhibitory control (Hoy, Fitzgerald, Bradshaw, Armatas, & Georgiou-Karistianis, 2004; Koerte et al., 2010). Thus, when “subtle” motor signs that are not considered abnormal during the early years persist into adolescence and beyond, they are often associated with continued executive dysfunction and cognitive inefficiency (Cole, Mostofsky, Larson, Denckla, & Mahone, 2008; D’Agati, Casarelli, Pitzianti, & Passarotti, 2010; Gidley Larson et al., 2007; Koerte et al., 2010; Vitiello, Ricciuti, Stoff, Behar, & Denckla, 1989).
Motor speed
Age-related improvements in motor speed are associated with motor system development (Denckla, 1973), with speed of performance typically reaching plateau by age 10 years (Gidley Larson et al., 2007). Observing distinct patterns of skill development, Martin et al. (2010) noted that there may be important developmental differences between two types of movements underlying speed of motor response: repetitive (less controlled) and sequenced (patterned, more controlled). Specifically, repetitive movements (e.g., finger tapping, foot tapping) represent a more basic, earlier developing function, while sequenced movements (e.g., finger succession, heel–toe successions) represent later developing, higher order skills, involving more widespread cerebral representation. Within child clinical populations, sequenced (but not repetitive) movements show associations with higher cognitive control skills, including IQ (Martin et al., 2010). Furthermore, relative to typically developing (TD) children, those with ADHD manifest deficits in speed on both repetitive and sequenced movements (Cole et al., 2008), although it remains unclear whether the developmental trajectory of these deficits (and thus sensitivity of the motor findings) is similar across both repetitive and sequenced movements. Examination of the early development of these skills in children with and without ADHD can potentially shed light on the unique neural substrates supporting different elements of motor control.
Subtle signs: motor overflow
Overflow movements, defined as co-movement of body parts not specifically needed to complete a task, are considered to represent failure of inhibition of prepotent movement. In TD children, these movements decline with age, in parallel with maturation (myelination) of the cortex and cortical–spinal tracts. Presence of most mirror overflow movements is expected in younger children but gradually diminishes by age 10–14 years (Addamo, Farrow, Hoy, Bradshaw, & Georgiou-Karistianis, 2007; Gidley Larson et al., 2007; Hoy et al., 2004), with rapid changes occurring between ages 5 and 8 years (Wolff, Gunnoe, & Cohen, 1983). Persistent or delayed reduction of overflow may reflect anomalous development of cortical–striatal systems involved in motor inhibition (Davis, Pass, Finch, Dean, & Woodcock, 2009; Gidley Larson et al., 2007), including cortical myelination (D’Agati et al., 2010), and/or deficits in interhemispheric transfer (corpus callosum)—particularly given the observation of reduced transcallosal inhibition (i.e., ipsilateral activation theory) and high excitatory response threshold in children with ADHD (Addamo et al., 2007; Gidley Larson et al., 2007). Failure to inhibit involuntary movements when developmentally appropriate may also indicate a higher risk for subsequent difficulties in attention and executive control—key features of ADHD (Davis et al., 2009; Mahone et al., 2006).
Motor coordination and balance: axial skills
Axial motor skills include balance and motor coordination (including gait control)—skills that have been traditionally linked to cerebellar function (Diamond, 2000). Through parallel circuitry with executive control systems, the cerebellum is believed to play a role in higher order cognitive processes, such as visuospatial functioning, learning and memory, language, executive functioning, and has been associated with behavioral deficits observed in ADHD (O’Halloran, Kinsella, & Storey, 2012). School-aged children with ADHD are commonly observed to have deficits in motor coordination and balance (Piek et al., 2004), while gait and balance skills in TD children show minimal improvement after age 7 years, suggesting that among these children, such skills reach maturity (adult level) in late preschool to early elementary school years (Gidley Larson et al., 2007). Taken together, these findings suggest that axial skills may be sensitive to developmental anomalies when assessed prior to age 7 years.
Summary
The purpose of this study was to concurrently examine the developmental trajectory of ADHD symptoms and motor skills (including those likely to discriminate children with and without ADHD during the preschool years), in order to better understand how these “delays” unfold and change over time, and which types of motor skills are best able to predict changes in behavioral function associated with ADHD. It was hypothesized that (relative to age- and sex-matched TD controls) preschool children with ADHD would manifest parallel deficits in motor control and ADHD symptoms and that the longitudinal trajectory of improvement over a 3-year observation period would be attenuated in those with ADHD and associated with patterns of symptom change.
Method
Study procedures
Study approval was granted from the Johns Hopkins Medicine Institutional Review Board. Parents of potential participants completed preenrollment telephone interviews that included a detailed description of study procedures and a medical/development screening to determine eligibility. Parents of all eligible participants provided written consent and all participants provided assent. During each study visit, participants completed a neuropsychological assessment battery, including an IQ test and the PANESS. Parents also completed behavioral rating scales at the time of each visit. The assessment battery completed on the initial visit included additional on-site eligibility screening (described below).
Participant inclusion and exclusion criteria
Participants were recruited from local preschools, daycare centers, community resource centers, pediatrician’s offices, websites, and local and national periodicals. Participants were enrolled into the study at visit one (V1) between ages 48 and 71 months. Each participant was subsequently assessed annually in two additional study waves: visit two (V2) between the ages 60 and 83 months, and visit three (V3) between the ages of 72 and 95 months. At study entry, participants were excluded for any of the following, established via review of medical/developmental history, and/or by study assessment: (1) intellectual disability, developmental language disorder, autism spectrum disorder, or measured FSIQ < 80; (2) visual impairment; (3) current treatment of any psychiatric disorder (other than ADHD) with psychotropic medications, and for ADHD other than stimulants; (4) neurological disorder (e.g., epilepsy, traumatic brain injury); (5) documented hearing loss ≥25 dB loss in either ear; and (6) evidence of current physical, sexual, or emotional abuse. Participants were also excluded from the present data analysis if they were not retained for all three annual study visits or if they were unable to complete all aspects of the PANESS at each visit.
ADHD group criteria
Once children met general inclusion/exclusion criteria, they were included in the ADHD group based on criteria modified from the NIH Preschool ADHD Treatment studies (Kollins et al., 2006). Diagnosis of ADHD was made using modified DSM-IV-TR criteria based on parent report on the Diagnostic Interview Schedule for Children-Young Child (YC-DISC; used for 4-year olds) (Lucas, Fisher, & Luby, 2008) or Diagnostic Interview for Children and Adolescents, Fourth Edition (DICA-IV; used for 5-year olds) (Reich, Shayka, & Taibleson, 1991), and the DSM-IV ADHD Scales (Scales L and M) of the Conners’ Rating Scales-Revised (CRS-R) (Conners, 1997). To be included in the ADHD group, children were required to have a positive ADHD diagnosis on the YC-DISC or DICA-IV, and T-score ≥ 65 on at least one of the DSM-IV ADHD Scales (Scale L—Inattention, Scale M—Hyperactivity-Impulsivity) on the Conners’ Parent Rating Scale-Revised (CPRS-R) or Conners’ Teacher Rating Scale-Revised (CTRS-R) (Conners, 1997). Additionally, for inclusion in the ADHD group, parents needed to report presence of symptoms for at least 6 months and evidence of cross-situational impairment (defined as parent report of problems at home and with peers, as not all children were enrolled in school). At study enrollment, all children enrolled in the ADHD group were medication naïve; however, if children were subsequently prescribed stimulant medication for ADHD management, parents were asked to refrain from giving the stimulant medication to the child on the day of and day prior to the second and third study visits.
TD group criteria
Upon meeting the general inclusion/exclusion criteria, children were enrolled in the TD group if they did not meet categorical diagnostic criteria for ADHD (based on parent interview or Conners’ Scales) and were free of immediate family members (sibling, parent) with ADHD. In addition, children in the control group were required to have T-scores < 65 on all DSM-related ADHD scales of the CPRS and CTRS.
Study assessment measures
CPRS-R and CTRS-R (Conners, 1997)
Dimensional ratings of ADHD symptom severity were obtained using the DSM-IV-oriented scales from the CPRS-R and CTRS-R, including Scale L (DSM-IV Inattentive) and Scale M (DSM-IV Hyperactive/Impulsive).
Revised physical and neurological assessment of subtle signs—PANNESS (Denckla, 1985)
The PANESS is a quantified motor examination comprised untimed and timed tasks. Untimed motor tasks include gaits on heels, toes, and sides of feet (and parallel overflow/postures); tandem gait forward/backward; standing/hopping on one foot; standing heel-to-toe with eyes closed; standing both feet together, arms outstretched with eyes closed (and choreiform). Timed tasks include a sequence of 20 toe taps, hand pats, and finger taps; 10 “heel-toe,” 10 hand pronate-supinate and tongue side-to-side; and 5 sequences of finger appositions (left and right sides). The PANESS has been found to have adequate test–retest reliability (Holden, Tarnowski, & Prinz, 1982), inter-rater reliability, and internal consistency (Vitiello et al., 1989).
Due to ongoing development of hand preference and increased prevalence of mixed hand dominance during the age range assessed in the study, performance of the right and left hands were combined into one variable for Finger Tapping and Finger Sequencing tasks (Denckla, 1973; Roeder et al., 2008). For the present study, Finger tapping speed was calculated using the total combined time of the 20 finger taps on the right + left hands. Finger sequencing speed was calculated as the combined time to complete five sequences of finger appositions on right + five sequences on left. Total overflow was calculated as the presence of overflow/posture during the specific untimed task assessing gaits on heels, toes, and sides of feet as well as the proximal, orofacial, and mirror overflow present during all timed tasks. Total axial movements were calculated as the sum of (1) gait errors (left + right) occurring during “stressed” gaits (heels, toes, sides of feet), (2) gait errors occurring during tandem walking (forward and backward), and (3) errors occurring during station and balance tasks (i.e., tandem stance, standing on one leg, hopping on one foot). For all variables assessed, higher scores indicate poorer performance.
Data analyses
The effects of group, visit, and the group-by-visit interaction for ADHD symptoms and motor skill variables were examined through linear mixed effects (LME) models, with posthoc inquiry of visit and group differences at V1 vs. V2 and V2 vs. V3.
Results
Details of demographic information are included in Table 1. The final sample included 47 children, each of whom was seen for three annual visits. When comparing the final study sample to excluded participants, there were no significant group differences in sex (p = .441), socioeconomic status (p = .883), or FSIQ (p = .527); however, the final sample was older at age of enrollment compared to those who were excluded (p = .004). The sample included 21 children with ADHD (11 boys) and 26 TD children (19 boys). Of the 20 participants who had Conners’ data at the time of V2 and V3, 14 of the participants continued to have a T-score ≥ 65 on at least one of the DSM-IV ADHD Scales (Scale L—Inattention, Scale M—Hyperactivity-Impulsivity) at both visits; 6 participants no longer had a T-score ≥ 65 on at least one of the DSM-IV ADHD Scales (Scale L—Inattention, Scale M—Hyperactivity-Impulsivity) on the CPRS-R at V2 and V3. Handedness was determined by demonstration of a series of unimanual tasks (e.g., writing, throwing, using scissors, combing hair). The ADHD group included 17 right-, 2 left-, and 2 mixed-handed children, while the TD group included 22 right-, 3 left-, and 1 mixed-handed participant. The sample was primary Caucasian (TD group: 85%, ADHD group: 90%). There were no significant group differences in sex [χ2(2) = 2.16, p = .222], racial distribution [χ2(2) = 0.89, p = .638], or handedness [χ2(2) = 0.65, p = .723] at baseline (V1) visit. Additionally, there were no significant group differences in age or Full Scale IQ, at any of the three visits. Compared to the TD group, children ADHD had significantly lower processing speed quotient at V1 and V2 (both p ≤ .05), but not at V3 (Table 1).
Table 1.
Participant demographic information at each visit.
| Visit 1 | Visit 2 | Visit 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||
| ADHD | TD | p | ADHD | TD | p | ADHD | TD | p | |||||||
|
|
|
|
|
|
|
||||||||||
| M (SD) | Min–max | M (SD) | Min–max | M (SD) | Min–max | M (SD) | Min–max | M (SD) | Min–Max | M (SD) | Min–Max | ||||
| Age (months) | 63.00 (6.89) | 49.92–71.87 | 60.37 (6.10) | 49.55– 71.74 | .171 | 74.23 (6.87) | 61.92– 84.72 | 72.38 (6.14) | 60.96– 83.04 | .334 | 86.17 (6.91) | 73.99– 95.88 | 83.85 (6.63) | 72.00– 95.04 | .249 |
| FSIQ | 108.86 (13.97) | 89–146 | 111.68 (15.01) | 79–134 | .516 | 108.81 (13.35) | 86–143 | 114.50 (13.06) | 74–135 | .148 | 108.33 (15.27) | 88–143 | 113.82 (13.84) | 87–140 | .322 |
| PSQ | 96.29 (17.29) | 68–128 | 105.24 (13.36) | 83–137 | .054 | 96.43 (19.85) | 64–128 | 107.04 (14.81) | 68–134 | .041 | 97.67 (21.10) | 73–143 | 108.94 (17.03) | 80–145 | .123 |
| CPRS-R L (raw) | 15.71 (5.91) | 4–24 | 2.28 (3.45) | 0–11 | <.001 | 4–22 | 3.16 (3.66) | 0–13 | <.001 | 12.90 (5.31) | 2–23 | 3.92 (5.46) | 0–21 | <.001 | |
| CPRS-R L (T-score) | 74.95 (13.16) | 48–90 | 44.80 (6.40) | 40–61 | <.001 | 65.10 (11.04) | 50–83 | 46.20 (6.73) | 40–65 | <.001 | 63.85 (10.68) | 46–90 | 46.96 (8.84) | 40–74 | <.001 |
| CPRS-R M (raw) | 15.76 (5.75) | 4–27 | 3.64 (4.05) | 0–12 | <.001 | 13.30 (6.42) | 2–26 | 3.96 (3.40) | 0–12 | <.001 | 13.75 (6.58) | 3–26 | 4.77 (5.30) | 0–21 | <.001 |
| CPRS-R M (T-score) | 70.43 (11.78) | 49–90 | 46.04 (6.77) | 39–60 | <.001 | 66.45 (13.10) | 47–89 | 47.92 (6.28) | 39–63 | <.001 | 68.35 (13.05) | 49–89 | 50.58 (9.82) | 41–80 | <.001 |
One participant had initial FSIQ of 79; however, since that individual also had VCI = 90, he was retained in the study. ADHD: Attention-deficit/hyperactivity disorder; TD: typically developing; FSIQ: Full Scale IQ; PSQ: processing speed quotient; CPRS-R: Conners’ Parent Rating Scale-Revised; L scale: DSM Inattention; M Scale: DSM Hyperactivity/Impulsivity.
Longitudinal group comparisons
ADHD inattention symptoms
LME analyses examining parent rating of ADHD symptoms (CPRS-R DSM-IV Inattention Scale—raw score) yielded the expected large effect for group [F(2, 131) = 164.4, p < .001], no effect for visit [F(2, 131) = 0.636, p = .531], and a significant group-by-visit interaction [F(2, 135) = 2.98, p = .054]. While there were significant group differences in inattention symptoms at all three visits (Table 1), the interaction effect was driven by diverging trajectories of symptom improvement. Within the ADHD group, there was evidence of marginally reduced symptom reduction between V1 and V2 [F(1,39) = 3.51, p = .069], but not for the TD group [F(1,48) = 0.77, p = .386], whereas neither the ADHD (p = .829) nor the TD group (p = .562) showed symptom change between V2 and V3 (Figure 1).
Figure 1.
Mean inattentive symptom scores on the Conners’ Parent Rating Scale between ADHD and TD children across all three visits.
ADHD hyperactivity–impulsivity symptoms
Across all three visits, the ADHD group was rated significantly higher for hyperactive–impulsive symptoms (CPRS-R DSM-IV Hyperactive-Impulsive Scale—raw score) [F(1, 131) = 125.3, p < .001]; however, there were no significant effects for visit [F(2, 131) = 0.47, p = .627] or the group-by-visit interaction [F(2, 131) = 1.21, p = .302], suggesting minimal change in hyperactive–impulsive symptoms for either group across visits. There were significant group differences in parent-rated hyperactive–impulsive symptoms at each of the three visits (Table 1).
Longitudinal group comparisons—motor skills
Finger tapping
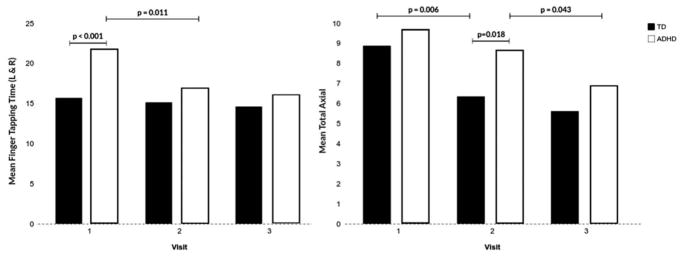
Examination of repetitive finger tapping speed across all visits revealed significant effects for group—ADHD slower than TD [F(1, 135) = 16.40, p < .001], visit—speed improving over time [F(2, 135) = 7.51, p = .001], and a group-by-visit interaction [F(2, 135) = 3.87, p = .023]. The interaction effect was marked by significant group differences (favoring the TD group) at V1 (p < .001), but not at V2 or V3 (Table 2). This pattern was also driven by significant improvement in speed between V1 and V2 within the ADHD group [F(1,40) = 7.20, p = .011], but not the TD group [F(1,50) = 0.35, p = .558]. Neither the ADHD (p = .536) nor TD groups (p = .586) showed significant change in finger tapping speed between V2 and V3 (Figure 2). In contrast, the ADHD group demonstrated significant change in finger tapping speed between V1 and V3 [F(1,40) = 8.89, p = .005], but the TD group did not [F(1,50) = 1.44, p = .236].
Table 2.
PANESS variables for ADHD and TD participants at each visit.
| Visit 1 | Visit 2 | Visit 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||
| ADHD | TD | p | ADHD | TD | p | ADHD | TD | p | |||||||
|
|
|
|
|
|
|
||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | ||||
| Total overflow | 12.81 | 4.15 | 11.04 | 4.63 | .179 | 9.86 | 5.26 | 7.92 | 4.21 | .168 | 9.62 | 5.58 | 8.81 | 4.79 | .594 |
| Finger tapping | 21.68 | 7.31 | 15.70 | 3.16 | <.001 | 16.86 | 3.81 | 15.15 | 3.60 | .121 | 16.03 | 4.68 | 14.62 | 3.34 | .232 |
| Finger sequence | 36.61 | 9.81 | 35.35 | 11.53 | .693 | 32.14 | 7.99 | 30.21 | 8.04 | .416 | 23.22 | 5.63 | 22.46 | 5.17 | .629 |
| Total axial | 9.67 | 2.92 | 8.88 | 2.94 | .368 | 8.62 | 2.82 | 6.35 | 3.38 | .018 | 6.86 | 2.63 | 5.62 | 3.62 | .195 |
PANESS: Revised Physical and Neurological Assessment of Subtle Signs; ADHD: attention-deficit/hyperactivity disorder; TD: typically developing.
Figure 2.
Mean performance scores on finger tapping and total axial on the PANESS between ADHD and TD children across all three visits.
Finger sequencing
LME analyses examining finger sequencing speed across all three visits revealed a significant effect for visit—speed improving over time [F(2, 135) = 29.37, p < .001], but not for group [F(1, 135) = 0.87, p = .354], or the group-by-visit interaction [F(2, 135) = 0.06, p = .945]. Across groups, speed improved significantly between V1 and V2 [F(1,90) = 5.93, p = .017], between V2 and V3 [F(1,90) = 34.64, p < .001], and also between V1 and V3 [F(1,90) = 55.12, p < .001]. Within both the ADHD and TD groups, finger sequencing speed improved significantly between V1 and V3 (p < .001).
Total overflow
LME analyses examining overflow across all three visits revealed a significant effect for visit—overflow improving over time [F(2, 135) = 5.67, p = .004], but not for group [F(1, 135) = 3.47, p = .065], or the group-by-visit interaction [F(2, 135) = 0.88, p = .829]. Across groups, overflow improved (reduced) significantly between V1 and V2 [F(1,90) = 10.26, p = .02], but not between V2 and V3 [F(1,90) = 0.10, p = .753]. There was a significant reduction in total overflow between V1 and V3 [F(1,90) = 7.40, p = .008] when combining groups; however, this was driven by the fact that between V1 and V3, the ADHD group demonstrated significant reduction of overflow (p = .042), but the TD group did not (p = .094).
Total axial
Examination of the total axial score yielded significant effects for group—favoring the TD group [F (1, 135) = 7.43, p = .007] and visit—performance improving over time [F(2, 135) = 11.27, p < .001], but no significant group-by-time interaction [F(2, 135) = 0.70, p = .497]. For the TD group, performance improved significantly between V1 and V2 [F(1,50) = 8.34, p = .006], but not between V2 and V3 [F(1,50) = 0.57, p = .456]. In contrast, the ADHD group did not show improvement between V1 and V2 [F(1,40) = 1.40, p = .244] but instead showed significant later improvement in performance between V2 and V3 [F(1,40) = 4.38, p = .043]. Both the ADHD group [F(1,40) = 10.72, p = .002] and TD group [F(1,50) = 12.75, p = .001] demonstrated improvement in total axial score between V1 and V3. Examination of group differences by visit revealed significantly poorer performance by the ADHD group at V2 (p = .018), but not at V1 (p = .368) or V3 (p = .195; Table 2, Figure 2).
Discussion
For preschool children with and without ADHD, motor control and speed improve rapidly between ages 4 and 7 years, with the most rapid changes occurring before age 6 years. By age 4 years, children with ADHD manifest significant deficits in repetitive motor speed and axial motor control, compared to TD children without ADHD. Moreover, while these skills continue to improve, the timing of most rapid improvement appears to occur in conjunction with developmental changes in core ADHD symptomatology. Conversely, overflow and sequenced movements (both affected in older children with ADHD) are not deficient (relative to age-matched controls) in preschool children with ADHD, suggesting a differential sensitivity of more “basic” vs. higher order elements of motor control during the preschool years. These observations support the idea that while attentional and motor control develop in parallel, these associations (like the skills themselves) show important developmental changes over time.
A distinction has been suggested between the brain systems associated with basic motor control and those involving more complex, higher cognitive order motor movements. In particular, tasks involving basic repetitive motor speed are typically mastered (i.e., reaching “adult” level of competence) at an earlier age, likely due to the timing of the maturation of associated motor/premotor brain regions and cortical systems. Conversely, neural networks involved in more complex motor movements, such as finger sequencing, are linked to brain systems that develop later, including more widespread (pre)frontal cortical systems (Martin et al., 2010; Roeder et al., 2008). Furthermore, due to the continued myelination of the nervous system and development of transcallosal inhibition, presence of overflow movements is not unexpected in younger children and not considered a biomarker sensitive to abnormality until after the age 10–14 years (Gidley Larson et al., 2007; Hoy et al., 2004; Koerte et al., 2010). In fact, it is often not until after age 9 years that children with CNS abnormalities demonstrate differences (relative to same age TD peers) in the presence of motor overflow movements (Cohen et al., 1967).
Based on the observation of different patterns of motor overflow and IQ in younger (before age 10 years) vs. older children (after age 10 years), the associations between motor and cognitive control are best understood through a developmental approach to assessment (Cohen et al., 1967). Using this perspective, it is critical to identify early signs of anomalous motor development in order to monitor for and predict later cognitive difficulties (Cohen et al., 1967; Nichols & Chen, 1981). Indeed, children with ADHD show delays in maturation of brain networks that are integral to development of higher cognitive and behavioral control, including maturational delays in gray and white matter volumes, basal ganglia structures, cortical thickness, and overall cerebral and cerebellar volume (Vaidya, 2012). The delay in cortical thickness of the cerebrum, most prominent in the prefrontal regions, can be seen in the fact that TD children reach peak cortical thickness around 7 years, but children with ADHD do not reach peak levels until age 10 years (Shaw et al., 2007). Moreover, the subsequent slower rate of cortical thinning seen in ADHD has also been linked to severity of hyperactive and impulsive symptom presentation, further supporting the neurological differences and behavioral manifestations of ADHD during the early years (Shaw et al., 2011).
The age range assessed in this study is a period of accelerated development with regard to behavioral presentation and motor skills, suggesting that during this time, children with ADHD may begin to manifest developmental differences compared to their TD peers. Consistent with existing research, motor skills continue to develop through childhood, but the rate of skill acquisition decelerates after age 6 years for both TD children and those with ADHD. Therefore, many of the skills assessed on the PANESS, such as repetitive finger tapping speed (Martin et al., 2010) and axial skills (Gidley Larson et al., 2007), have reached ceiling levels by age 7 years for TD children, but presence of overflow and finger sequencing speed continue to undergo rapid changes beyond age 7 years. However, due to developmental delay, ADHD children demonstrated increased deficits in basic motor speed and balance and coordination as well as increased overflow compared to TD children at the final visit of the study (age 7 years, 11 months), suggesting that these skills had not yet fully matured (plateaued) during the expected time period. Therefore, while overflow may be a sensitive biomarker of neurodevelopmental vulnerability in older children (Cohen et al., 1967), evaluation of basic motor speed, balance, and coordination may represent a more valid way to characterize CNS vulnerability at earlier ages (i.e., prior to age 7 years). Importantly, when critical motor anomalies are identified in the preschool years, there is greater opportunity for close monitoring and implementation of early intervention.
One limitation of this study was the use of modified DSM-IV criteria for the ADHD group assignment at time of study enrollment. Due to the necessity of cross-sectional impairment, the diagnostic criteria for ADHD are best utilized for a population that has regular access to an academic setting. However, due to the young age at study enrollment (48–71 months), many of the children were not enrolled in school and, thus, did not have regular access to teacher ratings. For these situations, parents were required to report problems within the home and a second setting (e.g., peers) in order to best meet the criteria for cross-sectional impairment. This study was also limited by the small sample size due to a desire to only include eligible participants who were able to complete the PANESS at all three study visits. Younger age at enrollment appeared to predict an increased risk for dropout during the study, which is not unexpected since younger children had more difficultly completing the entire PANESS (and other study components) at visit one. Future directions may include attempting to use a larger sample size of both ADHD and TD children. Additionally, it may be interesting to further assess behavioral differences between the children included in the sample and those who were excluded based on inability to complete the PANESS (both within the ADHD and TD groups). Such inquiry may include determining whether or not the inability to complete the PANESS at age 4 years is a predictor of increased symptom presentation and functional impairment during childhood and adolescence. Future research may also include assessing the same preschool cohort used in this sample after age 10 years in order to determine developmental trajectory of motor skills that had not plateaued by age 7 years (i.e., overflow and finger sequencing speed). Additionally, previous research indicated that the ability to hop on one foot at age 4 was the best predictor of “hyperactivity” at age 7 (Nichols & Chen, 1981); so, examination of this particular variable on the PANESS may provide further information regarding a sensitive marker during the preschool years for motor deficits and subsequent ADHD.
Overall, the current study provided important information regarding the developmental trajectory of motor skills and ADHD symptoms during the preschool years. Specifically, children with and without ADHD experience not only reduction of motor overflow but also consistent improvements in speed when performing more complex, high-order motor movements (i.e., finger sequencing) during the preschool years. However, children with ADHD demonstrate a developmental delay in improvements of basic motor speed (i.e., finger repetition) and axial skills (i.e., balance and coordination) prior to age 7 years. Such behavioral delays concomitant with the previously observed delays in the development and maturation of brain regions associated with ADHD (Shaw et al., 2007, 2011; Vaidya, 2012); this study provides further understanding of brain–behavior relationships during a period of rapid neurological development (i.e., preschool age) when the implementation of early interventions is critical to future impairment.
Acknowledgments
A portion of this study was presented at the Annual Meeting of the International Neuropsychological Society, February 4, 2017, New Orleans, Louisiana, USA. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS, or NIH.
Funding
This work was supported by the National Institutes of Health: [Grant Numbers 1R01 HD068425, U54 HD079123, UL1 TR000424]; Johns Hopkins Brain Sciences Institute.
References
- Addamo PK, Farrow M, Hoy KE, Bradshaw JL, Georgiou-Karistianis N. The effects of age and attention on motor overflow production–A review. Brain Research Reviews. 2007;54(1):189–204. doi: 10.1016/j.brainresrev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Taft LT, Mahadeviah MS, Birch HG. Developmental changes in overflow in normal and aberrantly functioning children. The Journal of Pediatrics. 1967;71(1):39–47. doi: 10.1016/s0022-3476(67)80228-2. [DOI] [PubMed] [Google Scholar]
- Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71(19):1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Conners’ rating scales - revised technical manual. North Tonawanda, New York: Multi-Health Systems Inc; 1997. [Google Scholar]
- D’Agati E, Casarelli L, Pitzianti M, Passarotti A. Overflow movements and white matter abnormalities in ADHD. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:441–445. doi: 10.1016/j.pnpbp.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Davis AS, Pass LA, Finch WH, Dean RS, Woodcock R. The canonical relationship between sensory-motor functioning and cognitive processing in children with attention-deficit/hyperactivity disorder. Archives of Clinical Neuropsychology. 2009;24:273–286. doi: 10.1093/arclin/acp032. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Development of speed in repetitive and successive finger-movements in normal children. Developmental Medicine Child Neurology. 1973;15:635–645. doi: 10.1111/j.1469-8749.1973.tb05174.x. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs. Psychopharmacology Bulletin. 1985;21(4):773–779. [PubMed] [Google Scholar]
- Denckla MB. Why assess motor functions “Early and often?”. Mental Retardation and Developmental Disabilities Research Reviews. 2005;11(3):1. doi: 10.1002/mrdd.20054. [DOI] [PubMed] [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH, Goldberg MC, Cutting LE, Denckla MB, Mahone EM. Effects of gender and age on motor exam in typically developing children. Developmental Neuropsychology. 2007;32(1):543–562. doi: 10.1080/87565640701361013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden EW, Tarnowski KJ, Prinz RJ. Reliability of neurological soft signs in children: Reevaluation of the PANESS. Journal of Abnormal Child Psychology. 1982;10(2):163–172. doi: 10.1007/BF00915938. [DOI] [PubMed] [Google Scholar]
- Hoy KE, Fitzgerald PB, Bradshaw JL, Armatas CA, Georgiou-Karistianis N. Investigating the cortical origins of motor overflow. Brain Research Reviews. 2004;46(3):315–327. doi: 10.1016/j.brainresrev.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Koerte I, Eftimov L, Laubender R, Esslinger O, Schroeder A, Ertl-Wagner B, … Danek A. Mirror movements in healthy humans across the lifespan: Effects of development and ageing. Developmental Medicine Child Neurology. 2010;52:1106–1112. doi: 10.1111/j.1469-8749.2010.03766.x. [DOI] [PubMed] [Google Scholar]
- Kollins S, Greenhill L, Swanson J, Wigal S, Abikoff H, McCracken J, … Bauzo A. Rationale, design, and methods of the Preschool ADHD Treatment Study (PATS) Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(11):1275–1283. doi: 10.1097/01.chi.0000235074.86919.dc. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Fisher P, Luby JL. Young Child DISC-IV: Diagnostic Interview Schedule for Children. New York, NY: Columbia University, Division of Children Psychiatry, Joy and William Ruane Center to Identify and Treat Mood Disorders; 2008. [Google Scholar]
- Mahone EM, Powell SK, Loftis CW, Goldberg MC, Denckla MB, Mostofsky SH. Motor persistence and inhibition in autism and ADHD. Journal of the International Neuropsychological Society. 2006;12:622–631. doi: 10.1017/S1355617706060814. [DOI] [PubMed] [Google Scholar]
- Martin R, Tigera C, Denckla MB, Mahone EM. Factor structure of paediatric timed motor examination and its relationship with IQ. Developmental Medicine and Child Neurology. 2010;52(8) doi: 10.1111/j.1469-8749.2010.03670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols P, Chen T. Minimal brain dysfunction: A prospective study. Hillside, New Jersey: Erlbaum Associates; 1981. [Google Scholar]
- O’Halloran C, Kinsella G, Storey E. The cerebellum and neuropsychological functioning: A critical review. Journal of Clinical and Experimental Neuropsychology. 2012;34(1):35–56. doi: 10.1080/13803395.2011.614599. [DOI] [PubMed] [Google Scholar]
- Piek JP, Dyck MJ, Nieman A, Anderson M, Hay D, Smith LM, … Hallmayer J. The relationship between motor coordination, executive functioning and attention in school aged children. Archives of Clinical Neuropsychology. 2004;19(8):1063–1076. doi: 10.1016/j.acn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. North Tonawanda: Multi-Health Systems; 1997. [Google Scholar]
- Roeder M, Mahone E, Gidley Larson J, Mostofsky S, Cutting L, Goldberg M, Denckla M. Left-right differences on timed motor examination in children. Child Neuropsychology. 2008;14:249–262. doi: 10.1080/09297040701370016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch J, Greenstein D, … Rapoport J. Attention-deficit/ hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liberpool M, Weddle C, Malek M, Sharp W, … Giedd J. Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: Support for a dimensional view of attention deficit hyperactivity disorder. American Journal of Psychiatry. 2011;168(2):143–151. doi: 10.1176/appi.ajp.2010.10030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ. Neurodevelopmental abnormalities in ADHD. Current Topics in Behavioral Neurosciences. 2012;9:49–66. doi: 10.1007/7854_2011_138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B, Ricciuti AJ, Stoff DM, Behar D, Denckla MB. Reliability of subtle (soft) neurological signs in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28(5):749–753. doi: 10.1097/00004583-198909000-00017. [DOI] [PubMed] [Google Scholar]
- Wolff PH, Gunnoe CE, Cohen C. Associated movements as a measure of developmental age. Developmental Medicine Child Neurology. 1983;25:417–429. doi: 10.1111/j.1469-8749.1983.tb13786.x. [DOI] [PubMed] [Google Scholar]