Abstract
Objectives:
To investigate the correlations among various temporomandibular joint (TMJ) findings on MRI and the relationships between MRI findings and symptoms.
Methods:
425 patients (850 TMJs) with temporomandibular joint disorders (TMDs) who underwent MRI were enrolled. Oblique sagittal proton density-weighted and T 2 weighted images in open- and closed-mouth positions were evaluated. MRI findings included disc configuration, disc position, condylar morphology, bone marrow pattern, and joint effusion. Symptoms included TMJ pain, TMJ noise, and limitation of mouth opening. For statistical analyses, Spearman's rank correlation coefficient and logistic regression analysis were applied.
Results:
Folded disc, disc displacement without reduction (DDWOR), and osteophytes had significant negative correlations with other normal MRI findings (p < 0.01). DDWOR and marrow edema were associated with TMJ pain. Conversely, osteophytes [odds ratio (OR): 0.52; 95% CI (0.30–0.90)] and combination-type condylar degeneration [OR: 0.45; 95% CI (0.24–0.83)] were associated with decreased risk of TMJ pain. Condylar flattening was positively associated with TMJ noise [OR: 5.25; 95% CI (1.44–19.07)] and negatively associated with limitation of mouth opening [OR: 0.34; 95% CI (0.11–0.99)]. High-grade joint effusion was significantly associated with TMJ pain and noise.
Conclusions:
DDWOR and high-grade joint effusion (an indicator of inflammation in the articular cavity) were associated with TMD symptoms. This finding suggests that treatment strategy for DDWOR and decreasing inflammation might lessen clinical TMD symptoms. Condylar degeneration was not associated with indicators of inflammation or TMJ symptoms. These results suggest that patients with TMD symptoms should undergo initial MRI to allow rapid selection of appropriate therapies.
Introduction
Temporomandibular joint disorders (TMDs) present with characteristic clinical signs and symptoms, including temporomandibular joint (TMJ) and facial pain, joint noise, and irregular jaw movement. MRI is widely used to evaluate TMJ characteristics, such as disc configuration, disc position, condylar morphology, bone marrow signal pattern, and the presence of joint effusion. Complicated associations among various MRI findings of the TMJ have been reported.1–7 Deformity of disc configuration is associated with internal derangement of the TMJ1–3 and condylar degenerative change.1 Internal derangement is associated with condylar degenerative change1, 4,5 and joint effusion.6, 7 However, these associations have involved only two or three MRI findings. A comprehensive study that includes more MRI findings and more detailed classification is necessary. An analysis of correlations among various changes in TMJ structures may reveal key abnormalities that are important for clinical treatment.
Relationships between the clinical signs and symptoms of TMD and MRI findings have also been reported.8–15 TMJ pain, the most common clinical symptom, correlates with internal derangement,7–10 bone marrow abnormality,9,11–13 condylar degenerative change,9, 10,14,15 and joint effusion.7, 9,10,13 Bone marrow edema has been associated with increased pain in TMDs.13 However, few reports have described the relationships between joint noise or irregular jaw movements and MRI findings. An evaluation of the relationships between TMD symptoms and MRI findings with detailed classifications may reveal which symptoms warrant MRI examination, thus reducing unnecessary radiographic examinations prior to MRI.
The aim of this this study was to comprehensively assess (1) the correlations among various MRI findings, including disc configuration, disc position, condylar morphology, bone marrow signal pattern, and joint effusion, and (2) the relationships between symptoms of TMD and MRI findings in a relatively large number of patients.
Methods and materials
Patients
The study included 850 TMJs in 425 participants (97 males and 328 females) with TMDs who underwent MRI at the Department of Occlusal and Oral Functional Rehabilitation at our university hospital from August 2011 to July 2014. Clinical diagnosis was made according to the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD).16 Patients with a history of TMJ trauma or systemic disease were excluded.
Clinical assessment included patient-reported functional pain in the TMJ region (TMJ pain), TMJ noise, and limitation of mouth opening. TMJ pain was defined as pain with jaw movement or during mastication. TMJ noise was defined as any joint noise during jaw opening or closing. Limitation of mouth opening was defined as jaw opening limitation severe enough to interfere with the ability to eat.
MRI
MRI was performed with head coils on one of four machines: 1.5 T Magnetom Vision (Siemens Healthineers, Erlange, Germany), 1.5 T Achieva (Philips Healthcare, Best, Netherlands), 3.0 T Magnetom Skyra (Siemens Healthineers, Erlange, Germany), 3.0 T Magnetom Verio (Siemens Healthineers, Erlange, Germany) (Table 1). The use of different machines for scanning did not affect the results of the study. Sagittal plane imaging parallel to the long axis of the mandibular ramus was carried out using proton density-weighted and T 2 weighted sequences in the open-mouth and closed-mouth positions. Multiple radiologists certified by the Society for Oral and Maxillofacial Radiology of our country evaluated the MRIs. All diagnoses were confirmed by at least two radiologists.
Table 1.
MRI parameters and coils
| TR/TE/TE a | FOV (cm) | Thickness (mm) | Matrix | NEX | Coil | |
| 1.5 T Magnetom Vision | 2000/14/85 | 6–13 | 3 | 160 × 512 | 2 | CP Head coil |
| 1.5 T Achieva | 1500/16/100 | 15–15 | 3 | 145 × 192 | 3 | Flex-M coil |
| 3.0 T Magnetom Skyra | 2940/22/98 | 9–12 | 3 | 110 × 192 | 2 | 20-channel Head coil |
| 3.0 T Magnetom Verio | 2940/22/98 | 9–12 | 3 | 108 × 192 | 2 | 20-channel Head coil |
TR/TE/TE, TR of both proton density-weighted and T 2 weighted sequences/ TE of proton density-weighted sequences/ TE of T 2 weighted sequences.
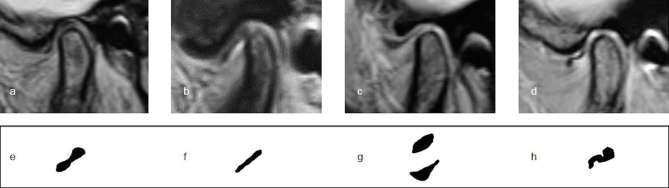
Disc configuration was classified according to Arayasantiparb et al2 as biconcave (“bow tie” shape with thick anterior and posterior bands and a narrow intermediate zone), flattened (anterior band, intermediate zone, and posterior band of the same thickness), convex (roundish disc shape with convex upper or lower surface), or folded (disc with any folded portion). All disc configurations were evaluated in the closed-mouth position (Figure 1).
Figure 1.
Types of disc configurations on proton density-weighted images. (a, e) Biconcave disc. (b, f) Flattened disc. (c, g) Concave disc. (d, h) Folded disc.
Internal derangements were classified according to Koh et al4 as normal (posterior band located above the apex of the condylar head in the closed-mouth position and thin intermediate zone located between the condyle and the articular eminence in the open-mouth position), disc displacement with reduction (DDWR; posterior band located anterior to the condylar head in the closed-mouth position, but with a normal disc–condyle relationship in the open-mouth position), or disc displacement without reduction (DDWOR; posterior band positioned anterior to the condyle in both the closed-mouth and open-mouth positions).
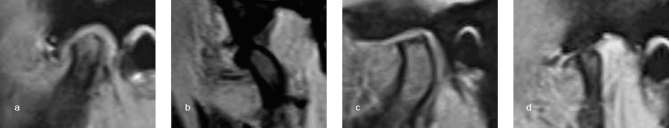
Degenerative condylar morphology was classified according to Roh et al6 as erosion (interruption or absence of cortical lining), sclerosis (dense and thickened variations of subchondral bone shown as thickening of cortical bone on proton density-weighted images), flattening (loss of round contour), osteophytes (marginal hypertrophic bone formation), or combination type (combinations of the previous morphologies) (Figure 2).
Figure 2.
Proton density-weighted images show condylar degenerative morphologies. (a) Erosion. (b) Sclerosis. (c) Flattening. (d) Osteophytes.
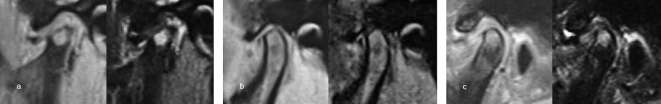
Condylar bone marrow signal pattern was classified according to Larheim et al17 as normal (homogeneous bright signal on proton density-weighted images and homogeneous intermediate signal on T 2 weighted images), marrow edema (increased signal on T 2 weighted images), marrow sclerosis (decreased signal on proton density-weighted and T 2 weighted images), or combined edema and sclerosis (Figure 3).
Figure 3.
Proton density-weighted images (left) and T 2 weighted images (right) show condylar bone marrow abnormalities. (a) Bone marrow edema. (b) Bone marrow sclerosis. (c) Bone marrow edema with sclerosis.
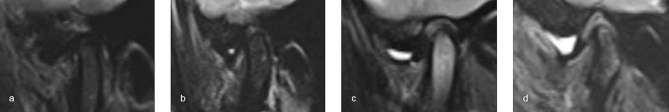
Joint effusions were classified into four degrees based on previous reports6, 17 as follows: Grade 0, no bright T 2 signal intensity in joint space; Grade 1, dots or lines of bright T 2 signal intensity along articular surface; Grade 2, bands of bright T 2 signal intensity; and Grade 3, collection with pooling of bright T 2 signal intensity in joint space (Figure 4).
Figure 4.
T 2 weighted images show joint effusions. (a) Grade 0. (b) Grade 1. (c) Grade 2. (d) Grade 3.
Data analysis
Descriptive analyses of central tendencies were reported as mean, median, and percentile. Inferential statistical analyses were evaluated with Spearman’s rank correlation coefficient and logistic regression analysis. All analyses were carried out with IBM SPSS Statistics Base 24.0 (Tokyo, Japan).
Results
The ratio of females to males was 3.38:1. The median patient age was 49 years (25th percentile: 29 years; 75th percentile: 63 years; range: 12–86 years). MRI findings are shown in Table 2. DDWR was seen in 42.8% of participants with internal derangement. The patterns of disc configuration, condylar morphology, bone marrow signal, and joint effusion were predominantly normal. TMJ pain and noise occurred in 44.1% (375/850 TMJs) and 45.8% (389/850 TMJs), respectively. Limitation of mouth opening was present 51.8% of participants (220/425 patients).
Table 2.
Characteristics of TMJs (n = 850)
| No. | % | ||
| Disc configuration | Biconcave | 257 | 30.2 |
| Flattened | 191 | 22.5 | |
| Convex | 89 | 10.5 | |
| Folded | 313 | 36.8 | |
| Internal derangement | Normal | 245 | 28.8 |
| DDWR | 364 | 42.8 | |
| DDWOR | 241 | 28.4 | |
| Condylar morphology | Normal | 584 | 68.7 |
| Flattening | 67 | 7.9 | |
| Erosion | 28 | 3.3 | |
| Sclerosis | 17 | 2.0 | |
| Osteophyte | 86 | 10.1 | |
| Combination | 68 | 8.0 | |
| Condylar bone marrow signal pattern | Normal | 726 | 85.4 |
| Edema | 67 | 7.9 | |
| Sclerosis | 12 | 1.4 | |
| Edema with sclerosis | 45 | 5.3 | |
| Joint effusion | Grade 0 | 371 | 43.6 |
| Grade 1 | 285 | 33.5 | |
| Grade 2 | 124 | 14.6 | |
| Grade 3 | 70 | 8.2 | |
TMJ, temporomandibular joint.
The correlations among MRI findings according to Spearman’s rank correlation coefficients are shown in Table 3. There was a strong positive correlation between biconcave disc and normal disc position (r = 0.706, p < 0.01). Folded disc had a moderate positive correlation with DDWOR (r = 0.467, p < 0.01), whereas flattened disc had a weak positive correlation with DDWR (r = 0.320, p < 0.01).
Table 3.
Correlations among various MRI findings in temporomandibular disorder, according to Spearman’s rank correlation coefficient (n = 850)
| Biconcave | Flattened | Convex | Folded | Normal ID | DDWR | DDWOR | Normal CM | Erosion | Sclerosis | Flattening | Osteophytes | Combination | Normal BM | BM edema | BM sclerosis | Edema with sclerosis | ||
| Normal ID | 0.706 | −.137 | −.209 | −.421 | ||||||||||||||
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | ||||||||||||||
| DDWR | −.352 | 0.320 | 0.154 | −0.040 | ||||||||||||||
| p-value | 0.00 | 0.00 | 0.00 | 0.25 | ||||||||||||||
| DDWOR | −.323 | −.214 | 0.041 | 0.467 | ||||||||||||||
| p-value | 0.00 | 0.00 | 0.24 | 0.00 | ||||||||||||||
| Normal CM | 0.190 | 0.072 | −.076 | −.195 | 0.250 | 0.112 | −.375 | |||||||||||
| p-value | 0.00 | 0.04 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||||
| Erosion | −.128 | 0.009 | −0.009 | 0.119 | −.140 | −0.045 | 0.191 | |||||||||||
| p-value | 0.00 | 0.79 | 0.80 | 0.00 | 0.00 | 0.19 | 0.00 | |||||||||||
| Sclerosis | −0.008 | −0.027 | 0.100 | −0.032 | −0.034 | −0.010 | 0.045 | |||||||||||
| p-value | 0.81 | 0.43 | 0.00 | 0.35 | 0.32 | 0.77 | 0.19 | |||||||||||
| Flattening | −.136 | −0.042 | 0.103 | 0.101 | −.133 | −0.026 | 0.162 | |||||||||||
| p-value | 0.00 | 0.22 | 0.00 | 0.00 | 0.00 | 0.45 | 0.00 | |||||||||||
| Osteophytes | −.123 | −.105 | 0.012 | 0.201 | −.201 | −.196 | 0.418 | |||||||||||
| p-value | 0.00 | 0.00 | 0.73 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||||
| Combination | −.111 | −0.067 | 0.067 | 0.121 | −.123 | −.127 | .262 | |||||||||||
| p-value | 0.00 | 0.05 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||||
| Normal BM | 0.156 | 0.071 | 0.054 | −.244 | 0.160 | 0.189 | −.369 | 0.390 | −.277 | −.086 | −.185 | −.351 | −.292 | |||||
| p-value | 0.00 | 0.04 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | |||||
| BM edema | −.107 | −0.053 | −0.043 | 0.175 | −.109 | −.156 | 0.281 | −.311 | 0.217 | 0.052 | .168 | .232 | .217 | |||||
| p-value | 0.00 | 0.12 | 0.21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | |||||
| BM sclerosis | −0.035 | 0.031 | −0.008 | 0.012 | −.076 | 0.017 | 0.057 | −.070 | 0.080 | 0.097 | −0.049 | 0.027 | 0.001 | |||||
| p-value | 0.30 | 0.36 | 0.81 | 0.73 | 0.03 | 0.61 | 0.09 | 0.04 | 0.02 | 0.01 | 0.16 | 0.44 | 0.98 | |||||
| Edema with sclerosis | −.098 | −0.064 | −0.029 | 0.168 | −.081 | −.120 | 0.213 | −.203 | 0.134 | 0.021 | .114 | .260 | .199 | |||||
| p-value | 0.00 | 0.06 | 0.39 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.54 | 0.00 | 0.00 | 0.00 | |||||
| JE Grade 0 | .206 | 0.168 | 0.017 | −.352 | 0.257 | 0.120 | −.390 | 0.185 | −.113 | −0.041 | −.101 | −.178 | −.149 | .196 | −.134 | 0.015 | −.155 | |
| p-value | 0.00 | 0.00 | 0.63 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.23 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.66 | 0.00 | |
| JE Grade 1 | −0.055 | −0.024 | 0.001 | 0.073 | −.078 | −0.010 | .090 | −0.037 | 0.027 | −0.020 | 0.039 | 0.056 | .072 | −0.031 | 0.033 | −0.022 | 0.021 | |
| p-value | 0.11 | 0.48 | 0.97 | 0.03 | 0.02 | 0.76 | 0.01 | 0.29 | 0.43 | 0.55 | 0.26 | 0.10 | 0.04 | 0.36 | 0.34 | 0.53 | 0.54 | |
| JE Grade 2 | −.091 | −.143 | 0.011 | 0.203 | −.138 | −0.055 | 0.198 | −.124 | 0.074 | 0.086 | 0.061 | 0.065 | 0.060 | −.150 | .089 | 0.007 | .126 | |
| p-value | 0.01 | 0.00 | 0.75 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.03 | 0.01 | 0.08 | 0.06 | 0.08 | 0.00 | 0.01 | 0.84 | 0.00 | |
| JE Grade 3 | −.160 | −.079 | −0.047 | 0.250 | −.153 | −.130 | 0.296 | −.112 | 0.062 | −0.001 | 0.037 | .141 | .068 | −.107 | .071 | 0.000 | .082 | |
| p-value | 0.00 | 0.02 | 0.18 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.97 | 0.28 | 0.00 | 0.05 | 0.00 | 0.04 | 0.99 | 0.02 | |
BM, bone marrow; CM, condylar morphology; DDWOR, disc displacement without reduction; DDWR, disc displacement with reduction; ID, internal derangement; JE, joint effusion.
DDWOR had weak negative correlations with normal disc configuration (r = −0.323, p < 0.01), condylar morphology (r = −0.375, p < 0.01), bone marrow signal pattern (r = −0.369, p < 0.01), and joint effusion (r = −0.390, p < 0.01) and had moderate positive correlations with osteophytes (r = 0.418, p < 0.01), marrow edema with and without sclerosis (r = 0.213, p < 0.01 and r = 0.281, p < 0.01, respectively), and Grade 3 joint effusion (r = 0.296, p < 0.01). Folded disc configuration had negative correlations with normal disc position (r = −0.421, p < 0.01), bone marrow signal pattern (r = −0.244, p < 0.01), and joint effusion (r = −0.352, p < 0.01). Osteophytes had positive correlations with DDWOR (r = −0.418, p < 0.01) and with marrow edema with and without sclerosis (r = 0.260, p < 0.01 and r = 0.232, p < 0.01, respectively).
Table 4 shows the odds ratios (ORs) and 95% confidence intervals (CIs) for MRI findings according to TMD symptom. TMJ pain was significantly associated with DDWOR [OR: 2.95; 95% (CI 1.85–4.68)], marrow edema [OR: 3.13; 95% CI (1.66–5.90)], Grade 2 joint effusion [OR: 1.80; 95% CI (1.13–2.87)], and Grade 3 effusion [OR: 2.46; 95% CI (1.33–4.55)]. However, osteophytes [OR: 0.52; 95% CI (0.30–0.90)] and combination-type condylar degeneration [OR: 0.45; 95% CI (0.24–0.83)] were negatively associated with TMJ pain. TMJ noise was significantly associated with DDWOR [OR: 1.95; 95% CI (1.24–3.06)], flattening of the condylar surface [OR: 5.25; 95% CI (1.44–19.07)], Grade 2 joint effusion [OR: 2.59; 95% CI (1.64–4.11)], and Grade 3 joint effusion [OR: 3.79; 95% CI (2.03–7.05)]. Limitation of mouth opening was positively associated with DDWR [OR: 1.91; 95% CI (1.36–2.67)] and DDWOR [OR: 1.97; 95% CI (1.27–3.17)] and was negatively associated with flattening of the condylar surface [OR: 0.34; 95% CI (0.11–0.99)].
Table 4.
Odds ratios for MRI findings according to temporomandibular joint symptom (n = 850)
| Pain | Noise | Limitation | ||||||||
| OR | (95% Cl) | p-value | OR | (95% Cl) | p-value | OR | (95% Cl) | p-value | ||
| Internal derangement | Normal | 1.00 | 1.00 | 1.00 | ||||||
| DDWR | 0.87 | (0.62–1.24) | 0.45 | 1.10 | (0.78–1.56) | 0.58 | 1.91 | (1.36–2.67) | 0.00 | |
| DDWOR | 2.95 | (1.85–4.68) | 0.00 | 1.95 | (1.24–3.06) | 0.00 | 1.97 | (1.27–3.07) | 0.00 | |
| Condylar morphology | Normal | 1.00 | 1.00 | 1.00 | ||||||
| Erosion | 0.59 | (0.33–1.06) | 0.08 | 1.07 | (0.62–1.83) | 0.81 | 1.14 | (0.68–1.92) | 0.62 | |
| Sclerosis | 0.42 | (0.17–1.05) | 0.06 | 0.93 | (0.40–2.16) | 0.86 | 1.61 | (0.70–3.74) | 0.26 | |
| Flattening | 1.46 | (0.51–4.17) | 0.48 | 5.25 | (1.44–19.07) | 0.01 | 0.34 | (0.11–0.99) | 0.05 | |
| Osteophytes | 0.52 | (0.30–0.90) | 0.02 | 0.91 | (0.54–1.54) | 0.74 | 1.11 | (0.67–1.83) | 0.69 | |
| Combination | 0.45 | (0.24–0.83) | 0.01 | 0.65 | (0.36–1.16) | 0.15 | 0.93 | (0.53–1.63) | 0.80 | |
| Bone marrow signal pattern | Normal | 1.00 | 1.00 | 1.00 | ||||||
| Edema | 3.13 | (1.66–5.90) | 0.00 | 1.67 | (0.92–3.05) | 0.09 | 1.06 | (0.60–1.87) | 0.84 | |
| Sclerosis | 3.13 | (0.81–12.17) | 0.10 | 1.44 | (0.41–5.03) | 0.57 | 1.05 | (0.31–3.50) | 0.94 | |
| Edema with sclerosis | 1.94 | (0.97–3.90) | 0.06 | 1.03 | (0.53–2.02) | 0.93 | 0.93 | (0.48–1.78) | 0.82 | |
| Joint effusion | Grade 0 | 1.00 | 1.00 | 1.00 | ||||||
| Grade 1 | 1.11 | (0.79–1.57) | 0.55 | 1.23 | (0.88–1.72) | 0.22 | 1.13 | (0.81–1.56) | 0.47 | |
| Grade 2 | 1.80 | (1.13–2.87) | 0.01 | 2.59 | (1.64–4.11) | 0.00 | 1.49 | (0.95–2.33) | 0.09 | |
| Grade 3 | 2.46 | (1.33–4.55) | 0.00 | 3.79 | (2.03–7.05) | 0.00 | 1.13 | (0.64–1.99) | 0.68 | |
Cl, confidence interval; DDWOR, anterior disc displacement without reduction; DDWR, anterior disc displacement with reduction; OR, odds ratio.
Adjusted for internal derangement, condylar morphology, bone marrow signal pattern and joint effusion.
Pain (Hosmer and Lemeshow test; p = 0.873); noise (Hosmer and Lemeshow test; p = 0.513); limitaion (Hosmer and Lemeshow test; p = 0.644).
Discussion
Correlations among MRI findings in the TMJ were comprehensively analyzed in a large number of patients in this study. Biconcave disc configuration correlated strongly with normal disc position, as previously reported.1 Positive correlations were found between DDWOR and folded disc and between DDWR and flattened disc. Given the hypothesis that internal derangement precedes disk deformation,18 DDWOR and DDWR appear to be responsible for folded and flattened deformation, respectively.
DDWOR had a moderate positive correlation with osteophytes and folded disc, and negatively correlated with other normal MRI findings. In contrast, there were no significant correlations between DDWR and abnormalities of the condylar surface, bone marrow signal pattern, or joint effusion. Moreover, the risk of each TMD symptom was significantly higher in joints with DDWOR. Conversely, the ORs for TMJ pain and noise were not higher in joints with DDWR. Both DDWR and DDWOR have been reported to increase the risks of condylar degenerative change.5, 6 The different findings for DDWR in this study are interesting. It appears that when the disc intervenes between the temporal fossa and the condylar surface in the open-mouth position, the damage during jaw function is minimized. Retaining DDWR status seems to be beneficial in maintaining a normal condylar surface, bone marrow, and joint effusion. These findings suggest that treatment strategy for DDWOR to reduce strain and/or injury to the TMJ may effectively lessen the clinical symptoms of TMDs.
Folded disc correlated with abnormalities of disc position, condylar morphology, and bone marrow signal pattern. Osteophytes correlated with folded disc, DDWOR, and bone marrow edema with and without sclerosis. High-grade (Grades 2 and 3) joint effusions, which reflect the inflammatory reaction in the articular cavity,19, 20 correlated with folded disc and DDWOR. Based on these observations, DDWOR, folded disc, and osteophytes appear to be the key MRI abnormalities in patients with TMDs. However, we found no significant correlation between condylar degeneration and joint effusion. This finding suggests that condylar degeneration is not related to inflammatory conditions in the articular cavity.
Patients with TMJ pain had higher ORs for DDWOR, bone marrow edema, and high-grade joint effusion. These findings suggest that abnormalities in the articular cavity and condylar bone marrow cause TMJ pain. Moreover, in contrast with previous studies,9, 14,15 osteophytes and combination-type condylar degeneration were associated with a decreased risk of TMJ pain. Isberg described changes in the soft tissues during the chronic progression of condylar degeneration, with soft-tissue proliferation allowing the contours to adapt morphologically to various mechanical stresses.21 In the late stages of condylar degeneration, osteophytes and combination-type degeneration seem to lessen TMJ pain because of the compensatory changes in the surrounding soft tissues.
In a small study involving autopsy specimens, Widmalm et al22 reported that clicking occurred in TMJs with DDWR, DDWOR, and arthrosis, while crepitation occurred only in joints with arthrosis and perforation. Our observations are similar to those findings, except that we only found significant associations with DDWOR and condylar flattening. Flattening change (disappearance of round contour) may lead to friction between the mandibular fossa and the condyle, causing crepitus. Significant increases in the risk of TMJ pain and noise occurred with Grade 2 and Grade 3 joint effusion. It appears that joint effusion, as an indicator of inflammation in the articular cavity, correlates with joint symptoms.
We found a significant relationship between limitation of mouth opening and both DDWR and DDWOR. Therefore, we infer that internal derangements mainly affect mandibular movements. In contrast, patients with condylar flattening tended not to have limitation of mouth opening.
Conclusion
In this study, condylar degeneration did not strongly affect TMD symptoms. However, DDWOR, bone marrow abnormalities, and high-grade joint effusion, which can only be observed with MRI examination, were risk factors for TMD symptoms. Panoramic radiography, tomography, and CBCT/CT, which mainly reveal condylar morphology, may not be very useful for evaluating the TMJ in most patients with TMDs. Initial MRI examination to evaluate the articular cavity should be considered in patients with TMD symptoms, allowing clinicians to choose appropriate therapies (medical therapy, physical therapy, or splinting) and reducing redundant medical radiation exposure and costs.
Contributor Information
Risa Matsubara, Email: de16012@s.okayama-u.ac.jp.
Yoshinobu Yanagi, Email: ya7@okayama-u.ac.jp.
Kazuhiro Oki, Email: kazu_z@md.okayama-u.ac.jp.
Miki Hisatomi, Email: tomi@md.okayama-u.ac.jp.
Babatunde O Bamgbose, Email: drtundebamgbose@yahoo.com.
Mariko Fujita, Email: gd421082@s.okayama-u.ac.jp.
Shunsuke Okada, Email: okadashunsuke@s.okayama-u.ac.jp.
Shogo Minagi, Email: minagi@md.okayama-u.ac.jp.
Junichi Asaumi, Email: asaumi@md.okayama-u.ac.jp.
REFERENCES
- 1. Westesson PL, Rohlin M. Internal derangement related to osteoarthrosis in temporomandibular joint autopsy specimens. Oral Surg Oral Med Oral Pathol 1984; 57: 17–22. doi: 10.1016/0030-4220(84)90251-2 [DOI] [PubMed] [Google Scholar]
- 2. Arayasantiparb R, Tsuchimochi M, Mitrirattanakul S. Transformation of temporomandibular joint disc configuration in internal derangement patients using magnetic resonance imaging. Oral Science International 2012; 9: 43–8. doi: 10.1016/S1348-8643(12)00025-0 [DOI] [Google Scholar]
- 3. Vogl TJ, Lauer HC, Lehnert T, Naguib NN, Ottl P, Filmann N, et al. . The value of MRI in patients with temporomandibular joint dysfunction: correlation of MRI and clinical findings. Eur J Radiol 2016; 85: 714–9. doi: 10.1016/j.ejrad.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 4. Koh KJ, Park HN, Kim KA. Relationship between anterior disc displacement with/without reduction and effusion in temporomandibular disorder patients using magnetic resonance imaging. Imaging Sci Dent 2013; 43: 245–51. doi: 10.5624/isd.2013.43.4.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dias IM, Cordeiro PC, Devito KL, Tavares ML, Leite IC, Tesch RS. Evaluation of temporomandibular joint disc displacement as a risk factor for osteoarthrosis. Int J Oral Maxillofac Surg 2016; 45: 313–7. doi: 10.1016/j.ijom.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 6. Roh HS, Kim W, Kim YK, Lee JY. Relationships between disk displacement, joint effusion, and degenerative changes of the TMJ in TMD patients based on MRI findings. J Craniomaxillofac Surg 2012; 40: 283–6. doi: 10.1016/j.jcms.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 7. Rudisch A, Innerhofer K, Bertram S, Emshoff R. Magnetic resonance imaging findings of internal derangement and effusion in patients with unilateral temporomandibular joint pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 92: 566–71. doi: 10.1067/moe.2001.116817 [DOI] [PubMed] [Google Scholar]
- 8. Emshoff R, Innerhofer K, Rudisch A, Bertram S. Clinical versus magnetic resonance imaging findings with internal derangement of the temporomandibular joint: an evaluation of anterior disc displacement without reduction. J Oral Maxillofac Surg 2002; 60: 36–41. doi: 10.1053/joms.2002.29071 [DOI] [PubMed] [Google Scholar]
- 9. Emshoff R, Brandlmaier I, Bertram S, Rudisch A. Relative odds of temporomandibular joint pain as a function of magnetic resonance imaging findings of internal derangement, osteoarthrosis, effusion, and bone marrow edema. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 95: 437–45. doi: 10.1067/moe.2003.95 [DOI] [PubMed] [Google Scholar]
- 10. Emshoff R, Brandlmaier I, Bertram S, Rudisch A. Risk factors for temporomandibular joint pain in patients with disc displacement without reduction - a magnetic resonance imaging study. J Oral Rehabil 2003; 30: 537–43. doi: 10.1046/j.1365-2842.2003.01111.x [DOI] [PubMed] [Google Scholar]
- 11. Sano T, Westesson PL, Larheim TA, Takagi R. The association of temporomandibular joint pain with abnormal bone marrow in the mandibular condyle. J Oral Maxillofac Surg 2000; 58: 254–7. doi: 10.1016/S0278-2391(00)90141-1 [DOI] [PubMed] [Google Scholar]
- 12. Sano T, Westesson PL, Yamamoto M, Okano T. Differences in temporomandibular joint pain and age distribution between marrow edema and osteonecrosis in the mandibular condyle. Cranio 2004; 22: 283–8. doi: 10.1179/crn.2004.035 [DOI] [PubMed] [Google Scholar]
- 13. Larheim TA, Westesson PL, Sano T. MR grading of temporomandibular joint fluid: association with disk displacement categories, condyle marrow abnormalities and pain. Int J Oral Maxillofac Surg 2001; 30: 104–12. doi: 10.1054/ijom.2000.0017 [DOI] [PubMed] [Google Scholar]
- 14. Kurita H, Kojima Y, Nakatsuka A, Koike T, Kobayashi H, Kurashina K. Relationship between temporomandibular joint (TMJ)-related pain and morphological changes of the TMJ condyle in patients with temporomandibular disorders. Dentomaxillofac Radiol 2004; 33: 329–33. doi: 10.1259/dmfr/13269559 [DOI] [PubMed] [Google Scholar]
- 15. Yajima A, Sano T, Otonari-Yamamoto M, Otonari T, Ohkubo M, Harada T, et al. . MR evidence of characteristics in symptomatic osteoarthritis of the temporomandibular joint: increased signal intensity ratio on proton density-weighted images of bone marrow in the mandibular condyle. Cranio 2007; 25: 250–6. doi: 10.1179/crn.2007.038 [DOI] [PubMed] [Google Scholar]
- 16. Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 1992; 6: 301–55. [PubMed] [Google Scholar]
- 17. Larheim TA, Katzberg RW, Westesson PL, Tallents RH, Moss ME. MR evidence of temporomandibular joint fluid and condyle marrow alterations: occurrence in asymptomatic volunteers and symptomatic patients. Int J Oral Maxillofac Surg 2001; 30: 113–7. doi: 10.1054/ijom.2000.0018 [DOI] [PubMed] [Google Scholar]
- 18. Westesson PL, Bronstein SL, Liedberg J. Internal derangement of the temporomandibular joint: morphologic description with correlation to joint function. Oral Surg Oral Med Oral Pathol 1985; 59: 323–31. doi: 10.1016/0030-4220(85)90051-9 [DOI] [PubMed] [Google Scholar]
- 19. Takahashi T, Nagai H, Seki H, Fukuda M. Relationship between joint effusion, joint pain, and protein levels in joint lavage fluid of patients with internal derangement and osteoarthritis of the temporomandibular joint. J Oral Maxillofac Surg 1999; 57: 1187–93. doi: 10.1016/S0278-2391(99)90483-4 [DOI] [PubMed] [Google Scholar]
- 20. Kaneyama K, Segami N, Yoshimura H, Honjo M, Demura N. Increased levels of soluble cytokine receptors in the synovial fluid of temporomandibular joint disorders in relation to joint effusion on magnetic resonance images. J Oral Maxillofac Surg 2010; 68: 1088–93. doi: 10.1016/j.joms.2009.10.027 [DOI] [PubMed] [Google Scholar]
- 21. Isberg A. Temporomandibular joint dysfunction: a practitioner’s guide. Oxford: The British Institute of Radiology.; 2001. 103–8. [Google Scholar]
- 22. Widmalm SE, Westesson PL, Brooks SL, Hatala MP, Paesani D. Temporomandibular joint sounds: correlation to joint structure in fresh autopsy specimens. Am J Orthod Dentofacial Orthop 1992; 101: 60–9. doi: 10.1016/0889-5406(92)70083-M [DOI] [PubMed] [Google Scholar]