Abstract
Background
The association between levels of acute kidney injury (AKI) during ICU admission and long-term mortality are not well defined.
Methods
We examined medical records of adult patients admitted to a large tertiary medical center with no history of end-stage renal disease who survived 60 days from ICU admission between 2001 and 2007. Demographic, clinical, physiologic, and date of death data were extracted.
Results
Among 15,048 patients, 12,399 (82.4%) survived 60 days from ICU admission and comprised the study population. AKI did not develop in 5,663 (45.7%) during ICU admission, whereas progressively severe levels of AKI as defined by Acute Kidney Injury Network (AKIN) criteria AKIN 1, AKIN 2, and AKIN 3 developed in 4,589 (37.0%), 1,613 (13.0%), and 534 (4.3%), respectively. Only 42.5% of patients with AKIN 3 survived 2 years from ICU admission. Patients with AKIN 3 had a 61% higher mortality risk 2 years from ICU discharge compared with patients in whom AKI did not develop. Patients with AKIN 1 and AKIN 2 had similar increased mortality risk 2 years from ICU admission (hazard ratio, 1.26 and 1.28, respectively). The level of estimated glomerular filtration rate on ICU discharge and chronic kidney disease were associated with long-term mortality.
Conclusions
Patients in whom AKI develops during ICU admission have significantly increased risks of death that extend beyond their high ICU mortality rates. These increased risks of death continue for at least 2 years after the index ICU admission.
Abbreviations
- AKI
acute kidney injury
- AKIN
Acute Kidney Injury Network
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- HR
hazard ratio
- ICD-9
International Classification of Diseases, Ninth Revision
- MDRD
Modification of Diet in Renal Disease
- MIMIC-II
Multiparameter Intelligent Monitoring in Intensive Care II
- RIFLE
risk, injury, failure, loss, end-stage renal disease
- RRT
renal replacement therapy
- SAPS
Simplified Acute Physiology Score
- SOFA
Sequential Organ Failure Assessment
Patients in whom acute kidney injury (AKI) develops while in the ICU have increased risks of death in the ICU, in the hospital, and in the short term.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 This increased risk has been found to be proportional to the stage of AKI.1, 2, 3, 6 However, most studies that have linked AKI to mortality have examined in-hospital mortality but not long-term outcomes.
In 2002, the Acute Dialysis Quality Initiative defined universal AKI criteria, 13 and in 2005, these were revised by the Acute Kidney Injury Network (AKIN), using updated serum creatinine and urine output criteria and including renal replacement therapy (RRT) data. 14 Several large studies have been performed to validate the new AKIN classification.1, 2, 15, 16 Some suggested that the risk, injury, failure, loss, end-stage renal disease (RIFLE) and AKIN criteria correlate with mortality risk.1, 2, 6, 13, 14 Ostermann et al 2 as well as Mandelbaum et al 1 found that although AKIN 3 criteria were a strong predictor for in-ICU mortality, AKIN 1 and AKIN 2 criteria had the same in-ICU mortality risk.
Relatively few studies examined the association between the severity of AKI and long-term outcomes. These studies showed that severe AKI that requires dialysis as well as AKI that does not require dialysis were associated with increased long-term mortality,17, 18, 19, 20, 21 but they did not use a validated AKI classification system (eg, AKIN, RIFLE). Lafrance and Miller 22 classified AKI by the AKIN criteria and found that long-term mortality risk was highest among the most severe cases of AKI.
The aim of the current study was to explore the effects of the severity of AKI in the ICU on long-term mortality among patients who survived their initial ICU encounter. To do so, we used an existing, prospectively collected, electronic repository of highly detailed patient data.
Materials and Methods
The Multiparameter Intelligent Monitoring in Intensive Care II Database
The Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC-II) project 23 was approved by the institutional review boards (IRB number 2001p-001699) of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center and granted a waiver of informed consent. The MIMIC-II database includes patients admitted to the ICUs at Beth Israel Deaconess Medical Center, a large, academic, tertiary medical center in Boston, Massachusetts, between January 2001 and September 2007 and is maintained by researchers at the Harvard-MIT Division of Health Sciences and Technology.
The MIMIC-II database includes physiologic information from bedside monitors; records of all laboratory values; admission records; discharge summaries; International Classification of Diseases, Ninth Revision (ICD-9), codes; general demographic data; and long-term mortality data derived from the Social Security Administration master file of deaths. A further description of the database is available at http://mimic.physionet.org.
Assembly of the Cohort
We examined medical records of patients aged ≥ 15 years who survived an ICU admission of > 24 h. Patients were excluded if they had an ICD-9 code for end-stage renal disease (ESRD). Additionally, patients who underwent RRT on the day of admission or who had a first-recorded serum creatinine level of > 4 mg/dL were suspected to have ESRD. Because the MIMIC-II database did not have a specific coding system for RRT, patients were considered to have undergone RRT if they had the words “end-stage renal disease” or “dialysis” (or equivalent, ie, “CVVH” [continuous venovenous hemofiltration], “CVVHD” [continuous venovenous hemodialysis], “RRT”) in text notes on the day of admission. We screened these patients' written admission notes and excluded those confirmed to have ESRD. Finally, we manually screened all the notes of patients who received RRT during ICU admission and excluded those who had ESRD.
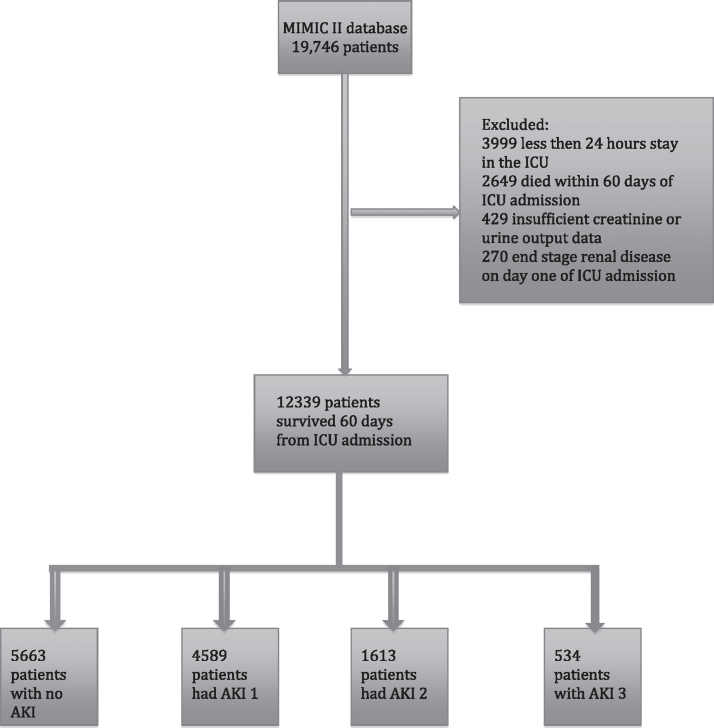
From this group of 15,048 patients, we excluded 2,649 who did not survive 60 days from ICU admission. Thus, the analysis focused on 60-day survivors (Fig 1).
Figure 1.
Patient distribution from the MIMIC II database. A total of 12,399 patients were classified by a combination of urine output and creatinine level measurements. AKI = acute kidney injury; MIMIC II = Multiparameter Intelligent Monitoring of Intensive Care II.
Definition of AKI
We classified patients into non-AKI and three AKI classes according the AKIN criteria (ie, AKIN 1, AKIN 2, AKIN 3).14, 15 AKIN class was determined by serum creatinine measurements from laboratory reports and urine output measurements recorded at least every 2 h during the patient's ICU stay. The observed time periods to detect creatinine elevation were divided to 48-h blocks. By AKIN classification (which is based on RIFLE classification), 14 creatinine elevation should be calculated relative to baseline creatinine level. To calculate the creatinine δ, we compared the highest creatinine rise to the lowest creatinine level during hospitalization. This approach was used previously when the AKI classifications were defined.2, 11, 16, 24 The most severe AKI stage was recorded for every patient. We also calculated the AKIN stage from the first creatinine level on ICU admission. Furthermore, we calculated the estimated glomerular filtration rate (eGFR) on ICU discharge (Modification of Diet in Renal Disease [MDRD] equation). 25 The study population was divided into five groups based on eGFR according to the chronic kidney disease (CKD) classification 26 (group 1, eGFR > 90 mL/min/1.73 m2; group 5, eGFR < 15 mL/min/1.73 m2).
Outcome Measures
The primary outcome measures were all-cause mortality within 1 and 2 years from ICU admission stratified by AKIN level (based on the peak creatinine level during the admission) among all patients admitted to the ICU and among 60-day survivors. Secondary outcomes were all-cause mortality within 60 days from ICU admission stratified by AKIN level and 2-year mortality risk among ICU survivors stratified by (1) AKIN level defined by the admission creatinine level, (2) eGFR on ICU discharge, and (3) presence of CKD.
Statistical Analysis
All data were extracted from the MIMIC-II database (version 2.5). The extracted data included baseline characteristics (age, sex, Elixhauser score), unit of admission, use of RRT, use of mechanical ventilation and vasopressors, laboratory results (ie, serum creatinine level), urine output measurements, ICD-9 codes, and 2-year survival. Acuity level on admission was assessed with the Simplified Acute Physiology Score (SAPS) 27 and Sequential Organ Failure Assessment (SOFA). 28 Both were calculated on admission day 1. Date of death was found and matched from Social Security death records as well as from hospital records.
The preferred method of analysis for continuous variables was parametric. Nonparametric procedures were used only if parametric assumptions could not be satisfied, even after data transformation attempts. Parametric model assumptions were assessed by normal plot or Shapiro-Wilks statistics for verification of normality and Levene test for verification of homogeneity of variances. Categorical variables were tested with Pearson χ2 test for contingency tables or Fisher exact test, as appropriate. Kaplan-Meier survival curves were plotted for the survival analysis stratified by AKI level and in patients with AKIN 3 stratified by RRT. Kaplan-Meier mortality rates were calculated for 1 and 2 years of follow-up.
Logistic regression models were used to assess the adjusted 60-day mortality risk. Cox proportional regression was used as a multivariate analysis for the prediction of 2-year mortality. The following variables were included in the model: sex, SAPS, Elixhauser score,29, 30 use of mechanical ventilation and vasopressors, sepsis, congestive heart failure, and malignancy. Parsimonious models were reported. All statistical tests and CIs, as appropriate, were performed at α = 0.05 (two sided). All P values reported were rounded to three decimal places. The data were analyzed with SPSS (IBM) software.
Results
There were 15,048 patients aged > 15 years who were admitted to the ICU with no ESRD. Of these patients, 12,399 (82.4%) survived 60 days from ICU admission (Fig 1); 5,663 (45.7%) had no AKI during their ICU stay; and AKIN 1, AKIN 2, and AKIN 3 developed in 4,589 (37.0%), 1,613 (13.0%), and 534 (4.3%), respectively. Table 1 shows the patient baseline characteristics. Patients with less severe AKI tended to have been hospitalized in the cardiovascular ICU (31.7% of all patients with AKIN 1 and 34.4% of all patients with AKIN 2), whereas most patients with AKIN 3 were admitted to the medical ICU (51.8%).
Table 1.
Baseline Characteristics of Patients in the ICU Who Survived 60 Days (n = 12,399)
| Variable | AKIN 0 (n = 5,663 [45.7%]) | AKIN 1 (n = 4,589 [37.0%]) | AKIN 2 (n = 1,613 [13.0%]) | AKIN 3 (n = 534 [4.3%]) | P Value |
|---|---|---|---|---|---|
| ICU unit | |||||
| Medical | 1,639 (28.9) | 1,371 (29.8) | 484 (30.0) | 277 (51.8) | < .001 |
| Surgical | 1,360 (24.0) | 1,053 (22.9) | 432 (26.8) | 126 (23.6) | … |
| Cardiac care | 1,178 (20.8) | 712 (15.5) | 142 (8.8) | 70 (13.1) | … |
| Cardiovascular | 1,486 (26.2) | 1,453 (31.7) | 555 (34.4) | 61 (11.4) | … |
| Male sex | 3,397 (60.0) | 2756 (60.1) | 832 (51.6) | 278 (52.2) | < .001 |
| Age, y | 60.76 ± 22.09 | 65.49 ± 20.85 | 65.31 ± 18.41 | 61.24 ± 18.20 | < .001a |
| Comorbidity | |||||
| Elixhauser score | 3.08 ± 6.22 | 5.15 ± 7.01 | 6.16 ± 7.14 | 8.94 ± 8.22 | < .001b |
| Diabetes uncomplicated | 918 (16.2) | 989 (21.6) | 366 (22.7) | 94 (17.6) | < .001 |
| Diabetes complicated | 134 (2.4) | 224 (4.9) | 85 (5.3) | 55 (10.3) | < .001 |
| Congestive heart failure | 618 (10.9) | 831 (18.1) | 330 (20.5) | 175 (32.8) | < .001 |
| Alcohol abuse | 363 (6.4) | 225 (4.9) | 83 (5.2) | 32 (6.0) | .01 |
| Cardiac arrhythmias | 691 (12.2) | 757 (16.5) | 325 (20.2) | 107 (20.1) | < .001 |
| Valvular disease | 326 (5.8) | 288 (6.3) | 118 (7.3) | 45 (8.4) | .02 |
| Hypertension | 1,886 (33.4) | 1,323 (28.9) | 484 (30.1) | 111 (20.8) | < .001 |
| CKD | 84 (1.5) | 188 (4.1) | 38 (2.4) | 93 (17.4) | < .001 |
| COPD | 796 (14.1) | 726 (15.8) | 271 (16.8) | 91 (17.1) | .01 |
| Liver failure | 174 (3.1) | 176 (3.8) | 85 (5.3) | 42 (7.9) | < .001 |
| Metastatic cancer | 153 (2.7) | 142 (3.1) | 59 (3.7) | 17 (3.2) | .23 |
| Psychosis | 181 (3.2) | 149 (3.2) | 54 (3.4) | 22 (4.1) | .72 |
| Depression | 253 (4.5) | 156 (3.4) | 57 (3.5) | 22 (4.1) | .04 |
| Drug abuse | 212 (3.8) | 121 (2.6) | 31 (1.9) | 26 (4.9) | < .001 |
Data are presented as No. (%) or mean ± SD. AKIN = Acute Kidney Injury Network; CKD = chronic kidney disease.
Between AKIN 0 and AKIN 1 and 2, between AKIN 1 and 3, and between AKIN 2 and 3 (P = .001).
Among all groups.
Table 2 presents patient hospitalization characteristics. Severity of illness on admission (SAPS I score) and the comorbidity index (Elixhauser score) increased from no AKI (11.76 and 3.08 points, respectively) to AKIN 3 (16.36 and 8.94 points, respectively). The prevalence of preexisting congestive heart failure, diabetes mellitus with complications, cardiac arrhythmias, valvular heart disease, COPD, liver failure, and IV drug abuse increased with the degree of renal failure, whereas the prevalence of hypertension decreased. The leading diagnoses on ICU admission for patients with AKIN 3 were exacerbation of congestive heart failure (40.4%), sepsis (36.0%), and pneumonia (39.4%). For AKIN 1, AKIN 2, and no AKI, the most prevalent diagnoses on admission were coronary artery diseases and congestive heart failure. One hundred seventy-nine patients (33.5%) with AKIN 3 needed RRT. The difference in the need for mechanical ventilation and vasopressor support was not clinically significant between AKIN 2 and AKIN 3.
Table 2.
Characteristics of Patient Hospitalization
| Variable | AKIN 0 (n = 5,663 [45.7%]) | AKIN 1 (n = 4,589 [37.0%]) | AKIN 2 (n = 1,613 [13.0%]) | AKIN 3 (n = 534 [4.3%]) | P Value |
|---|---|---|---|---|---|
| Diagnosis | |||||
| Postsurgery trauma | 1,360 (24.0) | 1,053 (22.9) | 432 (26.8) | 126 (23.6) | .02 |
| Sepsis | 282 (5.0) | 556 (12.1) | 292 (18.1) | 192 (36.0) | < .001 |
| Congestive heart failure | 977 (17.3) | 1,392 (30.3) | 523 (32.4) | 216 (40.4) | < .001 |
| Cirrhosis | 75 (1.3) | 108 (2.4) | 45 (2.8) | 24 (4.5) | < .001 |
| Pneumonia | 383 (6.8) | 750 (16.3) | 329 (20.4) | 157 (29.4) | < .001 |
| COPD | 503 (8.9) | 535 (11.7) | 180 (11.2) | 74 (13.9) | < .001 |
| GI bleeding | 311 (5.5) | 263 (5.7) | 88 (5.5) | 47 (8.8) | .02 |
| Diabetic ketoacidosis | 40 (0.7) | 69 (1.5) | 39 (2.4) | 17 (3.2) | < .001 |
| Neurologic event | 182 (3.2) | 180 (3.9) | 54 (3.3) | 8 (1.5) | .02 |
| Coronary artery disease | 2,304 (40.7) | 2,093 (45.6) | 663 (41.1) | 153 (28.7) | < .001 |
| ARDS | 84 (1.5) | 303 (6.6) | 177 (11.0) | 61 (11.4) | < .001 |
| RRT during admission | 0 (0.0) | 0 (0.0) | 0 (0.0) | 179 (33.5) | < .001 |
| Acuity score on admission | |||||
| SOFA | 4.27 ± 3.25 | 6.57 ± 3.58 | 7.66 ± 3.62 | 8.91 ± 4.19 | < .001a |
| SAPS I | 11.76 ± 4.79 | 14.72 ± 4.77 | 15.83 ± 4.75 | 16.36 ± 5.42 | < .001b |
| Treatment | |||||
| Mechanical ventilation | 2,647 (46.7) | 3,202 (69.8) | 1,267 (78.5) | 377 (70.6) | < .001 |
| Vasopressors | 1,613 (28.5) | 2,197 (47.9) | 923 (57.2) | 293 (54.9) | < .001 |
| Creatinine level | |||||
| Admission | 0.83 ± 0.33 | 1.10 ± 0.70 | 1.15 ± 0.83 | 2.71 ± 2.22 | < .001c |
| Maximum | 0.87 ± 0.34 | 1.29 ± 0.78 | 1.43 ± 0.94 | 3.99 ± 2.79 | < .001a |
| Discharge | 0.82 ± 0.32 | 1.05 ± 0.64 | 1.00 ± 0.62 | 2.47 ± 2.40 | < .001a |
| Mortality | |||||
| 1 y | 427 (7.6) | 540 (11.9) | 226 (14.1) | 120 (22.7) | < .001d |
| 2 y | 743 (13.1) | 875 (19.1) | 340 (21.1) | 171 (32.0) | < .001d |
Data are presented as No. (%) or mean ± SD. RRT = renal replacement therapy; SAPS I = Simplified Acute Physiology Score I; SOFA = Sequential Organ Failure Assessment. See Table 1 legend for expansion of other abbreviation.
Among all groups.
Between all groups other than 2 and 3.
Between all groups other than 2 and 1.
Kaplan-Meier mortality rates.
Primary Outcome
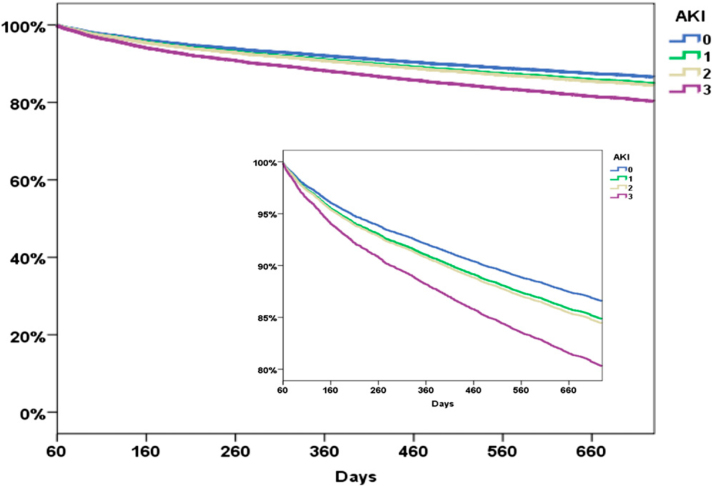
Two years from ICU admission, Kaplan-Meier mortality rates for patients with no AKI, AKIN 1, AKIN 2, and AKIN 3 were 21.4% (95% CI, 20.5%-23.8%), 34.3% (95% CI, 33.2%-35.5%), 37.8% (95% CI, 35.7%-40.0%%), and 57.5% (95% CI, 55.4%-62.1%), respectively. After adjustment for sex, SAPS and Elixhauser scores, use of mechanical ventilation and vasopressors, and admission diagnosis, Cox regression analysis showed an association between 2-year mortality risk from ICU admission and AKI severity (hazard ratios [HRs], 1.19, 1.17, and 1.53 for AKIN 1-3 respectively; P < .001 for all).
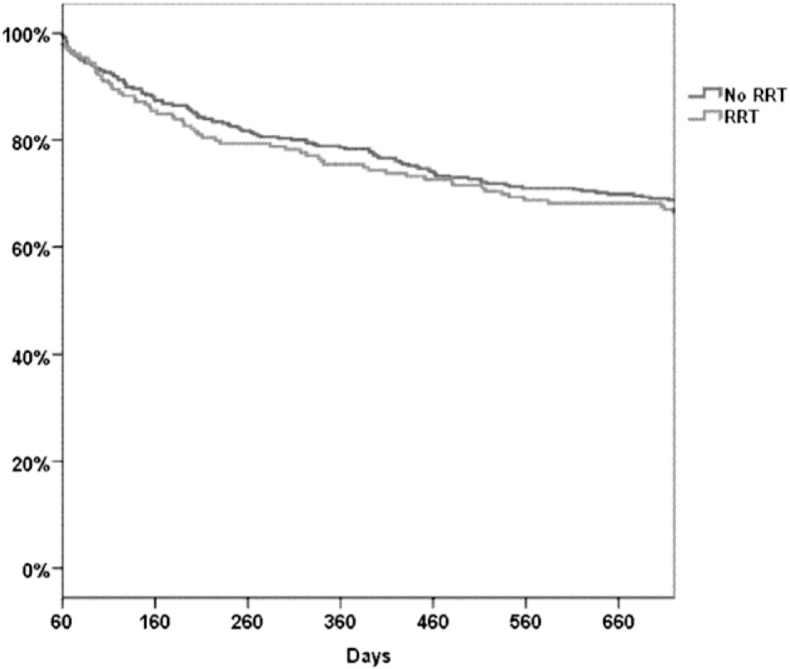
Table 3 and Figure 2 show 2-year mortality risk and 2-year survival curves among ICU survivors, respectively, both stratified by AKIN class and adjusted for baseline and clinical characteristics. For 60-day survivors, 1- and 2-year mortality rates increased significantly with AKIN level (Table 2). Patients in whom AKIN 3 developed and who survived 60 days after admission had a 61% higher adjusted mortality risk 2 years from ICU admission than those in whom AKI did not develop (P < .001). A similar 2-year survival time was found among patients who required RRT during their ICU admission and patients with AKIN 3 who did not require RRT (Fig 3). Patients in whom AKIN 1 and AKIN 2 developed had the same 2-year mortality risk (HR, 1.26 and 1.28, respectively, compared with no AKI; P < .001). After adjusting for CKD at baseline, AKIN 1 to AKIN 3 were still independently associated with higher 2-year mortality (HR, 1.12, 1.19, and 1.24, respectively, compared with no AKI; P < .05 for all). For each point increase in the Elixhauser comorbidity score, there was an 8% increase in mortality risk (P < .001). SAPS I showed a 2% increased mortality risk per point (P < .001).
Table 3.
Cox Proportional Hazards Regression Model for Variables Associated With 2-Year Mortality Among 60-Day Survivors
| Variable | HR (95% CI) | P Value |
|---|---|---|
| AKIN (vs 0)a | ||
| 1 | 1.26 (1.14–1.40) | < .001 |
| 2 | 1.28 (1.11–1.47) | .001 |
| 3 | 1.61 (1.30–1.99) | < .001 |
| Elixhauser score, per point | 1.08 (1.07–1.08) | < .001 |
| SAPS I, per point | 1.02 (1.01–1.03) | < .001 |
| Mechanical ventilation | 0.74 (0.65–0.83) | < .001 |
| Vasopressors | 0.82 (0.74–0.92) | < .001 |
| Sepsis | 1.44 (1.28–1.63) | < .001 |
| Congestive heart failure | 1.29 (1.16–1.42) | < .001 |
| Malignancy | 1.40 (1.24–1.58) | < .001 |
Figure 2.
Survival curves derived from a Cox proportional hazards regression model for survival in 2 y. See Figure 1 legend for expansion of abbreviation.
Figure 3.
Kaplan-Meier curves of patients with Acute Kidney Injury Network 3 criteria stratified by RRT received during ICU stay. RRT = renal replacement therapy.
Secondary Outcomes
The adjusted 60-day mortality rate was associated with the severity of AKI as well. Patients in whom AKIN 3 developed had an 85% higher adjusted mortality rate at 60 days from ICU admission compared with the no-AKI group (P < .001) (Table 4).
Table 4.
Cox Proportional Hazards Regression Model for Variables Associated With 60-Day Mortality
| Variable | OR (95% CI) | P Value |
|---|---|---|
| AKIN (vs 0)a | ||
| 1 | 1.31 (1.15–1.47) | < .001 |
| 2 | 1.28 (1.10–1.48) | .001 |
| 3 | 1.85 (1.48–2.32) | < .001 |
| Male sex | 0.89 (0.81–0.98) | .02 |
| SAPS I, per point | 1.13 (1.12–1.14) | < .001 |
| Elixhauser score, per point | 1.08 (1.08–1.09) | < .001 |
| RRT | 1.87 (1.37–2.54) | < .001 |
In ICU survivors, the level of eGFR on ICU discharge showed a strong association with mortality rate at 2 years. For the decrease in one eGFR class (from 5 [lowest eGFR] to 1 [normal eGFR] calculated with the MDRD equation), there was a 47% decrease in 2-year mortality risk (P < .001). CKD at baseline was found to be an independent risk factor for mortality 2 years after ICU survival (HR, 1.19; P < .001). However, in the same group of ICU survivors, AKIN levels based on the admission creatinine level and adjusted for the baseline characteristics were not associated with the long-term mortality (Table 5).
Table 5.
Cox Proportional Hazards Regression Model for AKIN Level Calculated From Creatinine Level on ICU Admission and 2-Year Mortality Among 60-Day Survivors
| Variable | HR (95% CI) | P Value |
|---|---|---|
| AKIN (vs 0)a | ||
| 1 | 1.09 (0.95–1.26) | .23 |
| 2 | 1.09 (0.85–1.40) | .49 |
| 3 | 1.07 (0.72–1.58) | .74 |
| Male sex | 0.89 (0.82–0.97) | .01 |
| SAPS I, per point | 1.02 (1.01–1.03) | < .001 |
| Elixhauser score, per point | 1.10 (1.09–1.10) | < .001 |
Discussion
The findings suggest that development of AKI during a stay in the ICU is a significant independent mortality risk factor not only for the short term but also for patients who survive their episode of critical illness. AKI remains associated with ongoing, substantial risks of mortality for at least 2 years after the ICU stay. Lower eGFR levels on ICU discharge and CKD were both associated with long-term mortality as well.
This ongoing decrement in survival has important individual and public health consequences. Moreover, it suggests that prevention of AKI could translate to increased long-term survival in critically ill patients, which is particularly true for those who meet AKIN 3 criteria.
Numerous studies linking the severity of AKI to mortality risk have focused on in-hospital mortality and did not address long-term outcomes.1, 2, 3, 6, 15, 16 Postdischarge mortality has been mainly evaluated in patients with severe AKI that required RRT.19, 20, 31, 32 These studies found a strong association between the need for RRT during ICU admission and both in-hospital mortality and long-term mortality risk. The present findings for AKIN 3 criteria are consistent with the reported findings. We found 1- and 2-year survival rates of 77.5% and 68%, respectively, for patients with AKIN 3 who survived 60 days from ICU admission. Compared with patients without AKI and after adjustment for baseline characteristics and severity for disease, AKIN 3 survivors had a 61% higher mortality rate 2 years from admission (Table 3).
To our knowledge, the present study is one of the first to assess the association between the degree of AKI, on the basis of both creatinine level and urine output, and long-term mortality risk among ICU survivors. Previous meta-analyses as well as retrospective studies looked at the degree of AKI during ICU admission and the association with long-term outcomes,17, 18, 22, 32, 33 but only a few of these studies used the newer AKI criteria (RIFLE or AKIN). 22 This may explain the heterogeneity of the findings. Bagshaw et al 19 used serum creatinine level during ICU admission without using the new AKI criteria and found significantly greater case fatality rates for any degree of kidney dysfunction, but there was no difference in the mortality rate 1 year from ICU admission for those who had elevated creatinine levels between 1.7 and 3.4 mg/dL compared with those with levels > 3.4 mg/dL. Ishani et al 33 followed 29,338 patients postcardiac surgery and defined AKI by the magnitude of creatinine level increase from preoperation to peak level after surgery. They found that even a mild increase in creatinine level after cardiac surgery is associated with an increase in mortality. This trend persisted for 5 years after surgery. Lafrance and Miller 22 were the first to use the AKIN criteria to assess the degree of AKI and the association with long-term (4-year) mortality among 82,711 hospitalizations of patients with AKI. AKIN 1-, AKIN 2-, and AKIN 3-adjusted mortality rates were 1.39, 1.51, and 1.71, respectively, compared with patients without AKI.
Patients with CKD at baseline had a 19% higher adjusted mortality rate 2 years from ICU admission. We also showed that eGFR level on ICU discharge had a strong negative association with long-term mortality. One class of improvement in eGFR was associated with an almost 50% decrease in the mortality risk. This association was demonstrated previously among patients with cardiovascular diseases and postsurgery.34, 35, 36
These studies, in addition to the present study, repeatedly showed that AKI during ICU admission is independently associated with short- and long-term mortality risk. Preventing or reversing AKI may translate to an increased survival rate among critically ill patients. Existing AKI classification schemes rely on serum creatinine level and urine output and practically use kidney function as a surrogate for kidney injury. Unfortunately, serum creatinine rises lag kidney insult.37, 38 Identifying sensitive, specific, and early kidney injury markers has the potential to revolutionize the timing and accuracy of the diagnosis of AKI. Several biomarkers have been proposed and are currently being investigated.37, 39, 40, 41, 42, 43, 44
It is also possible that the creatinine rise is a marker for the disease severity beyond that captured by clinical scores (SOFA and SAPS). Sicker patients have higher creatinine levels; hence, patients die with AKI rather than because of AKI. However, the current clinical knowledge implies that AKI is an independent contributor to the mortality risk.4, 6, 16, 45, 46, 47, 48, 49, 50 The proposed mechanisms include the risks of the secondary complications, such as fluid overload,51, 52, 53 inflammation,54, 55, 56 acidosis,57, 58 electrolyte abnormalities,57, 59 infections and inadequate antimicrobial therapy,60, 61 and inadequate metabolic and nutritional support. 62 However, without showing that the decrease in AKI incidence is associated with the mortality benefit, the possibility of AKI being a marker rather than a cause of clinical deterioration cannot be excluded.
Finally, we found that patients who received RRT during ICU admission had the same long-term survival as patients with AKIN 3 who did not receive RRT (Fig 3). This finding may imply that kidney injury per se and not the intervention itself (RRT) is the actual factor associated with mortality. Further studies are needed to better understand the pathogenesis and mechanism of kidney injury and its effect on outcome.
The study has several strengths. First, it used a large, comprehensive cohort of > 19,000 ICU patients. Second, the data are from an accurate, validated urine output assessment, which allowed us to evaluate the prognosis associated with the AKIN criteria in a more robust way than used in previous studies that used only creatinine measurements. 63 On the basis of a recent study published by our group that showed that both urine output- and creatinine-based definitions of AKI perform accurately in predicting mortality risk, 64 we defined AKI by the worst one of these two criteria. Finally, accurate follow-up to 2 years after discharge allowed us to examine the long-term consequences of AKI.
The study has a number of limitations as well. First, we did not have the true baseline creatinine levels and used the lowest value for the hospital admission. A number of studies demonstrated the inaccuracy of other commonly used methods for the calculation of baseline serum creatinine level (ie, MDRD equation), especially in patients with pre-AKI-reduced glomerular filtration rate.65, 66 The AKIN classification is based on the RIFLE classification, 14 which was validated by using the lowest creatinine level during admission as the baseline when the real baseline was unknown.2, 11, 16, 24 Second, the MIMIC-II database contains data from a period of 7 years (2001-2007), during which there were changes in management of critically ill patients and, therefore, possibly in patient outcome. Third, we did not have the data on patients who were RRT dependent on discharge. Finally, although the study included the data from > 19,000 patients and had strong statistical power, it was still a retrospective analysis and a single-center design with their characteristic limitations.
Conclusions
Even among patients who survive an episode of critical illness, AKI remains associated with ongoing, substantial risks of mortality for at least 2 years after the ICU stay. This ongoing decrement in survival has important individual and public health consequences. Moreover, it suggests that prevention of AKI could translate to increased long-term survival in critically ill patients, which is particularly true for those who meet AKIN 3 criteria.
Acknowledgments
Author contributions: Drs Fuchs, Lee, and Novack had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Fuchs: contributed to the study design, data analysis, and manuscript writing.
Dr Lee: contributed to the study design, data analysis, and manuscript writing.
Dr Novack: contributed to the study design, data analysis, and manuscript writing.
Dr Baumfeld: contributed to the data analysis and manuscript writing.
Dr Scott: contributed to the data analysis and manuscript writing.
Dr Celi: contributed to the study design and manuscript writing.
Dr Mandelbaum: contributed to the study design and manuscript writing.
Dr Howell: contributed to the study design and manuscript writing.
Dr Talmor: contributed to the study design and manuscript writing.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: This study was performed in the Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts.
Footnotes
Funding/Support: The authors have reported to CHEST that no funding was received for this study.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Mandelbaum T, Scott DJ, Lee J. Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med. 2011;39(12):2659–2664. doi: 10.1097/CCM.0b013e3182281f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostermann M, Chang R, Riyadh ICU Program Users Group Correlation between the AKI classification and outcome. Crit Care. 2008;12(6):R144. doi: 10.1186/cc7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abosaif NY, Tolba YA, Heap M, Russell J, El Nahas AM. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis. 2005;46(6):1038–1048. doi: 10.1053/j.ajkd.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104(4):343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 5.de Mendonça A, Vincent JL, Suter PM. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26(7):915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 6.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37(9):2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 7.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365(9457):417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 8.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1(1):43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 9.Liaño F, Pascual J, Madrid Acute Renal Failure Study Group Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 1996;50(3):811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 10.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 11.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 12.Welten GM, Schouten O, Chonchol M. Temporary worsening of renal function after aortic surgery is associated with higher long-term mortality. Am J Kidney Dis. 2007;50(2):219–228. doi: 10.1053/j.ajkd.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta RL, Kellum JA, Shah SV, Acute Kidney Injury Network Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joannidis M, Metnitz B, Bauer P. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35(10):1692–1702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 16.Hoste EA, Clermont G, Kersten A. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10(3):R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oeyen S, Vandijck D, Benoit D, Decruyenaere J, Annemans L, Hoste E. Long-term outcome after acute kidney injury in critically-ill patients. Acta Clin Belg Suppl. 2007;(2):337–340. doi: 10.1179/acb.2007.076. [DOI] [PubMed] [Google Scholar]
- 18.Bagshaw SM, Mortis G, Doig CJ, Godinez-Luna T, Fick GH, Laupland KB. One-year mortality in critically ill patients by severity of kidney dysfunction: a population-based assessment. Am J Kidney Dis. 2006;48(3):402–409. doi: 10.1053/j.ajkd.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Bagshaw SM, Laupland KB, Doig CJ. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9(6):R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgera S, Kraft AK, Siebert G, Luft FC, Neumayer HH. Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis. 2002;40(2):275–279. doi: 10.1053/ajkd.2002.34505. [DOI] [PubMed] [Google Scholar]
- 21.Wald R, Quinn RR, Adhikari NK, University of Toronto Acute Kidney Injury Research Group Risk of chronic dialysis and death following acute kidney injury. Am J Med. 2012;125(6):585–593. doi: 10.1016/j.amjmed.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21(2):345–352. doi: 10.1681/ASN.2009060636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeed M, Lieu C, Raber G, Mark RG. MIMIC II: a massive temporal ICU patient database to support research in intelligent patient monitoring. Comput Cardiol. 2002;29:641–644. [PubMed] [Google Scholar]
- 24.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73(5):538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 26.Burden R, Tomson C, Guideline Development Committee, Joint Specialty Committee on Renal Disease of the Royal College of Physicians of London and the Renal Association Identification, management and referral of adults with chronic kidney disease: concise guidelines. Clin Med. 2005;5(6):635–642. doi: 10.7861/clinmedicine.5-6-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Gall JR, Loirat P, Alperovitch A. A simplified acute physiology score for ICU patients. Crit Care Med. 1984;12(11):975–977. doi: 10.1097/00003246-198411000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Arts DG, de Keizer NF, Vroom MB, de Jonge E. Reliability and accuracy of Sequential Organ Failure Assessment (SOFA) scoring. Crit Care Med. 2005;33(9):1988–1993. doi: 10.1097/01.ccm.0000178178.02574.ab. [DOI] [PubMed] [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 31.Vaara ST, Pettilä V, Reinikainen M, Kaukonen KM, Finnish Intensive Care Consortium Population-based incidence, mortality and quality of life in critically ill patients treated with renal replacement therapy: a nationwide retrospective cohort study in Finnish intensive care units. Crit Care. 2012;16(1):R13. doi: 10.1186/cc11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishani A, Nelson D, Clothier B. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171(3):226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 34.Go AS, Yang J, Ackerson LM. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113(23):2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 35.Bihorac A, Yavas S, Subbiah S. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249(5):851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 36.Hillis GS, Croal BL, Buchan KG. Renal function and outcome from coronary artery bypass grafting: impact on mortality after a 2.3-year follow-up. Circulation. 2006;113(8):1056–1062. doi: 10.1161/CIRCULATIONAHA.105.591990. [DOI] [PubMed] [Google Scholar]
- 37.Belcher JM, Edelstein CL, Parikh CR. Clinical applications of biomarkers for acute kidney injury. Am J Kidney Dis. 2011;57(6):930–940. doi: 10.1053/j.ajkd.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 38.Doi K, Yuen PS, Eisner C. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20(6):1217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nedelkov D, Nelson RW. Analysis of human urine protein biomarkers via biomolecular interaction analysis mass spectrometry. Am J Kidney Dis. 2001;38(3):481–487. doi: 10.1053/ajkd.2001.26831. [DOI] [PubMed] [Google Scholar]
- 40.Hewitt SM, Dear J, Star RA. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004;15(7):1677–1689. doi: 10.1097/01.asn.0000129114.92265.32. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu-Tokiwa A, Kobata M, Io H. Serum cystatin C is a more sensitive marker of glomerular function than serum creatinine. Nephron. 2002;92(1):224–226. doi: 10.1159/000064453. [DOI] [PubMed] [Google Scholar]
- 42.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16(10):3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 43.Herget-Rosenthal S. One step forward in the early detection of acute renal failure. Lancet. 2005;365(9466):1205–1206. doi: 10.1016/S0140-6736(05)74787-5. [DOI] [PubMed] [Google Scholar]
- 44.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 45.Kellum JA, Angus DC. Patients are dying of acute renal failure. Crit Care Med. 2002;30(9):2156–2157. doi: 10.1097/00003246-200209000-00041. [DOI] [PubMed] [Google Scholar]
- 46.Metnitz PG, Krenn CG, Steltzer H. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 47.Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med. 2008;36(4):S146–S151. doi: 10.1097/CCM.0b013e318168c590. [DOI] [PubMed] [Google Scholar]
- 48.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275(19):1489–1494. [PubMed] [Google Scholar]
- 49.Lassnigg A, Schmid ER, Hiesmayr M. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36(4):1129–1137. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 50.Hobson CE, Yavas S, Segal MS. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 51.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouchard J, Soroko SB, Chertow GM, Program to Improve Care in Acute Renal Disease (PICARD) Study Group Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 53.Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6(5):966–973. doi: 10.2215/CJN.08781010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19(3):547–558. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gómez JL, Gunnerson KJ, Song M, Li J, Kellum JA. Effects of hypercapnia on BP in hypoalbuminemic and Nagase analbuminemic rats. Chest. 2007;131(5):1295–1300. doi: 10.1378/chest.06-2069. [DOI] [PubMed] [Google Scholar]
- 56.Kellum JA, Song M, Li J. Science review: extracellular acidosis and the immune response: clinical and physiologic implications. Crit Care. 2004;8(5):331–336. doi: 10.1186/cc2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellomo R, Cass A, Cole L, RENAL Replacement Therapy Study Investigators Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 58.Myburgh JA, Finfer S, Bellomo R, CHEST Investigators, Australian and New Zealand Intensive Care Society Clinical Trials Group Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 59.Funk GC, Lindner G, Druml W. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010;36(2):304–311. doi: 10.1007/s00134-009-1692-0. [DOI] [PubMed] [Google Scholar]
- 60.Vandijck DM, Reynvoet E, Blot SI, Vandecasteele E, Hoste EA. Severe infection, sepsis and acute kidney injury. Acta Clin Belg Suppl. 2007;(2):332–336. doi: 10.1179/acb.2007.075. [DOI] [PubMed] [Google Scholar]
- 61.Reynvoet E, Vandijck DM, Blot SI. Epidemiology of infection in critically ill patients with acute renal failure. Crit Care Med. 2009;37(7):2203–2209. doi: 10.1097/CCM.0b013e3181a03961. [DOI] [PubMed] [Google Scholar]
- 62.Demirjian S, Teo BW, Guzman JA. Hypophosphatemia during continuous hemodialysis is associated with prolonged respiratory failure in patients with acute kidney injury. Nephrol Dial Transplant. 2011;26(11):3508–3514. doi: 10.1093/ndt/gfr075. [DOI] [PubMed] [Google Scholar]
- 63.Wlodzimirow KA, Abu-Hanna A, Slabbekoorn M, Chamuleau RA, Schultz MJ, Bouman CS. A comparison of RIFLE with and without urine output criteria for acute kidney injury in critically ill patients. Crit Care. 2012;16(5):R200. doi: 10.1186/cc11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandelbaum T, Lee J, Scott DJ. Empirical relationships among oliguria, creatinine, mortality, and renal replacement therapy in the critically ill. Intensive Care Med. 2013;39(3):414–419. doi: 10.1007/s00134-012-2767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagshaw SM, Uchino S, Cruz D, Bellomo R, Morimatsu H, Morgera S. A comparison of observed versus estimated baseline creatinine for determination of RIFLE class in patients with acute kidney injury. Nephrol Dial Transplant. 2009;24(9):2739–2744. doi: 10.1093/ndt/gfp159. [DOI] [PubMed] [Google Scholar]
- 66.Rule AD. Understanding estimated glomerular filtration rate: implications for identifying chronic kidney disease. Curr Opin Nephrol Hypertens. 2007;16(3):242–249. doi: 10.1097/MNH.0b013e328057de8b. [DOI] [PubMed] [Google Scholar]