Abstract
Invasion of epithelial cells by Salmonella enterica requires expression of genes located in the pathogenicity island I (SPI-1). The expression of SPI-1 genes is very tightly regulated and activated only under specific conditions. Most studies have focused on the regulatory pathways that induce SPI-1 expression. Here, we describe a new regulatory circuit involving CRP-cAMP, a widely established metabolic regulator, in silencing of SPI-1 genes under non-permissive conditions. In CRP-cAMP-deficient strains we detected a strong upregulation of SPI-1 genes in the mid-logarithmic growth phase. Genetic analyses revealed that CRP-cAMP modulates the level of HilD, the master regulator of Salmonella invasion. This regulation occurs at the post-transcriptional level and requires the presence of a newly identified regulatory motif within the hilD 3’UTR. We further demonstrate that in Salmonella the Hfq-dependent sRNA Spot 42 is under the transcriptional repression of CRP-cAMP and, when this transcriptional repression is relieved, Spot 42 exerts a positive effect on hilD expression. In vivo and in vitro assays indicate that Spot 42 targets, through its unstructured region III, the 3’UTR of the hilD transcript. Together, our results highlight the biological relevance of the hilD 3’UTR as a hub for post-transcriptional control of Salmonella invasion gene expression.
Author summary
Salmonella infection is one of the major causes of foodborne illness worldwide. During infection, Salmonella expresses a set of virulence genes encoded in discrete regions of the genome. The expression of these genes is tightly regulated, being specific for different stages of the Salmonella infection process. While many regulatory mechanisms that lead to the activation of infection-related gene expression have been described, little is known about silencing mechanisms under conditions when expression is not needed and may rather represent a burden than a benefit for the bacterial fitness. Here, we report a condition-specific silencing mechanism of bacterial virulence. That is, the global transcriptional regulator CRP-cAMP represses, indirectly through a post-transcriptional mechanism, the expression of the major Salmonella virulence regulator HilD. In bacteria, post-transcriptional regulation has so far been mainly focused on 5’ untranslated regions (5’UTR). Remarkably, here we describe a molecular mechanism targeting the 3’untranslated region (3’UTR) of the mRNA of the major regulator of Salmonella virulence by a small non-coding RNA under the transcriptional control of the global regulator CRP-cAMP. Our data highlight the importance of 3’UTR in the regulation of gene expression in bacteria.
Introduction
Salmonella enterica serovar Typhimurium is a prevalent gastrointestinal pathogen. Upon arrival in the intestinal lumen, Salmonella is able to both invade epithelial cells and survive within phagocytic cells. Genomic studies revealed the presence of several pathogenicity islands in the Salmonella chromosome (SPIs). Among them, SPI-1 and SPI-2 are the best studied and known to encode factors required for invasion of non-phagocytic cells and survival within macrophages, respectively [1,2]. Induction of virulence programs is generally associated with significant energetic costs for the bacterial cell. For example, induction of SPI-1 under non-infectious conditions in vitro has a negative impact on cell physiology, resulting in a deleterious effect on Salmonella’s growth [3]. Consequently, the expression of virulence programs is generally tightly regulated and induction occurs only upon sensing of a variety of defined environmental and physiological signals.
The complex regulatory circuit that controls SPI-1 expression has attracted much attention [2,4] and become a model to understand how the activities of multiple molecular factors converge to achieve a precise timing of virulence gene activation. The majority of the multiple signal transduction systems that modulate SPI-1 regulation converge at the level of HilA expression, a SPI-1 encoded transcriptional regulator required for the expression of most SPI-1 genes [5]. Salmonella does not express HilA when it is growing exponentially in LB cultures, a condition stated as non-permissive in the present study. However, HilA expression is induced at early stationary phase, when growth conditions become nutrient-limiting [6], a condition here referred to as SPI-1-permissive. Transcription of hilA is controlled by three AraC-like transcriptional activators: HilD, HilC and RtsA. The first two are encoded within SPI-1 itself, while RtsA is encoded outside this locus [7]. HilD, HilC and RtsA form a feed-forward regulatory loop, whereby each activator induces the two other genes, but also auto-regulates its own expression [8]. This regulatory triad responds to a wide range of physiological and environmental stimuli that are sensed by a variety of cellular factors, including both global and specific regulators (as reviewed by Fabrega and Vila [2]). Within this triad, a prominent role has been attributed to HilD, the main target for signaling pathways controlling SPI-1 expression [8,9]. Regulatory mechanisms have been described, acting at all levels of hilD gene expression—transcriptional, post-transcriptional, translational and post-translational [10–13]. Most studies focused on the mechanisms required for full induction of SPI-1 genes, whereas very little is known on the regulatory pathways involved in the shutdown of the SPI-1 expression under non-permissive conditions. Here, we report that general transcription factor CRP is required to silence SPI-1 genes in exponential growing cells and propose a new regulatory axis formed by CRP and the broadly conserved small RNA (sRNA) Spot 42 that contributes to growth phase-specific activation of SPI-1 genes.
CRP is a global transcriptional regulator that acts as a metabolic sensor upon binding of intracellular cAMP (cyclic adenosine monophosphate), which is synthesized by the adenylate cyclase CyaA [14]. CRP-cAMP-deficient Salmonella strains are unable to secrete SPI-1 T3SS effector proteins and are avirulent in a mouse model, suggesting a role for CRP-cAMP in the regulation of Salmonella virulence [15,16]. Indeed, CRP-cAMP indirectly regulates virulence by affecting the post-transcriptional regulation of hilD. The sRNAs CsrB and CsrC are under the transcriptional control of Bar/SirA and are upregulated in a crp knockout mutant. CsrB and CsrC are antagonists of CsrA, a post-transcriptional repressor of hilD mRNA [10,17,18]. Therefore, in early stationary phase (permissive conditions for SPI-1 expression), CRP-cAMP generally promotes SPI-1 expression by indirectly repressing the activity of CsrA [17–19]. Here, we report that in mid-logarithmic growth phase (non-permissive conditions), CRP-cAMP represses hilD expression by a mechanism requiring Hfq and the 3’UTR of hilD mRNA. Given the established primary role of Hfq in mediating the base pairing interactions of sRNAs [20,21], it is tempting to speculate that hilD may be post-transcriptionally regulated by a CRP-cAMP controlled sRNA; this control, however, would be unusual in light of the fact that almost all Hfq-associated sRNAs characterized to date recognize mRNAs in the 5’ region. Of several candidates for CRP-cAMP-dependent sRNAs known in enteric bacteria [22], Spot 42 has been best characterized in Escherichia coli, where together with CRP-cAMP, it forms a multi-output feedforward loop to enact catabolite repression [23–27]. In Salmonella, Spot 42 has been known as one of the most abundant Hfq-associated sRNAs in fast-growing cells [28], but except for a repression of the sugar-related mglB mRNA [29] its activity has not been characterized.
By dissecting the molecular mechanism of CRP-cAMP-mediated SPI-1 repression in exponentially growing Salmonella, we here reveal novel mechanisms by which sRNAs target mRNAs. Our data point towards an unusual post-transcriptional stimulation of the hilD mRNA by Spot 42. Different from other trans-acting sRNA characterized, Spot 42-mediated activation occurs in the 3’ UTR of the hilD mRNA, adding to a growing appreciation of mRNA 3’ ends as sites for post-transcriptional control in bacteria.
Results
CRP-cAMP represses SPI-1 expression
To characterize the role of the metabolic sensor CRP-cAMP in SPI-1 expression, we monitored transcription of the main regulator HilA in wild-type and Δcrp derivative strains grown in LB at 37°C. The expression pattern was determined in mid-logarithmic cultures (OD600nm 0.4, non-permissive conditions for SPI-1 expression) and at early stationary phase (OD600nm 2.0, permissive conditions for SPI-1 expression) (Fig 1A). Consistent with previous reports, a growth phase dependent profile in SPI-1 expression was observed [6]. In the wild-type strain, hilA expression levels were 8-fold higher at early stationary phase when compared to mid-logarithmic cultures. Remarkably, we also observed a growth-phase dependent effect of the Δcrp mutation. In agreement with previous work [18], the Δcrp mutation reduces hilA transcription in early stationary phase. In mid-logarithmic cultures, however, the Δcrp mutation caused an upregulation of hilA expression (4-fold, as compared to the wild-type). Using a chromosomally encoded FLAG-tagged HilA variant, these transcriptional profiles were corroborated on the protein level. More HilA protein accumulated in the Δcrp mutant in mid-logarithmic cultures and less in early stationary cultures, relative to wild-type levels (Fig 1B).
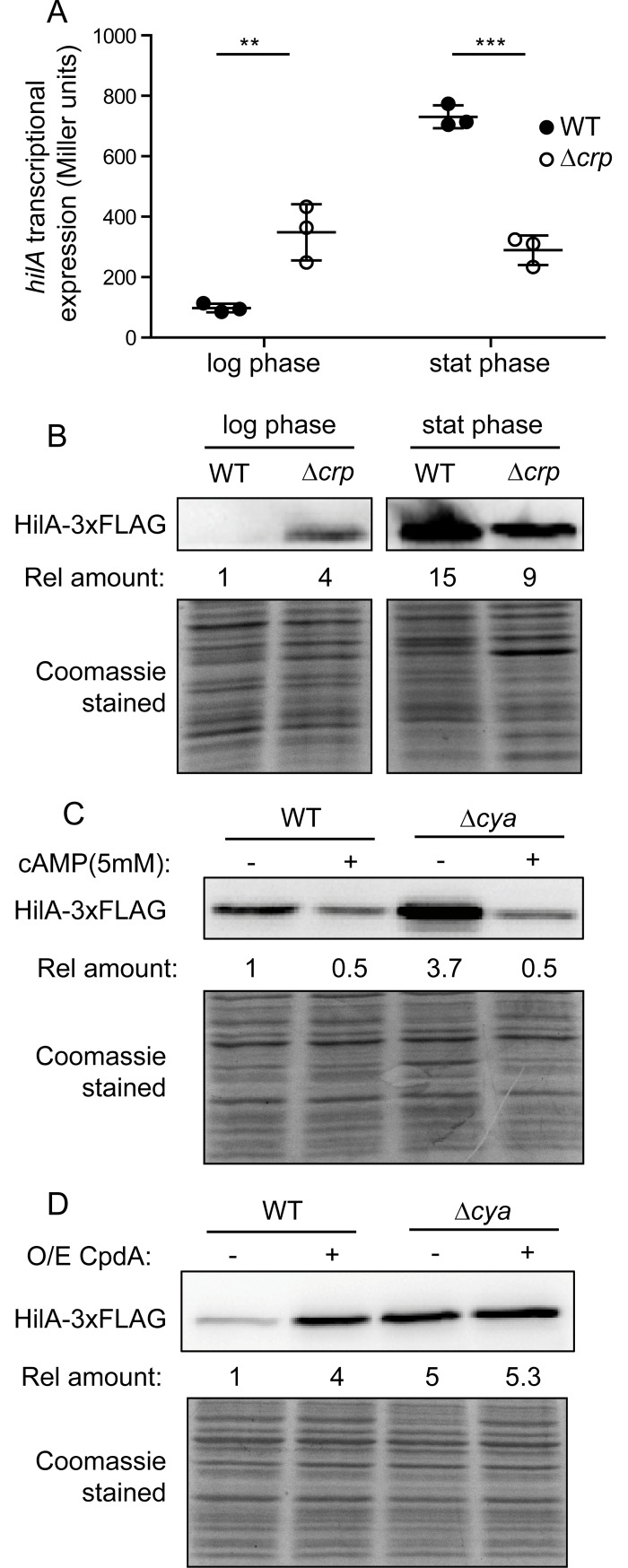
Fig 1. CRP-cAMP represses hilA expression in mid-logarithmic growing cells.
(A) Transcriptional expression of hilA in a wild-type (WT) and a Δcrp derivative strain. β-galactosidase activity from a hilA-lacZ fusion was assessed in LB cultures grown at 37°C up to either mid-logarithmic (OD600nm 0.4) or early stationary (OD600nm 2.0) phase of growth. Data from three independent experiments are averaged and the standard deviation is shown. **, p< 0.01; ***, p< 0.001. (B) Immuno-detection of HilA-3xFLAG protein was performed on whole cell extracts of the WT and Δcrp derivative strains, grown as in A. (C). Immuno-detection of HilA-3xFLAG on whole cell extracts from cultures of the WT and Δcya derivative strains in the absence (-) or presence (+) of cAMP (5 mM). Cultures were grown in LB at 37°C up to an OD600nm of 0.4. (D) Effect of over-expressing the cAMP-phosphodiesterase CpdA. Immunodetection of HilA-3xFLAG was performed on cultures of the WT and a Δcya derivative strain carrying either pTrc99a (-, control vector) or pCpdA (+, pTrc99a+cpdA). Cultures were grown as in C in LB supplemented with IPTG (0.1 mM). In B, C and D the relative amount of HilA-3xFLAG is indicated. In each case the reference value was set as one. Coomassie Blue staining of the whole cell extracts serve as loading controls. Full length images of the Western blots, including molecular mass markers, are shown in S12 Fig.
CRP is active upon binding of the cofactor cAMP [14]. Therefore, lack of either CRP or cAMP should have a similar effect on SPI-1 expression. HilA levels were monitored in a Δcya mutant strain, which is deficient in the synthesis of cAMP (Fig 1C). As expected, Δcya mutation caused an almost 4-fold increase in HilA levels in mid-logarithmic phase cells. Chemical complementation was performed by monitoring HilA abundance after addition of cAMP (Fig 1C). An 8-fold decrease in HilA levels was observed when cAMP was added to cultures of the Δcya strain. Interestingly, when cAMP was added to a culture of a cya+ (i.e. wild-type) strain, a 2-fold drop in HilA levels was observed. These results may indicate that the intracellular cAMP levels were not saturating all CRP molecules. Consequently, external addition of the cofactor to wild-type cultures would lead to an increase in the number of CRP-cAMP complexes, causing further repression of HilA expression.
To further corroborate the involvement of cAMP in the control of HilA expression, the intracellular levels of cAMP were lowered by ectopically over-expressing CpdA in Salmonella, a putative cAMP phosphodiesterase [30]. Over-expression of CpdA, confirmed by immunodetection (S1 Fig), caused a 4-fold increase in HilA expression in the wild-type background. This clearly depended on cAMP turnover, since CpdA over-expression had no effect in a Δcya strain (Fig 1D).
Impact on SPI-1-encoded effector proteins
HilA regulates the transcriptional expression of most SPI-1 genes, including those required for the synthesis of a type III secretion system (T3SS) and several effector proteins that are translocated to the host cell during Salmonella infection [2]. A ΔhilA mutation impairs secretion of SPI-1 effector proteins [31]. Comparative studies of the secreted protein profile between wild-type and ΔhilA strains were performed to identify protein bands corresponding to SPI-1 effectors (S2 Fig). Major protein bands exclusively detected in extracts of the wild-type strain were identified by LC-MS/MS as the SPI-1-encoded proteins SipA and SipC. The secretome of wild-type, Δcrp and Δcya derivative strains was characterized in LB cultures grown to mid-logarithmic and early stationary phase (Fig 2A). Consistent with previous reports [16], CRP-cAMP-deficient cells in early stationary phase showed a lower amount of those secreted proteins. Yet, CRP-cAMP-deficient Salmonella hyper-secreted SPI-1 effector proteins in mid-logarithmic phase cultures. The Δcrp-dependent overproduction in mid-logarithmic phase of the larger protein, the effector protein SipA, was confirmed by using a SipA-3xFLAG variant (Fig 2B).
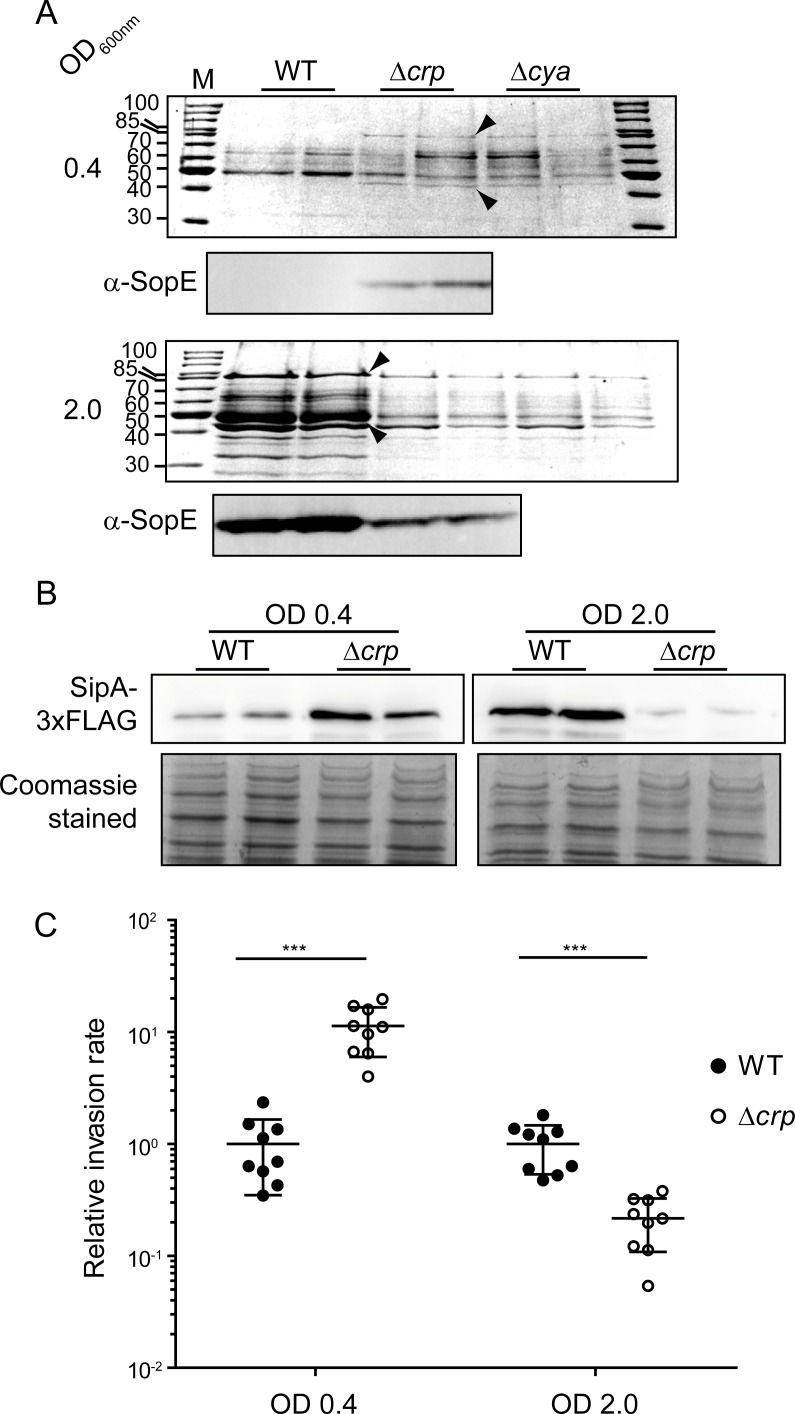
Fig 2. Effect of crp and cya deletion on the expression of SPI-1 effector proteins.
(A) Proteins from cell-free supernatants from cultures of the wild-type (WT), Δcrp and Δcya derivative strains grown up to mid-logarithmic (OD600nm 0.4) or early stationary (OD600nm 2.0) phase were TCA precipitated. The resulting extracts were analyzed by SDS-PAGE and Coomassie Blue staining. Size in kDa of the molecular mass marker (M) is indicated. Arrowheads indicate the protein bands corresponding to SipA (upper band) and SipC (lower band). Immunodetection of SopE protein (α-SopE) was performed in WT and Δcrp extracts. (B) Immunodetection of SipA-3xFLAG was performed on whole cell extracts from cultures of the WT and Δcrp derivative strains grown as in A. Coomassie Blue stainings of the whole cell extracts serve as loading controls. In A and B, extracts from two independent cultures of the same strain were analyzed. Full length images of the Western blots, including molecular mass markers, are shown in S12 Fig. (C) Invasion of HeLa cells. Cultures of the WT and Δcrp strains were grown as in A and assessed for invasion of HeLa cells. Invasion rates were calculated and given as relative rate, the reference value of WT was set to one in both mid-logarithmic and early stationary phase cultures. The invasion rates were 0.7 x 10−3 and 2.4 x 10−2 for the WT in mid-logarithmic and early stationary phase, respectively. ***, p< 0.001.
HilA has also been reported to regulate the expression of SopE, an effector protein that is encoded outside the SPI-1 locus but it is secreted by the SPI-1 encoded T3SS [32]. SopE levels were monitored in secreted protein extracts of wild-type and Δcrp mutant strains (Fig 2A). Again, the Δcrp strain secreted more SopE protein in the mid-logarithmic phase and less in early stationary phase, as compared to wild-type. The fact that CRP-cAMP-mediated repression of hilA expression has a concomitant effect on the expression and secretion of SPI-1 effector proteins highlights the biological relevance of CRP-cAMP in the control of Salmonella virulence under non-permissive conditions. In support of this notion, a Δcrp mutant grown to mid-logarithmic phase prior to infection, invaded HeLa cells more efficiently (>10-fold) than the wild-type (Fig 2C). In contrast, the wild-type strain showed a higher rate (>4.5-fold) than the Δcrp derivative when cultures were grown to early stationary phase prior to infection.
CRP-cAMP regulation of SPI-1 occurs upstream of HilA by repression of hilD, hilC and rtsA
Three AraC-like transcriptional activators, HilD, HilC and RtsA, are directly involved in hilA activation [8]. To determine at which level CRP-cAMP controls SPI-1 through HilA, the expression of hilD, hilC and rtsA mRNA was monitored. RNA was extracted from mid-logarithmic cultures (OD600nm 0.4) of both wild-type and Δcrp derivative strains and the relative amounts of mRNA of all three AraC-like regulators were determined by qRT-PCR. As shown in Fig 3A, in the Δcrp mutant higher transcripts levels of hilD, hilC and rtsA were detected than in the wild-type, indicating that the effect of CRP-cAMP on SPI-1 expression occurs upstream of HilA. The hilA transcript was also monitored by qRT-PCR as a control; as expected, it too over-accumulated in the Δcrp strain (S3 Fig).
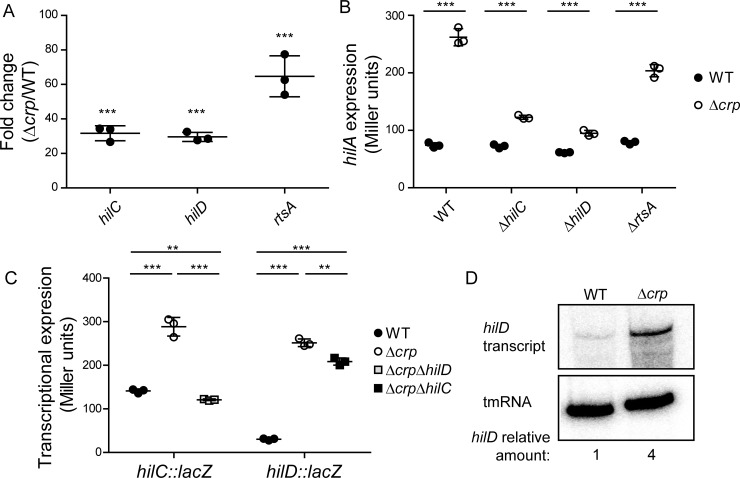
Fig 3. Effect of crp deletion on the expression of upstream activators of hilA.
(A) Relative quantification of hilD, hilC and rtsA mRNA by qRT-PCR. Total RNA samples were extracted from cultures of the wild-type (WT) and Δcrp derivative strains. Results are expressed as fold changes between WT and Δcrp. Detection of gapA (GAPDH) was used as an internal control (see Material and Methods). (B) hilA transcriptional expression (β-galactosidase activity) was monitored in crp+ and Δcrp strains in different genetic backgrounds: WT, ΔhilC, ΔhilD and ΔrtsA. (C) hilC and hilD transcriptional expression (β-galactosidase activity) was monitored. For hilC, WT, Δcrp and Δcrp ΔhilD derivative strains carrying a hilC-lacZ, chromosomal fusion were used. For hilD, WT, Δcrp and Δcrp ΔhilC carrying the hilD1235-lacZ (hilD+, 3’UTR+) were used. In A, B and C, data correspond to the average and standard deviation of three independent experiments. **, p< 0.01; ***, p< 0.001. (D) Northern blot analysis for hilD mRNA. Total RNA samples were extracted from cultures of the WT and Δcrp strains. tmRNA was detected as loading control. For quantification, the ratio [hilD mRNA/tmRNA] was calculated. Full length image of the northern blot is shown in S12 Fig. In all cases cultures were grown in LB at 37°C up to an OD600nm of 0.4.
HilD, HilC and RtsA form a feed-forward regulatory loop to stimulate hilA expression. In order to elucidate the direct target of CRP-mediated regulation of SPI-1, mutants of each of the three regulators in a crp proficient and deficient background were generated and hilA expression monitored in mid-logarithmic cultures (Fig 3B). In the crp+ strains, hilA expression was very low in all genetic backgrounds, further validating the silenced hilA expression during logarithmic growth. The hilA derepression in the absence of CRP was altered in the different mutants. Remarkably, in the absence of HilC and HilD the derepression of hilA transcription was greatly reduced. The expression of hilC and hilD was further studied using transcriptional lacZ fusions. As expected, both hilC and hilD were deregulated in a Δcrp mutant background (Fig 3C). Particularly, HilD seems to be required for the observed upregulation of hilC in the Δcrp background, whereas hilD induction does not require HilC. Taken together, these results suggest that HilD is the direct target of CRP-cAMP-mediated regulation of SPI-1 expression. Northern blot detection further corroborates an increase in the levels of hilD mRNA in the Δcrp strain as compared to wild-type in mid-logarithmic phase (Fig 3D).
CRP-cAMP-mediated transcriptional regulation of HilD requires the 3’UTR of the hilD transcript
The hilD mRNA possess an unusually long (310 nt) 3’UTR that has an overall negative effect on hilD expression [11]. If the 3’UTR is deleted, the hilD mRNA accumulates and the SPI-1 genes are induced concomitantly [11]. Of note, the above-described effect of CRP-cAMP on hilD transcription (Fig 3C) was elucidated using a hilD-lacZ fusion at position +1,235 (relative to the transcription start site), containing the hilD coding sequence and the full-length 3’UTR. To determine whether the hilD 3’UTR is important in CRP-mediated regulation, a proximal fusion at position +76 was constructed. Remarkably, the Δcrp mutation had no effect on this proximal fusion (Fig 4A). This indicates that either CRP-cAMP does not regulate hilD expression at the level of transcription initiation or that the HilD protein is required for the induction of transcription initiation in a crp-deficient strain. To discriminate between these two possibilities, a hilD-lacZ fusion at position +965 was constructed, carrying the whole hilD coding sequence but lacking the hilD 3’UTR. As shown in Fig 4A, the Δcrp mutation did not lead to a significant induction even when the full coding sequence was included (hilD965-lacZ), as compared to a 6-fold induction detected using the hilD1235-lacZ fusion which includes both the hilD coding sequence and its 3’UTR. Although we cannot fully rule out a potential effect of CRP on hilD transcription, the different behavior of the hilD965-lacZ and hilD1235-lacZ reporters clearly points towards the 3’UTR being crucial for CRP-mediated post-transcriptional regulation of hilD.
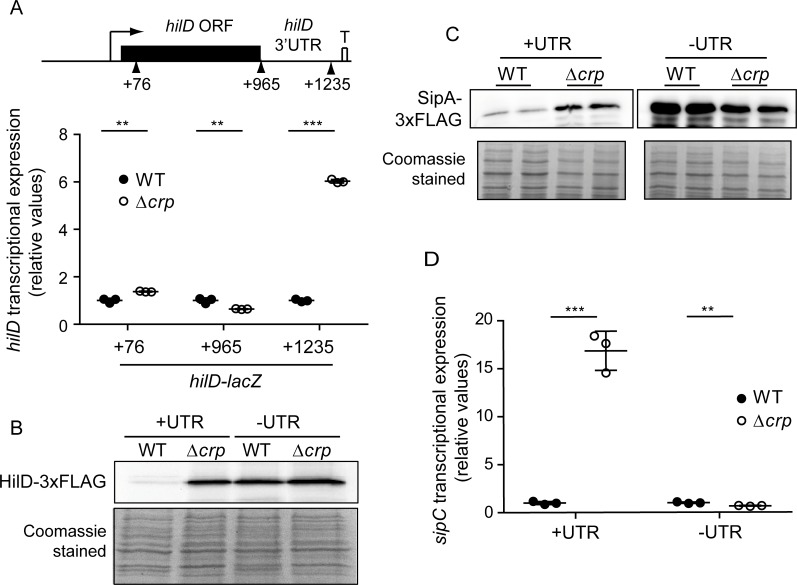
Fig 4. CRP-cAMP-mediated repression of HilD in mid-logarithmic growth requires the 3’UTR of hilD.
(A) hilD transcriptional expression was monitored using three different hilD-lacZ reporter fusions; these comprise either hilD to position +76 (within the hilD ORF), to position +965 (including the full-length hilD ORF), or to position +1,235 (including the hilD ORF and the hilD 3’UTR). Transcriptional studies were performed in the wild-type (WT) and Δcrp derivative strains grown in LB at 37°C up to an OD600nm of 0.4. The transcriptional expression is shown in relative values. In each case the reference (WT) value was set to one. Miller units in WT strains, hilD76-lacZ 63.3 +/- 5.9; hilD965-lacZ 5193.0 +/- 334.6; hilD1235-lacZ 38.3+/- 2.0. (B) Immunodetection of HilD-3xFLAG. Two different genetic constructs were used: one containing the hilD 3’UTR (+UTR) and the other lacking that region (-UTR). Immunodetection was assessed in whole cell extracts from cultures of the WT and Δcrp derivative strains grown as in A. (C) Immunodetection of the SPI-1 encoded SipA-3xFLAG protein was performed on whole cell extracts from two independent cultures of the WT and Δcrp derivative strains, either in the presence (+UTR) or the absence (-UTR) of the hilD 3’UTR. Cultures were grown as in A. In B and C, Coomassie Blue staining of the whole cell extracts serve as loading controls. Full length images of the Western blots, including molecular mass markers, are shown in S12 Fig. (D) sipC transcriptional expression was monitored in cultures grown as in A of the WT and Δcrp derivative strains carrying a hilD native gene or a derivative hilD lacking the 3’UTR. The transcriptional expression is shown in relative values. In each background (+UTR, -UTR) the activity reference of WT was set to one. Miller units in WT strains, UTR+ 73.6 +/- 11.8; UTR- 13783.0 +/- 1142.5. In A and D, the β-galactosidase activity was determined for three independent cultures, average and standard deviation is shown. **, p< 0.01; ***, p< 0.001.
The relevance of the 3’UTR in CRP-mediated regulation of HilD expression was supported by i) a Δcrp-dependent increase in the levels of HilD-3xFLAG protein was only detected when the hilD-3xFLAG mRNA contained the 3’UTR (Fig 4B) and ii) similarly, the Δcrp-dependent increase in SipA levels was only detected in strains carrying a hilD allele with the 3’UTR (Fig 4C). Additionally, the transcriptional expression of the SPI-1 gene sipC can be monitored as a proxy for HilD activity in the cell, since sipC upregulation in a crp mutant strain requires the presence of HilD (S4 Fig). Consistently, sipC-lacZ was upregulated in a Δcrp mutant background only when the hilD allele carried its native 3’UTR (Fig 4D). Our data also demonstrate that, according to the role attributed to the 3’UTR in the expression of hilD mRNA [11], there was an increase in the levels of HilD-3xFLAG, SipA-3xFLAG and sipC-lacZ when the 3’UTR was removed as compared to the parental strains carrying the native hilD mRNA containing the 3’UTR.
A small RNA is involved in CRP-mediated regulation of hilD
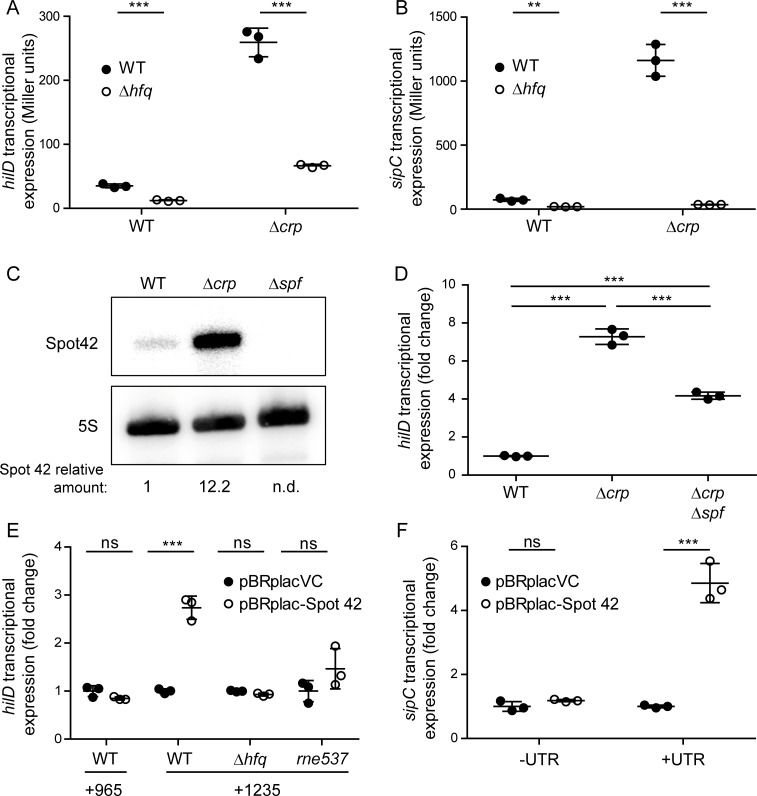
Based on the fact that CRP-cAMP is a transcriptional factor, it is surprising that the CRP-mediated regulation of HilD occurs at the post-transcriptional (and not the transcriptional) level, requiring the hilD 3’UTR. In other words, the data shown suggest that CRP-cAMP modulates hilD expression by an indirect mechanism. In line with previous reports [11,33], we found that the Δcrp-dependent activation of hilD expression, as judged by the hilD1235-lacZ fusion (containing the 3’UTR), was impaired in the absence of the sRNA chaperone Hfq (Fig 5A). Similarly, the drastic increase (16-fold) in sipC expression caused by the deletion of crp was abolished in the absence of Hfq (Fig 5B). We thus hypothesized that Hfq-dependent sRNA may be involved in the CRP-mediated regulation of hilD.
Fig 5. Spot 42 is under the control of CRP-cAMP in Salmonella and is involved in hilD regulation.
hilD (hilD1235-lacZ) (A) and sipC (sipC-lacZ) (B) transcriptional expression was monitored in the wild-type (WT) and Δcrp derivative strains in either an hfq+ or Δhfq genetic background. Cultures were grown in LB at 37°C up to an OD600nm of 0.4. (C) Northern blot analysis for Spot 42 sRNA. Total RNA samples were extracted from cultures of the WT, Δcrp and Δspf strains grown as in A. 5S rRNA was detected as loading control. For quantification, the ratio [Spot 42/5S] of three independent experiments was set to 1 in the wild-type strain. As a control, Spot 42 was not detected (n.d.) in a Spot 42 deficient strain. Full length images of the Northern blots, including molecular mass markers, are shown in S12 Fig. (D) hilD transcriptional expression was assessed in the WT, Δcrp and Δcrp Δspf derivative strains carrying a hilD1235-lacZ chromosomal fusion (+UTR). The transcriptional expression is presented in relative values, the reference value (WT) was set to one. Miller units WT hilD1235-lacZ, 40.6 +/- 1.3. (E) hilD transcriptional expression was assessed upon over-expression of Spot 42 (pBRplac-Spot 42). β-galactosidase activity was measured in WT strains carrying two different chromosomal transcriptional fusions: the hilD965-lacZ (lacking the 3’UTR) and the hilD1235-lacZ fusion (containing it). The transcriptional activity of hilD1235-lacZ was additionally assessed in both Δhfq and rne537 derivative strains. The transcriptional expression is shown as relative values; the reference values (from strains containing the pBRplacVC) were set to one. Miller units for pBRplacVC hilD965-lacZ 4381.6 +/- 490.3; for hilD1235-lacZ WT pBRplacVC 33.6 +/- 1.6, Δhfq pBRplacVC 12.1 +/- 0.1 and rne537 pBRplacVC 175.8 +/- 38.8. (F) sipC (sipC-lacZ) transcriptional expression was assessed in the WT strain upon over-expression of Spot 42 in presence (+UTR) or absence (-UTR) of the hilD 3’UTR. The transcriptional expression is presented as relative values. The reference values (from strains containing pBRplacVC) were set to one. Miller units in presence of (+UTR) 82.4 +/- 12.7, and in absence of (-UTR) 11967.1 +/- 507.0. In all cases, cultures were grown in LB at 37°C up to an OD600nm of 0.4. β-galactosidase activity was measured from three independent cultures, averages and standard deviations are shown. **, p< 0.01; ***, p< 0.001; ns, not significant.
In search for candidate sRNAs in Salmonella, we focused on Spot 42 (encoded by the spf gene) which is transcriptionally controlled by CRP-cAMP in the closely related species, E. coli [34]. Work by the Storz and Valentin-Hansen laboratories had established this sRNA to be a general repressor of sugar-related mRNAs during CRP-mediated catabolite repression [23]. In Salmonella, Spot 42 is highly abundant, with maximal expression in mid-logarithmic phase and reduced upon entry into stationary phase [35]. We tested by Northern blot whether Spot 42 is under CRP-cAMP control also in Salmonella (Fig 5C). In the mid-logarithmic growth phase a 12-fold upregulation of Spot 42 sRNA was detected in the CRP-deficient compared to the wild-type strain. This pattern was further validated using a chromosomal spf-lacZ fusion (S5 Fig). Additionally, spf expression was assessed at early stationary phase. Interestingly, the spf induction detected in the Δcrp derivative strain in mid-logarithmic phase was no longer observed in early stationary phase (S5 Fig), reflecting the divergent effects observed for CRP on SPI-1 expression in exponential versus early stationary phase.
To establish whether Spot 42 is involved in the CRP-cAMP-mediated regulation of hilD, expression studies in strains either deficient in Spot 42 or over-expressing the sRNA were performed. In the absence of CRP, a partial but significant drop in the upregulation of hilD in the Spot 42-deficient background (Δspf) was detected (Fig 5D). In contrast, ectopically expressing Spot 42 stimulated hilD expression. This suggests that Spot 42 is indeed involved in CRP-mediated repression of hilD. Importantly, over-expression of Spot 42 caused a 3-fold increase in hilD expression only when the 3’UTR was present, which implicates the hilD 3’UTR as a previously unknown target of this sRNA (Fig 5E). Consistently, a 2.5-fold increase in HilD-3xFLAG levels was detected upon the over-expression of Spot 42 (S6 Fig).
In agreement with previous data (Fig 5A, [29,35,36]), the positive effect of Spot 42 on hilD requires the chaperone Hfq (Fig 5E). As it has been shown before [29], Hfq binds to both Spot 42 and the hilD 3’UTR (S7 Fig). In addition, the major endoribonuclease RNase E has been suggested to play a role in 3’UTR mediated silencing of hilD expression [11]. Accordingly, hilD induction upon over-expression of Spot 42 was partially lost in the rne537 background encoding an RNase E with a truncated C-terminal domain that is defective in degradosome assembly [37] (Fig 5E). These results suggest that both Hfq and RNase E are involved in the Spot 42-mediated effect on hilD expression.
The involvement of Spot 42 in the control of SPI-1 gene expression was further assessed by examining the transcriptional activity of a sipC-lacZ reporter. Transcription was monitored in either strains carrying the native hilD (+UTR) or strains from which the hilD 3’UTR had been removed (-UTR). As shown in Fig 5F, there was a 5-fold induction of sipC-lacZ upon over-expression of Spot 42 in the +UTR background, whereas sipC transcription was unaffected when Spot 42 was over-expressed in a background lacking the hilD 3’UTR (-UTR).
To confirm that the hilD 3’UTR is targeted by Spot 42, the hilD 3’UTR was cloned downstream of the gfp coding sequence expressed from a constitutive promoter. Expression of this genetic reporter was monitored in either the presence or absence of Spot 42. Co-expression of the sRNA led to a nearly two-fold increase in fluorescence, suggesting that Spot 42 targets the hilD 3’UTR regardless of the genomic location of the latter (S8 Fig). Overall, these results led us to conclude that Spot 42 sRNA activates hilD expression (either directly or indirectly) in a manner that requires the presence of the hilD 3’UTR.
The unstructured region III of Spot 42 is required for hilD regulation
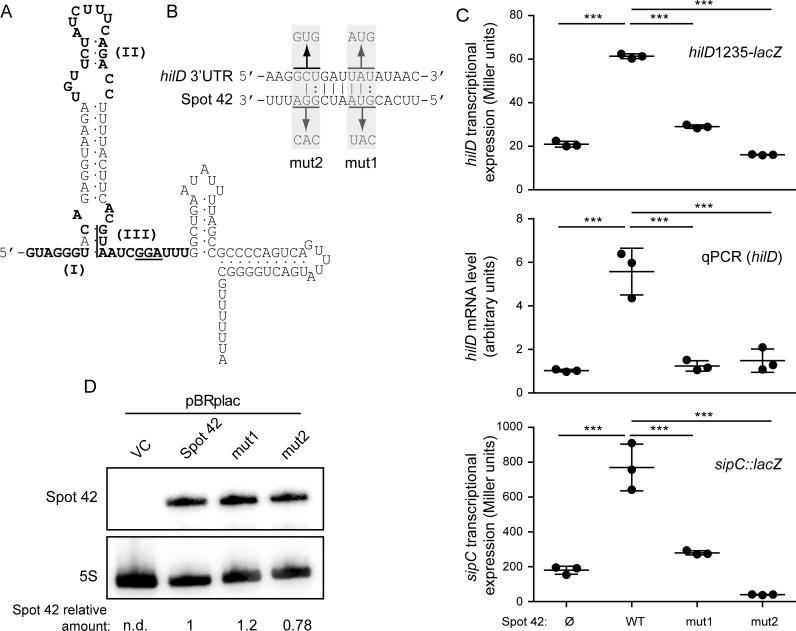
Spot 42 from E. coli and Salmonella share 98% sequence identity. Three unstructured regions (denoted I, II and III, Fig 6A) of Spot 42 have been identified in E. coli to participate in gene regulation through base-pairing interactions [24]. To dissect the mechanism of action of Spot 42 on hilD expression, we determined if specific regions within the sRNA were essential for regulation. The software IntaRNA [38], developed to search for putative interaction sites between two given RNA molecules, predicted an interaction between unstructured region III of Spot 42 and positions 1,129–1,138 of the hilD mRNA (i.e. a region within the 3’UTR). To test whether this unstructured region III of Spot 42 is required for the regulation of SPI-1 genes, two Spot 42 mutant variants were generated, spf-mut1 and spf-mut2 (Fig 6B). Over-expression of these Spot 42 derivatives was performed in strains carrying a deletion of the endogenous spf gene, and their effect on hilD expression was monitored by determination of i) hilD1235-lacZ expression, ii) hilD mRNA levels by qRT-PCR, and iii) sipC-lacZ expression as a readout for HilD activity.
Fig 6. Unstructured region III is required for Spot 42 mediated stimulation of hilD expression.
(A) Secondary structure prediction of Spot 42. The three unstructured regions (I, II, and III) are highlighted. The figure was adapted from [24]. (B) Putative interaction site between hilD 3’UTR and the unstructured region III of Spot 42 as predicted by IntaRNA software. The full spf sequence encoding for Spot 42 sRNA was used as an input sRNA sequence, and the 310 nt of the hilD 3’UTR were used as a target RNA sequence. The predicted base pairing and the altered nucleotides in the mut1 and mut2 are underlined and sequence substitutions are indicated. (C) Transcriptional expression of hilD and sipC was assessed upon over-expression of Spot 42WT, Spot 42mut1 or Spot 42mut2. As a control, strains carrying the empty vector pBRplac (Ø) were included. For monitoring hilD1235-lacZ and sipC-lacZ expression, β-galactosidase activity was determined. For relative hilD mRNA quantification, qRT-PCR was performed and the expression levels of strains carrying the pBRplac empty vector were set to 1. Detection of gapA (GAPDH) was used as an internal control (see Material and Methods). In all cases data correspond to the average and standard deviation of three independent experiments. ***, p< 0.001. (D) Northern blot analysis for Spot 42 sRNA and its derivative mutants mut1 and mut2. Total RNA samples were extracted from cultures of the Δspf strain carrying the control vector pBRplacVC or derivatives to over-express the different Spot 42 variants. 5S rRNA served as loading control. Full length images of the Northern blots, including molecular mass markers, are shown in S12 Fig. In C and D cultures were grown in LB at 37°C up to an OD600nm of 0.4.
In accordance with our previous results, over-expression of wild-type Spot 42 (Spot 42WT) upregulated hilD1235-lacZ and sipC-lacZ expression. Likewise, relative hilD mRNA levels were elevated upon Spot 42WT over-expression (Fig 6C). Conversely, over-expression of neither Spot 42mut1 (spf-mut1) nor Spot 42mut2 (spf-mut2) induced hilD1235-lacZ expression, hilD mRNA levels or sipC expression, demonstrating that mutations in region III disrupt the stimulatory effect of Spot 42 on hilD expression. (Fig 6C). The substitutions introduced to generate spf-mut1 (GUA-CAU) and spf-mut2 (GGA-CAC) have previously been described in E. coli, where those substitutions were shown to retain Spot 42 steady-state levels [37,38]. Similarly, Northern blots showed that these mutations did not dramatically affect Spot 42 stability in Salmonella either (Fig 6D), arguing that the reduced capability of the Spot 42 mutant variants to induce SPI-1 was not due to lowered sRNA levels. Taken together, the results indicate that the unstructured region III of Spot 42 is required for the regulation of hilD expression.
The in silico prediction suggests that region III of Spot 42 interacts within the hilD 3’UTR, between positions 1,129 and 1,138 of the hilD mRNA. Accordingly, we generated two chromosomal compensatory mutations in the hilD 3’UTR that restore the base pairing of Spot 42mut1 or Spot 42mut2 with the putative target sequence within hilD. The mutant alleles were designated hilD 3’UTRmut1 and hilD 3’UTRmut2, respectively (Fig 6B). sipC expression was used as a readout for HilD activity. Over-expression of Spot 42WT in both hilD 3’UTRmut1 and hilD 3’UTRmut2 genetic backgrounds induced expression of sipC-lacZ, indicating that substitution of those residues within the hilD 3’UTR did not impair the positive effect of Spot 42 on SPI-1 expression. Additionally, over-expression of either Spot 42mut1 or Spot 42mut2 in both hilD 3’UTRmut1 and hilD 3’UTRmut2 backgrounds did not reestablish the ability to induce sipC expression (S9 Fig). Despite unstructured region III of Spot 42 being responsible for hilD activation, these results suggest that the in silico predicted interaction site—positions 1,129–1,138 within hilD mRNA—is not the actual target site or, at least, not the unique interaction site with Spot 42. More complex interaction mechanisms cannot be ruled out such as multiple interactions sites of Spot 42 within the hilD 3’UTR.
Spot 42 targets the last 185 nt of the hilD 3’UTR
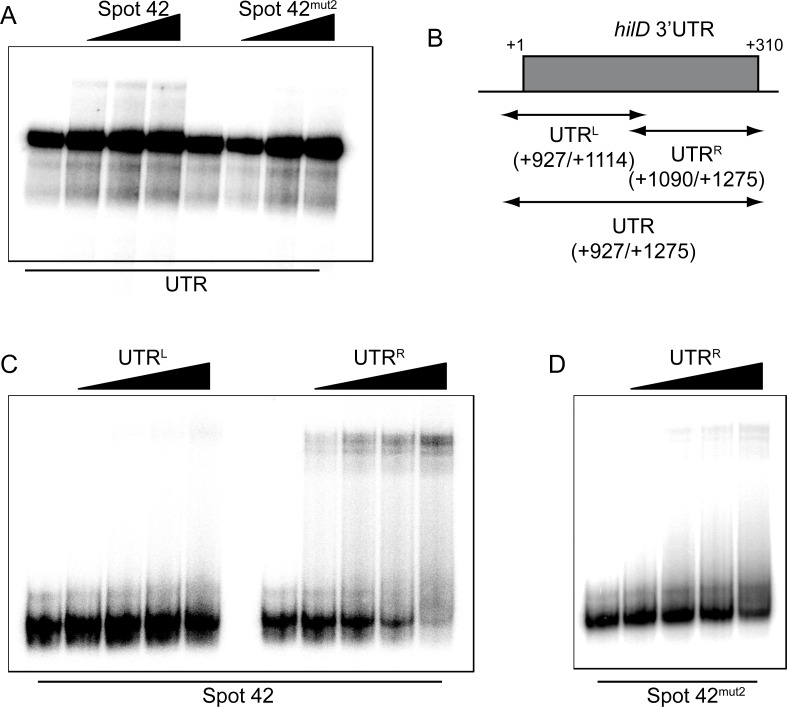
Biochemical approaches were used to confirm the physical interaction between Spot 42 and the hilD 3’UTR. The ability of Spot 42 to bind to the hilD 3’UTR-derived fragments was assessed by electrophoretic mobility shift assays (EMSAs). EMSAs of radiolabeled full-length hilD 3’UTR incubated with increasing concentrations of Spot 42 confirmed a direct interaction between the two RNA species (Fig 7A). Consistent with our in vivo data, the unstructured region III of Spot 42 is required for the interaction with the hilD 3’UTR, since the binding affinity of the mutant version of Spot 42 (spf-mut2) was markedly reduced. Next, the hilD 3’UTR was divided into two halves, UTRL and UTRR (Fig 7B). The UTRL fragment spans positions +927 to +1114 of the hilD mRNA (roughly the first half of the hilD 3’UTR), while UTRR covers the second half of it (position +1090 to +1275) and includes the putative interaction site with the unstructured region III of Spot 42 (Fig 6B) as well as two Hfq binding sites as inferred from CLIP-seq [29]. EMSAs with radiolabelled Spot 42 and increasing concentrations of either one of the two UTR fragments were conducted. A concentration dependent upshift of Spot 42 was only observed upon addition of the UTRR fragment with an apparent Kd of 80 nM but not upon addition of the UTRL fragment (Fig 7C), indicating that Spot 42 interacts with the second half of hilD 3’UTR. Again, Spot 42-UTRR interaction was specific as the affinity of the UTRR fragment to the mutant version of Spot 42 (spf-mut2) was markedly reduced (Fig 7D). Further supporting this notion, in the reverse experiment increasing concentrations of Spot 42 did not lead to a band shift of the UTRL but only of the UTRR fragment (S10 Fig). Our results indicate that the loss of hilD activation by Spot 42mut2 in vivo is due to the inability of this mutant sRNA version to directly interact with the hilD mRNA, specifically with the second half of its 3’UTR. Overall, this makes Spot 42 the first Hfq-associated sRNA that potentially activates a trans-encoded target gene via its 3’UTR.
Fig 7. Spot 42 interacts with the downstream part of the hilD 3’UTR.
(A) EMSA assay using 4 nM of hilD 3’UTR RNA radiolabeled incubated with increasing concentration of either Spot 42 or Spot 42mut2 RNA (0, 280, 560, 1700 nM). (B) Schematic representation of the hilD 3’UTR and the UTRL and UTRR fragments. EMSA using 4 nM of radiolabeled Spot 42WT (C) or Spot 42mut2 (D) incubated with increasing concentrations (0, 56, 280, 560, 1,700 nM) of UTRL or UTRR. All RNA transcripts used were obtained by T7 in vitro transcription. Samples were subjected to electrophoresis in a native gel and band shifts were observed upon drying and exposure of the gel.
Discussion
During the infection process, Salmonella relies on the expression of genes encoded on SPI-1 for epithelial cell invasion. Although SPI-1 is therefore crucial for Salmonella infection, it has a retarding effect on the growth rate, presumably as a consequence of the energetically high costs to produce the SPI-1 T3SS [3]. Accordingly, the expression of SPI-1 genes is tightly regulated [1]. Most relevant studies have focused on the regulatory pathways dedicated to induce SPI-1 under permissive conditions. However, as SPI-1 expression affects cell fitness, SPI-1 silencing mechanisms under non-permissive conditions, for instance in fast growing cells in the mid-logarithmic phase, are equally important. In this study, we identified CRP-cAMP, a metabolic sensor and global transcription factor [14,39], as a key player in the repression of SPI-1. CRP-cAMP is involved in a post-transcriptional regulatory circuit, controlling the expression of hilD by a mechanism dependent on its 3’UTR.
Coordination of metabolism and stress-related functions is crucial for the evolutionary success of bacterial populations. In pathogenic bacteria, the cross regulation between virulence factors, which can be considered within-host stress-related factors, and physiology is crucial for efficient colonization. A sudden shift between the expression of genes involved in active growth and genes involved in adaptation to stress might be required for rapid adaptation to changing conditions during the infection process. Secondary messengers such as cAMP, the intracellular levels of which can be altered by the action of both synthetases (adenylate cyclases) and degrading enzymes (phosphodiesterases), provide a rapid response system that can promote rapid changes in the expression profile. Although cAMP has traditionally been described as a regulator of metabolism, its role in the modulation of virulence-related functions has been extensively studied in several pathogens [40]. In E. coli, CRP-cAMP has been described to repress type 1 fimbriae expression during logarithmic growth [41]. Other secondary messengers, such as ppGpp, have also been reported to participate in the interplay between cell metabolism and virulence control [42]. Post-transcriptional regulation by small non-coding RNA confers to the cell another level for a rapid response to environmental conditions, in fact, a number of sRNAs have been found to play a relevant role in the metabolism-virulence crosstalk [43,44].
In this study we found that CRP-cAMP represses SPI-1 expression by modulating the expression of the regulator HilD (Figs 1–3). The role of HilD is not restricted to SPI-1; rather there is a complex cross-talk between HilD and master regulators of other virulence associated pathways. For example, it has been shown that HilD activates, under certain conditions, SPI-2 expression that is required for survival within macrophages [6,45]. Within macrophage-like cells, SPI-1 genes are downregulated and SPI-2 genes are induced [46]. CRP-cAMP is a regulator tailored to mediate rapid responses to environmental changes and may therefore be relevant for HilD-mediated regulation of virulence in response to the environmental conditions that Salmonella encounters through the infection process.
The CRP-mediated regulation of hilD does not occur at the transcriptional initiation level (Fig 4). Rather CRP-cAMP modulates hilD expression at the post-transcriptional level through the long 3’UTR (310 nt) of hilD. Post-transcriptional regulation is an extensively used mechanism to finely regulate virulence in bacterial pathogens [47,48]. The role of 5’UTRs in post-transcriptional gene expression control has been established and it is noteworthy that mRNAs of important SPI-1 regulators, such as invF, hilA and hilE, all carry long 5’UTRs [43,49–51]. In contrast, the involvement of 3’UTRs in post-transcriptional regulation is still poorly understood. Recently, it has been reported in Staphylococcus aureus that one-third of the cellular transcripts carry 3’UTRs longer than 100 nt [52]. In addition to be targets of regulation, 3’UTRs may provide regulators themselves, namely 3’UTR-derived sRNAs [36,53].
The 3’UTR of hilD constitutes a silencing module, since its deletion causes significant hilD upregulation. Although we are yet to elucidate the full molecular mechanism, the observed Hfq dependency suggested that one or several sRNAs are targeting the hilD 3’UTR [11]. Likewise, the CRP-mediated repression of hilD requires both the presence of the hilD 3’UTR and Hfq, indicating that CRP regulates hilD expression in an sRNA-mediated manner (Fig 4 and Fig 5).
Spot 42 is an integral member of the CRP-mediated gene expression network in E. coli [23–27] and its expression is repressed by CRP-cAMP in both E. coli and Salmonella ([47], Fig 5). Here we found that Spot 42 is involved in the CRP-mediated regulation of hilD expression, since the derepression of hilD in a Δcrp strain was diminished in the absence of Spot 42 and over-expressing Spot 42 caused a concomitant upregulation of hilD expression. Remarkably, Spot 42-mediated regulation targets the long hilD 3’UTR. The fact that the absence of Spot 42 did not completely abolish the hilD deregulation caused by the Δcrp mutation points at additional factors that could be involved in the described regulation. Although the nature of these factors remains fully elusive, it should be noted that these putative factors seem to also act through the hilD 3’UTR. Further studies will be required to determine whether other sRNAs or proteins plays a role in the CRP-mediated repression of hilD expression.
Both genetic and biochemical approaches point towards a direct interaction between Spot 42 and the hilD 3’UTR, involving the unstructured region III of Spot 42 (Figs 6 and 7). Although the exact target sequence within the hilD 3’UTR have not been identified, EMSA experiments indicate that the interaction occurs between Spot 42 and the downstream half of the 3’UTR (last 185 nt). This interaction is strongly diminished when the unstructured region III of Spot 42 is altered by base substitution in three positions previously described to be involved in base-pairing [23]. The recent finding that the transcription elongation factors GreA and GreB target the hilD 3’UTR to regulate hilD at permissive conditions [54] led us to speculate that transcriptional pausing might trigger a specific folding of the hilD 3’UTR important for post-transcriptional regulation. Overall, the regulation through the hilD 3’UTR seem to be complex and presumably several factors target the hilD 3’UTR. Although it has been proposed that intrinsic motifs in the long 3’UTR of hilD might confer susceptibility to degradation in a polynucleotide phosphorylase (PNP) and RNase E dependent manner, no effect in the stability of the hilD mRNA was detected [11]. Consistent with these data, we found no difference in the stability of the hilD mRNA between the wild-type and Δcrp strain in mid-logarithmic phase (S11 Fig). The exact mechanism by which these factors converge to regulate hilD expression should be the focus of future studies. Our results highlight the 3’UTR of hilD as a central hub in SPI-1 regulation and indicate that the whole hilD 3’UTR is required for the post-transcriptional regulation of hilD.
To our knowledge, there are no other examples of trans-encoded sRNAs targeting 3’UTRs. Of note, the cis-encoded sRNA GadY, which is encoded on the opposite strand of gadX 3’UTR, seems to positively regulate gadX through interaction with the gadX-gadW intergenic region [55]. Unlike Spot 42 and hilD which are expressed from regions in the chromosome over 1 Mb apart, GadY and gadX physically overlap. Global screens for Hfq-mediated sRNA-mRNA interactions [56,57] suggest, however, that 3’UTR targeting may be more common than currently appreciated.
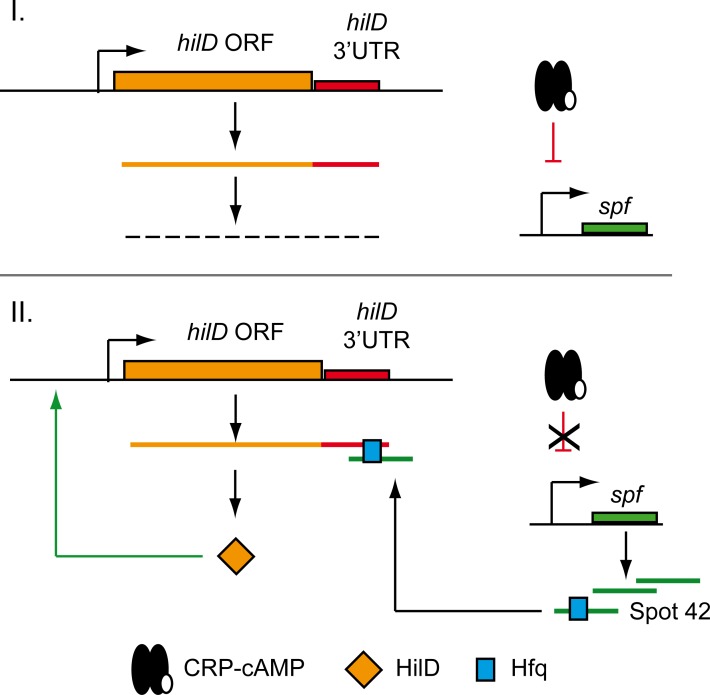
In conclusion, our findings imply a novel mechanism in the complex regulatory network of SPI-1 expression. Under non-permissive conditions, very low transcriptional expression from the hilD gene occurs. Additionally, CRP-cAMP represses the transcription of the sRNA Spot 42, thereby maintaining basal levels of hilD expression. Consequently, despite hilD transcription occurs, only low levels of HilD protein arise (Fig 8 panel I). In contrast, environmental and/or physiological signals may relieve CRP-dependent Spot 42 repression. Upon binding to its 3’UTR in an Hfq-dependent manner, Spot 42 may exerts a positive effect on hilD mRNA, thereby activating HilD protein expression. As HilD auto-activates itself by promoting its own transcription, expression of some copies of HilD protein would likely be sufficient to amplify the final output (Fig 8 panel II). Thus, CRP-cAMP seems to play a relevant role by coordinating post-transcriptional virulence control in Salmonella. Somewhat similar to the described Spot 42-mediated regulation of SPI-1, the sRNA PinT acts as a timer of virulence gene expression in Salmonella, regulating SPI-2 genes through the modulation of CRP-cAMP [58]. Altogether, this highlights the emerging importance of collaborative activities of general transcription factors and sRNAs to precisely adjust the costly expression of major virulence factors to internal and external metabolic cues [44].
Fig 8. Proposed model of CRP-cAMP-mediated repression of hilD expression.
I. Silencing of SPI genes (non-permissive SPI-1 conditions); II. Transition to SPI expression is triggered by Spot 42-mediated stimulation of hilD mRNA. Green and red lines indicate stimulation and repression, respectively.
Materials and methods
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in S1 Table.
Salmonella enterica serovar Typhimurium SL1344 and derivative strains were cultivated either in Luria Bertani broth (tryptone 10 g/l, yeast extract 5 g/l and sodium chloride 10 g/l). When required, the media was supplemented with ampicillin (Amp) 100 μg/ml, kanamycin (Km) 50 μg/ml, chloramphenicol (Cm) 15 μg/ml, or tetracycline (Tc) 15 μg/ml. Induction of genes cloned into pTRc99a was achieved by adding 0.1 mM IPTG.
Liquid cultures (20 ml of LB in 100 ml culture flasks) were inoculated to an OD600nm of 0.001 and incubated at 37°C with vigorous shaking (200 rpm). An OD600nm of 0.3–0.4 was considered mid-logarithmic phase of growth, while an OD600nm of 2.0 was considered early stationary phase of growth.
Genetic manipulations
The cpdA (SL1344_3157) gene was cloned into the IPTG inducible vector pTRc99a [59]. The cpdA coding sequence was PCR amplified by Phusion polymerase (Invitrogen) with the primers cpdA_XbaI_Fw and cpdA_SalI6xHis_rev (S2 Table), subsequently digested with XbaI and SalI and ligated into XbaI/SalI digested pTRc99a.
The spf gene encoding Spot 42 sRNA was cloned into pBRplac vector [60]. spf was PCR amplified with the primers spf_AatII_Fw and spf_EcoRI_rev (S2 Table), subsequently digested with AatII and EcoRI and ligated into AatII/EcoRI digested pBRplac. Mutations in Spot 42 (spf-mut1 and spf-mut2) were generated by assembly PCR and subsequent cloning in the pBRplac vector.
A gfp-hilD 3’ UTR construct was cloned in the backbone of plasmid pXG1 [61]. The hilD 3’UTR region was fused to gfp by overlapping PCR using chromosomal SV5015 and plasmid pXG1 as templates and the primers gfp_NheI_Fw, gfp_hilD_rev, hilD_gfp_Fw and hilD_XbaI_rev (S2 Table). The purified PCR fragment was subsequently digested with XbaI/NheI and ligated into an XbaI/NheI digested pXG1 vector, resulting in the plasmid pXG1 gfp-3’UTR.
Deletion mutants were generated by gene replacement as described by Datsenko and Wanner [62]. Briefly, antibiotic resistance cassettes carrying either KmR or CmR resistance genes were amplified from pKD4 and pKD3, respectively. Primers used include a 40 bp sequence complementary to the region where the insertion was desired. Purified fragments were electroporated into strains carrying pKD46. Positive clones were selected in presence of the required antibiotic. When desired, the antibiotic resistance cassette was removed by expression of the Flp recombinase from the pCP20 plasmid, as described [63].
Deletion mutants, where the antibiotic cassette was removed, were further used for the generation of reporter gene fusions. Transcriptional lacZ fusions were generated as described [64], the remaining FRT-site was used to integrate plasmid pKG136.
Epitope tagged proteins were constructed as follows: HilA, HilD and SipA 3xFLAG tagged proteins were generated by a λRed recombinase system as described [65]. When desired, the KmR cassette downstream of the 3xFLAG epitope was removed using the Flp recombinase (see above). In the HilD 3xFLAG construct retaining the KmR cassette, the hilD coding sequence and 3’UTR are split and not co-transcribed. Thus, the KmR cassette was removed, with the hilD 3’UTR now located right downstream of the 3xFLAG epitope. Oligonucleotides used to generate the constructs are listed in S2 Table. All strains were PCR confirmed and integrity of the sequence was checked by DNA sequencing.
Chromosomal modifications in the hilD 3’UTR region were generated by scarless mutation [66]. Briefly, a 1 kb fragment containing the hilD 3’UTR sequence was cloned into pGEM vector, desired point mutations were generated via the Quick change method using the hilD-UTR oligonucleotides listed in S2 Table. Subsequently, the vector was digested with SacI/XbaI and ligated into the suicide plasmid pDMS197 [67]. The derivative pDMS197 was propagated in S17-1 lambda pir and used as donor in matings with SV5015 Δspf sipC-lacZ. Trans-conjugants were selected for tetracycline resistance. Selected clones were grown in salt-free nutrient broth supplemented with 5% sucrose. Individual tetracycline-sensitive clones were checked by PCR and subsequent DNA sequencing to select the clones carrying the desired chromosomal mutation.
Collecting protein extracts
To obtain whole cell and secreted protein extracts, LB cultures were grown at 37°C and processed as previously described [54]. Samples in Laemmli sample buffer were subjected to SDS-PAGE separation. Normalization of the loading samples was performed based on the culture biomass (OD600nm). Coomassie stain was used to visualize protein bands.
Western blot assay
Protein extracts were subjected to SDS-PAGE separation, transfer to PVDF filter and subsequent immunodetection using monoclonal Anti-FLAG (Sigma), Anti-His (Sigma) or polyclonal Anti-SopE [68] as primary antibodies. Commercial polyclonal anti-mouse (Promega) and anti-rabbit (GE Healthcare) secondary antibodies conjugated to horseradish peroxidase were used. For detection, ECL Prime Western Blotting Detection Reagent (GE Healthcare) served as a substrate. Chemi-luminescence was detected using Chemidoc equipment (Bio-Rad). As a control prior to the immunodetection, all whole cell extract samples were analyzed by SDS-PAGE and Coomassie staining to ensure proper normalization of the loaded amounts.
Protein identification
For protein identification, the protein bands from Coomassie stained SDS-PAGE gels were trypsin digested and analyzed by LC-MS/MS by the Proteomic facility from the Scientific Park of Barcelona (PCB).
β-galactosidase assay
β-galactosidase activity was measured as described previously [69]. Activity determination was performed in technical duplicates for each of three biological replicates.
RNA isolation
For each strain, samples from three independent LB cultures grown at 37°C to mid-logarithmic phase (OD600nm 0.4) were processed. RNA was extracted by classical hot phenol method. RNA quality and concentration was assessed by an Agilent Technologies Bioanalyzer 2100.
qRT-PCR
Quantitative reverse transcription-PCR (qRT-PCR) was performed as previously described [54]. The relative amount of target cDNA was normalized using the gapA (GAPDH) gene as an internal control. Oligonucleotides used for qRT-PCR are listed in S2 Table.
Northern blot
Electrophoretic separation of total RNA samples was carried out in Tris-Borate-EDTA (TBE) 8% acrylamide gels containing 8.3 M urea. Samples were prepared by mixing 10 μl of RNA samples with 10 μl of urea dye (2x) loading buffer and incubated for 10 minutes at 65°C, immediately chilled on ice and loaded for electrophoretic separation at 30 mA for 2 hours.
RNAs were transferred to Hybond N+ (GE Healthcare) filters by semi-dry TBE based transfer for 2 hours at 400 mA. RNAs were subsequently fixed to the filter by UV crosslinking. Filters were then hybridized with radiolabeled oligos, sequences are given in S2 Table. Images of radioactive filters were obtained with the FLA-5100 imaging system (Fujifilm) and quantification was performed using Image J software.
Electrophoretic mobility shift assay (EMSA)
RNA-RNA interactions were detected by Electrophoretic Mobility Shift Assay (EMSA) as described in [70]. First, DNA templates for in vitro T7 RNA transcription were generated by PCR, primers used are listed in S2 Table. RNA was produced in vitro by following the Megascript transcription procedure from Ambion. Then, either the sRNA (Spot 42) or the target RNAs (hilD 3’UTR, UTRR or UTRL) was dephosphorylated and 5’ labeled with [(α-32P) ATP]. The putatively interacting RNAs were next incubated in structure buffer (Ambion): In a 10 μl final volume, 4 nM of the radiolabeled RNA was incubated with increasing concentrations of the unlabeled RNA (0, 56, 280, 560, 1700nM). Samples were incubated at 37°C for 1 hour and subjected to electrophoresis in a native 6% acrylamide gel. For specific RNA detection, acrylamide gels were dried and exposed. Images were obtained as for Northern blots.
GFP measurement
For single-cell analysis, cell cultures were grown to the desired conditions, pelleted and resuspended in PBS. The bacterial suspensions were then fixed in 4% formaldehyde. The fluorescence of 20,000 bacterial cells was measured by flow cytometry using preset parameters for GFP (excitation wavelength of 484 nm and emission wavelength of 512 nm). Measurements were performed in technical duplicates for each three biological replicates; average was used to compare GFP expression.
Invasion assay in HeLa epithelial cells
HeLa human epithelial cells (ATCC CCL2) were cultured in tissue culture medium (Dulbecco’s Modified Essential Medium (DMEM) supplemented with 10% fetal calf serum and 1mM glutamine). HeLa cells were seeded the day before the infection in 24-well plates containing 0.5 ml of DMEM per well and grown at 370°C, 5% CO2. Bacterial cells grown at 37°C to different phases of growth were prepared in DMEM. The bacterial mixture was added to HeLa cells to reach a multiplicity of infection (MOI) of 75 bacteria per eukaryotic cell. 30 minutes post-infection HeLa cells were washed twice with phosphate buffered saline (PBS) and incubated in fresh DMEM medium containing 100 μg/ml gentamicin for 90 minutes. Numbers of viable intracellular bacteria were obtained after lysis of infected cells with 1% Triton X-100, and subsequent plating. Infections were carried out in triplicate. Invasion rate is defined as the intracellular bacteria recovered versus viable bacteria used to infect the HeLa cells (initial inoculum). Invasion rates were normalized to bacterial culture of a wild type strain.
Statistical analysis
GraphPad Prism 5.0 software was used for data analysis. Two-tailed Student’s t-test were carried out and p-values < 0.05 were considered significant.
Supporting information
Upper panel. Immunodetection of CpdA-6xHis was performed in extracts of the wild-type (WT) and a Δcya derivative strain carrying either pTrc99a (-, control vector) or pCpdA (+, pTrc99a+cpdA). Cultures were grown in LB supplemented with IPTG (0.1 mM) at 37°C up to an OD600nm of 0.4. The band corresponding to the CpdA protein is indicated with an arrowhead. Lower panel. Coomassie Blue staining of the whole cell extracts serve as loading controls. M: molecular mass markers (kDa).
(TIF)
Cell-free supernatants from two independent cultures of the WT and ΔhilA strain grown up to early stationary phase (OD600nm 2.0) were TCA precipitated. The resulting extracts were analyzed by SDS-PAGE and Coomassie staining. Arrowheads indicate presumed secreted effector proteins from Salmonella. Size in kDa of molecular mass marker bands (M) are indicated.
(TIF)
Relative quantification by qRT-PCR of hilA mRNA in a Δcrp derivative strain compared to wild type (WT). The reference value (WT) was set as one. Detection of gapA (GAPDH) was used as an internal control (see Materials and Methods). RNA samples were extracted from cultures of the WT and Δcrp derivative strains grown in LB at 37°C up to an OD600nm of 0.4. The average and standard deviation from three independent experiments are shown. *** p< 0.001.
(TIF)
Transcriptional expression of sipC-lacZ was monitored in the wild type (WT) and Δcrp derivative strains in either a hilD+ or hilD- genetic background. Cultures were grown in LB at 37°C up to an OD600nm of 0.4. The β-galactosidase activity from three independent experiments was averaged and the standard deviation is shown. ***, p< 0.001; ns, not significant.
(TIF)
Transcriptional expression of spf in the wild type (WT) and Δcrp derivative strains was monitored by β-galactosidase activity determination of a spf-lacZ chromosomal fusion. LB cultures were grown at 37°C up to either mid-logarithmic (OD600nm 0.4) or early stationary (OD600nm 2.0) phase. Data from three independent experiments are averaged and the standard deviation is shown. ***, p< 0.001; ns, not significant.
(TIF)
A. Immunodetection of HilD-3xFLAG was performed on whole cell extracts from cultures of the wild type (WT) strain (+UTR) carrying either the pBRplacVC (control vector, reference value) or pBRplac-Spot 42. Coomassie Blue staining of the whole cell extracts serve as loading controls. B. Merged image of white light caption for detection of the molecular mass marker and the chemiluminiscence detected bands in an extract from WT carrying the pBRplacVC. Molecular mass markers in kDa. C. The band corresponding to HilD-3xFLAG (indicated with an arrowhead) is easily identified as the protein band over-accumulated in Δcrp as compared to WT.
(TIF)
In vitro transcribed RNA was radiolabeled. 4 nM of the radiolabeled RNA was incubated with increasing concentration of purified Hfq (0, 1.3, 4, 13, 40, 130 nM) and subjected to electrophoresis in a native gel. Band shift was observed upon drying and exposure of the gel.
(TIF)
GFP fluorescence assessment by flow cytometry of GFP-hilD 3’UTR upon overexpression of the sRNA Spot 42. Cultures of Δspf strains carrying the construct pXG1gfp-hilD3’UTR in presence (pBRplac-Spot42) or absence of the sRNA Spot 42 (pBRplacVC) grown in LB at 37°C up to an OD600nm of 0.4. Data from three independent experiments are averaged and the standard deviation is shown. ***, p< 0.001.
(TIF)
Transcriptional expression of sipC-lacZ was monitored in two different hilD backgrounds: hilD 3’UTRmut1 and hilD 3’UTRmut2. sipC-lacZ expression was assessed upon overexpression of either Spot 42WT, Spot 42mut1 and Spot 42mut2. β-galactosidase activity was determined for three independent cultures, average and standard deviation are shown. ***, p< 0.001; ns, not significant. In all cases, bacterial cultures were grown in LB at 37°C up to an OD600nm of 0.4.
(TIF)
EMSA assay using 4 nM of either UTRL or UTRR fragments incubated with increasing concentration (0, 56, 280, 560, 1700 nM) of Spot 42 RNA. All RNA molecules used were obtained by T7 in vitro transcription. Samples were subjected to electrophoresis in a native gel and band shift was observed upon drying and exposure of the gel.
(TIF)
hilD mRNA was detected by Northern blot. Culture of wild type (WT) and Δcrp were grown to mid-logarithmic phase (OD600nm 0.4), rifampicin was added (500 μg/ml) and samples were taken for total RNA extraction at 2, 4, 8, 16 and 32 min. Samples before rifampicin addition (time 0) were taken. RNA radiolabeled probe complementary to the first 300 nt of hilD mRNA was generated by in vitro T7 RNA transcription and used for hilD mRNA detection. tmRNA was detected as loading control. Full length image of the Northern blot is shown in S12 Fig.
(TIF)
(PDF)
(PDF)
Acknowledgments
We are indebted to Professor Josep Casadesús (Universidad de Sevilla) for fruitful discussions, for providing several Salmonella strains and his help in the determination of Spot 42 levels, to Dr. Bustamante (Universidad Nacional Autónoma de México) for providing the HilA-3xFLAG reporter strain, to Prof. Hughes (University of Utah) for providing the HilD-3xFLAG reporter strain, to Prof. Gottesman (NIH) for the pBRpLac vector, and to Prof. Hardt (ETH Zurich) for generous gift of SopE antiserum.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Spanish Ministry of Economy and Competitiveness (www.mineco.gob.es/) [BIO2010-15417, BIO2013-44220-R, AGL2013-45339-R]; the RecerCaixa program (http://www.acup.cat/es/project/recercaixa) [2012/ACUP/00048] and the Catalonian government (http://agaur.gencat.cat/es/inici/)[2017SGR499]. YEM and TGC were recipient of APIF grant from the University of Barcelona. YEM was recipient of an EMBO STF (http://www.embo.org/) [6903]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ellermeier JR, Slauch JM. Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol. 2007;10: 24–29. doi: 10.1016/j.mib.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 2.Fàbrega A, Vila J. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev. 2013;26: 308–341. doi: 10.1128/CMR.00066-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturm A, Heinemann M, Arnoldini M, Benecke A, Ackermann M, Benz M, et al. The cost of virulence: retarded growth of Salmonella Typhimurium cells expressing type III secretion system 1. PLoS Pathog. 2011;7: e1002143 doi: 10.1371/journal.ppat.1002143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Que F, Wu S, Huang R. Salmonella pathogenicity Island 1(SPI-1) at work. Curr Microbiol. 2013;66: 582–587. doi: 10.1007/s00284-013-0307-8 [DOI] [PubMed] [Google Scholar]
- 5.Jones BD. Salmonella invasion gene regulation: a story of environmental awareness. J Microbiol. 2005;43 Spec No: 110–117. [PubMed] [Google Scholar]
- 6.Bustamante VH, Martínez LC, Santana FJ, Knodler LA, Steele-Mortimer O, Puente JL. HilD-mediated transcriptional cross-talk between SPI-1 and SPI-2. Proc Natl Acad Sci U S A. 2008;105: 14591–14596. doi: 10.1073/pnas.0801205105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellermeier CD, Slauch JM. RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J Bacteriol. 2003;185: 5096–5108. doi: 10.1128/JB.185.17.5096-5108.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellermeier CD, Ellermeier JR, Slauch JM. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2005;57: 691–705. doi: 10.1111/j.1365-2958.2005.04737.x [DOI] [PubMed] [Google Scholar]
- 9.Boddicker JD, Knosp BM, Jones BD. Transcription of the Salmonella invasion gene activator, hilA, requires HilD activation in the absence of negative regulators. J Bacteriol. 2003;185: 525–533. doi: 10.1128/JB.185.2.525-533.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez LC, Yakhnin H, Camacho MI, Georgellis D, Babitzke P, Puente JL, et al. Integration of a complex regulatory cascade involving the SirA/BarA and Csr global regulatory systems that controls expression of the Salmonella SPI-1 and SPI-2 virulence regulons through HilD. Mol Microbiol. 2011;80: 1637–1656. doi: 10.1111/j.1365-2958.2011.07674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Garrido J, Puerta-Fernández E, Casadesús J. A eukaryotic-like 3’ untranslated region in Salmonella enterica hilD mRNA. Nucleic Acids Res. 2014;42: 5894–5906. doi: 10.1093/nar/gku222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olekhnovich IN, Kadner RJ. Role of nucleoid-associated proteins Hha and H-NS in expression of Salmonella enterica activators HilD, HilC, and RtsA required for cell invasion. J Bacteriol. 2007;189: 6882–6890. doi: 10.1128/JB.00905-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinosa E, Casadesús J. Regulation of Salmonella enterica pathogenicity island 1 (SPI-1) by the LysR-type regulator LeuO. Mol Microbiol. 2014;91: 1057–1069. doi: 10.1111/mmi.12500 [DOI] [PubMed] [Google Scholar]
- 14.Kolb A, Busby S, Buc II, Garges S, Adhya S. Transcriptional Regulation by cAMP and its Receptor Protein. Annu Rev Biochem. 1993;62: 749–797. doi: 10.1146/annurev.bi.62.070193.003533 [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Kelly SM, Bollen WS, Curtiss R. Characterization and immunogenicity of Salmonella typhimurium SL1344 and UK-1 delta crp and delta cdt deletion mutants. Infect Immun. 1997;65: 5381–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z-W, Hsuan S-L, Liao J-W, Chen T-H, Wu C-M, Lee W-C, et al. Mutations in the Salmonella enterica serovar Choleraesuis cAMP-receptor protein gene lead to functional defects in the SPI-1 Type III secretion system. Vet Res. 41: 5 doi: 10.1051/vetres/2009053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teplitski M, Goodier RI, Ahmer BMM. Pathways leading from BarA/SirA to motility and virulence gene expression in Salmonella. J Bacteriol. 2003;185: 7257–7265. doi: 10.1128/JB.185.24.7257-7265.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teplitski M, Goodier RI, Ahmer BMM. Catabolite repression of the SirA regulatory cascade in Salmonella enterica. Int J Med Microbiol. 2006;296: 449–466. doi: 10.1016/j.ijmm.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 19.Altier C, Suyemoto M, Lawhon SD. Regulation of Salmonella enterica serovar typhimurium invasion genes by csrA. Infect Immun. 2000;68: 6790–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat Rev Microbiol. 2011;9: 578–589. doi: 10.1038/nrmicro2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Updegrove TB, Zhang A, Storz G. Hfq: the flexible RNA matchmaker. Curr Opin Microbiol. 2016;30: 133–138. doi: 10.1016/j.mib.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuss AM, Heroven AK, Waldmann B, Reinkensmeier J, Jarek M, Beckstette M, et al. Transcriptomic Profiling of Yersinia pseudotuberculosis Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs. PLOS Genet. 2015;11: e1005087 doi: 10.1371/journal.pgen.1005087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beisel CL, Storz G. The base-pairing RNA spot 42 participates in a multioutput feedforward loop to help enact catabolite repression in Escherichia coli. Mol Cell. 2011;41: 286–297. doi: 10.1016/j.molcel.2010.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beisel CL, Updegrove TB, Janson BJ, Storz G. Multiple factors dictate target selection by Hfq-binding small RNAs. EMBO J. 2012;31: 1961–1974. doi: 10.1038/emboj.2012.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Møller T, Franch T, Udesen C, Gerdes K, Valentin-Hansen P. Spot 42 RNA mediates discoordinate expression of the E. coli galactose operon. Genes Dev. 2002;16: 1696–1706. doi: 10.1101/gad.231702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright PR, Richter AS, Papenfort K, Mann M, Vogel J, Hess WR, et al. Comparative genomics boosts target prediction for bacterial small RNAs. Proc Natl Acad Sci U S A. 2013;110: 3487–3896. doi: 10.1073/pnas.1303248110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desnoyers G, Massé E. Noncanonical repression of translation initiation through small RNA recruitment of the RNA chaperone Hfq. Genes Dev. 2012;26: 726–739. doi: 10.1101/gad.182493.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kröger C, Dillon SC, Cameron ADS, Papenfort K, Sivasankaran SK, Hokamp K, et al. The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci U S A. 2012;109: 1277–1286. doi: 10.1073/pnas.1201061109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmqvist E, Wright PR, Li L, Bischler T, Barquist L, Reinhardt R, et al. Global RNA recognition patterns of post‐transcriptional regulators Hfq and CsrA revealed by UV crosslinking in vivo. EMBO J. 2016;35: 991–1011. doi: 10.15252/embj.201593360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imamura R, Yamanaka K, Ogura T, Hiraga S, Fujita N, Ishihama A, et al. Identification of the cpdA gene encoding cyclic 3’,5’-adenosine monophosphate phosphodiesterase in Escherichia coli. J Biol Chem. 1996;271: 25423–25429. [DOI] [PubMed] [Google Scholar]
- 31.Mizusaki H, Takaya A, Yamamoto T, Aizawa S-I. Signal Pathway in Salt-Activated Expression of the Salmonella Pathogenicity Island 1 Type III Secretion System in Salmonella enterica Serovar Typhimurium. J Bacteriol. 2008;190: 4624–4631. doi: 10.1128/JB.01957-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrbar K, Friebel A, Miller SI, Hardt W-D. Role of the Salmonella pathogenicity island 1 (SPI-1) protein InvB in type III secretion of SopE and SopE2, two Salmonella effector proteins encoded outside of SPI-1. J Bacteriol. 2003;185: 6950–6967. doi: 10.1128/JB.185.23.6950-6967.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sittka A, Pfeiffer V, Tedin K, Vogel J. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol. 2007;63: 193–217. doi: 10.1111/j.1365-2958.2006.05489.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polayes DA, Rice PW, Garner MM, Dahlberg JE. Cyclic AMP-cyclic AMP receptor protein as a repressor of transcription of the spf gene of Escherichia coli. J Bacteriol. 1988;170: 3110–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kröger C, Colgan A, Srikumar S, Händler K, Sivasankaran SK, Hammarlöf DL, et al. An Infection-Relevant Transcriptomic Compendium for Salmonella enterica Serovar Typhimurium. Cell Host Microbe. 2013;14: 683–695. doi: 10.1016/j.chom.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 36.Chao Y, Papenfort K, Reinhardt R, Sharma CM, Vogel J. An atlas of Hfq-bound transcripts reveals 3’ UTRs as a genomic reservoir of regulatory small RNAs. EMBO J. 2012;31: 4005–4019. doi: 10.1038/emboj.2012.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viegas SC, Mil-Homens D, Fialho AM, Arraiano CM. The Virulence of Salmonella enterica Serovar Typhimurium in the Insect Model Galleria mellonella Is Impaired by Mutations in RNase E and RNase III. Appl Environ Microbiol. 2013;79: 6124–6133. doi: 10.1128/AEM.02044-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busch A, Richter AS, Backofen R. IntaRNA: efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics. 2008;24: 2849–2856. doi: 10.1093/bioinformatics/btn544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balsalobre C, Johansson J, Uhlin BE. Cyclic AMP-dependent osmoregulation of crp gene expression in Escherichia coli. J Bacteriol. 2006;188: 5935–5944. doi: 10.1128/JB.00235-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonough KA, Rodriguez A. The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol. 2012;10: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller CM, Aberg A, Straseviçiene J, Emody L, Uhlin BE, Balsalobre C. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP. PLoS Pathog. 2009;5: e1000303 doi: 10.1371/journal.ppat.1000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS. ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev. 2010;74: 171–199. doi: 10.1128/MMBR.00046-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliva G, Sahr T, Buchrieser C. Small RNAs, 5’ UTR elements and RNA-binding proteins in intracellular bacteria: impact on metabolism and virulence. FEMS Microbiol Rev. 2015;39: 331–349. doi: 10.1093/femsre/fuv022 [DOI] [PubMed] [Google Scholar]
- 44.Papenfort K, Vogel J. Small RNA functions in carbon metabolism and virulence of enteric pathogens. Front Cell Infect Microbiol. 2014;4: 91 doi: 10.3389/fcimb.2014.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrone BL, Stringer AM, Wade JT. Identification of HilD-regulated genes in Salmonella enterica serovar Typhimurium. J Bacteriol. 2014;196: 1094–1101. doi: 10.1128/JB.01449-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hautefort I, Thompson A, Eriksson-Ygberg S, Parker ML, Lucchini S, Danino V, et al. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell Microbiol. 2008;10: 958–84. doi: 10.1111/j.1462-5822.2007.01099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansson J, Cossart P. RNA-mediated control of virulence gene expression in bacterial pathogens. Trends Microbiol. 2003;11: 280–285. [DOI] [PubMed] [Google Scholar]
- 48.Gripenland J, Netterling S, Loh E, Tiensuu T, Toledo-Arana A, Johansson J. RNAs: regulators of bacterial virulence. Nat Rev Microbiol. 2010;8: 857–866. doi: 10.1038/nrmicro2457 [DOI] [PubMed] [Google Scholar]
- 49.Akbar S, Schechter LM, Lostroh CP, Lee CA. AraC/XylS family members, HilD and HilC, directly activate virulence gene expression independently of HilA in Salmonella typhimurium. Mol Microbiol. 2003;47: 715–728. [DOI] [PubMed] [Google Scholar]
- 50.Lucas RL, Lee CA. Roles of hilC and hilD in regulation of hilA expression in Salmonella enterica serovar Typhimurium. J Bacteriol. 2001;183: 2733–2745. doi: 10.1128/JB.183.9.2733-2745.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim S, Yun J, Yoon H, Park C, Kim B, Jeon B, et al. Mlc regulation of Salmonella pathogenicity island I gene expression via hilE repression. Nucleic Acids Res. 2007;35: 1822–1832. doi: 10.1093/nar/gkm060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruiz de los Mozos I, Vergara-Irigaray M, Segura V, Villanueva M, Bitarte N, Saramago M, et al. Base pairing interaction between 5’- and 3’-UTRs controls icaR mRNA translation in Staphylococcus aureus. PLoS Genet. 2013;9: e1004001 doi: 10.1371/journal.pgen.1004001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chao Y, Vogel J. A 3′ UTR-Derived Small RNA Provides the Regulatory Noncoding Arm of the Inner Membrane Stress Response. Mol Cell. 2016;61: 352–363. doi: 10.1016/j.molcel.2015.12.023 [DOI] [PubMed] [Google Scholar]
- 54.Gaviria-Cantin T, El Mouali Y, Le Guyon S, Römling U, Balsalobre C. Gre factors-mediated control of hilD transcription is essential for the invasion of epithelial cells by Salmonella enterica serovar Typhimurium. Mecsas J, editor. PLOS Pathog. 2017;13: e1006312 doi: 10.1371/journal.ppat.1006312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Opdyke JA, Kang J-G, Storz G. GadY, a Small-RNA Regulator of Acid Response Genes in Escherichia coli. J Bacteriol. 2004;186: 6698–6705. doi: 10.1128/JB.186.20.6698-6705.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melamed S, Peer A, Faigenbaum-Romm R, Gatt YE, Reiss N, Bar A, et al. Global Mapping of Small RNA-Target Interactions in Bacteria. Mol Cell. 2016;63: 884–897. doi: 10.1016/j.molcel.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waters SA, McAteer SP, Kudla G, Pang I, Deshpande NP, Amos TG, et al. Small RNA interactome of pathogenic E. coli revealed through crosslinking of RNase E. EMBO J. 2017;36: 374–387. doi: 10.15252/embj.201694639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westermann AJ, Förstner KU, Amman F, Barquist L, Chao Y, Schulte LN, et al. Dual RNA-seq unveils noncoding RNA functions in host–pathogen interactions. Nature. 2016;529: 496–501. doi: 10.1038/nature16547 [DOI] [PubMed] [Google Scholar]
- 59.Amann E, Ochs B, Abel KJ. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69: 301–315. [DOI] [PubMed] [Google Scholar]
- 60.Guillier M, Gottesman S. Remodelling of the Escherichia coli outer membrane by two small regulatory RNAs. Mol Microbiol. 2006;59: 231–247. doi: 10.1111/j.1365-2958.2005.04929.x [DOI] [PubMed] [Google Scholar]
- 61.Urban JH, Vogel J. Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res. 2007;35: 1018–1037. doi: 10.1093/nar/gkl1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97: 6640–6645. doi: 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158: 9–14. [DOI] [PubMed] [Google Scholar]
- 64.Ellermeier CD, Janakiraman A, Slauch JM. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene. 2002;290: 153–161. [DOI] [PubMed] [Google Scholar]
- 65.Uzzau S, Figueroa-Bossi N, Rubino S, Bossi L. Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci U S A. 2001;98: 15264–15269. doi: 10.1073/pnas.261348198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cota I, Blanc-Potard AB, Casadesús J. STM2209-STM2208 (opvAB): a phase variation locus of Salmonella enterica involved in control of O-antigen chain length. PLoS One. 2012;7: e36863 doi: 10.1371/journal.pone.0036863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hautefort I, Proença MJ, Hinton JCD. Single-copy green fluorescent protein gene fusions allow accurate measurement of Salmonella gene expression in vitro and during infection of mammalian cells. Appl Environ Microbiol. 2003;69: 7480–7491. doi: 10.1128/AEM.69.12.7480-7491.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirold S, Rabsch W, Rohde M, Stender S, Tschäpe H, Rüssmann H, et al. Isolation of a temperate bacteriophage encoding the type III effector protein SopE from an epidemic Salmonella typhimurium strain. Proc Natl Acad Sci U S A. 1999;96: 9845–9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller J.H. 1992. Short Course in Bact.Genet. CSH Press; o:429. [Google Scholar]
- 70.Pernitzsch SR, Tirier SM, Beier D, Sharma CM. A variable homopolymeric G-repeat defines small RNA-mediated posttranscriptional regulation of a chemotaxis receptor in Helicobacter pylori. Proc Natl Acad Sci. 2014;111: 501–510. doi: 10.1073/pnas.1315152111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Upper panel. Immunodetection of CpdA-6xHis was performed in extracts of the wild-type (WT) and a Δcya derivative strain carrying either pTrc99a (-, control vector) or pCpdA (+, pTrc99a+cpdA). Cultures were grown in LB supplemented with IPTG (0.1 mM) at 37°C up to an OD600nm of 0.4. The band corresponding to the CpdA protein is indicated with an arrowhead. Lower panel. Coomassie Blue staining of the whole cell extracts serve as loading controls. M: molecular mass markers (kDa).
(TIF)
Cell-free supernatants from two independent cultures of the WT and ΔhilA strain grown up to early stationary phase (OD600nm 2.0) were TCA precipitated. The resulting extracts were analyzed by SDS-PAGE and Coomassie staining. Arrowheads indicate presumed secreted effector proteins from Salmonella. Size in kDa of molecular mass marker bands (M) are indicated.
(TIF)
Relative quantification by qRT-PCR of hilA mRNA in a Δcrp derivative strain compared to wild type (WT). The reference value (WT) was set as one. Detection of gapA (GAPDH) was used as an internal control (see Materials and Methods). RNA samples were extracted from cultures of the WT and Δcrp derivative strains grown in LB at 37°C up to an OD600nm of 0.4. The average and standard deviation from three independent experiments are shown. *** p< 0.001.
(TIF)
Transcriptional expression of sipC-lacZ was monitored in the wild type (WT) and Δcrp derivative strains in either a hilD+ or hilD- genetic background. Cultures were grown in LB at 37°C up to an OD600nm of 0.4. The β-galactosidase activity from three independent experiments was averaged and the standard deviation is shown. ***, p< 0.001; ns, not significant.
(TIF)
Transcriptional expression of spf in the wild type (WT) and Δcrp derivative strains was monitored by β-galactosidase activity determination of a spf-lacZ chromosomal fusion. LB cultures were grown at 37°C up to either mid-logarithmic (OD600nm 0.4) or early stationary (OD600nm 2.0) phase. Data from three independent experiments are averaged and the standard deviation is shown. ***, p< 0.001; ns, not significant.
(TIF)
A. Immunodetection of HilD-3xFLAG was performed on whole cell extracts from cultures of the wild type (WT) strain (+UTR) carrying either the pBRplacVC (control vector, reference value) or pBRplac-Spot 42. Coomassie Blue staining of the whole cell extracts serve as loading controls. B. Merged image of white light caption for detection of the molecular mass marker and the chemiluminiscence detected bands in an extract from WT carrying the pBRplacVC. Molecular mass markers in kDa. C. The band corresponding to HilD-3xFLAG (indicated with an arrowhead) is easily identified as the protein band over-accumulated in Δcrp as compared to WT.
(TIF)
In vitro transcribed RNA was radiolabeled. 4 nM of the radiolabeled RNA was incubated with increasing concentration of purified Hfq (0, 1.3, 4, 13, 40, 130 nM) and subjected to electrophoresis in a native gel. Band shift was observed upon drying and exposure of the gel.
(TIF)
GFP fluorescence assessment by flow cytometry of GFP-hilD 3’UTR upon overexpression of the sRNA Spot 42. Cultures of Δspf strains carrying the construct pXG1gfp-hilD3’UTR in presence (pBRplac-Spot42) or absence of the sRNA Spot 42 (pBRplacVC) grown in LB at 37°C up to an OD600nm of 0.4. Data from three independent experiments are averaged and the standard deviation is shown. ***, p< 0.001.
(TIF)
Transcriptional expression of sipC-lacZ was monitored in two different hilD backgrounds: hilD 3’UTRmut1 and hilD 3’UTRmut2. sipC-lacZ expression was assessed upon overexpression of either Spot 42WT, Spot 42mut1 and Spot 42mut2. β-galactosidase activity was determined for three independent cultures, average and standard deviation are shown. ***, p< 0.001; ns, not significant. In all cases, bacterial cultures were grown in LB at 37°C up to an OD600nm of 0.4.
(TIF)
EMSA assay using 4 nM of either UTRL or UTRR fragments incubated with increasing concentration (0, 56, 280, 560, 1700 nM) of Spot 42 RNA. All RNA molecules used were obtained by T7 in vitro transcription. Samples were subjected to electrophoresis in a native gel and band shift was observed upon drying and exposure of the gel.
(TIF)
hilD mRNA was detected by Northern blot. Culture of wild type (WT) and Δcrp were grown to mid-logarithmic phase (OD600nm 0.4), rifampicin was added (500 μg/ml) and samples were taken for total RNA extraction at 2, 4, 8, 16 and 32 min. Samples before rifampicin addition (time 0) were taken. RNA radiolabeled probe complementary to the first 300 nt of hilD mRNA was generated by in vitro T7 RNA transcription and used for hilD mRNA detection. tmRNA was detected as loading control. Full length image of the Northern blot is shown in S12 Fig.
(TIF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.