Abstract
Aims/hypothesis
Prior studies suggest white matter growth is reduced and white matter microstructure is altered in the brains of young children with type 1 diabetes when compared with brains of non-diabetic children, due in part to adverse effects of hyperglycaemia. This longitudinal observational study examines whether dysglycaemia alters the developmental trajectory of white matter microstructure over time in young children with type 1 diabetes.
Methods
One hundred and eighteen children, aged 4 to < 10 years old with type 1 diabetes and 58 age-matched, non-diabetic children were studied at baseline and 18 months at five Diabetes Research in Children Network clinical centres. We analysed longitudinal trajectories of white matter using diffusion tensor imaging. Continuous glucose monitoring profiles and HbA1c levels were obtained every 3 months.
Results
Axial diffusivity was lower in children with diabetes at baseline (p = 0.022) and at 18 months (p = 0.015), indicating that differences in white matter microstructure persist over time in children with diabetes. Within the diabetes group, lower exposure to hyperglycaemia, averaged over the time since diagnosis, was associated with higher fractional anisotropy (p = 0.037). Fractional anisotropy was positively correlated with performance (p < 0.002) and full-scale IQ (p < 0.02).
Conclusions/interpretation
These results suggest that hyperglycaemia is associated with altered white matter development, which may contribute to the mild cognitive deficits in this population.
Keywords: Brain development, Paediatric diabetes, White matter
Introduction
The long-term impact of type 1 diabetes on microvascular complications is well documented [1] but effects on the developing brain are less understood. Early childhood is a period of rapid myelination and brain development, with total brain volume reaching approximately 90% of adult brain volume by the age of 6 years [2–4]. Grey matter volume continues to increase until approximately 11 years of age but subsequently decreases through adolescence, whereas white matter volume, driven by the ongoing myelination of axons, gradually increases into early adulthood [4]. Although previous studies have found altered grey and white matter volume and white matter microstructure in children and adolescents with type 1 diabetes, related to dysglycaemia [5–9], no longitudinal studies have examined how diabetes and associated dysglycaemia affects white matter microstructural measures during development.
Brain white matter microstructure can be estimated using the MRI-based technique of diffusion tensor imaging (DTI). This imaging modality allows the detection of white matter structural alterations before gross anatomical changes occur and become apparent on structural (anatomical) MRI. DTI measures the movement of water molecules within tissue and is sensitive to the orientation of axons and cell membranes as well as the fraction of intracellular vs extracellular water [10, 11]. The primary DTI metrics for assessing white matter microstructure are fractional anisotropy (increases with myelination and fibre tract coherence; decreases with extracellular diffusion), radial diffusivity (measures water diffusivity perpendicular to the local axonal direction and decreases with myelination) and axial diffusivity (measures water diffusivity parallel to the axonal direction and may decrease with cell swelling, more neurofilaments within axons or axonal branching). Mean diffusivity (measures degree of overall water diffusivity) is an average of these effects in white matter and is more commonly used in isotropic media such as grey matter or cerebrospinal fluid. By examining patterns of altered DTI metrics in white matter tracts, myelin and axonal integrity can be inferred in controlled experiments [12, 13]. However, in general, a change in diffusivity is not specific to a particular microstructural change and results also may be influenced by other factors, such as packing density, complex fibre architecture and water content [11, 14].
In animal studies, myelination and fibre integrity are adversely affected by hyperglycaemia. Streptozotocin-induced diabetic rats display reduced myelination [15] and altered intracellular water shifts in excised sciatic nerves [16]. Using DTI, we and others have observed that children with type 1 diabetes had lower axial diffusivity when compared with age-matched non-diabetic children and that these differences were related to metrics of hyperglycaemia [8, 9, 17].
Furthermore, in two studies [9, 17], greater radial diffusivity and lower fractional anisotropy correlated with greater hyperglycaemia, suggesting hyperglycaemia-induced myelin damage. Thus, hyperglycaemia adversely affects myelination and nerve fibre structure and integrity in animal models and is associated with lower fractional anisotropy and greater radial diffusivity in cross-sectional human studies. Because of long-standing concerns that the brains of young children with type 1 diabetes are vulnerable to the adverse effects of hyperglycaemia, we in the Diabetes Research in Children Network (DirecNet) carried out a large, prospective brain imaging study of children with and without type 1 diabetes who were aged 4 to < 10 years at baseline. We reported that the growth of grey and white matter volume was slower in the developing brains of the young children with diabetes in this cohort over a period of 18 months [18, 19]. It was noteworthy that the reduced rate of increase in white matter volume was more closely associated with exposure to hyperglycaemia than hypoglycaemia, as measured by serial continuous glucose monitoring profiles and HbA1c. In the present study, we examined the hypothesis that hyperglycaemia will continue to adversely affect white matter development over an 18 month period.
Methods
Study population
Details of the study population and measurements are given elsewhere [17]. Briefly, 144 children aged 4 to < 10 years with type 1 diabetes for at least 6 months and 68 age-matched, non-diabetic children (HbA1c < 42 mmol/mol [<6%], fasting glucose < 6.1 mmol/l [< 110 mg/dl], no history of dysglycaemia) were recruited from 2010 to 2011 from five paediatric diabetes clinical centres participating in the DirecNet consortium: Nemours Children’s Health System, Jacksonville; Stanford University; University of Iowa; Washington University in St Louis and Yale University. The past medical history of all participants was screened to exclude the following criteria: disorders that could have affected neurologic development, history of intellectual or significant learning disabilities, psychiatric treatment, premature birth (< 34 weeks’ gestation) or low birthweight (< 2000 g). Participants with diabetes were at least 6 months old at diagnosis and were receiving insulin therapy for at least 1 month. Studies were approved by the individual clinical centres’ Institutional Review Boards and a National Institutes of Health-designed Data Safety Monitoring Board. Written informed consent was obtained from the legal guardian of each participant and assent was obtained from the participants when appropriate. In children with diabetes, HbA1c was recorded at baseline and every 3 months throughout the study and a continuous glucose monitor (CGM) was worn every 3 months (either Medtronic [Medtronic, Northridge, CA, USA] iPro or Dexcom [Dexcom, San Diego, CA, USA] G4 for up to 6 or 7 days, respectively).
Imaging data acquisition
A detailed description of the MRI is given elsewhere [17]. Briefly, MRI of the brain was carried out at baseline and again at 18 months. All imaging sites used a Siemens [Siemens Medical Solutions, Malvern, PA, USA] 3T Tim Trio whole-body MRI system and a standard 12-channel head coil. An identical imaging protocol was uploaded to every scanner, as described in previously published work [17]. Diffusion-weighted echo planar scans were acquired in the axial plane with 30 directions, TR/TE 8800/99 ms, b value 1000 s/mm2, voxel size 2 × 2 × 2 mm and duration 4 h 59 min. Sagittal T1 images of the brain were acquired using a magnetisation-prepared rapid gradient echo (MP-RAGE) pulse sequence with TR/TE/TI 2300/2.98/900 ms, flip angle 9°, voxel size 1 × 1 × 1 mm and duration 4 h 54 min. Scans were repeated twice, with more scans added to obtain good image quality if necessary. Participants were awake and unsedated and received training designed to help them succeed with the motion restriction requirements of MRI [20]. A second MRI session was performed on a separate day if the initial scan could not be successfully completed or if image quality was deemed unacceptable after the first attempt. Participants with diabetes were required to have glucose levels between 3.9 and 16.7 mmol/l (70 and 300 mg/dl) within the 60 min preceding all scan sessions. Multisite calibrations were performed by scanning the same two adult human phantoms on every machine to confirm the repeatability of measurements across sites and over time at each site. The calibration checks for DTI found variations < 3%, which is a typical level of reproducibility across identical scanners [21].
Data analysis
Image processing
For each individual, the diffusion-weighted data were automatically reconstructed into white matter pathways. Each diffusion image was quality-checked using DTIprep software (version 1.2.7 for MacOSX, https://www.nitrc.org/projects/dtiprep) [22] and aligned to the T1-weighted structural images. The white matter fibre tract locations for each individual were computed by a maximum likelihood estimate using the ball-and-stick model variables at each voxel [23] combined with a priori knowledge of tract locations in the brain using TRActs Constrained by Underlying Anatomy (TRACULA) software (version 5.3.2014, http://surfer.nmr.mgh.harvard.edu/pub/dist/freesurfer/5.3.0-tracula-addons/) [24]. This software computes statistics for 18 white matter tracts and a total motion index of the head motion during the DTI scan [25]. Using the tracts as a white matter skeleton, the software finds axial, radial and mean diffusivity values and fractional anisotropy values at 100 points along each tract, from which the mean values for each tract were computed. A preliminary analysis showed that the average diffusivities of the 16 intrahemispheric tracts were highly correlated with one another, consistent with previous normative results of white matter development [26]. The 16 tracts include the bilateral anterior thalamic radiation, cingulum angular bundle, cingulate, cortical spinal tract, arcuate, inferior longitudinal fasciculus, superior longitudinal fasciculus and uncinate fasciculus. The two excluded tracts were the forceps major and minor. Because our previous analyses of this cohort showed diabetes to have widespread effects on grey matter growth, white matter growth and diffusivity [17–19], we used the summary statistic of brain mean computed as an average of the 16 intrahemispheric tracts at baseline and 18 months for axial, radial and mean diffusivity and fractional anisotropy. The longitudinal DTI analysis used only participants who had a high-quality DTI scan at both time points.
Glycaemia exposure during the study
HbA1c was measured using a DCA Vantage Analyzer (Siemens Healthcare Diagnostics, Norwood, MA, USA) at each participating site. Lifetime average exposure to hyperglycaemia was calculated as the average of all HbA1c measurements > 42 mmol/mol (> 6%) from diagnosis to baseline [18]. Similarly, the interval average exposure to hyperglycaemia was calculated as the average of all HbA1c measurements > 42 mmol/mol (> 6%) over the study interval, using the trapezoidal rule from all available HbA1c measurements (usually seven per individual). CGM data, obtained quarterly during the study in children with diabetes, were analysed for the percentage of time blood glucose was < 3.9 mmol/l (< 70 mg/dl) and > 10 mmol/l (> 180 mg/dl) and measures of blood glucose variation, including average mean amplitude of blood glucose excursions (MAGE).
Cognitive assessments
Cognitive testing was performed in diabetic and non-diabetic participants using either the Wechsler Preschool and Primary Scale of Intelligence (for children < 6 years old) or the Wechsler Intelligence Scale for Children, 4th edition (for children ≥6 years old), which have been published elsewhere [27, 28]. For participants with type 1 diabetes, a blood glucose level of 3.9–16.7 mmol/l (70–300 mg/dl) was required within 60 min prior to the testing session. The Wechsler Abbreviated Scale of Intelligence was used to assess parental full-scale intelligence quotient.
Statistical analyses
The study size for DirecNet was powered to detect group differences between diabetic and non-diabetic individuals and to detect hyperglycaemic effects within the diabetes group. Descriptive statistics of the baseline variables are presented for the diabetes and non-diabetes groups using means and SDs for the continuous variables and frequencies and percentages for categorical variables. We performed two-sample t tests for demographic continuous variables and χ2 tests for categorical variables to determine group differences. To assess whether the two groups (diabetic vs non-diabetic individuals) differed over time (baseline to 18 months) in key outcomes of interest (mean axial diffusivity, fractional anisotropy, mean diffusivity, radial diffusivity), we performed longitudinal mixed effects modelling using a random intercept model and maximum likelihood estimation with robust SEs (Mplus version 8 [Muthén & Muthén, Los Angeles, CA, USA]). Using the same method, within the group with diabetes, we examined the effect of blood glucose measure on DTI outcomes over time. Both analyses were conducted with and without conditional on age, sex and site as a way of sensitivity analysis. The effects of adjusting or not adjusting for these covariates on our key variables of interest (i.e. effects of group and blood glucose measure on DTI) were minimal and did not change our conclusions. The results reported in this paper are based on the model conditional on age, sex and site. Most participants were scanned on the same scanner at both time points, although 14 individuals changed between two sites. These participants were grouped as a seventh site in the analysis. Significance was set at p < 0.05 for each of the four mixed effects statistical models.
Results
Of the 212 individuals initially studied at baseline, 176 completed the procedures at 18 months, with good DTI scan quality at both time points (118 with diabetes). There were no differences in demographic characteristics between the diabetic and non-diabetic participants for age, sex and site (Table 1). Moreover, there were no significant differences in head motion between groups, thus reducing the risk of spurious results due to head motion [25].
Table 1.
Clinical characteristics of the study participants at baseline and 18 months
| Characteristic | Participants with diabetes (N=118) | Participants without diabetes (N=58) | All participants (N=176) |
|---|---|---|---|
| Age, years | |||
| Baseline | 7.07±1.64 | 7.01±1.76 | 7.05±1.67 |
| 18 months | 8.54±1.62 | 8.55±1.76 | 8.54±1.66 |
| Sex | |||
| Male | 66 (55.9) | 33 (56.9) | 99 (56.3) |
| Female | 52 (44.1) | 25 (43.1) | 77 (43.8) |
| HbA1c | |||
| Baseline, mmol/mol | 62.8±10 | ||
| Baseline, % | 7.9±0.87 | ||
| 18 months, mmol/mol | 62.8±10 | ||
| 18 months, % | 7.9±0.94 | ||
| Duration of diabetes, years | |||
| Baseline | 2.99±2.01 | ||
| 18 months | 4.52±2.00 | ||
| Age at diabetes onset, years | 4.1±1.9 | ||
| DKA history | |||
| Baseline | 40 (34) | ||
| Interval | 4 (3.4) | ||
| Severe hypoglycaemia history | |||
| Baseline | 19 (16) | ||
| Interval | 5 (4.2) | ||
| Lifetime average glycaemic exposure, % | 2.1±0.8 | ||
| Interval average glycaemic exposure, % | 1.9±0.8 | ||
Data are shown as mean±SD or n (%)
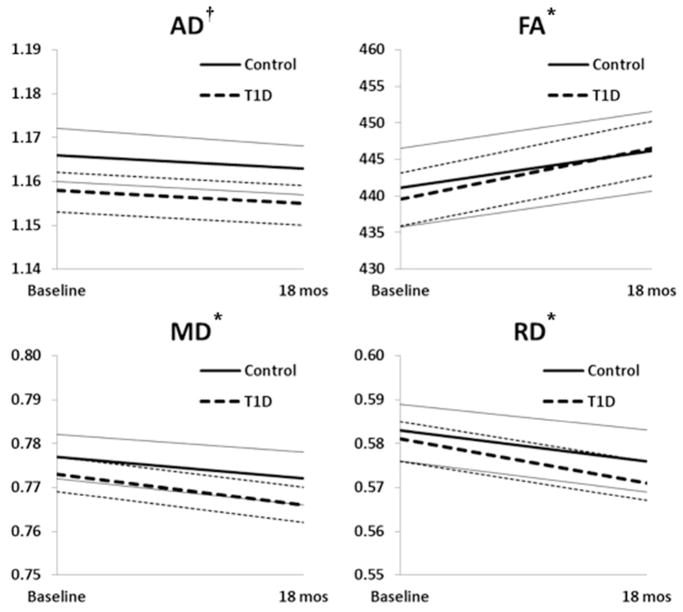
Estimated longitudinal trajectories of axial, radial and mean diffusivity and fractional anisotropy conditional on age, sex and site are shown in Fig. 1. Details of the longitudinal analysis results based on mixed effects modelling are shown in Table 2. Fractional anisotropy (a putative measure of myelination) increased from baseline to 18 months for diabetes (p < 0.001) and non-diabetes (p = 0.003) groups. Mean (p < 0.001 and p = 0.004) and radial (p < 0.001 and p = 0.002) diffusivity decreased significantly over time in both the diabetes and non-diabetes groups, respectively. There was no significant change in axial diffusivity in either group, but participants with diabetes displayed lower axial diffusivity compared with non-diabetic participants at baseline (p = 0.022) and at 18 months (p = 0.015) (Fig. 1a). There was no between-group difference in fractional anisotropy, mean diffusivity or radial diffusivity at baseline or at 18 months and there was no significant between-group difference when comparing rate of change of fractional anisotropy, mean diffusivity or radial diffusivity from baseline to 18 months.
Fig. 1.
Estimated trajectories of axial (a), radial (b) and mean (c) diffusivity and fractional anisotropy (d) from baseline to 18 months. Dashed black lines, diabetes group; solid black lines, group without diabetes; grey dotted lines, 95% CIs for diabetes group; grey solid lines, 95% CIs for the group without diabetes. *p<0.05, with vs without diabetes at baseline and 18 months in axial diffusivity only; no between-group differences were noted at baseline or 18 months in radial diffusivity, mean diffusivity or fractional anisotropy. †p<0.05, 18 months vs baseline for radial diffusivity, mean diffusivity and fractional anisotropy within groups for participants with and without diabetes (p values are shown in Table 2)
Table 2.
Effects of type 1 diabetes on longitudinal trajectories of axial diffusivity, fractional anisotropy, mean diffusivity and radial diffusivity using mixed effects modelling
|
|
|
|
|
|||||
|---|---|---|---|---|---|---|---|---|
| Comparison | Axial diffusivity (10−3 mm2/s) | Fractional anisotropy (×1000) | Mean diffusivity (10−3 mm2/s) | Radial diffusivity (10−3 mm2/s) | ||||
|
|
|
|
|
|||||
| Estimate | p value | Estimate | p value | Estimate | p value | Estimate | p value | |
|
|
|
|
|
|||||
| Baseline vs 18 months | ||||||||
| Diabetes group | −0.003 | 0.107 | 6.922 | <0.001 | −0.007 | <0.001 | −0.009 | <0.001 |
| Control group | −0.003 | 0.194 | 4.965 | 0.003 | −0.005 | 0.004 | −0.006 | 0.002 |
| Diabetes vs without diabetes | ||||||||
| At baseline | −0.008 | 0.022 | −1.612 | 0.582 | −0.004 | 0.194 | −0.002 | 0.563 |
| At 18 months | −0.008 | 0.015 | 0.346 | 0.905 | −0.006 | 0.070 | −0.005 | 0.207 |
| Effect on slope | 0.000 | 0.982 | 1.957 | 0.325 | −0.002 | 0.403 | −0.003 | 0.271 |
| Baseline age effect | ||||||||
| At baseline | −0.004 | <0.001 | 5.648 | <0.001 | −0.007 | <0.001 | −0.008 | <0.001 |
| At 18 months | −0.004 | <0.001 | 3.319 | <0.001 | −0.005 | <0.001 | −0.005 | <0.001 |
| Effect on slope | 0.000 | 0.971 | −2.329 | <0.001 | 0.002 | 0.003 | 0.003 | <0.001 |
| Sex effect | ||||||||
| At baseline | −0.006 | 0.082 | −2.289 | 0.419 | −0.002 | 0.555 | 0.000 | 0.962 |
| At 18 months | −0.006 | 0.052 | 0.430 | 0.878 | −0.004 | 0.181 | −0.003 | 0.401 |
| Effect on slope | 0.000 | 0.906 | 2.719 | 0.144 | −0.002 | 0.328 | −0.003 | 0.200 |
Estimates of effect are shown with corresponding p values
Within and between-group comparisons were made. Baseline age effect and sex effect at baseline and 18 months were also assessed, as was effect on slope (change from baseline to 18 months for baseline age and sex)
Baseline age in both groups was a significant predictor of all four brain measures at baseline and at 18 months, with older participants having a higher fractional anisotropy and lower axial, mean and radial diffusivity at both time points (Table 2). Baseline age was also significantly associated with how the brain measures change over time, such that fractional anisotropy increased at a lower rate and mean diffusivity and radial diffusivity decreased at a lower rate in older children. The baseline age had little impact on how axial diffusivity changed over time. There was no significant sex effect on any of the brain measures and no correlation between the occurrence of diabetic ketoacidosis (DKA) prior to enrolment and any of the brain measures. Too few episodes of DKA occurred during the length of the study (n = 4) to assess its effect on changes in the diffusion measures.
We examined how the trajectories of brain measures within the diabetes group were related to exposure to hyperglycaemia, with baseline age and sex as covariates (Table 3). Lower lifetime exposure to hyperglycaemia was associated with higher fractional anisotropy at 18 months (p = 0.037). However, lower interval exposure to hyperglycaemia was not significantly correlated with the slope of fractional anisotropy (p > 0.7) and nor was disease duration significantly correlated with fractional anisotropy at 18 months (p > 0.7; data not shown). The effects of age on the trajectories of four brain measures in the diabetic participants, in whom fractional anisotropy increased and mean and radial diffusivity decreased significantly over time, were similar to the trajectories in the non-diabetic participants (Table 2). Further, although there were no significant differences across sex in the overall analysis (Table 2), there was a significant sex difference in fractional anisotropy in the diabetes group, with female participants having lower fractional anisotropy at baseline (Table 3). There were no significant correlations between diffusion data and the CGM measures of hypoglycaemia or glycaemic variation. Similarly, the correlation with hyperglycaemia (glucose > 10 mmol/l [> 180 mg/dl]) was in the same direction as for average hyperglycaemia exposure but was not significant (p = 0.14).
Table 3.
Effects of average hyperglycaemia exposure, baseline age and sex on axial diffusivity, fractional anisotropy, mean diffusivity and radial diffusivity using mixed effects modelling
|
|
|
|
|
|||||
|---|---|---|---|---|---|---|---|---|
| Comparison | Axial diffusivity (10−3 mm2/s) | Fractional anisotropy (×1000) | Mean diffusivity (10−3 mm2/s) | Radial diffusivity (10−3 mm2/s) | ||||
|
|
|
|
|
|||||
| Estimate | p value | Estimate | p value | Estimate | p value | Estimate | p value | |
|
|
|
|
|
|||||
| Hyperglycaemia | ||||||||
| Lifetime effect at 18 months | −0.001 | 0.677 | −4.781 | 0.037 | 0.003 | 0.300 | 0.005 | 0.087 |
| Interval effect on slope | 0.001 | 0.720 | −0.643 | 0.712 | 0.002 | 0.334 | 0.002 | 0.276 |
| Baseline age | ||||||||
| Effect at baseline | −0.004 | 0.007 | 6.241 | <0.001 | −0.007 | <0.001 | −0.008 | <0.001 |
| Effect at 18 months | −0.004 | 0.001 | 3.349 | 0.002 | −0.005 | <0.001 | −0.005 | <0.001 |
| Effect on slope | 0.000 | 0.713 | −2.884 | <0.001 | 0.002 | 0.022 | 0.003 | 0.001 |
| Sex effect | ||||||||
| Effect at baseline | −0.003 | 0.450 | −7.010 | 0.038 | 0.003 | 0.410 | 0.006 | 0.146 |
| Effect at 18 months | −0.004 | 0.270 | −5.668 | 0.091 | 0.002 | 0.614 | 0.005 | 0.235 |
| Effect on slope | −0.001 | 0.758 | 1.298 | 0.569 | −0.001 | 0.652 | −0.001 | 0.655 |
Estimate of effects are shown with corresponding p values
At 18 months, there were significant correlations in diabetic participants between fractional anisotropy and performance IQ (r = 0.29, p < 0.002) and full-scale IQ (r = 0.23, p < 0.02) but not verbal IQ (r = 0.09, p = 0.34). In the non-diabetic volunteers, however, none of the IQ test scores were significantly correlated with fractional anisotropy (performance IQ, r = 0.109 [p =0.42]; full-scale IQ, r = 0.149 [p = 0.27]; verbal IQ, r = 0.153 [p = 0.26]). Furthermore, there were no between-group differences in any of the IQ tests at 18 months.
Discussion
The developing brain in young children with type 1 diabetes is susceptible to the effects of hyperglycaemia. In our study, children in both the diabetes and non-diabetes groups had significantly lower fractional anisotropy at baseline than at 18 months. However, there were no significant between-group differences in rate of change for fractional anisotropy, mean diffusivity and radial diffusivity (i.e. slope effects) (Table 2), even though the estimates of fractional anisotropy (Table 2) in the diabetes group appeared able to ‘catch up’ with those of the non-diabetes group over time. Surprisingly, for the group with type 1 diabetes, the rate of change of fractional anisotropy was not correlated with degree of hyperglycaemia and neither were axial, radial or mean diffusivity. This finding suggests that hyperglycaemia did not affect the rate of microstructural white matter development during the 18 months of the study. Similarly, we found no significant correlation between fractional anisotropy and disease duration. If hyperglycaemia were hypothesised to cause cumulative damage starting at the age of diagnosis, one would expect to see a correlation of fractional anisotropy with disease duration and a correlation of fractional anisotropy slope with the interval average hyperglycaemia. Instead, our findings suggest that brain myelination in children is affected by average excess levels of glucose rather than by a cumulative hyperglycaemic effect from the age of diagnosis. However, these results need further exploration and confirmation in a separate cohort.
In both the diabetic and the non-diabetic children, significant changes were seen in fractional anisotropy and radial diffusivity from baseline to 18 months, suggesting that white matter development continued in the diabetes group as well as in the non-diabetes group. However, within the diabetes group, fractional anisotropy was lower in association with higher average lifetime exposure to hyperglycaemia, suggesting that typical white matter development may be impaired when young brains undergo greater exposure to hyperglycaemia. This result is consistent with a previous cross-sectional study that demonstrated a negative correlation between hyperglycaemia and fractional anisotropy in several brain regions [17]. These data suggest myelination is likely continuing in the diabetes group but is adversely affected by increased hyperglycaemia. Increased fractional anisotropy is often associated with myelination, but is also affected by additional factors such as alignment of cell membranes and other cell processes [29]. In animal models, loss of myelin increases radial diffusivity and reduces fractional anisotropy [12]. Metabolic pathways involving non-enzymatic glycation may induce inflammation, cellular oxidative stress and axonal atrophy [30], potentially explaining, at least in part, how increased hyperglycaemia may be associated with slower myelination.
Our study did not find a significant difference in fractional anisotropy between individuals with and without diabetes, yet hyperglycaemia was associated with lower fractional anisotropy within the diabetes group at 18 months. One possible explanation is that the between-group result may be confounded by reduced diffusivity [31, 32] in conjunction with reduced myelination (i.e. the diabetes group has both reduced myelination and reduced diffusivity relative to the non-diabetes group). Reduced diffusivity lowers axial, mean and radial diffusivity and increases fractional anisotropy [13, 14]. In contrast, reduced myelination and associated altered white matter microstructural integrity would be expected to decrease fractional anisotropy, increase radial diffusivity and moderately increase mean diffusivity [33]. When both reduced diffusivity and reduced myelination effects are present, the combined effects could result in significantly reduced axial diffusivity for those with diabetes, less prominent differences in mean diffusivity and radial diffusivity and little difference in fractional anisotropy between groups. This would effectively mask an underlying between-group effect on fractional anisotropy. While the imaging data in the current study can only suggest this possible interpretation, more advanced two-shell DTI methods can model these components separately and could be applied in future studies [13]. Previously, cross-sectional analyses have revealed that children with type 1 diabetes have lower axial diffusivity than healthy children [8, 9, 17]. We found this difference persists for 18 months in young children. Similarly, axial diffusivity did not significantly change over 18 months in either group, consistent with the very slow rates of change previously reported in healthy children and young adolescents [26, 33]. The significant between-group effect for axial diffusivity must be interpreted with caution, however. Biological processes related to insulin insufficiency or C-peptide levels [34, 35] may reduce white matter coherence and adversely affect axial diffusivity. In addition, overall tissue diffusivity is reduced in animal models of diabetes [31, 32], indicating that brain cell swelling could also reduce axial diffusivity.
A significant positive correlation was found between fractional anisotropy and performance IQ at 18 months for the participants with diabetes. These correlations are consistent with previous results reported in typically developing children, where higher IQ was associated with higher fractional anisotropy [26, 36, 37]. Our data are particularly interesting because higher fractional anisotropy was associated with better blood glucose control (i.e. lower lifetime average exposure to hyperglycaemia). Cognitive results for this cohort were previously reported [28]; executive function was negatively correlated with average exposure to hyperglycaemic. Thus, our data suggest that reduced fractional anisotropy (and hence possibly reduced myelination) due to hyperglycaemia may contribute to the mild cognitive deficiencies previously reported in this population.
Our results show that young children with early-onset type 1 diabetes are susceptible to adverse neural effects of hyperglycaemia. It does not appear, however, that hyperglycaemia causes an increasing deficit in myelination over time. It is possible that brain growth in childhood can partially compensate for the effects of hyperglycaemia. Thus, the degree of hyperglycaemia, the resiliency of brain growth and younger age at diagnosis may be key in determining how vulnerable the brain is to the effects of hyperglycaemia. This vulnerability concept is supported by Ferguson et al [38], who demonstrated that adults with early-onset diabetes (before 7 years of age) had mild brain atrophy and diminished intellectual ability compared with those diagnosed with type 1 diabetes later in childhood or during adolescence. Furthermore, Siller et al [39] reported lower volume in the left temporal–parietal–occipital cortex in individuals with type 1 diabetes soon after diagnosis, when compared with healthy individuals. In that study, higher HbA1c at diagnosis (and thus greater exposure to hyperglycaemia) was associated with lower white matter volume, lower right posterior parietal cortical thickness and greater right occipital cortical thickness [39]. One potential explanation for the findings of aberrant white matter development in young children with type 1 diabetes is that oligodendrocytes responsible for myelination are predominantly generated early in development (e.g. before the age of 5 years) in the corpus callosum [40] and thus glycaemic insults before that age potentially reduce this oligodendrocyte population.
There were no significant between-group differences noted in radial diffusivity, mean diffusivity or fractional anisotropy in our study participants but there were significant differences within both diabetes and non-diabetes groups when comparing these measures at 18 months vs baseline (Fig. 1b–d). These three brain measures each changed as expected over the course of the study as has been reported in non-diabetic populations [26]. A limitation of this study is that we did not report results for individual fibre tracts. This approach was used because of the expected widespread effects of dysglycaemia and the assumption that overall averages would be the most sensitive measurement of potential brain alterations.
In summary, the brains of children with type 1 diabetes have demonstrable differences in axial diffusivity at baseline and 18 months later, suggesting differences in white matter structure that persist over time. When these differences develop in the course of diabetes is unknown, but age at onset of diabetes may play a role. The early presence and persistence of differences in white matter between children with and without diabetes might indicate differences in the metabolic state of the brain between these groups of children. Additionally, and most importantly, the presence of diabetes does not seem to influence the rate of subsequent white matter development–overall, the fibre tracts of individuals with type 1 diabetes seem to develop at the same rate as those of healthy individuals regardless of degree of hyperglycaemia exposure. Last, lower exposure to hyperglycaemia was correlated with better white matter maturation, further emphasising the importance of achieving optimal blood glucose control in young children with diabetes. Additional studies with longer follow-up are needed to better delineate the effects of diabetes on the brain from a functional and anatomical standpoint and to determine how these findings are clinically relevant in the care of children with type 1 diabetes.
Research in context.
What is already known about this subject?
Children with type 1 diabetes have reduced white matter growth and altered white matter microstructure relative to healthy controls
These abnormalities appear to be due, at least in part, to the adverse effects of hyperglycaemia
What is the key question?
Do the adverse effects of dysglycaemia on white matter development persist over an 18 month period in children with type 1 diabetes?
What are the new findings?
Axial diffusivity was lower in children with diabetes at baseline and 18 months. Thus, differences in white matter microstructure appear to persist over time
Lower exposure to hyperglycaemia was associated with higher fractional anisotropy (a proxy measure for myelination)
Fractional anisotropy was positively correlated with performance and full-scale IQ
How might this impact on clinical practice in the foreseeable future?
These results emphasise the importance of minimising hyperglycaemia in children with type 1 diabetes, given the putative negative effects on brain development during childhood
Acknowledgments
The authors thank the participants and their families, as well as the clinical and imaging staff at all investigator sites. They also thank the external collaborators at University of California at San Francisco (San Francisco, CA, USA), El Camino Hospital (Mountain View, CA, USA) and University of Florida/Shands-Jacksonville Medical Center (Jacksonville, FL, USA) for the use of their imaging facilities. The authors also thank K. Winer, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, for advice and support.
Funding
This research was supported by the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (DIRECNET HD-41906, HD-41908, HD-41915, HD-41918, HD-56526, R01-HD-078463).
Abbreviations
- CGM
Continuous glucose monitor
- DirecNet
Diabetes Research in Children Network
- DKA
Diabetic ketoacidosis
- DTI
Diffusion tensor imaging
Appendix
The Diabetes Research in Children Network (DirecNet) Clinical Centers, Principal Investigators (PI), Co-Investigators (I) and Coordinators (C) are as follows.
Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA, USA: Eva Tsalikian, MD (PI); Michael J. Tansey, MD (I); Julie Coffey, MSN (C); Joanne Cabbage (C) and Sara Salamati (C)
Nemours Children’s Health System, Jacksonville, FL, USA: Nelly Mauras, MD (PI); Larry A. Fox, MD (I); Allison Cato, PhD (I); Kim Englert, RN, BSN, CDE (C) and Kaitlin Sikes ARNP, MSN (C)
Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA, USA: Bruce A. Buckingham, MD (PI); Darrell M. Wilson, MD (I); Tandy Aye, MD (I); Kimberly Caswell, ARNP (C) and Ellen Ambers RN (C)
Department of Pediatrics, Yale University School of Medicine, New Haven, CT, USA: Stuart A. Weinzimer, MD (PI); William V. Tamborlane, MD (I); Amy Steffen, BS (C); Kate Weyman, MSN (C); Melinda Zgorski, BSN (C) and Jodie Ambrosino, PhD (I)
Washington University in St Louis, St Louis, MO, USA: Neil H. White, MD, CDE (PI); Ana Maria Arbelaez, MD (I); Lucy Levandoski, PA-C (C); Angie Starnes, RN, BSN, CDE (C) and Tamara Hershey, PhD (I)
Image Coordinating Center, Stanford University, Stanford, CA, USA: Allan L. Reiss, MD; Naama Barnea-Goraly, MD; Matthew J. Marzelli, BS; Paul K. Mazaika, PhD and Daniel X. Peng, BS
Coordinating Center, Jaeb Center for Health Research, Tampa, FL, USA: Roy Beck, MD, PhD (PI); Craig Kollman, PhD (Co-I) and Katrina Ruedy, MPH (Co-I)
Data and Safety Monitoring Board: Mark Sperling, MD; Dorothy M. Becker, MBBCh; Patricia Cleary, MS; Carla Greenbaum, MD and Antoinette Moran, MD
Footnotes
See Appendix for a complete list of Diabetes Research in Children Network (DirecNet) investigators.
Contribution statement
Each listed author has participated in the work represented by the article. Participation has included: (1) substantial contributions to the conception and design of the work or the acquisition, analysis or interpretation of data and (2) drafting the article or revising it critically for important intellectual content. All authors have given final approval of the version to be published. LAF and PM are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data availability
The data that support the findings of this study are available from the corresponding author in anonymised form upon reasonable request.
Duality of interest
LAF reports a research device agreement with Johnson & Johnson (Animas, Inc.); NM reports a research device supply agreement with her institution from Medtronic and Lifescan, research grant support from Medtronic and consultancy from PicoLife Technologies; BAB is a consultant for Dexcom and has received research support from and conducted research studies for Dexcom and Medtronic. WVT, AMA and KE report consultancy from PicoLife Technologies. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
References
- 1.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 3.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 4.Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- 5.Bjorgaas MR. Cerebral effects of severe hypoglycemia in young people with type 1 diabetes. Pediatr Diabetes. 2012;13:100–107. doi: 10.1111/j.1399-5448.2011.00803.x. [DOI] [PubMed] [Google Scholar]
- 6.Marzelli MJ, Mazaika PK, Barnea-Goraly N, et al. Neuroanatomical correlates of dysglycemia in young children with type 1 diabetes. Diabetes. 2014;63:343–353. doi: 10.2337/db13-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perantie DC, Koller JM, Weaver PM, et al. Prospectively determined impact of type 1 diabetes on brain volume during development. Diabetes. 2011;60:3006–3014. doi: 10.2337/db11-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antenor-Dorsey JA, Meyer E, Rutlin J, et al. White matter microstructural integrity in youth with type 1 diabetes. Diabetes. 2013;62:581–589. doi: 10.2337/db12-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aye T, Barnea-Goraly N, Ambler C, et al. White matter structural differences in young children with type 1 diabetes: a diffusion tensor imaging study. Diabetes Care. 2012;35:2167–2173. doi: 10.2337/dc12-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 11.Walhovd KB, Johansen-Berg H, Karadottir RT. Unraveling the secrets of white matter--bridging the gap between cellular, animal and human imaging studies. Neuroscience. 2014;276:2–13. doi: 10.1016/j.neuroscience.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 13.Jelescu IO, Zurek M, Winters KV, et al. In vivo quantification of demyelination and recovery using compartment-specific diffusion MRI metrics validated by electron microscopy. Neuroimage. 2016;132:104–114. doi: 10.1016/j.neuroimage.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. 2009;62:717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- 15.Malone JI, Lowitt S, Korthals JK, Salem A, Miranda C. The effect of hyperglycemia on nerve conduction and structure is age dependent. Diabetes. 1996;45:209–215. doi: 10.2337/diab.45.2.209. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki E, Yasuda K, Yasuda K, et al. 1H-NMR analysis of nerve edema in the streptozotocin-induced diabetic rat. J Lab Clin Med. 1994;124:627–637. [PubMed] [Google Scholar]
- 17.Barnea-Goraly N, Raman M, Mazaika P, et al. Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care. 2014;37:332–340. doi: 10.2337/dc13-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauras N, Mazaika P, Buckingham B, et al. Longitudinal assessment of neuroanatomical and cognitive differences in young children with type 1 diabetes: association with hyperglycemia. Diabetes. 2015;64:1770–1779. doi: 10.2337/db14-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazaika PK, Weinzimer SA, Mauras N, et al. Variations in brain volume and growth in young children with type 1 diabetes. Diabetes. 2016;65:476–485. doi: 10.2337/db15-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnea-Goraly N, Weinzimer SA, Ruedy KJ, et al. High success rates of sedation-free brain MRI scanning in young children using simple subject preparation protocols with and without a commercial mock scanner--the Diabetes Research in Children Network (DirecNet) experience. Pediatr Radiol. 2014;44:181–186. doi: 10.1007/s00247-013-2798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vollmar C, O’Muircheartaigh J, Barker GJ, et al. Identical, but not the same: intra-site and inter-site reproducibility of fractional anisotropy measures on two 3.0T scanners. Neuroimage. 2010;51:1384–1394. doi: 10.1016/j.neuroimage.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oguz I, Farzinfar M, Matsui J, et al. DTIPrep: quality control of diffusion-weighted images. Front Neuroinform. 2014;8:4. doi: 10.3389/fninf.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 24.Yendiki A, Panneck P, Srinivasan P, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform. 2011;5:23. doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2014;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clayden JD, Jentschke S, Munoz M, et al. Normative development of white matter tracts: similarities and differences in relation to age, gender, and intelligence. Cereb Cortex. 2012;22:1738–1747. doi: 10.1093/cercor/bhr243. [DOI] [PubMed] [Google Scholar]
- 27.Cato MA, Mauras N, Ambrosino J, et al. Cognitive functioning in young children with type 1 diabetes. J Int Neuropsychol Soc. 2014;20:238–247. doi: 10.1017/S1355617713001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cato MA, Mauras N, Mazaika P, et al. Longitudinal evaluation of cognitive functioning in young children with type 1 diabetes over 18 months. J Int Neuropsychol Soc. 2016;22:293–302. doi: 10.1017/S1355617715001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Concha L. A macroscopic view of microstructure: using diffusion-weighted images to infer damage, repair, and plasticity of white matter. Neuroscience. 2014;276:14–28. doi: 10.1016/j.neuroscience.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Sugimoto K, Yasujima M, Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr Pharm Des. 2008;14:953–961. doi: 10.2174/138161208784139774. [DOI] [PubMed] [Google Scholar]
- 31.Haraldseth O, Jones RA, Skottner A. A quantitative in-vivo MR imaging study of brain dehydration in diabetic rats and rats treated with peptide hormones. Magn Reson Imaging. 1997;15:203–210. doi: 10.1016/s0730-725x(96)00344-x. [DOI] [PubMed] [Google Scholar]
- 32.Glaser N, Ngo C, Anderson S, Yuen N, Trifu A, O’Donnell M. Effects of hyperglycemia and effects of ketosis on cerebral perfusion, cerebral water distribution, and cerebral metabolism. Diabetes. 2012;61:1831–1837. doi: 10.2337/db11-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krogsrud SK, Fjell AM, Tamnes CK, et al. Changes in white matter microstructure in the developing brain--a longitudinal diffusion tensor imaging study of children from 4 to 11years of age. Neuroimage. 2016;124:473–486. doi: 10.1016/j.neuroimage.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai XJ, Xu HQ, Lu Y. C-peptide and diabetic encephalopathy. Chin Med Sci J. 2011;26:119–125. doi: 10.1016/s1001-9294(11)60031-x. [DOI] [PubMed] [Google Scholar]
- 35.Caballero AE. Endothelial dysfunction, inflammation, and insulin resistance: a focus on subjects at risk for type 2 diabetes. Curr Diab Rep. 2004;4:237–246. doi: 10.1007/s11892-004-0074-9. [DOI] [PubMed] [Google Scholar]
- 36.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunst B, Benedek M, Koschutnig K, Jauk E, Neubauer AC. Sex differences in the IQ-white matter microstructure relationship: a DTI study. Brain Cogn. 2014;91:71–78. doi: 10.1016/j.bandc.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson SC, Blane A, Wardlaw J, et al. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care. 2005;28:1431–1437. doi: 10.2337/diacare.28.6.1431. [DOI] [PubMed] [Google Scholar]
- 39.Siller AF, Lugar H, Rutlin J, et al. Severity of clinical presentation in youth with type 1 diabetes is associated with differences in brain structure. Pediatr Diabetes. 2017;18:686–695. doi: 10.1111/pedi.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeung MS, Zdunek S, Bergmann O, et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]