Abstract
Objectives:
This study aimed to search for scientific evidence concerning the accuracy of dental development for estimating the pubertal growth spurt.
Methods:
It was conducted according to the statements of PRISMA. An electronic search was performed in six databases, including the grey literature. The PICOS strategy was used to define the eligibility criteria and only observational studies were selected.
Results:
Out of 1,416 identified citations, 10 articles fulfilled the criteria and were included in this systematic review. The association between dental development and skeletal maturity was considered strong in seven studies, and moderate in two, although the association with the pubertal growth spurt had been verified in only four articles. According to half of the studies, the tooth that provided the greater association with the ossification centres was the lower canine. The meta-analysis performed also indicated a positive association, being stronger in females [0.725 (0.649–0.808)]. However, when the method used for dental evaluation was considered, it was possible to verify greater correlation coefficients for Nolla [0.736 (0.666–0.814)] than for Demirjian [0.631 (0.450–0.884)], at the boys sample. The heterogeneity test reached high values (Q = 51.00), suggesting a potential bias within the studies.
Conclusions:
Most of individual studies suggested a strong correlation between dental development and skeletal maturation, although the association with the peakof pubertal growth spurtwas clearly cited only in some of them. However, due to the high heterogeneity found among the studies included in this meta-analysis, a pragmatic recommendation about the use of dental stages is not possible.
Introduction
Assessment of craniofacial growth status, whether the pubertal growth spurt of the patient has been reached or completed, has a considerable impact on diagnosis, objectives, treatment planning and the eventual outcome of orthodontic treatment. This is especially evident when clinical considerations are based strongly on the increased or decreased rates of craniofacial growth, such as the timing and use of extraoral traction, the use of functional appliances, extraction vs nonextraction, and the selection and execution of orthodontic retention.1–3 Considerable variations in the development among individuals of the same chronological age have led to the concept of biologic age, which can be measured by some indicators, such as the somatic, sexual, skeletal and dental maturation stages.4
Various areas of the skeleton have been used for assessing skeletal maturity, such as the cervical vertebrae and the hand-wrist.5 The hand-wrist pattern of ossification has been commonly used, and most investigators have found significant correlation among maturation stages derived from hand-wrist radiographs, changes in height during pubertal growth period, and facial growth.6–8 Various methods have been developed to compute bone age score from these radiographs by comparing the maturity of the bones to idealized standards. One of the most commonly used method is based on Greulich and Pyle atlas,9 its last edition published in 1959. This atlas contains reference standard images of the left wrist and hand from birth till 18 years for females and 19 years for males, and also explanation regarding the gradual age-related changes observed in the bone structure with each standard image. Another widely used method is based on the study of Fishman,6 published in 1982, who used a system with four stages of bone maturation, all found at six anatomical sites located on the thumb, third finger, fifth finger, and radius.
Biologic age calculated by assessing dental maturity has also extensively been applied, and can be determined by the stages of tooth eruption or the stages of tooth formation, the latter being considered a more reliable criterion.10–12 Nolla,10 in 1960, established a series of standardized drawings depicting 10 stages of tooth calcification. In 1963, Moorrees et al11 developed an atlas in which distinct stages of dentition of various teeth were specified. In another method, devised by Demirjian et al12 in 1973, each tooth was assigned a maturity score based on the level of dentition, and a total maturity score was calculated by adding up all the individual scores and used to calculate the dental age.
If a strong association exists between skeletal maturity and dental development, the stages of dental calcification might be used as a first-level diagnostic tool to estimate the timing of the pubertal growth spurt.4 The ease of recognizing dental developmental stages, together with the availability of intraoral or panoramic radiographs in most orthodontic or paediatric practices, are reasons for attempting to assess physiologic maturity without resorting to hand-wrist radiographs. This fact would make it easier for the clinician to refer the patient to orthodontic treatment in a more timely time. As there are still many controversies surrounding the accuracy of dental development for estimating the pubertal growth spurt, the present systematic review aims to investigate and exposure if there is a scientific evidence that supports the use of this method.
Methods and materials
Protocol and registration
This systematic review was performed according to the statements of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA),13 with guidance from the Cochrane Handbook for Systematic Reviews of Interventions.14 The systematic review protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) database, under the number CRD42016046297 (http://www.crd.york.ac.uk/PROSPERO).
Eligibility criteria
The present research aimed to answer the following focused question: Is dental development as accurate for estimating the pubertal growth spurt as the hand-wrist maturation stages?
It used the population, intervention, comparison, outcome and study design (PICOS) strategy to define the eligibility criteria, and the research question was based on the following elements: population, dental development stages; intervention, estimation of the pubertal growth spurt; comparison, hand-wrist maturation stages; outcome, dental development is accurate for estimating the pubertal growth spurt and study design, observational studies.
Only observational studies that evaluated the accuracy of dental development stages for estimating the pubertal growth spurt in comparison to the hand-wrist maturation analysis were included. No language or publication year and status were imposed. Exclusion criteria were applied in two phases, and (1) studies in which the subject of interest was not addressed, (2) abstracts or indexes, (3) letters to editors, (4) literature reviews, (5) personal or short communications, (6) book chapters, (7) patents, (8) studies in which the assessment of skeletal maturity was not performed using Greulich and Pyle or Fishman methods, (9) studies in which dental maturity was not assessed based on the stages of tooth formation and (10) studies that used a sample of syndromic patients or with systemic disorders were excluded.
Information sources
In order to identify relevant studies, a systematic search was conducted in the following electronic databases: PubMed (including MedLine), Science Direct (only journals, excluding books and reference works), SciELO and LILACS. A grey literature search was performed through Google Scholar and OpenGrey to avoid potential selection bias. For Google Scholar, the first 100 records of the used search strategy were analysed, not including patents or citations. Additional studies were obtained from a well-published expert in pubertal growth spurt.
Search
The Medical Subject Headings (MeSH) was used to select the descriptors “Age Determination by Skeleton”, “Tooth”, “Hand Bones”, “Age Determination by Teeth”, “Growth Spurt”, “Skeletal Maturation” and “Hand”. Boolean operators (OR and AND) were used to combine the descriptors. This search was performed in December 2016. The full electronic search strategy is illustrated in the Table 1. Additionally, all obtained references were exported to Mendeley™ Desktop 1.13.3 (Mendeley Ltd., London, England) software, in order to track potential duplicate records.
Table 1.
Electronic databases and applied search strategy.
| Database | Search strategy (December, 2016) |
| PubMed https://www.ncbi.nlm.nih.gov/pubmed/ | (“tooth”[MeSHTerms] OR “tooth”[AllFields]) AND “skeletal”[AllFields] AND “maturation”[AllFields](“age determination by teeth”[MeSHTerms] OR (“age”[AllFields]) AND “determination”[AllFields] AND “teeth”[AllFields]) OR “age determination by teeth”[AllFields] AND (“age determination by skeleton”[MeSHTerms] OR (“age”[AllFields] AND “determination”[AllFields] AND “skeleton”[AllFields]) OR “age determination by skeleton”[AllFields])(“age determination by teeth”[MeSHTerms] OR (“age”[AllFields] AND “determination”[AllFields] AND “teeth”[AllFields]) OR “age determination by teeth”[AllFields]) AND (“growth and development”[Subheading] OR (“growth”[AllFields] AND “development”[AllFields]) OR “growth”[AllFields]) OR “growth”[MeSHTerms] AND “spurt”[AllFields] |
| Science Direct www.sciencedirect.com/ | Age Determination by Skeleton OR Skeletal Maturation AND Tooth AND Growth Spurt“Age Determination by Teeth” AND “Hand Bones” AND “Skeletal Maturation” AND “Growth Spurt” |
| SciELO www.scielo.org/ | “Skeletal Maturation” AND “Tooth”Age Determination by Skeleton OR Age Determination by TeethHand Bones OR Growth Spurt“Growth Spurt” AND “Tooth” |
| LILACS lilacs.bvsalud.org | Age Determination by Skeleton AND ToothHand Bones AND ToothAge Determination by Teeth AND Age Determination by SkeletonAge Determination by Teeth AND Growth Spurt |
| Google Scholar scholar.google.com.br/ | “Age Determination by Teeth” AND “Hand Bones” AND “Growth Spurt” |
| OpenGrey www.opengrey.eu/ | “Age Determination by Teeth”“Skeletal Maturation OR Hand” |
Study selection
The data collection was independently performed by two reviewers (RSG and APBL), in two different phases. First, titles and abstracts were carefully read to exclude articles out of the scope of this research. At this stage, both reviewers were blinded for authorship information and journals’ names, and studies in which the subject of interest was not addressed, abstracts or indexes, letters to editors, literature reviews, personal or short communications, book chapters and patents, as previously cited, were excluded. Those whose titles matched the eligibility criteria but did not have abstracts available were also obtained and analysed. Then, in Phase 2, remaining studies had their fulltext analysed in order to verify whether they fulfill the others eligibility criteria, and then studies in which the assessment of skeletal maturity was not performed using Greulich and Pyle or Fishman methods, studies in which dental maturity was not assessed based on the stages of tooth formation, and studies that used a sample of syndromic patients or with systemic disorders were also excluded. In specific cases, when article did not present complete data to judge eligibility, author was contacted by e-mail in order to obtain more detailed information. When mutual agreement between the two reviewers was not reached, a third reviewer (LRP) was involved to make a final decision. Rejected studies and reasons for its exclusion were separately recorded.
Data collection process and data items
After the screening was done, texts of selected articles were reviewed and data were extracted in a standardized way. One author (RSG) collected the required information from the selected articles; a second author (AF) cross-checked the information to confirm the quality of extracted data. Any disagreement was resolved by discussion with a third author (MAVB). When additional assistance was necessary to make a final decision, a fourth author (GOC) was summoned. Attempts were made to contact the authors of the selected studies to retrieve missing information.
After filtration, full-text articles underwent data systematic extraction. Data were extracted regarding the study population (size, sex and age), distribution by sex and age, skeletal maturation, skeletal imaging, dental maturation, dental imaging, evaluated teeth and main outcome, besides authorship, year of publication and country of origin.
Risk of bias in individual studies
The risk of bias of the included articles was independently performed by two reviewers (MAVB and AF), according to the PRISMA recommendation.13 This evaluation prioritized the clear description of data and reviewers were blinded for authorship information and journals’ names, avoiding any potential bias or conflict of interest.
Meta-Analysis of Statistical Assessment and Review Instrument (MAStARI) critical appraisal tool15 was used. Nine questions were used to evaluate the risk of bias: (1) was the study based on a random or pseudorandom sample? (2) Were the criteria for inclusion in the sample clearly defined? (3) Were confounding factors identified and strategies to deal with them stated? (4) Were outcomes assessed using objective criteria? (5) If comparisons are being made, was there sufficient description of the groups? Originally, this was the question, but in the present study the comparisons were interpreted as the association between dental and skeletal development within age groups; (6) was the follow up carried out over a sufficient time period? (7) Were the outcomes of people who withdrew described and included in the analysis? (8) Were the outcomes measured in a reliable way? And (9) was an appropriate statistical analysis used? Then, it was rated the risk of bias as being high, when the study reached up to 49% score “yes”, moderate, when it reached 50 to 69% score “yes”, and low, when it reached more than 70% score “yes”.
Outcome measures and data analysis
The association between dental calcification stages and hand-wrist maturational indicators, for estimating the pubertal growth spurt, assessed by the correlation coefficient, was the main outcome evaluated.
It was performed an association meta-analysis between the correlation coefficients of both the dental calcification stages and the hand-wrist maturational indicators of the included articles (r value). The outcome measure considered the Pearson and/or Spearman correlation coefficients, with a statistically significance set as p < 0.05. Forest plot graphs were generated using STATA 12.0 software (StataCorp LLC, College Station, TX). The results were analysed considering male and female genders, separately, and also with no gender distinction.
The heterogeneity among the included articles was calculated using the Cochran Q-test. Values greater than K-1, with k representing the number of studies, were considered indicators of substantial heterogeneity, and a random effect model was indicated. The statistically significance level was set as p < 0.05.
Results
Study selection
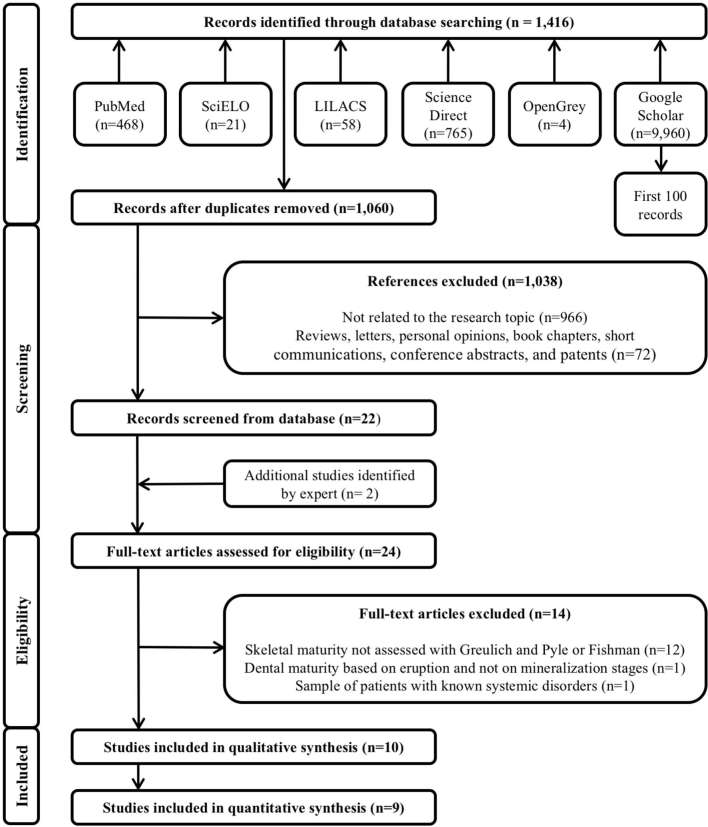
The systematic search performed within six electronic databases resulted in 1,416 references, of which 356 were duplicates. After removal of duplicate references, 1,060 titles had their titles and abstracts carefully read in Phase 1, in order to eliminate studies that did not match the objectives of this systematic review or were literature reviews, letters, personal opinions, book chapters, short communications, conference abstracts and patents. A total of 1,038 references were excluded. Then, in Phase2, the 22 remaining titles were selected for a full manuscript reading and assessment of the eligibility criteria. At this stage, two additional articles were suggested by experts. After reading the full text of the 24 references, only 10 articles16–25 met the eligibility criteria and were included in the present systematic review. Then, in the qualitative analysis these 10 articles were included and in the quantitative synthesis nine references were used. The excluded articles and the reasons for exclusion can be seen in Supplementary Material 1, supplementary material available only. A flowchart depicting the selection process based in the PRISMA diagram13 is provided in Figure 1.
Figure 1.
Flowchart showing the results of the search process.
Study characteristics
All articles consisted of observational studies published from 1987 to 2015. Six of them were written in English,16–19,21,22 while two were in Portuguese,20,25 and two in Spanish.23,24 Research groups from seven different countries performed the studies, namely Mexico,16 United States,17 Thailand,18 Turkey,19 Brazil,20,25 India21,22 and Peru.23,24 Table 2 provides a summary of their characteristics.
Table 2.
Characteristics of studies included in the systematic review and meta-analysis.
| Authorship, year of publication, country of origin | N | Sex | Age (years) | Distribution by sex and age (years) | Skeletal development | Skeletal imaging | Dental development | Dental imaging | Teeth # |
| Sierra,161987,Mexico | 153 | 72 ♂81♀ | 8–12 | 7.6–8: 4♂6♀8.1–8.5: 3♂3♀8.6–9: 9♂11♀9.1–9.5: 11♂8♀9.6–10: 10♂9♀10.1–10.5: 10♂4♀10.6–11: 4♂11♀11.1–11.5: 7♂8♀11.6–12: 11♂13♀12.1–12.5: 3♂8♀ | Greulich and Pyle, 19599 | Hand-wrist radiographs | Nolla, 196010 | Panoramic radiographs | 13, 14, 15, 1723, 24, 25, 2733, 34, 35, 3743, 44, 45, 47 |
| Coutinho et al,171993,USA | 415 | 200♂215♀ | 7–16.5 | 7–8.5: 13♂18♀8.6–9.5: 27♂32♀9.6–10.5: 38♂34♀10.6–11.5: 27♂43♀11.6–12.5: 38♂36♀12.6–13.5: 30♂27♀13.6–14.5: 19♂15♀14.6–16.5: 58♂10♀ | Greulich and Pyle, 19599 | Hand-wrist radiographs | Demirjian et al, 197312 | Panoramic radiographs | 33, 43 |
| Krailassiri et al, 182002 Thailand | 361 | 139♂222♀ | 7–19 | n/m | Fishman, 19826 | Hand-wrist radiographs | Demirjian et al, 197312 | Panoramic radiographs | 33, 34, 35, 37, 38 |
| Sağlam and Gazilerli,192002,Turkey | 422 | 146♂276♀ | 7.6–14 | 7.6–8: 2♂4♀8.1–8.5: 5♂4♀8.6–9: 4♂7♀9.1–9.5: 2♂11♀9.6–10: 3♂12♀10.1–10.5: 7♂20♀10.6–11: 12♂16♀11.1–11.5: 10♂30♀11.6–12: 15♂37♀12.1–12.5: 24♂39♀12.6–13: 22♂25♀13.1–13.5: 23♂43♀13.6–14:17♂28♀ | Fishman, 19826 | Hand-wrist radiographs | Demirjian et al, 197312 | Panoramic and periapical radiographs | 23, 24, 2533, 34, 35, 37 |
| Lima et al,202006, Brazil | 40 | n/m | 7–16 | n/m | Fishman, 19826 | Hand-wrist radiographs | Nolla, 196010 | Panoramic radiographs | 37, 47 |
| Gupta et al, 212007, India | 50 | 25♂25♀ | 9–15 | n/m | Fishman, 19826 | Hand-wrist radiographs | Moorrees et al, 196311 | Panoramic radiographs | 12, 11, 21, 2231, 32, 33, 34, 35, 36, 37, 3841, 42, 43, 44, 45, 46, 47, 48 |
| Bala et al,222010 India | 160 | 80♂80♀ | 8–14 | 8–8.99: 10♂ 10♀9–9.99: 11♂ 11♀10–10.99: 15♂ 15♀11–11.99: 12♂ 12♀12–12.99: 10♂ 10♀13–13.99: 11♂ 11♀14–14.99: 11♂ 11♀ | Greulich and Pyle, 19599 | Hand-wrist radiographs and radiographs of the middle phalanx of the third finger | Nolla, 196010 | Periapical radiographs | 13 |
| Guillén et a,l232011 Peru | 182 | 91♂91♀ | 9–16 | n/m | Fishman, 19826 | Hand-wrist radiographs | Demirjian et al, 197312 | Periapical radiographs | 33, 34, 35, 37 |
| Ríos Villasis and Soldevilla Galarza242014, Peru | 72 | 41♂31♀ | 9–15 | n/m | Fishman, 19826 | Hand-wrist radiographs | Demirjian et al, 197312 | Panoramic radiographs | 33, 34, 3543, 44, 45 |
| Notaroberto et al,252015 Brazil | 55 | 24♂31♀ | 7–17 | n/m | Greulich and Pyle, 19599 | Hand-wrist radiographs | Demirjian et al, 197312 | Panoramic radiographs | 31, 32, 33, 34, 35, 36, 37 |
N, sample size; ♂, males; ♀, females; n/m, not mentioned; #, tooth position in the dental arch coded according to the Federation Dentaire Internationale.
From the eligible articles, only one performed sample size calculation23 and three were careful to report the examiner calibration,17,23,24 although observer reproducibility tests (intra or interexaminer) were carried out in most of them.16–20,23 Some assessments were made by one examiner,16,19,25 and others by two20,23,24 or three.17,18 In some studies, interpretations of data were also discussed among the examiners until agreement was reached.18,23 Just one article mentioned about the approval by the ethical committee,23 although other two report that the informed consent was obtained from parents.22,24
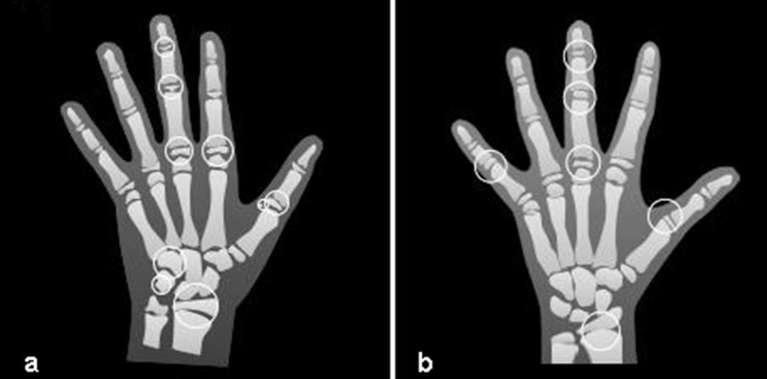
Skeletal development was assessed using hand-wrist radiographs in all the studies, with Greulich and Pyle9 method in four studies,16,17,22,25 while in six studies18–21,23,24 the method of Fishman6 was used. Additionally, one study22 used the radiograph of the middle phalanx of the third finger to perform this evaluation. The evaluation of dental development phases was performed using panoramic radiographs in most of the researches,16–18,20,21,24,25 while two22,23 used periapical radiographs and one19 used both. The mineralization stages proposed by Demirjian et al12 were used in six studies,17–19,23–25 stages defined by Nolla10 were used in three,16,20,22 and described by Moorrees et al11 in just one.21 Illustrations with anatomical sites and stages of tooth formation used for skeletal and dental evaluation, based on the studies cited above, can be seen in Figures 2 and 3.
Figure 2.
Nine anatomical sites located on the thumb, second finger, third finger, hamato, pisiform and radius, adapted from Greulich and Pyle atlas (a), and six anatomical sites located on the thumb, third finger, fifth finger and radius, adapted from Fishman (b).
Figure 3.
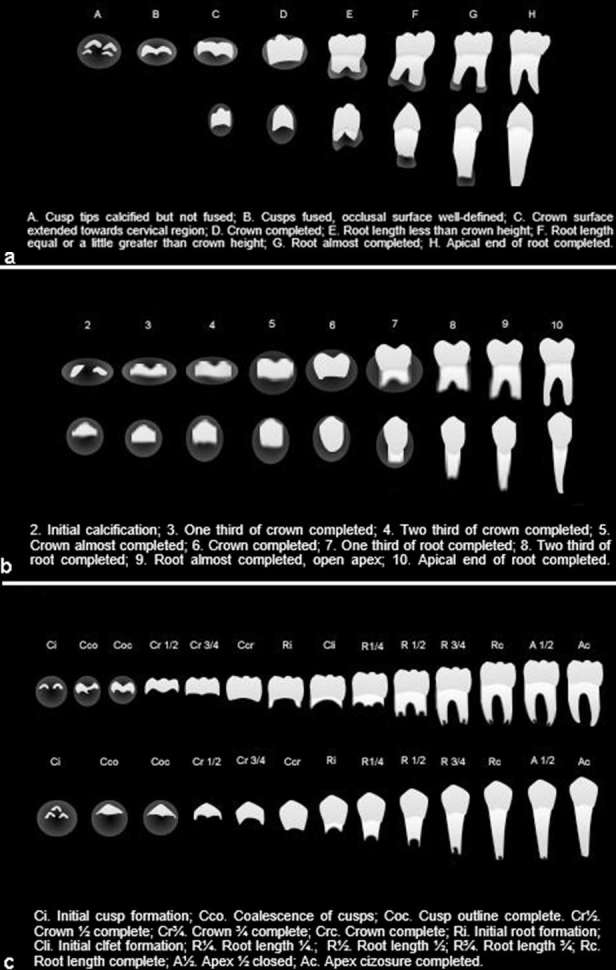
Stages of tooth development adapted from Demirjian et al12 (a), Nolla10 (b) and Moorrees et al11(c).
The teeth chosen to perform the assessment varied widely, but just one article20 was designed without the use of the canines. Most of the studies used the canines, premolars and second molars,16,18,19,21,23–25 some upper and lower16,19,24 and others only lower.18,21,23,25 On the other hand, one article17 used just the lower canines and other22 used just the upper right canine. Upper and lower incisors were used in just one research21 and lower incisors only in another one.25
Risk of bias within studies
The methodological risk of bias evaluation using the MAStARI critical appraisal tool15 is shown in Table 3, where it is possible to visualize the answers to the nine formulated questions. None of the included articles fulfilled all the criteria proposed in that checklist. In fact, nine studies scored low risk of bias,16–19,21–25 while just one reached high risk.20 It is important to mention that questions 6 and 7 of checklist were considered not applicable (N/A) because none of the researches was designed as a cohort study and did not require clinical follow-up.
Table 3.
Risk of bias assessed by the Meta-Analysis of Statistical Assessment and Review Instrument (MAStARI).
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | % Yes | Risk of bias |
| Sierra,16 | -- | √ | √ | √ | √ | N/A | N/A | √ | √ | 85.71% | + |
| Coutinho et al17 | -- | √ | √ | √ | √ | N/A | N/A | √ | √ | 85.71% | + |
| Krailassiri et al18 | -- | √ | √ | √ | -- | N/A | N/A | √ | √ | 71.42% | + |
| Sağlam and Gazilerli19 | -- | √ | √ | √ | √ | N/A | N/A | √ | √ | 85.71% | + |
| Lima et al20 | -- | √ | -- | -- | -- | N/A | N/A | √ | √ | 42.85% | +++ |
| Gupta et al21 | √ | √ | √ | √ | -- | N/A | N/A | √ | √ | 85.71% | + |
| Bala et al22 | -- | √ | √ | √ | √ | N/A | N/A | √ | √ | 85.71% | + |
| Guillén et al23 | -- | √ | √ | √ | -- | N/A | N/A | √ | √ | 71.42% | + |
| Ríos Villasis and Soldevilla Galarza24 | -- | √ | √ | √ | -- | N/A | N/A | √ | √ | 71.42% | + |
| Notaroberto et al25 | -- | √ | √ | √ | -- | N/A | N/A | √ | √ | 71.42% | + |
(Q1)Was the study based on a random or pseudorandom sample? (Q2) Were the criteria for inclusion in the sample clearly defined? (Q3) Were confounding factors identified and strategies to deal with them stated? (Q4) Were outcomes assessed using objective criteria? (Q5)If comparisons are being made, was there sufficient description of the groups? Originally, this was the question, but in the present study the comparisons were interpreted as the association between dental and skeletal development within age groups; (Q6) was the follow up carried out over a sufficient time period? (Q7) Were the outcomes of people who withdrew described and included in the analysis? (Q8) Were the outcomes measured in a reliable way? (Q9) Was an appropriate statistical analysis used?
√, yes; --, no; U, unclear; N/A, not applicable; +++, high; ++, moderate; +, low.
Results of individual studies and meta-analysis
As previously mentioned, the total sample for qualitative evaluation involved 10 manuscripts. Their main outcomes are described in Table 4. The association between dental development and skeletal maturity was mentioned as being strong in most of the studies,16–18,22–25 and moderate in two,19,20 although the association with the pubertal growth spurt has been cited as true in just four articles.17,18,20,23 On the other hand, only one article19 was categorical in stating that tooth development is not a reliable indicator to determine the pubertal growth spurt. Besides, authors of two researches21,25 found a more significant correlation between chronological age and skeletal maturation than between this one and dental age.
Table 4.
Description of the main outcomes of studies.
| Authorship year of publication, country of origin | Outcome |
| Sierra, 1987, Mexico16 | Strong correlation was verified between the ossification centres and the lower canine, followed closely by the upper first premolar, in both sexes. The second premolar seemed the least reliable. |
| Coutinho et al, 1993,USA17 | Close association was observed between lower canine development and skeletal maturity. Canine stage F (root length equal or a little greater than crown height) indicated the beginning of puberty. Its intermediate period between stages F and G (root formation almost completed) identified the early phase of the pubertal growth spurt. |
| Krailassiri et al, 2002, Thailand18 | Tooth calcification stages might be clinically used as a maturity indicator of the pubertal growth period. Canine stage F (root length equal or a little greater than crown height) for both sexes indicated the onset of the accelerating growth period. The second molar stage E (root length less than crown height) for females and stage G (root formation almost completed) for males were indicative of the period of very rapid growth velocity. |
| Sağlam and Gazilerli, 2002, Turkey19 | Tooth development is not a reliable indicator to determine the pubertal growth spurt. However, the highest correlation between dental and skeletal development was observed considering the upper first premolar, in girls, and the lower second molar, in boys. The lowest correlation was observed within the lower first premolar, in girls, and the upper canine, in boys. |
| Lima et al, 2006, Brazil20 | Just a moderate correlation was observed between lower second molar formation and skeletal maturity. The association of this indicator with the cervical vertebrae development observed at the lateral cephalometric radiograph was clinically recommended for determination of pubertal growth spurt. |
| Gupta et al, 2007, India21 | The correlation between chronological and skeletal ages was more significant than the correlation between this one and dental age. The authors did not describe clearly the level of correlation and did not make inferences on the use of dental development for assessing the pubertal growth spurt. |
| Bala et al, 2010,India 22 | Close correlation was observed between dental age and skeletal development in children of 12–14 years of age of both sexes. |
| Guillén et al, 2011, Peru23 | Dental calcification stages can be clinically useful to assess the skeletal maturity phases. The lower canine figured as the tooth with higher correlation to the skeletal development, while the lower first premolar reached the lowest correlation rate. Canine stage G (root formation almost completed), in both sexes, coincided with the peak of the pubertal growth spurt. |
| Ríos Villasis and Soldevilla Galarza, 2014, Peru24 | Strong correlation was observed between the stages of skeletal maturity and the stages of calcification of lower canines, and first and second premolars. Among these teeth, the lower left canine presented the highest correlation, while the lower left second premolar showed the lowest one. |
| Notaroberto et al, 2015, Brazil25 | The highest correlation occurred between skeletal and chronological ages, in both sexes, although there has also been a strong correlation between skeletal and dental ages. The authors did not describe clearly the level of correlation considering different teeth and did not make inferences on the use of dental stages for assessing the pubertal growth spurt. |
The tooth that provided the greater association between its developmental stages and the skeletal maturity was the canine. According to half of the studies,16–18,23,24 a strong association was observed between the ossification centres and lower canine development. In addition, Gupta et al,21 using only upper right canines, also found a close association between this tooth development and skeletal age. The other two teeth cited as having stronger associations with skeletal maturation stages were the upper first premolar19 and the lower second molar.20
At the quantitative analysis, one article21 was not included because it was not reported the correlation value (r) between skeletal and dental methods. In contrast, Bala et al22 article was considered twice in the meta-analysis because it presented different correlation values depending on the method of skeletal evaluation they used (hand-wrist or middle phalanx of the third finger radiographs). Then, although the total sample for quantitative evaluation involved nine manuscripts, the final meta-analysis was based on the use of 10 values. Later, analyses were performed separately for each sex. For this evaluation, only six articles16–19,22,23 were included, because three studies20,24,25 did not present separated results according to the sex.
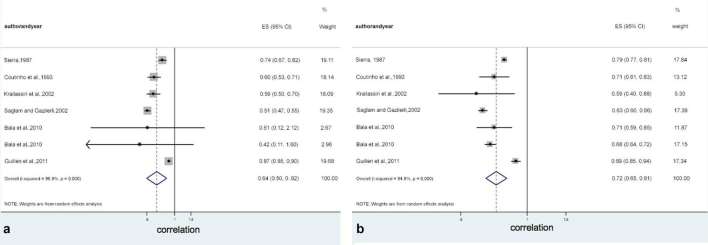
Apart the methods, the meta-analysis performed in this study indicated a positive association between dental development and skeletal maturity, with differences considering males and females. Tables 5 and 6, and Figure 4 show the outcomes obtained separately based on sex. Stronger association was detected in females [0.725; 95% CI (0.649–0.808)] compared to males [0.640; 95% CI (0.498–0.821)].
Table 5.
Overall correlation rank between dental development and skeletal maturation, and subgroup analysis according to the methodological characteristics to male sample.
| Methodological characteristics | Studies | Boys correlation rank | Heterogeneity (I2) |
| Overall correlation | 7 | 0.640 (0.498–0.821) | 96.8% |
| Dental method | |||
| Nolla10 | 3 | 0.736 (0.666–0.814) | 0.0% |
| Demirjian et al12 | 4 | 0.631 (0.450–0.884) | 98.4% |
| Teeth | |||
| Canine | 4 | 0.669 (0.566–0.792) | 42.1% |
| Premolar | 1 | 0.590 (0.498–0.699) | – |
| Molar | 2 | 0.667 (0.395–0.895) | 99.4% |
Table 6.
Overall correlation rank between dental development and skeletal maturation, and subgroup analysis according to the methodological characteristics to female sample.
| Methodological characteristics | Studies | Girls correlation rank | Heterogeneity (I2) |
| Overall correlation | 7 | 0.725 (0.649–0.808) | 94.9% |
| Dental method | |||
| Nolla10 | 3 | 0.729 (0.645–0.826) | 91.0% |
| Demirjian et al12 | 4 | 0.711 (0.560–0.902) | 96.8% |
| Teeth | |||
| Canine | 5 | 0.761 (0.689–0.841) | 91.9% |
| Premolar | 2 | 0.629 (0.599–0.661) | 0.0% |
| Molar | – | – | – |
Figure 4.
Overall correlation rank between dental development and skeletal maturation to male (a) and female (b) samples.
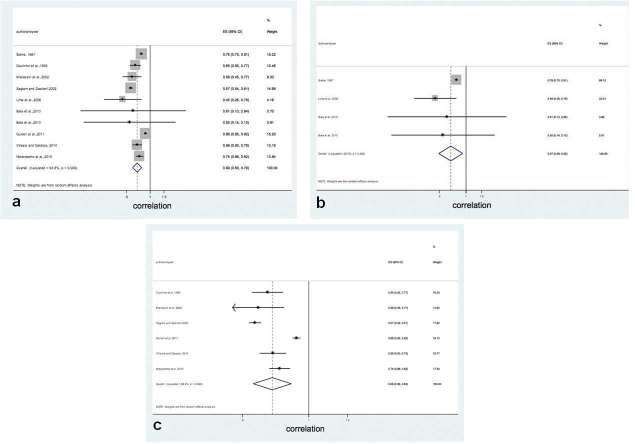
Finally, stratified meta-analysis was performed considering the method used to evaluate the stages of dental developmental (Nolla10 or Demirjian et al12), and the group of teeth (canine, premolar and molar) which presented the strongest correlation with skeletal maturity. Considering the method used for dental evaluation, at the male sample (Table 5) it was possible to verify greater correlation coefficients for Nolla [0.736; 95% CI (0.666–0.814)] than for Demirjian et al [0.631; 95% CI (0.450–0.884)]. Figure 5 shows the positive correlation between dental developmental stages and skeletal maturity for both Nolla10 and Demirjian et al12 methods. It is also possible to verify at Tables 5 and 6 that, considering the group of teeth, both in the male and female samples, the canine presented the greatest values, especially in the female sample.
Figure 5.
Overall correlation rank between dental development and skeletal maturation and subgroup analysis according to methodological characteristics: (a) both methods; (b) only Nolla;10 and (c) only Demirjian et al.12
The heterogeneity test reached high values (Q = 51.00) both in the male and female samples, suggesting a potential bias within the publications, which was confirmed by metafunnel analysis (Figure 6).
Figure 6.
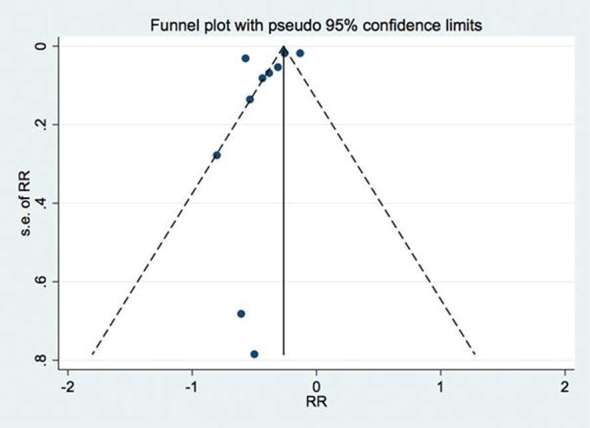
Graph publication bias, indicating the heterogeneity between studies.
Discussion
This systematic review and meta-analysis aimed to evaluate the accuracy of dental calcification stages for estimating the skeletal maturation, especially the pubertal growth spurt, in comparison to skeletal development. Considerable variations exist between individual children in the ages at which they attain similar developmental events and this fact has led to the concept of biologic or physiologic age. The relationship between skeletal maturity and the peak of pubertal growth spurt is well established.26–29 However, for clinicians contemplating the initiation of orthopaedic treatment, it remains questionable whether associations based on dental stages are strong enough to make reliable skeletal maturation prediction.
Dental development has been described in the literature as a valuable alternative.16–18,22–24 Although dental eruption is the most conspicuous and easily determined indicator of dental maturation, it is much more variable in its timing than the calcification sequence in the dentition.10 For this reason, this research was limited to consider only the manuscripts that adopted this form of assessment of dental development. According to Sierra,16 many clinicians traditionally use the adductor sesamoid of the thumb to analyse skeletal maturity, but it is characterized by great variability in the time of onset calcification. Then, using the eight ossification centre method,9 this author16 found a high correlation between calcification of lower canine and skeletal age, ranging from 0.67 to 0.82, being slightly higher for females. In a very similar research, Coutinho et al17 found a quite high association between calcification of lower canine and skeletal maturity indicators, ranging from 0.53 to 0.85.
Considering the stages of dental calcification, assessed according to Demirjian et al,12 some authors state that there are a close relationship between the lower canine developmental stages and the pubertal growth spurt. Coutinho et al17 verified that the beginning of the spurt is indicated by canine stage F, and that the canine stage G occurs approximately 1 year before the peak in boys, and 5 months in girls. The study of Krailassiri et al18 points in the same direction, being reported that the lower canine stage F indicated the onset of a period of accelerating growth. In other research, Guillén et al found that the peak of pubertal growth spurt coincided in 100% with the lower canine stage G.
Although Sağlam and Gazilerli19 have stated that dental stages is not reliable to predict the pubertal growth spurt, and Gupta et al21 and Notaroberto et al25 have found a weak correlation between dental age and skeletal maturation, the results of this meta-analysis showed high correlation between dental development and skeletal age, for both sexes, slightly higher for the females. Despite the high correlation values, caution should be exercised when stating that the dental development can be used to replace the skeletal age obtained from the hand-wrist radiograph. It is possible to verify the potential bias within the manuscripts showed by the metafunnel graph (Figure 6).
Another aspect that should be observed is that it is very difficult to perform a close comparison of the various studies because of the many differences in methodology, especially related to the methods used to assess the skeletal and the dental ages, and this is probably the main reason for some contradictory studies found at the literature. In this systematic review, only observational studies that evaluated the accuracy of dental development stages for estimating the skeletal development in comparison to the hand-wrist maturation analysis were included. Although various methods have been developed to compute bone age score from these radiographs, two methods were selected, namely Greulich and Pyle9 and Fishman.6 These methods have a larger number of scales and use a greater number of ossification centres for classification. According to Sierra,16 this greater number makes it possible to select the centres that exhibit the least variability in timing of the onset of ossification.
The same care was taken in relation to the method used for assessing the dental age. Although the literature reports several possibilities, the stages of tooth formation are considered a more reliable criterion.10–12 In this review, it was possible to verify that the method proposed by Demirjian et al12 was the most used, adopted in six researches,17–19,23–25 followed by the method described by Nolla,10 used in three articles.16,20,22 Another method, developed by Moorrees et al,11 was chosen in just one article.21 This lack of standardization in the choice of the method used for evaluating the dental stages may be one factor responsible for the high heterogeneity found among the studies included in this meta-analysis. Through the stratified analysis of the data, it was possible to verify a slight difference at the male sample between the correlation coefficient values for Nolla10 method in comparison to the Demirjian et al12 method.
Another important point that should be highlighted is the statistical analysis of choice to validate the use of dental development as an indicator of skeletal maturity, since the Pearson and the Spearman correlation coefficients provide exclusively the association between two variables. A high correlation coefficient only shows that there is a strong association between the two measures, but it does not have the capacity to indicate if values obtained by one method are the same as those obtained by another method. Therefore, the development of additional studies with more precise statistical analyses is necessary to verify the reliability of the use of other methods than the skeletal development, such as the dental calcification stages, to determine the skeletal maturation and the biological age of the subject.
Conclusion
Most of theindividual studies suggested a strong correlation between dental development and skeletal maturation, although the association with the peak of pubertal growth spurt was clearly cited only in some of them. However, due to the high heterogeneity found among the studies included in this meta-analysis, a pragmatic recommendation about the use of dental stages is not possible. Further standardized observational studies are needed to increase the strength of evidence and to confirm the accuracy of dental calcification stages for estimating the pubertal growth spurt.
Conflicts of Interest
All authors disclose any financial and personal relationships with other people or organizations that could inappropriately influence this work.
Contributor Information
MarcosAlan Vieira Bittencourt, Email: alan_orto@yahoo.com.br.
GrazielaOro Cericato, Email: graziela.cericato@imed.edu.br.
Ademir Franco, Email: franco.gat@gmail.com.
RafaelaSilva Girão, Email: rafaela_girao89@hotmail.com.
Anderson Paulo Barbosa Lima, Email: dr.andersondentista@hotmail.com.
LuizRenato Paranhos, Email: paranhos.lrp@gmail.com.
REFERENCES
- 1. Grave K. The use of the hand and wrist radiograph in skeletal age assessment; and why skeletal age assessment is important. Aust Orthod J 1994; 13: 96. [PubMed] [Google Scholar]
- 2. Kopecky GR, Fishman LS. Timing of cervical headgear treatment based on skeletal maturation. Am J Orthod Dentofacial Orthop 1993; 104: 162–9. doi: 10.1016/S0889-5406(05)81006-6 [DOI] [PubMed] [Google Scholar]
- 3. Moore RN, Moyer BA, DuBois LM. Skeletal maturation and craniofacial growth. Am J Orthod Dentofacial Orthop 1990; 98: 33–40. doi: 10.1016/0889-5406(90)70029-C [DOI] [PubMed] [Google Scholar]
- 4. Dhiman S, Maheshwari S, Verma SK. Assessment of maturity in orthodontics: a review. Journal of Advanced Clinical & Research Insights 2015; 2: 100–3. doi: https://doi.org/10.15713/ins.jcri.54 [Google Scholar]
- 5. Hassel B, Farman AG. Skeletal maturation evaluation using cervical vertebrae. Am J Orthod Dentofacial Orthop 1995; 107: 58–66. doi: 10.1016/S0889-5406(95)70157-5 [DOI] [PubMed] [Google Scholar]
- 6. Fishman LS. Radiographic evaluation of skeletal maturation. A clinically oriented method based on hand-wrist films. Angle Orthod 1982; 52: 88–112. doi: [DOI] [PubMed] [Google Scholar]
- 7. Fishman LS. Chronological versus skeletal age, an evaluation of craniofacial growth. Angle Orthod 1979; 49: 181–9. doi: [DOI] [PubMed] [Google Scholar]
- 8. Silveira AM, Fishman LS, Subtelny JD, Kassebaum DK. Facial growth during adolescence in early, average and late maturers. Angle Orthod 1992; 62: 185–90. doi: [DOI] [PubMed] [Google Scholar]
- 9. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2 ed California: The British Institute of Radiology.; 1959. 256. [Google Scholar]
- 10. Nolla CM. The development of the permanent teeth. J Dent Child 1960; 27: 254–63. [Google Scholar]
- 11. Moorrees CF, Fanning EA, Hunt EE. Age variation of formation stages for ten permanent teeth. J Dent Res 1963; 42: 1490–502. doi: 10.1177/00220345630420062701 [DOI] [PubMed] [Google Scholar]
- 12. Demirjian A, Goldstein H, Tanner JM. A new system of dental age assessment. Hum Biol 1973; 45: 211–27. [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62: e1–e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 14. Leeflang MM, Deeks JJ, Takwoingi Y, Macaskill P. Cochrane diagnostic test accuracy reviews. Syst Rev 2013; 2: 82: 82. doi: 10.1186/2046-4053-2-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Joana Briggs Institute. Joanna briggs institute reviewers’ manual. 2014 edition Adelaide: The British Institute of Radiology.; 2014. [Google Scholar]
- 16. Sierra AM. Assessment of dental and skeletal maturity. A new approach. Angle Orthod 1987; 57: 194–208. doi: [DOI] [PubMed] [Google Scholar]
- 17. Coutinho S, Buschang PH, Miranda F. Relationships between mandibular canine calcification stages and skeletal maturity. Am J Orthod Dentofacial Orthop 1993; 104: 262–8. doi: 10.1016/S0889-5406(05)81728-7 [DOI] [PubMed] [Google Scholar]
- 18. Krailassiri S, Anuwongnukroh N, Dechkunakorn S. Relationships between dental calcification stages and skeletal maturity indicators in Thai individuals. Angle Orthod 2002; 72: 155–66. doi: [DOI] [PubMed] [Google Scholar]
- 19. Sahin Sağlam AM, Gazilerli U. The relationship between dental and skeletal maturity. J Orofac Orthop 2002; 63: 454–62. doi: 10.1007/s00056-002-0029-1 [DOI] [PubMed] [Google Scholar]
- 20. Lima KTF, Sales RD, Soares EA, Cruz HN, Soares RPF. Comparação entre três métodos para a determinação da maturação esquelética. Odontol Clín Cient 2006; 5: 49–55. [Google Scholar]
- 21. Gupta B, Anegundi R, Sudha P. Comparison of dental age of Hubli Dharwad children by Moore’s method with the skeletal age and chronological age. Internet J Dent Sci 2007; 6: 1–7. [Google Scholar]
- 22. Bala M, Pathak A, Jain RL. Assessment of skeletal age using MP3 and hand-wrist radiographs and its correlation with dental and chronological ages in children. J Indian Soc Pedod Prev Dent 2010; 28: 95–9. doi: 10.4103/0970-4388.66746 [DOI] [PubMed] [Google Scholar]
- 23. Arriola Guillén LE, Peña Carmelo U, Pardo Bancalari M, Guillén LEA, Carmelo UP, Bancalari MP. Concordancia entre estadíos de calcificación dentaria y maduración esquelética en niños y adolescentes de una localidad peruana. Revista Estomatológica Herediana 2011; 21: 131–6. doi: https://doi.org/10.20453/reh.v21i3.215 [Google Scholar]
- 24. Ríos Villasis LK, Soldevilla Galarza L. Relación entre los estadios de maduración esqueletal y calcificación dentaria. Avances en Odontoestomatología 2014; 30: 23–8. doi: 10.4321/S0213-12852014000100003 [DOI] [Google Scholar]
- 25. Notaroberto DFC, Goldner MTA, Martins MM, Mendes AM. Correlação entre as idades cronológica, dentária e esquelética em pacientes submetidos ao tratamento ortodôntico. Orthod Sci Pract 2015; 8: 165–72. [Google Scholar]
- 26. Björk A, Helm S. Prediction of the age of maximum puberal growth in body height. Angle Orthod 1967; 37: 134–43. doi: [DOI] [PubMed] [Google Scholar]
- 27. Grave KC, Brown T. Skeletal ossification and the adolescent growth spurt. Am J Orthod 1976; 69: 611–9. doi: 10.1016/0002-9416(76)90143-3 [DOI] [PubMed] [Google Scholar]
- 28. Hägg U, Taranger J. Skeletal stages of the hand and wrist as indicators of the pubertal growth spurt. Acta Odontol Scand 1980; 38: 187–200. doi: 10.3109/00016358009004719 [DOI] [PubMed] [Google Scholar]
- 29. Houston WJ. Relationships between skeletal maturity estimated from hand-wrist radiographs and the timing of the adolescent growth spurt. Eur J Orthod 1980; 2: 81–93. doi: 10.1093/ejo/2.2.81 [DOI] [PubMed] [Google Scholar]