Summary
Despite the significance of red blood cell (RBC) alloimmunization, the lack of standardized registries in the US has prevented the completion of large studies. Data from 3.5 years of the Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) recipient database, containing information from 12 hospitals, were studied. A RBC alloantibody responder had an antibody identified at any point during the study, and a non-responder had a negative antibody screen at least 15 days post-RBC transfusion. Demographics, blood type, ICD9/10 codes, and other potential correlates were evaluated. Of 319 177 (2.07%) screened patients, 6597 had a total of 8892 clinically significant RBC alloantibodies identified, with 75% being in the Rh or Kell families. Alloimmunization was more common in females (2.38%) than males (1.68%), and in RhD negative (2.82%) than RhD positive (1.94%) patients. Age, sex, RhD status and race were associated with being a responder, and certain diagnoses (including sickle cell disease or trait, systemic lupus erythematosus, rheumatoid arthritis and myelodysplastic syndrome) were more common among responders than non-responders. Data collected in this multi-centre recipient database provide the largest RBC alloimmunized patient cohort studied in the US, with previously known demographic and disease associations of responder status confirmed, and new associations identified.
Keywords: red blood cells, alloimmunization, antibodies, transfusion
Transfusion of red blood cells (RBCs) is a therapeutic and lifesaving treatment for many patients. In the United States, approximately 13 million units of red cells are transfused annually (Whitaker et al, 2016). Transfusion, however, is not without risk. One adverse event that may occur with the transfusion of RBCs is the haemolytic transfusion reaction, which can result in mortality. Furthermore, an estimated one in 1200 transfused patients experience a delayed haemolytic transfusion reaction, which results in a diminished haemoglobin increment and can lead to bystander haemolysis in some patient populations (Pirenne et al, 2017). Cost prediction models suggest that billions of dollars are spent annually to prevent, investigate and treat haemolytic reactions (Kacker et al, 2014a,b).
The formation of a new red cell alloantibody, the pathophysiological agent for haemolytic transfusion reactions, requires differences in surface proteins (antigens) between the RBC of the blood donor and the recipient (Reid & Lomas-Francis, 2004). Given that the only antigens routinely matched between donors and RBC recipients are those in the ABO system and the RhD antigen, exposure to foreign RBC antigens occurs frequently. Nevertheless, historic studies indicate that the overall prevalence of alloimmunization is actually relatively small (Hoeltge et al, 1995), with few patients forming RBC alloantibodies despite the receipt of multiple RBC units (Schonewille et al, 2006; Higgins & Sloan, 2008; Zalpuri et al, 2012).
In addition to being induced by transfusion, RBC alloantibodies may be induced by pregnancy, where they may be detrimental to fetuses and newborns. Fetal anaemia, hyperbilirubinaemia, and even hydrops fetalis/death may result from maternal RBC alloimmunization, with as many as 1 in 300 to 1 in 600 births being affected to some extent by maternal RBC alloimmunization (Hendrickson & Delaney, 2016). One study, completed at a tertiary care referral centre, reported that as many as 1 in 80 pregnancies had maternal alloantibodies (Smith et al, 2013). Efforts to prevent pregnancy-associated RBC alloimmunization are currently limited to those cases involving the paternal RhD antigen, and treatments for hydropic fetuses are limited to intrauterine exchange transfusion, maternal treatment with intravenous immunoglobulin or, potentially, maternal therapeutic plasma exchange.
The explanation for why only a select group of patients (responders) form alloantibodies to donor RBCs despite multiple exposures through transfusion or pregnancy is both complex and poorly understood. Given the associated morbidity and mortality in transfusion and pregnancy, determining specific factors associated with increased risk for RBC alloimmunization has been a focus of active investigation. Separate studies have identified epidemiological factors, such as sex, race and certain medical conditions, to be independently predictive of alloantibody formation (Rosse et al, 1990; Seyfried & Walewska, 1990; Stiegler et al, 2001; Verduin et al, 2012; Chou et al, 2013; Lin et al, 2017). However, as alloimmunization is a relatively rare event, these studies have been limited by sample size.
The National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) has developed a transfusion recipient database that combines inpatient and outpatient electronic health records from four major blood centres and 12 community and academic hospitals in the United States. Over the course of the 3.5 years queried for this manuscript, this database has captured the results of over 300 000 antibody screens from over 600 000 patients. This current analysis aims to: (i) characterize the epidemiology of RBC alloimmunization in the United States within the REDS-III recipient database; (ii) define responder alloantibody specificity, and (iii) investigate independent recipient risk factors associated with RBC alloantibody formation.
Methods
Database structure
In brief, the REDS-III transfusion recipient database involves the collection of recipient laboratory, transfusion and clinical data from 12 hospitals served by four geographically diverse US blood centres located in Connecticut, Pennsylvania, Wisconsin and California. Each blood centre serves as a ‘hub’ with ‘spokes’ comprised of 2–4 selected hospitals including a mix of academic medical centres and community-based hospitals. Institutional Review Board approval for constructing this database was obtained by each participating institution and the data coordinating centre (DCC). Data collected in the REDS-III transfusion recipient database are subject to the NHLBI Policy for Data Sharing from Clinical Trials and Epidemiological Studies, for eventual release as a public use data set. The data fields captured in this database and its quality control have recently been described (Karafin et al, 2017).
Study variables
The present analysis includes data collected from the recipient database from 1 January 2013 to 30 June 2016. Data evaluated for this analysis included patient age, primary and other diagnoses, ABO type, sex, ethnicity, antibody screen results and antibody panel results. History concerning transfusion or pregnancy prior to 2013 was not available. To categorize admission diagnoses and comorbidities, International Classification of Diseases, ninth and tenth revision (ICD-9 and ICD-10) codes were grouped into Healthcare Cost and Utilization Project Clinical Classifications Software (HCUP-CCS) categories (https://www.hcupus.ahrq.gov/).
For the purposes of this study, a “responder” was defined as any patient with at least one detectable clinically significant RBC alloantibody that could have been induced by transfusion or pregnancy. Participating hospitals each used their own methods for determining the presence of RBC antibody and identifying its specificity. Methods used included solid phase, gel, and tube polyethylene glycol (PEG)-enhanced screening/antibody identification. Table S1 lists all RBC antibodies collected in the database and their classification for this study. Clinically significant alloantibodies included: D, C, c, E, e, Cw, G, V, f, Jsa, Kpa, K, k, Jka, Jkb, Fya, Fyb, M (IgG only), S, s, U and Lutheran. Patients with only warm autoantibodies, cold autoantibodies and non-specific antibodies were not counted as responders. Moreover, all women in whom an anti-D was the sole antibody detected were excluded from being classified as responders and were not included in the subsequent analyses, due to the inability to separate those who recently received Rh immune globulin from those who had an authentic transfusion- or pregnancy-induced alloantibody.
A ‘non-responder’ was defined as a patient with at least one RBC alloantibody screen completed 15 days or later after the recorded issue date of a RBC transfusion at one of the study hospitals, with no alloantibodies detected at that screen or at any other RBC antibody screen completed during the 3.5-year study period.
Statistical analysis
Categorical variables were summarized as frequencies with percentages, and continuous variables were summarized as means with standard deviations (s.d.) or medians with interquartile ranges (IQRs), as appropriate. The primary outcome of interest in this study was the presence of an alloantibody (responder status), and its association with other patient characteristics, such as patient age, sex, ethnicity, ABO type and diagnosis. Descriptive statistics were used to summarize the sampled characteristics. Patient-related factors were compared between responders and non-responders by a student’s t-test for continuous variables and Fisher’s exact test for categorical variables. Separate bivariate and multivariate logistic regression were used to model the odds of being an alloantibody responder, while considering the above-mentioned variables as possible covariates. A backward elimination model selection procedure was employed to identify statistically significant covariates to be added into the model. A statistical significance (alpha) level of 0.05 was used throughout. SAS/STAT software Version 9.4 of the SAS System for Windows was used to perform all statistical analyses.
Results
Patients studied
Over the period 2013–2016, data from 612 417 unique patients (inpatients and outpatients) were entered into the REDS-III recipient database. Approximately half of the individuals (319 177) in this database had a RBC antibody screen and were included for this analysis.
There were 6597 unique patients (2.07% of all patients with an antibody screen) with at least one clinically significant RBC alloantibody detected and defined as ‘responders’. Another 30 569 patients met the ‘non-responder’ definition (Fig 1). The % responders, defined as the percentage of responders/[(responders + non-responders)], is 17.8% overall.
Fig 1.
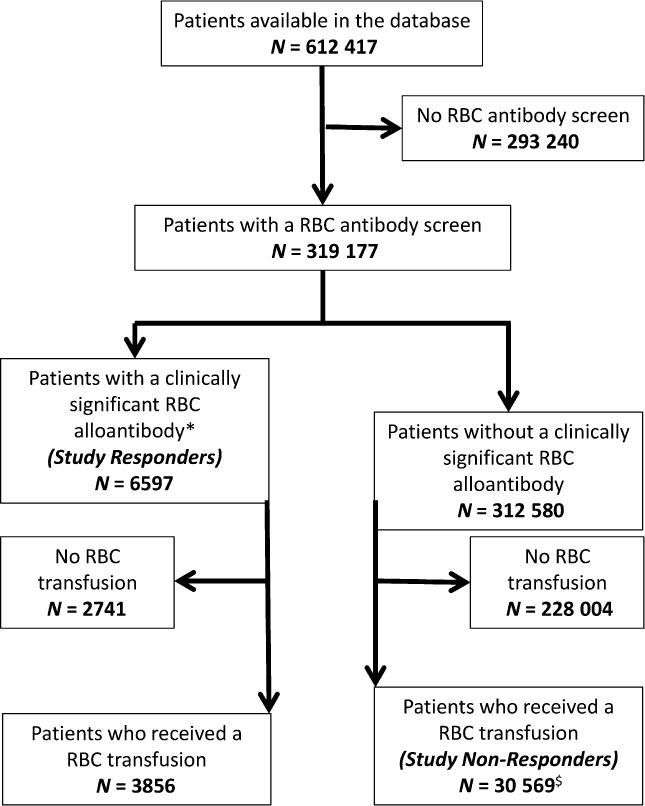
Study population overview. *Please refer to Table S1 for RBC alloantibody classification; anti-D antibodies in females were excluded. $ Patients who did not have an antibody screen completed 15 or more days post-RBC transfusion were excluded. RBC, red blood cell.
RBC antibody screening data
A total of 698 856 RBC antibody screens were completed over the course of the study. 33 352 antibody screens were positive (4 77%), with approximately 51% (17 097) of these positive screens ultimately resulting in an identified RBC alloantibody categorized as clinically significant in this study (Figure S1A and Table S1). Responders had a mean and median number of antibody screens of 5 and 2, respectively, with non-responders having 8 and 5, respectively.
A total of 3856 of the 6597 alloimmunized patients (58.45%) received a RBC transfusion during the study duration, with 2185 of the 6597 alloimmunized patients (33.12%) also having a RBC alloantibody screen at least 15 days post-transfusion. Taking the total denominator (32 754) of patients documented to have received a RBC transfusion and to have had a RBC alloantibody screen in that same time window into consideration, the alloimmunization rate subsequent to RBC transfusion is 2185/32 754 = 6.67% (Figure S1B).
A total of 8892 unique clinically significant antibodies were detected in the 6597 alloimmunized patients, including 4749 (53.41%) in the Rh family (2588 anti-E, 780 anti-C, 556 anti-c, 105 anti-e, and 525 anti-D (in males) among others), 1,937 (21.78%) in the K family, 912 (10.26%) in the Kidd family, 669 (7.44%) in the Duffy family, in 349 (3.92%) the MNS family, and 471 (5.3%) of other specificities (Fig 2). A total of 3291 antibodies were detected in the 2386 alloimmunized males, and 5601 antibodies were detected in the 4211 alloimmunized females. Two-hundred and ninety-eight (4.54%) of the responders also had warm autoantibodies detected, and 101 (1.59%) had cold autoantibodies detected. In contrast, only 0.46% of the non-responder patients had warm autoantibodies and 0.28% had cold autoantibodies detected. A total of 723 and 436 unique subjects, respectively, in the entire study, had warm or cold autoantibodies detected. Thus, 298/723 (41.22%) of patients with warm autoantibodies and 101/436 (23.17%) of patients with cold autoantibodies also had underlying RBC alloantibodies.
Fig 2.
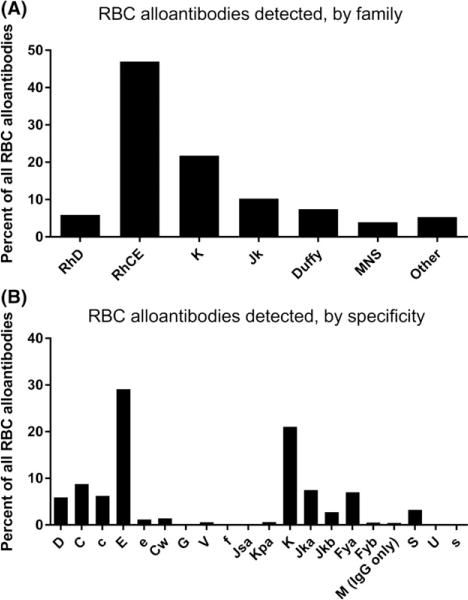
RBC alloantibody family and specificity. Red blood cell (RBC) alloantibodies detected by (A) family and (B) specificity.
Demographics of responders and non-responders
RBC alloimmunization and sex
In the entire study cohort, 284 485/612 419 patients (46.45%) were male and 327 630/612,419 (53.5%) were female. 2386/141 920 (1.68%) of males who underwent RBC alloantibody screening during the study period had RBC alloantibodies, and 4211/177 231 (2.38%) screened females had RBC alloantibodies. Limiting the analysis to patients who were RBC transfused and antibody screened during the 3.5-year study duration showed that 1498/41 865 = 3.58% of males were responders, compared to 2358/46 567 = 5.06% of females. A total of 36.2% of the 6597 alloimmunized patients were male, and 63.8% were female (Table I and Figure S2). A comparison of [responders/(responders + non-responders)] showed that 13.44% [2386/(2386 + 15 362)] of males by this calculation had RBC alloantibodies. This rate was significantly lower than females, where this same calculation showed that 21.69% [4211/(4211 + 15 206)] had RBC alloantibodies. The actual RBC alloimmunization rate in females was even higher than these numbers depict, as anti-D antibodies in all females were excluded from analysis given the inability to distinguish passively administered anti-D from transfusion- or pregnancy-induced anti-D in the database.
Table I.
Demographics of responders and non-responders.
| Demographic | Total, N | Patients with a RBC antibody screen, n | Responders, n | Non-Responders, n | *χ2 P value |
|---|---|---|---|---|---|
| All | 612417 | 319177 | 6597 | 30569 | |
| Sex | |||||
| Male | 284486 | 141920 | 2386 | 15362 | <0.0001 |
| Female | 327629 | 177231 | 4211 | 15206 | |
| Age category | |||||
| 6 months–12 months | 1078 | 251 | 1 | 38 | <0.0001 |
| 13 months–10 years | 12283 | 2596 | 25 | 403 | |
| 11 years–20 years | 25014 | 10096 | 73 | 577 | |
| 21 years–30 years | 63906 | 35381 | 351 | 1426 | |
| 31 years–40 years | 68969 | 39706 | 476 | 1723 | |
| 41 years–50 years | 70480 | 33871 | 676 | 3118 | |
| 51 years–60 years | 107270 | 55505 | 1210 | 6234 | |
| 61 years–70 years | 112088 | 62955 | 1421 | 7626 | |
| 71 years–80 years | 81854 | 45587 | 1335 | 5674 | |
| 81 years+ | 69468 | 33226 | 1029 | 3749 | |
| Race | |||||
| White | 415568 | 222231 | 4964 | 21638 | <0.0001 |
| Black | 88109 | 43167 | 988 | 5068 | |
| Asian | 14520 | 8306 | 87 | 721 | |
| Other | 94220 | 45473 | 558 | 3142 | |
| Ethnicity | |||||
| Hispanic or Latino | 48740 | 25592 | 430 | 2023 | 0.3505 |
| Not hispanic or Latino | 536481 | 278240 | 5812 | 27031 | |
| Other | 27196 | 15345 | 355 | 1515 | |
| Blood group | |||||
| A | 119682 | 117877 | 2429 | 11395 | 0.177 |
| B | 44504 | 43722 | 860 | 4287 | |
| O | 144821 | 141988 | 2968 | 13503 | |
| AB | 14213 | 13998 | 284 | 1363 | |
| Rh factor | |||||
| Rh(D) positive | 280816 | 275914 | 5365 | 26622 | <0.0001 |
| Rh(D) negative | 42256 | 41614 | 1175 | 3920 |
RBC, red blood cell.
χ2 analysis shows differences in responder/non-responder status based on demographic variables.
RBC alloimmunization and race/ethnicity
A total of 222 231 Whites, 43 167 Blacks, 8306 Asians and 45 473 patients of other descent had RBC antibody screens during the study period. Of these, 4964 Whites (2.23%), 988 Blacks (2.29%), 87 Asians (1.05%), 558 and patients of other descent (1.23%) had detectable RBC alloantibodies. A comparison of [responders/(responders + non-responders)] showed alloimmunization rates differed significantly by race; 18 66% [4964/(4964 + 21 638)] of Whites, 16.31% [988/(988 + 5068)] of Blacks, 10.77% [87/(87 + 721)] of Asians, and 14.96% [558/(558 + 3142)] patients of other descent by this calculation had RBC alloantibodies (Figure S3).
A total of 25 592 Hispanics and 278 240 non-Hispanics had RBC antibody screens during the study period. Of these, 430 Hispanics (1.68%) and 5812 non-Hispanics (2.09%) had detectable RBC alloantibodies. A comparison of responders and non-responders [responders/(responders + non-responders)] showed that 17.53% [430/(430 + 2023)] of Hispanics and 17.7% [5812/5812 + 27 031] of non-Hispanics by this calculation had RBC alloantibodies (Figure S3).
RBC alloimmunization and recipient Rh blood type
A total of 275 914 RhD positive patients and 41 614 RhD negative patients had antibody screens during the study period. Of these, 5365 RhD positive patients (1.94%) and 1175 RhD negative patients (2.82%) had detectable RBC alloantibodies. A comparison of %responders shows that 16.77% [5365/(5365 + 26,622)] of RhD positive patients and 23.06% [1175/(1175 + 3920)] of RhD negative patients by this calculation had RBC alloantibodies (Figure S4). Aside from anti-D, anti-C was significantly more likely to be detected in RhD negative patients (making up 27% of all antibodies) than in RhD positive patients (making up 4.9% of all antibodies).
RBC alloimmunization and recipient age
Few RBC alloimmunization studies have included both children and adults. The fact that some of the 12 hospitals studied in this database cared for paediatric patients allowed a comparison of alloimmunization rates by recipient age at the time of study entry. Only one child between 6 and 12 months of age (of 251 screened, 0.4%) had a RBC alloantibody (Table I). Only 25 children between 13 months and 10 years of age (of 2596 screened, 0.96%) and 73 children between 11 and 20 years of age (of 10 096 screened, 0.72%) were alloimmunized. These alloimmunization rates are not low solely due to a lack of RBC exposure, as a comparison of % responders also shows low rates of alloimmunization in the paediatric population (2.56%, 5.84%, and 11.25%, respectively for 6–12 months, 13 months-10 years, and 11–20 years) (Fig 3).
Fig 3.
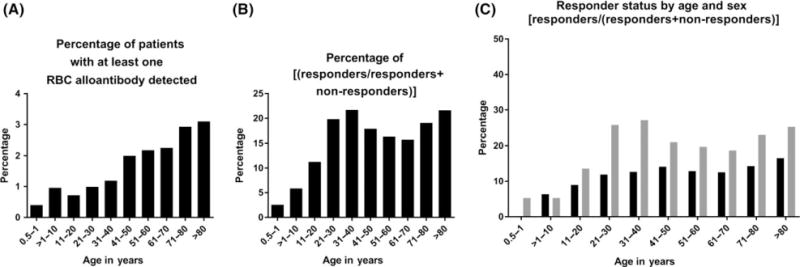
RBC alloantibody presence by recipient age at study entry. (A) Percent of patients of an age range with at least one red blood cell (RBC) alloantibody identified over the study duration. (B) Relationship of responders/[(responders + non-responders)] of an age range over the study duration. (C) Relationship of responders/[(responders + non-responders)], by age range and sex, with black bars indicating males and grey bars indicating females.
In addition to childhood, alloimmunization rates were also studied in adults by decade, unadjusted by the number of RBC transfusions (Table I). When evaluating the number of alloimmunized patients as a fraction of the total screened patients, alloimmunization rates increased incrementally by decade. A comparison of % responders shows higher rates of responder status in patients in the 31–40 year (21.7%) and 81 years and older (21.6%) groups, with differences observed by sex (Fig 3).
Multi-variate analysis of responder and non-responder demographic data
A multi-variate analysis was completed, to evaluate demographic variables and their potential interactions in responders and non-responders. Variables that retained their statistical significance regarding responder and non-responder status included age, race, sex, and RhD status (chi square <0.001); ABO type was marginally significant (chi square 0.0495). Figure 4 shows the odds ratio of being a responder, using the groups RhD positive, blood group A, male sex, white race and age 61–70 years as comparators.
Fig 4.
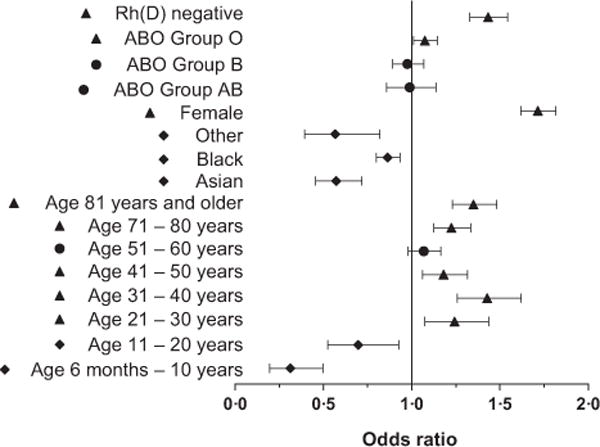
Odds ratio of being a responder compared to a non-responder. Forest plot of the odds ratio by multi-variate analysis of being a responder compared to a non-responder, with error bars depicting 95% Wald confidence limits. The comparator baseline values used include those with the largest population per category (RhD positive, blood group A, male sex, white race, age 61–70 years). Triangles = more likely to be responders; diamonds = less likely to be responders.
Transfusion documentation
A total of 3856 (58.45%) (1498 males and 2358 females) of the 6597 responders were transfused with RBCs at some point during the study, with a mean, standard deviation (s.d.) and median of RBCs transfused of 9.1, 18.6 and 4. All 30 569 of the non-responders were, by definition, transfused with RBCs at some point during the study duration, with a mean, s.d., and median of RBCs transfused to be 9.3, 14.4, and 5 during the study period.
Patients with more than one RBC alloantibody detected
The majority of alloimmunized patients had a single alloantibody detected. However, 1511 of the 6597 alloimmunized patients (22.9%) had more than one alloantibody detected, with the maximum number of distinct alloantibodies identified per patient being 9. Five-hundred and ninety-seven of 2386 alloimmunized males (25.02%) and 914 of 4,211 alloimmunized females (21.7%) had more than one antibody detected. By using the total screened unique patients as a denominator, 597/141,920 (0.42%) of male patients formed more than one alloantibody, compared to 914/177,231 (0.52%) of female patients.
Responders, non-responders, and diagnoses
The primary diagnosis (ICD9/10) at the first encounter of all 600 000 + patients in the database, including 6597 responders and 30 569 non-responders, was evaluated and these diagnoses were grouped into broad categories. The categories were defined as cardiovascular, gastrointestinal, genitourinary, haematology/oncology, infection, musculoskeletal, neurological, pulmonary, obstetrical, and other (Table S2). A 2 × 2 table was created for each of these broad diagnostic categories, and an odds ratio was completed for the likelihood of being alloimmunized in the presence of one of these categories (Fig 5A).
Fig 5.
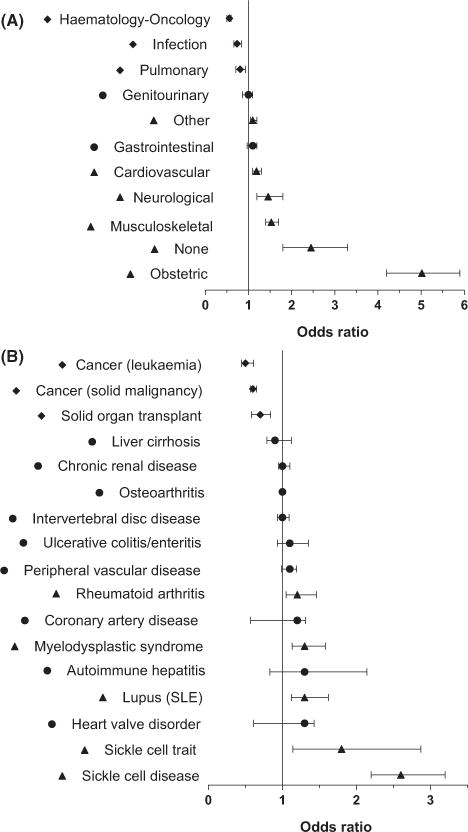
Odds ratio of being a responder compared to a non-responder by diagnosis. Forest plot of the odds ratio by univariate analysis of being a responder (or not) in the presence of (A) a broad diagnostic category at study entry or (B) a particular ICD9/10 diagnosis. Error bars depict 95% confidence limits. Triangles = more likely to be responders; diamonds = less likely to be responders.
To investigate the likelihood of being a responder or a non-responder in the presence of a specific diagnosis or chronic illness, the database was queried for RBC transfused patients with an ICD9/10 code of interest recorded at any point during the study duration. A 2 × 2 table was created for each chronic disease diagnostic code of interest, and P-values were calculated using a Fisher’s exact test to evaluate the association between responder status and clinical diagnosis (Table S3). An odds ratio was then completed for the likelihood of being alloimmunized in the presence of a specific diagnosis (Fig 5B). The odds of having a diagnosis of sickle cell disease or trait, systemic lupus erythematosus, rheumatoid arthritis, or myelodysplastic syndrome were significantly higher for responders than non-responders, whereas the odds of having a diagnosis of leukaemia, solid tumours, or solid organ transplant were significantly higher for non-responders than responders.
Discussion
The newly created recipient database of the National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) (Karafin et al, 2017), containing inpatient and outpatient electronic health records, including demographic, laboratory, and transfusion data on all patients treated at 12 community and academic hospitals in the United States, allowed this study to be completed. Data from more than 300,000 patients who underwent type and screen evaluation over a 3.5-year period were evaluated, making this study, to our knowledge, the largest RBC alloimmunization epidemiological study ever completed in the US.
The overall RBC alloimmunization rates described in this study range from 2% to 6%, depending on whether the denominator includes all screened patients or all confirmed transfused and screened patients; these rates are consistent with those previously reported in retrospective studies (Schonewille & Brand, 2005; Tormey et al, 2008; Stack & Tormey, 2016) and are lower than those published in prospective studies (Redman et al, 1996) due to the phenomenon of antibody evanescence (Tormey & Stack, 2009; Stack & Tormey, 2016). This study found, by both univariate and multivariate analyses, that recipient variables are critically important in impacting humoral immune responsiveness to transfused RBCs. Some findings of this study, including the high rates of RBC alloimmunization in patients with sickle cell disease (Chou & Fasano, 2016) and myelodysplastic syndrome (Lin et al, 2017) and the low rates of RBC alloimmunization in patients with leukaemia (Evers et al, 2017), have been known for many years. Other findings, such as the high rates of RBC alloimmunization in females (Verduin et al, 2015a) and in patients with a diagnosis related to pregnancy, have been touched upon in smaller studies but not extensively studied. The finding of increasing alloimmunization rates with increasing age is logical due to increasing transfusion (Zalpuri et al, 2012) and pregnancy lifetime exposure, and is unlikely to be explained by disease since those associated with increased rates of alloimmunization were not more prevalent in older individuals (data not shown). Additional findings, including the higher likelihood of a White than a Black or an Asian individual (independent of RhD status) being an antibody responder, as well as the relatively high rates of RBC alloimmunization in patients with sickle cell trait, are being described for the first time. Follow-up studies are warranted, however, given the small sample size of the Asian and sickle cell trait subsets. The fact that RhD status impacts RBC alloimmunization, as previously documented in smaller studies, (Al-Mousawi et al, 2015) is largely due to RhD negative transfusion recipients having a higher likelihood of exposure to non-self donor antigens in the Rh family, such as C and D, and potentially due to the receipt of RhD positive RBCs in emergent situations.
The magnitude of the female RBC alloimmunization response rate, though not fully unexpected, suggests that additional studies in this area are warranted. The age distribution of female responders, with more than 50% spanning the childbearing years of 21–40 years, suggests that many RBC alloantibodies were likely pregnancy- and not transfusion-associated. The odds ratio of being a responder (versus a non-responder) was highest among females with a pregnancy-related ICD9/ICD10 code in their first encounter in the database than with any other broad diagnostic grouping, further strengthening this pregnancy/RBC alloimmunization association. These data are consistent with the recently published multinational Blood Group Antigen Matching on Gestational Outcomes (AMIGO) study, (Delaney et al, 2017) which reported pregnancy and not transfusion as the sensitizing event in most cases of maternal alloimmunization. Verduin et al (2015b) suggests that pregnancy associated alloantibodies have lower rates of evanescence than transfusion associated antibodies, potentially contributing to our findings. We cannot readily comment upon differences in transfusion-associated alloimmunization rates in females versus males, given the relatively short time-period studied and the lack of lifetime pregnancy/transfusion histories.
Alloimmunity and autoimmunity are closely linked. The present study found an association between alloimmunization and systemic lupus erythematosus, rheumatoid arthritis, and ulcerative colitis. Further, 41.22% of patients with warm autoantibodies also had detectable alloantibodies. Ramsey and Smietana (1995) identified autoimmune disease as a risk factor for the formation of multiple or unusual RBC alloanti-bodies and subsequently, Papay et al (2012) described a 2.5-fold increased risk of RBC alloimmunization in transfused patients with inflammatory bowel disease compared to controls with non-inflammatory diseases. Recently, Ryder et al (2016) reported that 16% of primarily male alloimmunized patients treated at a Veterans Administration hospital had a chronic autoimmune disorder, compared to 8% of non-alloimmunized patients. Multiple studies have also described a close association between RBC alloantibodies and RBC autoantibodies (Dhawan et al, 2014; Nickel et al, 2015). Animal studies (Gibb et al, 2017a,b) suggest that a potential unifying hypothesis for the connection between some types of autoimmunity (Crow, 2014) and alloimmunization may involve type 1 interferon signalling.
The limitations of a large retrospective epidemiological study, such as this, deserve consideration. First, the US medical system does not have a single source medical record system, and as such, we cannot account for lifetime transfusions, pregnancies or medical conditions. Second, despite stringent quality control, some fields that would have been helpful for this RBC alloimmunization analysis, including whether an anti-D was passively acquired or was actively formed, could not be interpreted due to a lack of uniformity in the way that the hospital sites internally reported these data. Third, it is likely that retrospective studies under-estimate the RBC alloimmunization risk by at least 50% and probably more, given antibody evanescence patterns. (Stack & Tormey, 2016) Record fragmentation, due to the use of multiple hospitals by alloimmunized individuals, also contributes to an underestimation of RBC alloimmunization (Unni et al, 2014).
In summary, this study confirmed known risk factors associated with alloimmunization and identified novel associations. The continued interrogation of the rich REDS-III recipient database will allow for additional in-depth studies of RBC alloimmunization as well as other transfusion-associated outcomes, with a long term goal of improving transfusion safety.
Supplementary Material
Table S1. RBC antibody classification.
Table S2. Primary diagnosis at first encounter of responders and non-responders.
Table S3. Chronic disease status of responders and non-responders.
Fig S1. RBC alloantibody screen overview.
Fig S2. Sex and RBC alloimmunization.
Fig S3. Race, ethnicity, and RBC alloimmunization.
Fig S4. RhD status and RBC alloimmunization.
Acknowledgments
Funding/Support: The authors were supported by research contracts from the National Heart, Lung, and Blood Institute (NHLBI Contracts HHSN268201100005I and HHSN268201100004I for the Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). The funding source designated an investigator-led steering committee, which independently oversaw the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Appendix 1 The NHLBI Recipient Epidemiology Donor Evaluation Study-III (REDS-III), domestic component, is the responsibility of the following persons
Hubs
A.E. Mast and J.L. Gottschall, BloodCenter of Wisconsin (BCW), Milwaukee, WI
D.J. Triulzi and J.E. Kiss, University of Pittsburgh and The Institute for Transfusion Medicine (ITXM), Pittsburgh, PA
E.L. Murphy and E. St. Lezin, University of California, San Francisco (UCSF), San Francisco, CA
E.L. Snyder, Yale University School of Medicine, New Haven, CT and R.G Cable, American Red Cross Blood Services, Farmington CT
Data coordinating centre
D. J. Brambilla and M. T. Sullivan, RTI International, Rockville, MD
Publication Committee Chairman
R. Y. Dodd, American Red Cross, Holland Laboratory, Rockville, MD
Steering Committee Chairman
S. H. Kleinman, University of British Columbia, Victoria, BC, Canada
National Heart, Lung, and Blood Institute, National Institutes of Health
S. A. Glynn and K. Malkin
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article.
Author contributions
MSK and JEH designed the study, wrote, and edited the manuscript. MW and RGH organized the data collection and analysis for the manuscript. MW completed the statistical analyses. All authors analysed the data and contributed to the final version of the manuscript. The authors would like to thank Pablo Cure of the NHLBI as well as the REDS-III Publications Committee for their helpful comments.
References
- Al-Mousawi MM, Al-Allawi NA, Alnaqshabandi R. Predictors of red cell alloimmunization in Kurdish multi transfused patients with hemoglobinopathies in Iraq. Hemoglobin. 2015;39:423–426. doi: 10.3109/03630269.2015.1077460. [DOI] [PubMed] [Google Scholar]
- Chou ST, Fasano RM. Management of patients with sickle cell disease using transfusion therapy: guidelines and complications. Hematology/Oncology Clinics of North America. 2016;30:591–608. doi: 10.1016/j.hoc.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–1071. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol. 2014;192:5459–5468. doi: 10.4049/jimmunol.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney M, Wikman A, van de Watering L, Schonewille H, Verdoes JP, Emery SP, Murphy MF, Staves J, Flach S, Arnold DM, Kaufman RM, Ziman A, Harm SK, Fung M, Eppes CS, Dunbar NM, Buser A, Meyer E, Savoia H, Abeysinghe P, Heddle N, Tinmouth A, Traore AN, Yazer MH. Blood Group Antigen Matching Influence on Gestational Outcomes (AMIGO) study. Transfusion. 2017;57:525–532. doi: 10.1111/trf.13977. [DOI] [PubMed] [Google Scholar]
- Dhawan HK, Kumawat V, Marwaha N, Sharma RR, Sachdev S, Bansal D, Marwaha RK, Arora S. Alloimmunization and autoimmunization in transfusion dependent thalassemia major patients: study on 319 patients. Asian J Transfus Sci. 2014;8:84–88. doi: 10.4103/0973-6247.137438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers D, Zwaginga JJ, Tijmensen J, Middelburg RA, de Haas M, de Vooght KM, van de Kerkhof D, Visser O, Pequeriaux NC, Hudig F, van der Bom JG. Treatments for hematologic malignancies in contrast to those for solid cancers are associated with reduced red cell alloimmunization. Haematologica. 2017;102:52–59. doi: 10.3324/haematol.2016.152074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb DR, Liu J, Natarajan P, Santhanakrishnan M, Madrid DJ, Eisenbarth SC, Zimring JC, Iwasaki A, Hendrickson JE. Type I IFN Is necessary and sufficient for inflammation-induced red blood cell alloimmunization in mice. J Immunol. 2017a;199:1041–1050. doi: 10.4049/jimmunol.1700401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb DR, Liu J, Santhanakrishnan M, Natarajan P, Madrid DJ, Patel S, Eisenbarth SC, Tormey CA, Stowell SR, Iwasaki A, Hendrickson JE. B cells require Type 1 interferon to produce alloantibodies to transfused KEL-expressing red blood cells in mice. Transfusion. 2017b;57:2595–2608. doi: 10.1111/trf.14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson JE, Delaney M. Hemolytic disease of the fetus and newborn: modern practice and future investigations. Transfusion Medicine Reviews. 2016;30:159–164. doi: 10.1016/j.tmrv.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112:2546–2553. doi: 10.1182/blood-2008-03-146415. [see comment] [DOI] [PubMed] [Google Scholar]
- Hoeltge GA, Domen RE, Rybicki LA, Schaffer PA. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Archives of Pathology & Laboratory Medicine. 1995;119:42–45. [PubMed] [Google Scholar]
- Kacker S, Ness PM, Savage WJ, Frick KD, Shirey RS, King KE, Tobian AA. Cost-effectiveness of prospective red blood cell antigen matching to prevent alloimmunization among sickle cell patients. Transfusion. 2014a;54:86–97. doi: 10.1111/trf.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacker S, Ness PM, Savage WJ, Frick KD, Shirey RS, King KE, Tobian AA. Economic evaluation of a hypothetical screening assay for alloimmunization risk among transfused patients with sickle cell disease. Transfusion. 2014b;54:2034–2044. doi: 10.1111/trf.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karafin MS, Bruhn R, Westlake M, Sullivan MT, Bialkowski W, Edgren G, Roubinian NH, Hauser RG, Kor DJ, Fleischmann D, Gottschall JL, Murphy EL, Triulzi DJ. Demographic and epidemiologic characterization of transfusion recipients from four US regions: evidence from the REDS-III recipient database. Transfusion. 2017;57:2903–2913. doi: 10.1111/trf.14370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Saskin A, Wells RA, Lenis M, Mamedov A, Callum J, Buckstein R. Prophylactic RhCE and Kell antigen matching: impact on alloimmunization in transfusion-dependent patients with myelodysplastic syndromes. Vox Sanguinis. 2017;112:79–86. doi: 10.1111/vox.12455. [DOI] [PubMed] [Google Scholar]
- Nickel RS, Horan JT, Fasano RM, Meyer E, Josephson CD, Winkler AM, Yee ME, Kean LS, Hendrickson JE. Immunophenotypic parameters and RBC alloimmunization in children with sickle cell disease on chronic transfusion. American Journal of Hematology. 2015;90:1135–1141. doi: 10.1002/ajh.24188. [DOI] [PubMed] [Google Scholar]
- Papay P, Hackner K, Vogelsang H, Novacek G, Primas C, Reinisch W, Eser A, Mikulits A, Mayr WR, Kormoczi GF. High risk of transfusion-induced alloimmunization of patients with inflammatory bowel disease. American Journal of Medicine. 2012;125:e711–e718. doi: 10.1016/j.amjmed.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Pirenne F, Bartolucci P, Habibi A. Management of delayed hemolytic transfusion reaction in sickle cell disease: prevention, diagnosis, treatment. Transfusion Clinique et Biologique. 2017;24:227–231. doi: 10.1016/j.tracli.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Ramsey G, Smietana SJ. Multiple or uncommon red cell alloantibodies in women: association with autoimmune disease. Transfusion. 1995;35:582–586. doi: 10.1046/j.1537-2995.1995.35795357881.x. [DOI] [PubMed] [Google Scholar]
- Redman M, Regan F, Contreras M. A prospective study of the incidence of red cell allo-immunisation following transfusion. Vox Sanguinis. 1996;71:216–220. doi: 10.1046/j.1423-0410.1996.7140216.x. [DOI] [PubMed] [Google Scholar]
- Reid ME, Lomas-Francis C. The Blood Group Antigen Facts Book. Elsevier Academic Press; Amsterdam: 2004. [Google Scholar]
- Rosse WF, Gallagher D, Kinney TR, Castro O, Dosik H, Moohr J, Wang W, Levy PS. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76:1431–1437. [PubMed] [Google Scholar]
- Ryder AB, Hendrickson JE, Tormey CA. Chronic inflammatory autoimmune disorders are a risk factor for red blood cell alloimmunization. British Journal of Haematology. 2016;174:483–485. doi: 10.1111/bjh.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonewille H, Brand A. Alloimmunization to red blood cell antigens after universal leucodepletion. A regional multicentre retrospective study. British Journal of Haematology. 2005;129:151–156. doi: 10.1111/j.1365-2141.2005.05408.x. [DOI] [PubMed] [Google Scholar]
- Schonewille H, van de Watering LM, Loomans DS, Brand A. Red blood cell alloantibodies after transfusion: factors influencing incidence and specificity. Transfusion. 2006;46:250–256. doi: 10.1111/j.1537-2995.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- Seyfried H, Walewska I. Analysis of immune response to red blood cell antigens in multitransfused patients with different diseases. Materia Medica Polona. 1990;22:21–25. [PubMed] [Google Scholar]
- Smith HM, Shirey RS, Thoman SK, Jackson JB. Prevalence of clinically significant red blood cell alloantibodies in pregnant women at a large tertiary-care facility. Immunohematology. 2013;29:127–130. [PubMed] [Google Scholar]
- Stack G, Tormey CA. Detection rate of blood group alloimmunization based on real-world testing practices and kinetics of antibody induction and evanescence. Transfusion. 2016;56:2662–2667. doi: 10.1111/trf.13704. [DOI] [PubMed] [Google Scholar]
- Stiegler G, Sperr W, Lorber C, Fabrizii V, Hocker P, Panzer S. Red cell antibodies in frequently transfused patients with myelodysplastic syndrome. Annals of Hematol-ogy. 2001;80:330–333. doi: 10.1007/s002770100308. [DOI] [PubMed] [Google Scholar]
- Tormey CA, Stack G. The persistence and evanescence of blood group alloantibodies in men. Transfusion. 2009;49:505–512. doi: 10.1111/j.1537-2995.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- Tormey CA, Fisk J, Stack G. Red blood cell alloantibody frequency, specificity, and properties in a population of male military veterans. Transfusion. 2008;48:2069–2076. doi: 10.1111/j.1537-2995.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- Unni N, Peddinghaus M, Tormey CA, Stack G. Record fragmentation due to transfusion at multiple health care facilities: a risk factor for delayed hemolytic transfusion reactions. Transfusion. 2014;54:98–103. doi: 10.1111/trf.12251. [DOI] [PubMed] [Google Scholar]
- Verduin EP, Brand A, Schonewille H. Is female sex a risk factor for red blood cell alloimmunization after transfusion? A systematic review. Transfusion Medicine Reviews. 2012;26:342–353. 353 e341–345. doi: 10.1016/j.tmrv.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Verduin EP, Brand A, Middelburg RA, Schonewille H. Female sex of older patients is an independent risk factor for red blood cell alloimmunization after transfusion. Transfusion. 2015a;55:1478–1485. doi: 10.1111/trf.13111. [DOI] [PubMed] [Google Scholar]
- Verduin EP, Brand A, van de Watering LM, Claas FH, Oepkes D, Lopriore E, Doxiadis II, Schonewille H. Factors associated with persistence of red blood cell antibodies in woman after pregnancies complicated by fetal alloimmune haemolytic disease treated with intrauterine transfusions. British Journal of Haematology. 2015b;168:443–451. doi: 10.1111/bjh.13130. [DOI] [PubMed] [Google Scholar]
- Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB blood collection, utilization, and patient blood management survey. Transfusion. 2016;56:2173–2183. doi: 10.1111/trf.13676. [DOI] [PubMed] [Google Scholar]
- Zalpuri S, Zwaginga JJ, le Cessie S, Elshuis J, Schonewille H, van der Bom JG. Red-blood-cell alloimmunization and number of red-blood-cell transfusions. Vox Sanguinis. 2012;102:144–149. doi: 10.1111/j.1423-0410.2011.01517.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. RBC antibody classification.
Table S2. Primary diagnosis at first encounter of responders and non-responders.
Table S3. Chronic disease status of responders and non-responders.
Fig S1. RBC alloantibody screen overview.
Fig S2. Sex and RBC alloimmunization.
Fig S3. Race, ethnicity, and RBC alloimmunization.
Fig S4. RhD status and RBC alloimmunization.


