Abstract
Leptospirosis is a zoonotic bacterial disease that affects more than one million people worldwide each year. Human infection is acquired through direct or indirect contact with the urine of an infected animal. A wide range of animals including rodents and livestock may shed Leptospira bacteria and act as a source of infection for people. In the Kilimanjaro Region of northern Tanzania, leptospirosis is an important cause of acute febrile illness, yet relatively little is known about animal hosts of Leptospira infection in this area. The roles of rodents and ruminant livestock in the epidemiology of leptospirosis were evaluated through two linked studies. A cross-sectional study of peri-domestic rodents performed in two districts with a high reported incidence of human leptospirosis found no evidence of Leptospira infection among rodent species trapped in and around randomly selected households. In contrast, pathogenic Leptospira infection was detected in 7.08% cattle (n = 452 [5.1–9.8%]), 1.20% goats (n = 167 [0.3–4.3%]) and 1.12% sheep (n = 89 [0.1–60.0%]) sampled in local slaughterhouses. Four Leptospira genotypes were detected in livestock. Two distinct clades of L. borgpetersenii were identified in cattle as well as a clade of novel secY sequences that showed only 95% identity to known Leptospira sequences. Identical L. kirschneri sequences were obtained from qPCR-positive kidney samples from cattle, sheep and goats. These results indicate that ruminant livestock are important hosts of Leptospira in northern Tanzania. Infected livestock may act as a source of Leptospira infection for people. Additional work is needed to understand the role of livestock in the maintenance and transmission of Leptospira infection in this region and to examine linkages between human and livestock infections.
Author summary
Leptospirosis is a globally important disease that is transmitted from animals to people and affects more than 1 million people worldwide each year. Leptospirosis is an important cause of febrile illness in northern Tanzania but little is known about the animal hosts of Leptospira infection for people in this area. This study aimed to evaluate the role of rodents and ruminant livestock (cattle, sheep and goats) in the epidemiology of Leptospira infection in northern Tanzania. The results of our study showed that ruminant livestock but not rodents are commonly infected with pathogenic Leptospira infection. Genetic typing identified four distinct types of Leptospira in livestock, including three types that were only identified in cattle, and one type that was identified in cattle, goats and sheep sampled in our study. These results indicate that livestock are a potential source of infection for people in Tanzania. This finding is important as a large proportion of the human population are employed in farming activities or keep ruminant livestock at home. Further work is needed to understand which Leptospira types are transmitted in our setting and to understand how livestock infection contributes to human disease.
Introduction
Leptospirosis is a zoonotic disease caused by infection with a pathogenic serovar of Leptospira bacteria. Worldwide, leptospirosis is estimated to affect more than one million people and result in the loss of 2.9 million Disability Adjusted Life Years (DALYs) each year [1]. The greatest burden of leptospirosis occurs in tropical and sub-tropical areas, where people live in close contact with animal hosts and warm humid conditions facilitate environmental survival of the bacteria [1, 2]. The clinical presentation of leptospirosis ranges from a mild febrile illness to severe disease with secondary manifestations including renal failure, multiple organ dysfunction, and severe pulmonary haemorrhagic syndrome (SPHS) [3]. The reported median case fatality ratio is around 2% for uncomplicated leptospirosis and 12–40% in patients with more severe disease manifestations such as jaundice and renal failure [4]. Under-reporting of leptospirosis is thought to be common, particularly as human leptospirosis can be difficult to distinguish clinically from other tropical causes of fever such as malaria or dengue fever [5, 6].
Human infection with Leptospira occurs following direct or indirect contact with the urine of an infected mammalian host [5]. To date, more than 250 pathogenic Leptospira serovars belonging to 10 different Leptospira species have been described, which infect a wide variety of animal hosts [7, 8]. Rodents are common hosts of pathogenic Leptospira and are often considered as the most important source of human infection [3, 5]. However, many other animals including companion animals, production livestock species such as cattle and pigs, or wildlife can also carry the infection [9]. In settings where multiple hosts and serovars are present, determining the epidemiology of leptospirosis and identifying sources of human infection can be complex and challenging.
Acute leptospirosis is an important cause of human febrile disease in Tanzania. Hospital-based surveillance conducted in the Kilimanjaro Region of northern Tanzania demonstrated acute leptospirosis in 2–9% of febrile admissions [10, 11]. Estimates of the population-level incidence of leptospirosis in the Kilimanjaro Region vary over time with 11–18 cases per 100,000 per year in 2012–14 [11] and 75–102 cases per 100,000 per year in 2007–08 [12]. A large number of different Leptospira serogroups have been implicated in human disease although the most common predominant serogroups vary by year and by study [11]. Little is known about sources of infection for people in northern Tanzania. Leptospira bacteria have been isolated from cattle, pigs and a variety of small mammal species elsewhere in Tanzania [13]. However, the roles of these animal hosts as a source of infection for people in the Kilimanjaro Region remains unclear.
This study was performed to identify hosts of pathogenic Leptospira bacteria in northern Tanzania. To assess the role of rodents in the epidemiology of Leptospira infection, a cross-sectional survey of peri-domestic rodents was conducted in two districts with a high reported incidence of human leptospirosis. Sampling of cattle, sheep and goats was also performed in local slaughterhouses. The prevalence of Leptospira infection was determined by qPCR testing of kidney samples. Molecular typing of Leptospira bacteria was performed to characterise circulating Leptospira species and genotypes in animal hosts. Here, we discuss the results of these studies and their implications for our understanding of human and animal Leptospira infection in northern Tanzania.
Methods
Ethics statement
Ethical approval for the study was granted by the Tanzania Commission for Science and Technology (COSTECH 2012-471-ER-2005-141); Kilimanjaro Christian Medical Centre (KCMC) Ethics Committee (537); National Institute of Medical Research (NIMR), Tanzania (NIMR/HQ/R.8a/Vol.IX/1499); Tanzania Wildlife Research Institute (TAWIRI); University of Glasgow College of Medicine, Veterinary Medicine and Life Sciences Ethics Committee (200120020), and University of Glasgow Faculty of Veterinary Medicine Ethics and Welfare Committee (01a/13 & 02a/13). Written consent for study participation was obtained for each participating household. Rodent sampling was performed in accordance with UK and international guidelines for humane euthanasia [14, 15].
Description of the study site
The study was conducted in the Kilimanjaro Region in northern Tanzania. The climate in this region follows a pattern of long rains from March to May and short rains from October to December with the coolest months coinciding with the long dry season from June to September. The region has a population of 1.64 million people, and an estimated population density of 124 people per km2 (national average: 51 per km2) [16]. The region is divided into seven districts. Two districts, Moshi Municipal and Moshi Rural (Fig 1), were chosen as the site of the study due to the high reported incidence of human leptospirosis [12] and on-going febrile disease surveillance at local hospitals (Fig 1).
Fig 1. Map of Tanzania showing the administrative regions of Tanzania (main map) and the location of the Moshi Municipal and Moshi Rural Districts within the Kilimanjaro Region (inset).
Maps were made using Quantum Geographic Information System (QGIS) open access software [19]. Shapefiles were obtained from Tanzania National Bureau of Statistics [20].
Moshi Municipal District is the administrative centre of the Kilimanjaro Region. In the 2012 Tanzania National Census, the district was classified as urban and had a population of approximately 184,000 people [16]. Moshi Rural District has a population of approximately 467,000 people and is dominated by small-scale agriculture and smallholder livestock farming [16]. The environment ranges from lush high-altitude mountainous areas where coffee, bananas, and avocados dominate cash crop production, to drier low-altitude pasture land and plains where maize and beans are cultivated. Subsistence livestock farming is common. In the most recent livestock census (2008), the populations of ruminant livestock reported were 139,000 cattle and 353,000 small ruminants (sheep and goats combined) for Moshi Rural District and 2,100 cattle and 7,300 small ruminants for Moshi Municipal District (population size given to nearest 100) [17].
Selection of study villages for cross-sectional sampling
A cross-sectional survey was performed to determine the prevalence of Leptospira infection in peri-domestic rodents within the catchment area of two hospitals (Kilimanjaro Christian Medical Centre (KCMC) or Mawenzi Regional Referral Hospital (MRRH)) that previously identified a high prevalence of acute leptospirosis in patients with febrile illness [10, 11]. The geographical sampling frame was composed of villages within Moshi Municipal and Moshi Rural Districts from which people had sought health care and been enrolled in fever surveillance studies at KCMC and MRRH in the preceding years (2012–2014). One village was selected by convenience as a pilot village (2013) and eleven study villages were selected at random (Fig 2). Consent for study participation was obtained from the Village Chairperson of each study village, who also provided a list of sub-villages within their villages. A single sub-village was selected at random as the sampling location within each study village. The population size of selected sub-villages ranged from 916 to 4320 people (Moshi Municipal: 1039–4320 people; Moshi Rural: 916–3926 people) [18]. Using a reported average household size of 4, this equates to approximately 229 to 1080 households per sub-village (Moshi Municipal: 260–1080 households; Moshi Rural: 229–935 households) [16, 18].
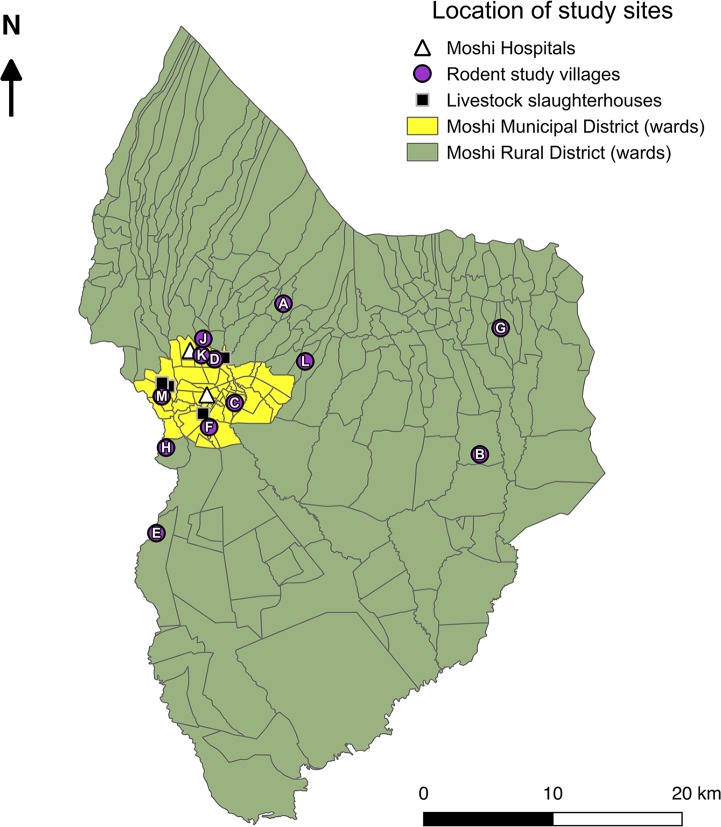
Fig 2. Map of Moshi Municipal and Moshi Rural Districts showing representative locations of rodent study villages and study slaughterhouses in relation to the two study hospitals (Kilimanjaro Christian Medical Centre (KCMC) and Mawenzi Regional Referral Hospital (MRRH).
Maps were made using Quantum Geographic Information System (QGIS) open access software [19]. Districts shapefiles were obtained from Tanzania National Bureau of Statistics [20].
Mapping of cross-sectional study
Study maps (Figs 1 & 2) were made using Quantum Geographic Information System (QGIS) open access software [19]. Shapefiles for Tanzania country boundaries, regions and districts from the most recent census were obtained from Tanzania National Bureau of Statistics [16, 20]. A single representative location for each study village was defined by recording the GPS co-ordinates for the administrative centre of each sampled sub-village.
Rodent trapping and sampling
Rodent trapping was performed in three sampling periods: 1) May-June 2013 (wet season); 2) May-June 2014 (wet season); and 3) August-September 2014 (dry season). The target sample size was 50 rodents per sub-village to give sufficient power (α = 0.95, β = 0.8) to detect a minimum Leptospira infection prevalence of 10% [21–24]. Based on a predicted average trap success of 12.5% [22, 25], 100 traps were set for a target of four nights to give a trapping effort of 400 trap nights per sub-village with the exception of the pilot village (A), where only 50 traps were used. Following initial trapping (villages A & B), the number of nights was increased to an average of eight (trapping effort of 800 trap nights) per sub-village due to lower than expected trapping success.
Sampling transects were established in each sub-village using a method based on the World Health Organization (WHO) Expanded Program for Immunization (EPI) random walk method for cluster sampling [26, 27]. The administrative centre of each sub-village was used as the starting point for sampling transects. The direction of each transect was determined at random within the sub-village (defined by spinning a pen in the field) and ran from the centre of the sub-village to its peripheral boundary. Households were recruited along each transect ensuring a minimum distance of 50 metres between each household until 20 households had been recruited.
Five rodent traps were set in each participating household. In 2013, four large Sherman traps (HB Sherman Traps, Tallahassee, USA. Dimensions: 7.6 x 8.9 x 22.9 cm) and one small Sherman trap (dimensions: 5.1 x 6.4 x 22.9 cm) were set in each household. In 2014, the trapping approach was adjusted and one large Sherman trap per household was replaced with a Tomahawk trap (Tomahawk Live Trap, Hazelhurst, USA. Model 602; dimensions 12.7 x 12.7 x 40.6 cm). Traps were placed in kitchens, food storage areas, and animal housing areas within each household and in sheltered outdoor areas within each compound (e.g. adjacent to animal houses, fence lines and in log piles). A stiff mixture of peanut butter and oats and chopped carrots was used to bait Sherman traps. Dried fish was used to bait Tomahawk traps. Traps were checked and reset each morning. Traps containing rodents were removed and replaced. Trapped rodents were euthanised by terminal halothane anaesthesia and cervical dislocation. The species of each trapped rodent was determined by observation of phenotypic characteristics and measurement of morphometric features [28, 29]. Rodent sex and age class (mature or immature) was determined based on external sexual characteristics [29]. A full necropsy and tissue sampling was performed. For detection of Leptospira infection, one kidney from each rodent was collected and preserved in 70–96% ethanol at room temperature prior to testing by real-time PCR (qPCR).
For a subset of rodents, kidney tissue was also collected for Leptospira culture. Culture was attempted opportunistically during the randomised cross-sectional survey in Villages C, D, E & M based on availability of culture media. In addition, to maximise the chance of Leptospira culture success, the village with the highest trap success in the cross-sectional survey (Village F) was re-visited in September 2014 for repeat rodent trapping and sampling for culture. In this village, trapping was repeated in the 20 previously recruited households using the same strategy (100 traps x 8 nights). Rodent sampling was performed as described above, and kidney tissue was collected for qPCR and culture.
Slaughterhouse sampling of ruminant livestock
Ruminant livestock (cattle, goats and sheep) was sampled in slaughterhouses within the Moshi Municipal District. Five slaughterhouses were selected for livestock sampling in liaison with the District Veterinary Officer based on high slaughter throughput (ranging from 14 and 210 cattle per week), accessibility of location and cooperation from livestock field officers responsible for meat hygiene inspection at each of the slaughterhouses. GPS co-ordinates were recorded at each participating slaughterhouse (Fig 2). The target sample size for cattle (n = 323) was selected to give the study sufficient power to estimate the prevalence of infection with a precision of 5% based on seroprevalence estimates of 30% [30]. Goat and sheep sampling was performed opportunistically at the same slaughterhouses.
Livestock sampling was performed between May 2013 and September 2014. A maximum of ten animals per species were sampled per slaughterhouse per day. The source (region, district and market of origin), approximate age (adult vs. juvenile), gender, and breed (indigenous, exotic or cross-breed) were recorded for each animal. Kidney samples were collected during evisceration into a clean, labelled, single-use Ziplok bag. Following surface sterilisation with a flamed blade, samples of kidney tissue (approximately 3 x 1 x 1 cm) spanning the cortico-medullary junction were taken using a sterile blade and placed directly into 70–96% ethanol prior to testing by qPCR. Samples of kidney tissue were also collected for Leptospira culture from an opportunistically selected subset of cattle and goats.
DNA extraction and qPCR testing for Leptospira infection
The prevalence of renal Leptospira infection in livestock and rodents was determined by qPCR testing. DNA was extracted from 25 milligrams (mg) of kidney tissue preserved in ethanol using the QIAamp DNA Mini Kit spin-column protocol for DNA purification from tissues (Qiagen, Maryland, USA). The DNA concentration was quantified using a NanoDrop spectrophotometer (ThermoScientific, Waltham, MA) and stored at -20°C prior to qPCR testing. DNA extracts were tested for pathogenic Leptospira spp. using a lipL32 TaqMan qPCR assay run on the ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA) as previously described [31, 32]. Amplification of a 245 bp product was performed using the primer set: lipL32-45F (5’-AAG CAT TAC CGC TTG TGG TG-3’) and lipL32-286R (5’-GAA CTC CCA TTT CAG CGA TT-3’), and a 19-bp 5’FAM-labelled probe with a 3’BHQ quencher dye (FAM-5’-AA AGC CAG GAC AAG CGC CG-‘3-BHQ1). Low concentration ROX (50nmol/L) was added to the final reaction mix as a passive reference to improve the diagnostic sensitivity and specificity of the assay [33]. DNA extracts were diluted 1:10 in PCR grade water to reduce the effects of PCR inhibitors. Amplifications were performed using 5μl of diluted template DNA (approximately 50 to 150ng) per 25μl qPCR reaction. Samples were tested in duplicate. Two replicates of a Leptospira positive control, L. interrogans serovar Copenhageni Strain Wijnberg were also run per reaction plate. Control DNA was sourced from the WHO/FAO/OIE Collaborating Leptospirosis Reference Laboratory in Amsterdam and tested at a concentration of 1 pg of DNA (approximately equal to 102 genomic equivalents) per 25μl qPCR reaction. In addition, two replicates of a non-template extraction control, and two replicates of PCR-grade water were included on each test plate. Reaction profiles were analysed using Applied Biosystems 7500 System Sequence Detection (SDS) Software Version 1.2.4 (Applied Biosystems, Carlsbad, CA 2001–2004). A qPCR plate run was considered valid when all negative controls were negative and at least one replicate of the Leptospira positive controls amplified with cycle threshold (Ct) value < 40. Samples were considered positive when at least one test well amplified the lipL32 target with a Ct value < 40.
Typing of Leptospira from qPCR-positive samples
For qPCR-positive samples, the infecting Leptospira species was determined through amplification and sequencing of a conserved 470-bp segment of the secY gene previously shown to have phylogenetic discriminatory power for pathogenic Leptospira species [34, 35]. PCR assays optimized for use in eastern Africa were run at the University of Aberdeen following published protocols [36]. Amplifications were performed using 5μl undiluted template DNA in a 25μl PCR reaction using the primer set: secYFd (5’-ATG CCG ATC ATY TTY GCT TC-3’) and secYR3 (5’-TTC ATG AAG CCT TCA TAA TTT CTC A-3’). All PCR assays included one non-template control (PCR grade water) per five test samples and a positive control of DNA extracted from a pure isolate of L. interrogans or L. borgpetersenii. PCR products were visualised by gel electrophoresis on a 1.5% agarose gel and purified using the QIAquick PCR Purification Kit following manufacturer’s instructions (Qiagen, Maryland, USA). Purified products were quantified using a Nanodrop ND1000 spectrophotometer (ThermoScientific, Massachusetts, USA) and sequenced by Eurofins Genomics GmbH (Ebersburg, Germany).
Leptospira culture
Leptospira culture was performed from kidney tissue samples collected from a total of 98 rodents, 100 cattle, and 49 goats. Following kidney collection, the renal capsule was sterilised using a hot flamed blade and approximately 25 mg of kidney tissue was dissected across the cortico-medullary junction. Tissue was immediately homogenised in 1ml of Ellinghausen-McCullough-Johnson-Harris (EMJH) culture media supplemented with 0.4mg/ml of fluorouracil (5’FU) (EMJH-5FU media) supplied by the WHO/FAO/OIE Collaborating Leptospirosis Reference Laboratory in Amsterdam. A ten-fold dilution series (1:10, 1:100, 1:1000) was prepared in three tubes with 5 ml of EMJH-5FU. Inoculated aliquots of culture media were shipped to the WHO/FAO/OIE Collaborating Leptospirosis Reference Laboratory in Amsterdam for Leptospira isolation. Cultures were incubated at 30°C and checked for Leptospira growth by dark-field microscopy every four weeks for three months and then again after six months of incubation. Positive cultures were confirmed by secY qPCR [37] and sub-cultured in EMJH media prior to typing.
Typing of Leptospira isolates
Leptospira isolated by culture were typed using serological and genetic methods at the WHO/FAO/OIE Collaborating Leptospirosis Reference Laboratory in Amsterdam. Serological typing of pathogenic Leptospira isolates was performed by microscopic agglutination test in two stages. First, a panel of polyclonal rabbit antisera raised against 24 Leptospira serogroups was used to determine the serogroup of isolates [38]. Subsequently, a panel of 18 serovar-specific mouse monoclonal antibodies was used to determine the isolate serovar [39, 40]. Sequence type was determined using a multi-locus sequence typing (MLST) scheme targeting seven Leptospira housekeeping genes (glmU, pntA, sucA, tpiA, pfkB, mreA and caiB) following published protocols [41]. PCR amplicons were sequenced by Macrogen Europe (Amsterdam, Netherlands). Trimmed sequences were aligned against reference sequences for the MLST scheme (obtained from PubMLST; Leptospira Scheme #1: http://pubmlst.org/leptospira/) to generate a unique allelic profile for each isolate [42, 43]. Finally, each allelic profile was compared to an online database of 223 profiles to determine the sequence type (ST) and Leptospira serovar [41].
Phylogenetic analysis
Phylogenetic analysis was performed using MEGA7.0 software [44]. Leptospira secY sequences from qPCR positive samples and Leptospira isolates obtained in this study were trimmed and then aligned using the ClustalW algorithm in MEGA with secY sequences from 128 Leptospira reference serovars obtained through GenBank [34, 45]. The model test function in MEGA was used to select the most appropriate nucleotide substution model for the aligned sequences. Phylogenetic analysis was performed using a maximum likelihood method with 500 bootstrap repeats to generate the final phylogenetic tree.
Statistical analysis
Adjusted trap success was used as a measure of relative rodent abundance in each sub-village [46]. Adjusted trap success was calculating by dividing the total number (n) of rodents caught per sub-village by the corrected number of trap nights (Total number of trap nights (number of traps x number of nights) minus lost trap nights (sum of number of closed, damaged or lost traps / 2) and expressed as a percentage). Statistical analysis was performed in R [47]. Two-sample T-tests were used to compare the adjusted trap success and proportion of households with rodents between the two study districts. Binomial confidence intervals for point prevalence estimates (Wilson method) were calculated using the Hmisc package [48]. Fisher’s exact tests were performed to compare the prevalence of infection between animal species, and between sex and age groups within-species.
Results
Cross-sectional surveillance of peri-domestic rodents
Overall, five villages in Moshi Municipal District and seven villages in Moshi Rural District were selected for inclusion in this study. A summary of selected village details is given in Table 1. During the randomised cross-sectional survey, 351 rodents were trapped across the 12 selected villages. Rodents were trapped in 60.0% of all participating households. The adjusted trap success by village ranged from 1.94 to 10.4% (median = 4.42%). Overall, no significant differences were observed in the adjusted trap successes (two sample t-test: p = 0.690) or average proportion of households with trapped rodents (two-sample t-test: p = 0.124) between the two study districts. In addition, a further 33 rodents (R. rattus: n = 21, 63.6% and M. musculus: n = 12, 36.4%) were trapped from 80.0% of households during repeat sampling in village F (adjusted trap success of 4.42%).
Table 1. Summary of rodent trapping effort and success by village.
| Village ID | A (Pilot) | B | C | D | E | F | F2 ‡ | G | H | J | K | L | M | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| District | Moshi Rural | Moshi Rural | Moshi Municipal | Moshi Municipal | Moshi Rural | Moshi Municipal | Moshi Municipal | Moshi Rural | Moshi Rural | Moshi Rural | Moshi Municipal | Moshi Rural | Moshi Municipal | - |
| Season and year | Wet 2013 | Wet 2013 | Wet 2013 | Wet 2013 | Wet 2013 | Wet 2014 | Dry 2014 | Wet 2014 | Wet 2014 | Wet 2014 | Dry 2014 | Dry 2014 | Dry 2014 | - |
| Sampling nights per village (n) | 3 | 4** | 7 | 10 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 88 |
| Adjusted trap nights per village (n) | 143* | 304 | 650 | 932 | 738 | 731 | 747 | 773 | 748 | 742 | 722 | 751 | 751 | 7985 |
|
Rodents trapped (n) |
14 | 13 | 31 | 25 | 39 | 76 | 33 | 15 | 35 | 20 | 23 | 22 | 38 | 351 |
| Adjusted trap success (%) | 9.79 | 4.28 | 4.77 | 2.68 | 5.28 | 10.8 | 4.42 | 1.94 | 4.69 | 2.70 | 3.19 | 2.93 | 5.06 | 4.42% |
| Households with trapped rodents | 60.0% | 45.0% | 60.0% | 50.0% | 50.0% | 90.0% | 80.0% | 40.0% | 60.0% | 65.0% | 55.0% | 60.0% | 65.0% | 60.0% |
*For pilot village sampling, 5 traps were placed in 10 households for a total of 3 sampling nights.
**For the first night in Village B, traps were set at only 10 households. A further 10 households were recruited the following day.
‡Repeat sampling was conducted in village F (shown as F2) at the end of the study period to increase the chance of Leptospira culture success.
In total, 384 rodents were trapped in this study and were tested for Leptospira infection. Of these, 221 (57.6%) were female and 225 (58.6%) were classified as sexually mature based on external sexual characteristics. The most common species trapped was the black rat (Rattus rattus) (n = 320, 85.1%). Other species included house mice (Mus musculus: n = 44, 11.5%); multimammate mice (Mastomys natalensis: n = 8, 2.08%); spiny mice (Acomys spp.: n = 6, 1.56%); African pygmy mice (Mus minutoides: n = 3, 0.781%); and striped bush squirrels (Paraxerus flavovittis: n = 3, 0.781%).
Slaughterhouse sampling of livestock
Kidney samples were collected from 452 cattle, 167 goats, and 89 sheep. Cattle were sampled at all five slaughterhouses included in this study (median per site = 70; range = 6–273). Opportunistic sampling of sheep was performed at three slaughterhouses (median = 40; range = 2–47) and goats at two slaughterhouses (range = 12–141, slaughterhouse information not recorded for 14 animals). Based on visual assessment of physical characteristics, 439 (97.1%) cattle, 165 (98.8%) goats and 88 (98.9%) sheep were classified as indigenous breeds. The majority of animals were male (cattle: n = 370, 81.9%; goats: n = 117, 70.1%; and sheep: n = 47, 53.8% of sheep) and 93.2% of animals were adult (cattle: n = 405, 89.6%; goats: n = 135, 80.8%; and sheep: n = 77, 86.5%).
Almost all ruminant livestock sampled in this study originated from areas outside the core study districts of Moshi Municipal and Moshi Rural (S1 Table). Of 452 cattle sampled, 381 (84.3%) originated from the Manyara Region (Fig 1), mainly from the districts of Mbulu (n = 296) and Babati (n = 65). Of five cattle that originated from the Kilimanjaro Region, only one originated from either of the Moshi districts (Moshi Rural District, n = 1). All small ruminants sampled in this study originated from either the Arusha or Manyara Regions (S1 Table).
Leptospira qPCR results
Renal infection with pathogenic Leptospira spp. was detected by lipL32 qPCR in 32 (7.1%) cattle, 2 (1.2%) goats, and 1 (1.1%) sheep (Table 2). Leptospira infection was not detected in any of 384 rodent kidney samples tested by lipL32 qPCR (Table 2). Statistically significant differences in the prevalence of infection were detected in pairwise comparisons between cattle and small ruminants, and cattle and rodents (Fisher’s Exact Test, p < 0.05). The odds ratio (OR) of cattle Leptospira infection was 6.26 (95% confidence interval (CI): 1.57–54.5) when compared to goat infection; and 6.75 (95% CI: 1.10–278) when compared to sheep infection. Compared to rodents, cattle were also significantly more likely to be infected with Leptospira (95% CI: 7.41 –Inf). No significant differences in infection prevalence were observed in pairwise comparisons between goats, sheep or rodents (Fisher’s Exact Test, p > 0.05). For ruminant livestock species, no significant differences were observed in infection prevalence by qPCR between male and female, or adult or juvenile animals (Fisher’s exact tests; p > 0.05).
Table 2. Results of Leptospira lipL32 qPCR testing of kidneys from peri-domestic rodents and ruminant livestock (cattle, goats and sheep).
| Animal host | Number tested by lipL32 qPCR |
Leptospira prevalence [Binomial 95% confidence interval] |
|---|---|---|
| Rodents | 384 | 0.00% [0.0–0.99%] |
| Cattle | 452 | 7.08% [5.06–9.82%] |
| Goats | 167 | 1.20% [0.33–4.26%] |
| Sheep | 89 | 1.12% [0.06–6.09%] |
Leptospira culture results and isolate typing
Leptospira was successfully isolated from four cattle kidneys from the subset of cattle tested by Leptospira culture (n = 100). All four Leptospira isolates derived from cattle kidneys were typed as L. borgpetersenii serovar Hardjo (Hardjo-bovis), serogroup Sejroe (ST 152) [43]. No Leptospira growth was detected from the subset of rodents (n = 98) or goat samples (n = 49) that were tested for Leptospira infection by culture.
Phylogenetic analysis from qPCR-positive samples
Identification of infecting Leptospira species by amplification and sequencing of the secY gene was successful for 19 (54.3%) of 35 qPCR-positive kidney samples (Table 3). L. borgpetersenii was the most common infecting Leptospira species and was identified in 13 (72.2%) of 17 cattle samples with secY sequence available for analysis. Phylogenetic analysis revealed two distinct clades of L. borgpetersenii sequence (Fig 3). Sequences from eight cattle samples showed 100% sequence identity with L. borgpetersenii serovar Hardjo isolates obtained in this study (Fig 3: Isolate C0097 and C0101). Sequences from five cattle samples formed a separate clade within the L. borgpetersenii species, which was distinct from all reference sequences.
Table 3. Infecting Leptospira species based on secY sequencing from qPCR positive samples from cattle, goats and sheep.
| Leptospira species | Cattle | Goats | Sheep |
|---|---|---|---|
| Leptospira borgpetersenii | 13 | 0 | 0 |
| Leptospira kirschneri | 1 | 1 | 1 |
| Unidentified Leptospira species | 3 | 0 | 0 |
| secY sequence not available | 15 | 1 | 0 |
| Total qPCR positive samples | 32 | 2 | 1 |
Fig 3. Phylogenetic tree showing the relatedness of the Leptospira secY gene (434-bp fragment) derived from qPCR-positive livestock samples.
The phylogenetic tree was constructed using the maximum likelihood method based on the Tamura-Nei nucleotide substitution model [73]. The tree with the highest log likelihood is shown and drawn to scale with branch lengths measured in the number of substitutions per site. Sequences from this study are labelled with unique identifiers (C0025-C0658); host species; and GenBank accession numbers (MF955862 to MF955882). Sequence from reference Leptospira serovars are also shown [34]. Expanded clades show reference serovars closely related to study genotypes. More distantly related species clades are collapsed and shown with species labels only. Host and country locations shown for Africa isolates are show in parentheses. Sequences from this study that show 100% identity with L. borgpetersenii serovar Hardjo are highlighted in blue; non-Hardjo L. borgpetersenii sequences are highlighted in pink; L. kirschneri sequences are highlighted in green and sequences without an attributed species are highlighted in orange. Abbreviations: (sv) serovar; DRC (Democratic Republic of Congo).
Leptospira kirschneri, was identified in qPCR-positive samples from one cattle, one goat, and one sheep. Sequences from small ruminants (Fig 3: C0417 and C0481) and one bovine (Fig 3: C0059) showed 100% identity to each other as well as to several reference serovars including three serovars isolated human leptospirosis cases in the Democratic Republic of Congo (DRC: Kambale (EU358030), Ndambari (EU358001) and Ndahambukuje (EU358002)).
Infecting Leptospira species could not be determined by secY sequence analysis for a clade of three cattle samples (Fig 3: C0221, C0223 and C0236). In the final phylogenetic tree, the clade containing these sequences appeared most closely related to L. kirschneri but showed only 95% similarity with the closest available reference sequences. GenBank searches also failed to identify any more similar Leptospira species or serovars.
Discussion
In this investigation of animal hosts of pathogenic Leptospira in northern Tanzania, Leptospira infection was detected in ruminant livestock but not in rodents sampled in two districts with a high reported incidence of human leptospirosis [10, 11]. No evidence of infection was detected in any of 384 peri-domestic rodents trapped in a cross-sectional survey conducted across a two-year period at 12 randomly selected sites. In contrast, slaughterhouse sampling of ruminant livestock detected Leptospira infection in cattle (7.06%), goats (1.20%) and sheep (1.11%). Two infecting Leptospira species were detected in ruminant livestock, including L. borgpetersenii in cattle and L. kirschneri in cattle, goats and sheep. A novel Leptospira genotype was also detected in cattle that showed relatively little sequence similarity (95%) to known Leptospira species.
The absence of Leptospira infection in the rodents is a notable finding of this study. Worldwide, rodents are frequent carriers of pathogenic Leptospira bacteria [3, 6] and are often described as the most common source of human infection [3]. However, the lack of detectable infection in our study, which was conducted in two districts where the incidence of human leptospirosis is known to be high [10, 11], indicates that peri-domestic rodents are not a major source of Leptospira infection for people in this area. Although these results were unexpected, we consider that they are robust. Diagnostic protocols used to test rodent samples were consistent with those used in other species (e.g. cattle) that yielded positive results. Rodent sampling was performed at 12 randomly selected villages over a two-year period and the total sample size achieved by our study (n = 384) had sufficient statistical power to demonstrate freedom from infection at the 95% confidence level, even allowing for a low prevalence of infection (e.g. 1.0%) [21, 49].
The reasons for a lack of detectable Leptospira infection in the rodents sampled in our study are unclear. Rattus rattus, the most common species sampled in our study, is globally widespread invasive rodent species that has been demonstrated as a carrier host of Leptospira infection in other settings [23, 50, 51]. Infection has been reported in these species in other African countries [52], including in a study conducted by the authors (KJA, JEBH, AA, RAH) in neighbouring Kenya, where Leptospira was detected in R. rattus (9.1%; n = 33) in the Kibera slums [22]. However, to date, no published studies of R. rattus in Tanzania (e.g. [13, 24, 53]) have demonstrated Leptospira infection by culture or PCR. Therefore, despite their prominent role in other settings, there is very little evidence to suggest that this species are important hosts of Leptospira in northern Tanzania.
To date, Leptospira infection has only been reported in indigenous rodent species such as the African pouched rats (Cricetomys spp.) and multimammate mice (Mastomys natalensis) [13, 54] that typically live outside of domestic environments. Although both rodent species are reported to live in the Kilimanjaro Region [28], Cricetomys was not trapped in our study and M. natalensis was trapped in very low numbers (n = 8) that may have been insufficient to detect low levels of infection in this host population. Another notable absence in the study was the lack of Norway rats (Rattus norvegicus), which is considered the definitive maintenance host of several Leptospira serovars including L. interrogans serovars Copenhagenii and Icterohaemorrhagiae worldwide [9, 55]. The apparent absence of key maintenance hosts of rodent-associated Leptospira serovars such as Cricetomys or R. norvegicus at our study sites is one possible explanation for the lack of infection in the rodents trapped and tested in this study.
In contrast, cattle Leptospira infection appears to be widespread across Tanzania. In this study, bovine Leptospira infection was detected in cattle originating from Manyara, Arusha, Dodoma, Singida and Tanga Regions (S1 Table). Infection has also been reported in cattle sampled in the Morogoro Region [56]. A degree of caution should be exercised in extrapolating estimates of cattle Leptospira prevalence from slaughterhouse studies to the source population. Selection biases for animals sent for slaughter and the potential for increased probability of infection associated with mixing of animals in markets and during transport may increase the prevalence of some infections in slaughterhouse populations [57, 58]. Further sampling of resident livestock in the study districts is necessary to understand the local prevalence and epidemiology of infection in these populations.
Demonstration of renal Leptospira carriage in small ruminant hosts in this study is a novel finding for sub-Saharan Africa. Leptospira infection is well-documented in small ruminants in other parts of the world (e.g. goats in Brazil [59] and sheep in New Zealand [60]) but there have been few studies of small ruminants as hosts of Leptospira infection in the African continent. Goats and sheep are important production livestock in Tanzania [61]. Small ruminant ownership is common and people live in close contact with their livestock in our study area [62]. Detection of renal infection in goats and sheep demonstrates that small ruminants in this setting also carry and shed pathogenic Leptospira in this setting and corroborates serological findings from elsewhere in Tanzania [63]. Small ruminants therefore also have the potential to act as sources of infection for people.
Multiple species and genotypes of pathogenic Leptospira were detected in infected ruminant livestock sampled in this study. Leptospira borgpetersenii was the predominant species infecting cattle. L. borgpetersenii serovar Hardjo was isolated from four cattle, supporting previous serological evidence for the presence of this serovar in Tanzania [63–66]. L. borgpetersenii sequence was also detected in 13 (76.5%) of 17 qPCR cattle with successful secY amplification. Sequences derived from eight qPCR-positive cattle samples were identical to those from L. borgpetersenii serovar Hardjo isolates. A second L. borgpetersenii genotype was detected in 5 (29.4%) cattle samples, which showed only 98% identity to the most similar reference serovars. GenBank BLAST searches identified Leptospira qPCR-positive samples with identical secY sequences in cattle from Brazil (KP862647.1) [67]. The presence of this L. borgpetersenii type in multiple international cattle populations suggests that this Leptospira type could be globally widespread in cattle.
Leptospira kirschneri was the second Leptospira species identified in ruminant livestock species. L. kirschneri sequences derived from cattle, goats and sheep in this study showed 100% identity to each other and to seven other reference serovars (serovars Bim, Bogvere, Kambale, Mozdok, Ndambari, Ndahambukujue, Tsaratsovo). Two serovars, L. kirschneri serovar Grippotyphosa and L. kirschneri serovar Sokoine, have previously been isolated from Tanzanian cattle and showed a high degree of similarity to L. kirschneri genotypes detected in this study (> 99%) [13, 56]. Notably, a clade of novel secY sequences was also detected in cattle qPCR-positive samples that could not be attributed to any Leptospira species by phylogenetic analysis. Sequences derived from three cattle infections were identical to each other but distinct from any reference sequences used in the phylogenetic analysis for this study. BLAST searches conducted in GenBank also failed to identify any similar sequences from other studies. Two possible explanations exist to describe the relationship of this clade of novel sequences to the rest of the Leptospira genus. First, these sequences could represent a divergent clade of L. kirschneri, which is the most similar known Leptospira species. However, sequence variation of 5% in the secY gene is the reported threshold of the difference observed between Leptospira species [34]. Therefore, an alternative explanation is that this clade represents a new and previously undescribed Leptospira species. Further work is needed to determine the species and fully characterise this novel Leptospira genotype.
The secY single-locus genotyping approach is this study provides a robust initial assessment of the diversity of Leptospira species circulating in Tanzanian livestock. The high degree of similarity between some of the livestock sequences identified in this study and sequences from human infections elsewhere in sub-Saharan Africa (e.g. DRC and Kenya, see Fig 3) suggests that livestock may be an important source of Leptospira infection for people across the eastern and central African region. To date, there are no secY sequences derived from human Leptospira infection in northern Tanzania, limiting our ability to use genomic data to compare infecting Leptospira species between human and livestock populations. Serological data from human cases in Tanzania does exists [10, 11] but the poor correlation between genotype and serogroup for Leptospira bacteria limits our ability to robustly link these data to attribute sources of Leptospira infection [7, 68]. However, epidemiological studies have identified milking cattle, feeding and cleaning cattle and handling cattle waste as significant risk factors for human Leptospira infection in Moshi and neighbouring regions [69, 70]. These findings suggest that cattle are indeed an important source of Leptospira infection for people in northern Tanzania and provide a strong rationale for further investigation linked human and cattle populations to better understand the relationship between human and bovine infection.
Overall, our study makes a substantial contribution to the growing body of evidence that livestock play an important role in the epidemiology of human leptospirosis in sub-Saharan Africa. Although the contribution of other species cannot be ruled out, contact with livestock has been demonstrated as an important risk factor for human Leptospira infection in northern Tanzania [70]. Occupational exposure to infected livestock is known to be an important risk factor for human leptospirosis in other settings [71] and currently more than 75% of the Tanzanian population is estimated to be employed in the agriculture sector [61]. Given the importance of leptospirosis as a cause of human febrile illness in Tanzania [72], quantifying the contribution of livestock-associated leptospirosis to human health and understanding the factors that support the maintenance and transmission of pathogenic Leptospira in livestock populations are important priorities for future leptospirosis and public health research.
Supporting information
(DOCX)
Acknowledgments
The authors would like to thank the District Medical and Veterinary Officers of Moshi Municipal and Moshi Rural Districts, study village executive officers, sub-village chairpeople, householders and butchers that participated in this project. Special thanks go to Godfrey Laiser, Lemuta Laiser, Isaya Rumas, Fadhili Mshana, Raphael Mahemba, Denice Luwumba, Francis William, Raymond Edward and Juvenile Urio for their assistance with sample collection. We are grateful to Cynthia Asiyo, Frank Michael and Francis Karia for administrative support. We would also like to thank the Kilimanjaro Christian Medical Centre, Kilimanjaro Clinical Research Institute and the Tanzania Wildlife Research Institute for crucial infrastructure support in Tanzania.
Data Availability
Datasets supporting this manuscript are available through: http://dx.doi.org/10.5525/gla.researchdata.582. Unique sequences generated through this study are available through GenBank (accession numbers MF955862 to MF955882).
Funding Statement
This work was supported by the Wellcome Trust (grant number 096400/Z/11/Z; https://wellcome.ac.uk/). JEBH, VPM, JAC, and SC received support from the Research Councils UK, UK Department for International Development, and UK Biotechnology and Biological Sciences Research Council (BBSRC) (grant numbers BB/J010367/1, BB/L018926, BB/L017679, BB/L018845; http://www.bbsrc.ac.uk/). JAC and VPM also received support from the US National Institutes of Health (NIH)-National Science Foundation (NSF) Ecology and Evolution of Infectious Disease program (R01TW009237; https://www.fic.nih.gov/programs/pages/ecology-infectious-diseases.aspx). MM received support from the BBSRC East of Scotland Bioscience Doctoral Training Partnership (http://www.eastscotbiodtp.ac.uk/). MJM received support from a University of Otago Frances G. Cotter Scholarship and a University of Otago MacGibbon PhD Travel Fellowship (http://www.otago.ac.nz/). VPM and JAC received support from the US National Institutes of Health National Institute for Allergy and Infectious (grant number R01 AI121378; https://www.niaid.nih.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898 doi: 10.1371/journal.pntd.0003898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abela-Ridder B, Sikkema R, Hartskeerl RA. Estimating the burden of human leptospirosis. Int J Antimicrob Agents. 2010;36(Suppl. 1):S5–S7. [DOI] [PubMed] [Google Scholar]
- 3.Haake DA, Levett PN. Leptospirosis in humans In: Adler B, editor. Leptospira and Leptospirosis. Curr Top Microbiol Immunol. 387 Berlin: Springer; 2015. p. 65–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor AJ, Paris DH, Newton PN. A systematic review of the mortality from untreated leptospirosis. PLoS Negl Trop Dis. 2015;9(6):e0003866 doi: 10.1371/journal.pntd.0003866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326. doi: 10.1128/CMR.14.2.296-326.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartskeerl R, Collares-Pereira M, Ellis W. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17(4):494–501. doi: 10.1111/j.1469-0691.2011.03474.x [DOI] [PubMed] [Google Scholar]
- 7.Levett PN. Systematics of Leptospiraceae In: Adler B, editor. Leptospira and Leptospirosis. Curr Top Microbiol Immunol. 387 Berlin: Springer-Verlag; 2015. p. 11–20. [DOI] [PubMed] [Google Scholar]
- 8.Bourhy P, Collet L, Brisse S, Picardeau M. Leptospira mayottensis sp. nov., a pathogenic Leptospira species isolated from humans. Int J Syst Evol Microbiol. 2014;64(12):4061–7. doi: 10.1099/ijs.0.066597–0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis WA. Animal Leptospirosis In: Adler B, editor. Leptospira and Leptospirosis Curr Top Microbiol Immunol. 387 Berlin: Springer; 2015. p. 99–137. doi: 10.1007/978-3-662-45059-8_6 [DOI] [PubMed] [Google Scholar]
- 10.Biggs HM, Bui DM, Galloway RL, Stoddard RA, Shadomy SV, Morrissey AB, Bartlett JA, Onyango JJ, Maro VP, Kinabo GD, Saganda W, Crump JA. Leptospirosis among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hyg. 2011;85(2):275–81. doi: 10.4269/ajtmh.2011.11-0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maze MJ, Biggs HM, Rubach MP, Galloway RL, Cash-Goldwasser S, Allan KJ, Halliday JEB, Hertz JT, Saganda W, Lwezaula BF, Cleaveland S, Mmbaga BT, Maro VP, Crump JA. Comparison of the estimated incidence of acute leptospirosis in the Kilimanjaro Region of Tanzania between 2007–08 and 2012–14. PLoS Negl Trop Dis. 2016;10(12):e0005165 doi: 10.1371/journal.pntd.0005165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biggs HM, Hertz JT, Munishi OM, Galloway RL, Marks F, Saganda W, Maro VP, Crump JA. Estimating leptospirosis incidence using hospital-based surveillance and a population-based health care utilization survey in Tanzania. PLoS Negl Trop Dis. 2013;7(12):e2589 doi: 10.1371/journal.pntd.0002589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mgode GF, Machang'u RS, Mhamphi GG, Katakweba A, Mulungu LS, Durnez L, Leirs H, Hartskeerl RA, Belmain SR. Leptospira serovars for diagnosis of leptospirosis in humans and animals in Africa: common Leptospira isolates and reservoir hosts. PLoS Negl Trop Dis. 2015;9(12):e0004251 doi: 10.1371/journal.pntd.0004251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Home Office. Guidance on the Operation of the Animals (Scientific Procedures) Act 1986. 2014.
- 15.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrakin MA, Meyer R, Miller D, Shearer J, Yanong R. The AVMA Guidelines for the Euthanasia of Animals. 2013 ed. Schaumburg, IL: American Veterinary Medical Association; 2014. [Google Scholar]
- 16.Tanzania National Bureau of Statistics. Population and Housing Census. 2012. [Google Scholar]
- 17.The United Republic of Tanzania Ministry of Livestock and Fisheries Development. Basic data for livestock and fisheries sectors. 2013. [Google Scholar]
- 18.Tanzania National Bureau of Statistics. Population and Housing Census. 2002. [Google Scholar]
- 19.QGIS Development Team. QGIS Geographic Information System 2.18 ed: Open Source Geospatial Foundation Project; 2017. [Google Scholar]
- 20.Tanzania National Bureau of Statistics. 2012 Population and Housing Census of Tanzania Shapefiles (Levels 1 and 2) Tanzania: Tanzania National Bureau of Statistics; 2013. [Google Scholar]
- 21.Sergeant ESG. Epitools epidemiological calculators: AusVet Animal Health Services and Australian Biosecurity Cooperative Research Centre for Emerging Infectious Disease 2016. Available from: http://epitool.ausvet.com.au/.
- 22.Halliday JE, Knobel DL, Allan KJ, Bronsvoort BMdC, Handel I, Agwanda B, Cutler SJ, Olack B, Ahmed A, Hartskeerl RA. Urban leptospirosis in Africa: a cross-sectional survey of Leptospira infection in rodents in the Kibera urban settlement, Nairobi, Kenya. Am J Trop Med Hyg. 2013;89(6):1095–102. doi: 10.4269/ajtmh.13-0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahelinirina S, Leon A, Harstskeerl RA, Sertour N, Ahmed A, Raharimanana C, Ferquel E, Garnier M, Chartier L, Duplantier JM, Rahalison L, Cornet M. First isolation and direct evidence for the existence of large small-mammal reservoirs of Leptospira sp. in Madagascar. PLoS One. 2010;5(11):e14111 doi: 10.1371/journal.pone.0014111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mgode GF, Mhamphi G, Katakweba A, Paemelaere E, Willekens N, Leirs H, Machang'u RS, Hartskeerl RA. PCR detection of Leptospira DNA in rodents and insectivores from Tanzania. Belg J Zool. 2005;135:17–9. [Google Scholar]
- 25.Halliday JEB. Animal Sentinel Surveillance: Evaluating domestic dogs as sentinels for zoonotic pathogen surveillance: University of Edinburgh; 2010. [Google Scholar]
- 26.Milligan P, Bennett S. Comparison of two cluster sampling methods for health surveys in developing countries. Int J Epidemiol. 2004;33(3):469–76. doi: 10.1093/ije/dyh096 [DOI] [PubMed] [Google Scholar]
- 27.Bostoen K, Chalabi Z. Optimization of household survey sampling without sample frames. Int J Epidemiol. 2006;35(3):751–5. doi: 10.1093/ije/dyl019 [DOI] [PubMed] [Google Scholar]
- 28.The Field Museum. Mammals of Tanzania: Order Rodentia Chicago. 2011. Available from: http://archive.fieldmuseum.org/tanzania/.
- 29.Cunningham D, Moors P. A guide to the identification and collection of New Zealand Rodents Wellington: New Zealand Wildlife Service Department; 1996. [Google Scholar]
- 30.Swai ES, Schoonman L. A survey of zoonotic diseases in trade cattle slaughtered at Tanga city abattoir: a cause of public health concern. Asian Pac J Trop Biomed. 2012;2(1):55–60. doi: 10.1016/S2221-1691(11)60190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn Microbiol Infect Dis. 2009;64(3):247–55. doi: 10.1016/j.diagmicrobio.2009.03.014 [DOI] [PubMed] [Google Scholar]
- 32.Stoddard RA. Detection of pathogenic Leptospira spp. through real-time PCR (qPCR) targeting the LipL32 gene In: Wilks M, editor. PCR Detection of Microbial Pathogens. 2nd ed. 2013. p. 257–66. [DOI] [PubMed] [Google Scholar]
- 33.Galloway RL, Hoffmaster AR. Optimization of LipL32 PCR assay for increased sensitivity in diagnosing leptospirosis. Diagn Microbiol Infect Dis. 2015;82(3):199–200. http://dx.doi.org/10.1016/j.diagmicrobio.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Victoria B, Ahmed A, Zuerner RL, Ahmed N, Bulach DM, Quinteiro J, Hartskeerl RA. Conservation of the S10-spc-α locus within otherwise highly plastic genomes provides phylogenetic insight into the genus Leptospira. PLoS ONE. 2008;3(7):e2752 doi: 10.1371/journal.pone.0002752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed N, Devi SM, De los Á Valverde M, Vijayachari P, Machang'u RS, Ellis WA, Hartskeerl RA. Multilocus sequence typing method for identification and genotypic classification of pathogenic Leptospira species. Ann Clin Microbiol Antimicrob. 2006;5(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dietrich M, Wilkinson DA, Soarimalala V, Goodman SM, Dellagi K, Tortosa P. Diversification of an emerging pathogen in a biodiversity hotspot: Leptospira in endemic small mammals of Madagascar. Mol Ecol. 2014;23(11):2783–96. doi: 10.1111/mec.12777 [DOI] [PubMed] [Google Scholar]
- 37.Ahmed A, Engelberts MF, Boer KR, Ahmed N, Hartskeerl RA. Development and validation of a real-time PCR for detection of pathogenic Leptospira species in clinical materials. PLoS One. 2009;4(9):e7093 doi: 10.1371/journal.pone.0007093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faine S. Leptospira and leptospirosis: CRC Press Inc.; 1994. [Google Scholar]
- 39.Hartskeerl R, Smits HL, Korver H, Goris M, Terpstra WJ. International course on laboratory methods for the diagnosis of leptospirosis 5th ed The Netherlands: Royal Tropical Institute; 2006. [Google Scholar]
- 40.Hartskeerl R, Smythe LD. The role of leptospirosis reference laboratories In: Adler B, editor. Leptospira and Leptospirosis. Curr Top Microbiol Immunol. 387 Berlin: Springer; 2015. p. 273–86. doi: 10.1007/978-3-662-45059-8_11 [DOI] [PubMed] [Google Scholar]
- 41.Boonsilp S, Thaipadungpanit J, Amornchai P, Wuthiekanun V, Bailey MS, Holden MT, Zhang C, Jiang X, Koizumi N, Taylor K, Galloway R, Hoffmaster AR, Craig S, Smythe LD, Hartskeerl RA, Day NP, Chantratita N, Feil EJ, Aanensen DM, Spratt BG, Peacock SJ. A single multilocus sequence typing (MLST) scheme for seven pathogenic Leptospira species. PLoS Negl Trop Dis. 2013;7(1):e1954 doi: 10.1371/journal.pntd.0001954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jolley KA, Maiden MC. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11(1):1–11. doi: 10.1186/1471-2105-11-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jolley KA, Maiden MC. Leptospira spp. MLST Databases University of Oxford; 2016. Available from: http://pubmlst/org/leptospira. [Google Scholar]
- 44.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2015;33(7):1870–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benson DA, Karsch-Mizrachi I, Clark K, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2011. doi: 10.1093/nar/gkr1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson L, Clark F. Correction for Sprung Traps in Catch/Effort Calculations of Trapping Results. J Mammal. 1973;54(1):295–8. [Google Scholar]
- 47.R Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 48.Harrell FE, Dupont C,. Hmisc: Harrell Miscellaneous. Version 3.17–4 ed. 2016.
- 49.Cameron AR, Baldock FC. A new probability formula for surveys to substantiate freedom from disease. Prev Vet Med. 1998;34(1):1–17. [DOI] [PubMed] [Google Scholar]
- 50.Desvars A, Michault A, Chiroleu F. Influence of risk factors on renal leptospiral load in naturally infected wild black rats. Acta Trop. 2013;125(3):258–61. doi: 10.1016/j.actatropica.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 51.Marshall R, Manktelow B. Fifty years of leptospirosis research in New Zealand: a perspective. N Z Vet J. 2002;50(3):61–3. [DOI] [PubMed] [Google Scholar]
- 52.Allan KJ, Biggs HM, Halliday JEB, Kazwala RR, Maro VP, Cleaveland S, Crump JA. Epidemiology of leptospirosis in Africa: a systematic review of a neglected zoonosis and a paradigm for ‘One Health’ in Africa. PLoS Negl Trop Dis. 2015;9(9):e0003899 doi: 10.1371/journal.pntd.0003899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belmain S, editor Rats and Human Health in Africa: Proceedings of an international workshop on rodent-borne diseases and the RatZooMan research project RatZooMan Workshop; 2006; Malenlane, Republic of South Africa: University of Greenwich. [Google Scholar]
- 54.Machang'u RS, Mgode GF, Assenga J, Mhamphi G, Weetjens B, Cox C, Verhagen R, Sondij S, Goris MG, Hartskeerl RA. Serological and molecular characterization of Leptospira serovar Kenya from captive African giant pouched rats (Cricetomys gambianus) from Morogoro Tanzania. FEMS Immunol Med Microbiol. 2004;41(2):117–21. doi: 10.1016/j.femsim.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 55.Kosoy M, Khlyap L, Cosson JF, Morand S. Aboriginal and invasive rats of genus rattus as hosts of infectious agents. Vector Borne Zoonotic Dis. 2015;15(1):3–12. doi: 10.1089/vbz.2014.1629 [DOI] [PubMed] [Google Scholar]
- 56.Mgode GF, Machang'u RS, Goris MG, Engelbert M, Sondij S, Hartskeerl RA. New Leptospira serovar Sokoine of serogroup Icterohaemorrhagiae from cattle in Tanzania. Int J Syst Evol Microbiol. 2006;56(3):593–7. doi: 10.1099/ijs.0.63278–0 [DOI] [PubMed] [Google Scholar]
- 57.Cleaveland S, Shaw D, Mfinanga S, Shirima GM, Kazwala R, Eblate E, Sharp M. Mycobacterium bovis in rural Tanzania: risk factors for infection in human and cattle populations. Tuberculosis (Edinb). 2007;87(30–43). [DOI] [PubMed] [Google Scholar]
- 58.McKenna S, Keefe GP, Barkema H, McClure J, VanLeeuwen JA, Hanna P, Sockett D. Cow-level prevalence of paratuberculosis in culled dairy cows in Atlantic Canada and Maine. J Dairy Sci. 2004;87(11):3770–7. doi: 10.3168/jds.S0022-0302(04)73515-8 [DOI] [PubMed] [Google Scholar]
- 59.Martins G, Lilenbaum W. Leptospirosis in sheep and goats under tropical conditions. Trop Anim Health Prod. 2014;46(1):11–7. doi: 10.1007/s11250-013-0480-6 [DOI] [PubMed] [Google Scholar]
- 60.Vallee E, Heuer C, Collins-Emerson JM, Benschop J, Wilson PR. Serological patterns, antibody half-life and shedding in urine of Leptospira spp. in naturally exposed sheep. N Z Vet J. 2015;63(6):301–12. doi: 10.1080/00480169.2015.1049668 [DOI] [PubMed] [Google Scholar]
- 61.Food and Agriculture Organization of the United Nations. Livestock Sector Brief: United Republic of Tanzania Livestock Information Sector Analysis and Policy Branch, editor. Rome, Italy: Food and Agriculture Organization of the United Nations (FAO); 2005. [Google Scholar]
- 62.Allan KJ. Leptospirosis in northern Tanzania: investigating the role of rodents and ruminant livestock in a neglected public health problem: University of Glasgow; 2016. [Google Scholar]
- 63.Assenga JA, Matemba LE, Muller SK, Mhamphi GG, Kazwala RR. Predominant leptospiral serogroups circulating among humans, livestock and wildlife in Katavi-Rukwa ecosystem, Tanzania. PLoS Negl Trop Dis. 2015;9(3):e0003607 doi: 10.1371/journal.pntd.0003607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swai ES, Schoonman L, Machang'u R. Prevalence and factors associated with of bovine leptospirosis in small scale dairy farms in Tanga Region, Tanzania. Bull Anim Health Prod Afr. 2005;53(1):51–9. [Google Scholar]
- 65.Machang'u RS, Mgode G, Mpanduji D. Leptospirosis in animals and humans in selected areas of Tanzania. Belg J Zool. 1997;127(Suppl.1):97–104. [Google Scholar]
- 66.Karimuribo ED, Swai ES, Kyakaisho PK. Investigation of a syndrome characterised by passage of red urine in smallholder dairy cattle in East Usambara Mountains, Tanzania. J S Afr Vet Assoc. 2008;79(2):89–94. [DOI] [PubMed] [Google Scholar]
- 67.Hamond C, Pestana CP, Medeiros MA, Lilenbaum W. Genotyping of Leptospira directly in urine samples of cattle demonstrates a diversity of species and strains in Brazil. Epidemiol Infect. 2016;144(1):72–5. doi: 10.1017/S0950268815001363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guernier V, Allan KJ, Goarant C. Advances and challenges in barcoding pathogenic and environmental Leptospira. Parasitology. 2017;18:1–13. doi: 10.1017/S0031182017001147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maze MJ, Cash-Goldwasser S, Rubach MP, Biggs HM, Galloway RL, Sharples KJ, Allan KJ, Halliday JEB, Cleaveland S, Shand MC, Muiruri C, Kazwala RR, Saganda W, Lwezaula BF, Mmbaga BT, Maro VP, Crump JA. Risk factors for acute leptospirosis in northern Tanzania. PLoS Negl Trop Dis. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schoonman L, Swai ES. Risk factors associated with the seroprevalence of leptospirosis, amongst at-risk groups in and around Tanga city, Tanzania. Ann Trop Med Parasitol. 2009;103(8):711–8. doi: 10.1179/000349809X12554106963393 [DOI] [PubMed] [Google Scholar]
- 71.Mwachui MA, Crump L, Hartskeerl R, Zinsstag J, Hattendorf J. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843 doi: 10.1371/journal.pntd.0003843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, Ooi EE, Maro VP, Saganda W, Kinabo GD, Muiruri C, Bartlett JA. Etiology of severe non-malaria febrile illness in northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7(7):e2324 doi: 10.1371/journal.pntd.0002324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol Biol Evol. 1992;9(4):678–87. doi: 10.1093/oxfordjournals.molbev.a040752 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Datasets supporting this manuscript are available through: http://dx.doi.org/10.5525/gla.researchdata.582. Unique sequences generated through this study are available through GenBank (accession numbers MF955862 to MF955882).