Abstract
BACKGROUND
Since the publication of the 2006 American College of Chest Physicians (CHEST) cough guidelines, a variety of tools has been developed or further refined for assessing cough. The purpose of the present committee was to evaluate instruments used by investigators performing clinical research on chronic cough. The specific aims were to (1) assess the performance of tools designed to measure cough frequency, severity, and impact in adults, adolescents, and children with chronic cough and (2) make recommendations or suggestions related to these findings.
METHODS
By following the CHEST methodologic guidelines, the CHEST Expert Cough Panel based its recommendations and suggestions on a recently published comparative effectiveness review commissioned by the US Agency for Healthcare Research and Quality, a corresponding summary published in CHEST, and an updated systematic review through November 2013. Recommendations or suggestions based on these data were discussed, graded, and voted on during a meeting of the Expert Cough Panel.
RESULTS
We recommend for adults, adolescents (≥ 14 years of age), and children complaining of chronic cough that validated and reliable health-related quality-of-life (QoL) questionnaires be used as the measurement of choice to assess the impact of cough, such as the Leicester Cough Questionnaire and the Cough-Specific Quality-of-Life Questionnaire in adult and adolescent patients and the Parent Cough-Specific Quality of Life Questionnaire in children. We recommend acoustic cough counting to assess cough frequency but not cough severity. Limited data exist regarding the performance of visual analog scales, numeric rating scales, and tussigenic challenges.
CONCLUSIONS
Validated and reliable cough-specific health-related QoL questionnaires are recommended as the measurement of choice to assess the impact of cough on patients. How they compare is yet to be determined. When used, the reporting of cough severity by visual analog or numeric rating scales should be standardized. Previously validated QoL questionnaires or other cough assessments should not be modified unless the new version has been shown to be reliable and valid. Finally, in research settings, tussigenic challenges play a role in understanding mechanisms of cough.
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; CB, consensus-based; CER, comparative effectiveness review; CHEST, American College of Chest Physicians; COI, conflict of interest; CQLQ, Cough-Specific Quality-of-Life Questionnaire; LCQ, Leicester Cough Questionnaire; PCQ, Pediatric Cough Questionnaire; PC-QOL, Parent Cough-Specific Quality of Life Questionnaire; PICOTS, population of interest, interventions, comparators, outcomes, timing of outcomes, and settings; QoL, quality of life; VAS, visual analog scale
Summary of Recommendations
-
1
In adult and adolescent patients (≥ 14 years of age) complaining of chronic cough, we recommend that validated and reliable health-related quality of life (QoL) questionnaires be used as the measurement of choice to assess the impact of cough on patients (Grade 1B).
-
2
In adults and adolescents with chronic cough, we recommend the Cough-Specific Quality-of-Life Questionnaire and Leicester Cough Questionnaire, as they are the most extensively studied and commonly used previously validated and reliable cough-specific health-related QoL questionnaires to assess the impact of cough (Grade 1B).
-
3
In children (< 14 years of age) with chronic cough, we recommend that validated and reliable health-related QoL questionnaires be used as the measurement of choice to assess the impact of cough (Grade 1B).
-
4
In children (< 14 years of age) with chronic cough, we recommend the Parent Cough-Specific Quality of Life Questionnaire, the most extensively studied and commonly used previously validated and reliable health-related QoL questionnaire, as the measurement of choice to assess the impact of cough (Grade 1B).
-
5
To standardize the development, utilization, and reporting of cough-specific QoL questionnaires, we suggest that cough counting alone not be used to establish validity of the questionnaires (consensus based [CB]).
-
6
To standardize the development, use, and reporting of cough severity by visual analog scales (VASs) or numeric rating scales, we suggest that they be used in standard fashion (CB).
-
7
To ensure the integrity of health-related QoL questionnaires and other patient-reported outcomes that have been shown to be valid and reliable, we suggest that a modified version should not be used and reported unless the modified version has been shown to be reliable and valid (CB).
-
8
In adult and adolescent patients with cough of any duration, we suggest that tussigenic challenges have a role in research settings to understand mechanisms of cough (CB).
-
9
In patients of all ages, we recommend acoustic cough counting to assess cough frequency but not cough severity (Grade 1B).
Cough, particularly chronic cough, is a common symptom. 1 Although the possible causes of this symptom are numerous, assessment of its etiologic factors should follow a systematic approach, as stated in previous guidelines.2, 3, 4 Furthermore, it is recognized that the assessment of antitussive medications should follow specific rules and use valid instruments. 5 Research outcomes often measured in studies of cough include one or more of the following concepts: cough severity, cough impact on quality of life (QoL), cough frequency, or cough sensitivity. Most often, measures of frequency and severity of cough and cough impact on QoL have not been based on the use of standardized or valid measures. Therefore, more-precise assessments could help to determine the actual impact of cough on patients and allow for valid evaluation of outcomes, providing reliable measurement of the effect of antitussive therapies.
In this regard, the American College of Chest Physicians (CHEST) Expert Cough Panel initially reviewed the 2006 cough guidelines on this topic to develop the current updated recommendations and suggestions. 2 In the former guideline, recommendations stressed the need for optimally evaluating chronic cough and the efficacy of cough-modifying agents by using both subject self-reporting and objective methods because they have the potential to measure different aspects of the problem. It was proposed to use a valid and reliable cough-specific health-related QoL instrument as well as visual analog scales (VASs). At the time of the 2006 publication, 6 some health-related QoL instruments had been psychometrically tested but VASs had not; thus, the cough-specific health-related QoL instruments were recommended as the primary subjective outcome measure. Regarding objective methods, tussigenic challenges were recommended before and after interventions to assess the effect of therapy on cough sensitivity only in disease states in which cough reflex sensitivity was known to be heightened. 6
Because a large number of studies and analyses have been published on cough assessment in the past decade, the CHEST Expert Cough Panel believed it necessary to review the current status of the previous recommendations and assess the need to develop new ones to advance the field in this area. The purpose of the present committee was to evaluate instruments used by investigators in clinical research on chronic cough. The specific aims were to (1) assess the performance of tools designed to measure cough frequency, severity, and impact in adults, adolescents, and children with chronic cough and (2) make recommendations or suggestions related to these findings.
Methods
The methodology used by the CHEST Guidelines Oversight Committee to select the Expert Cough Panel chair and the international panel of experts and to perform the synthesis of the evidence to develop the recommendations and suggestions has been previously published.7, 8 In addition to the quality of the evidence, the recommendation grading also includes a strength of recommendation dimension. In the context of practice recommendations, a strong recommendation applies to almost all patients, whereas a weak recommendation is conditional and only applies to some patients. In the context of research recommendations, such as the ones in this guideline, we intended for a strong recommendation (grade 1) to imply that we recommend using a particular cough assessment in almost all the cases and instances where such a tool is being considered. The strength of recommendation here is based on consideration of three factors: balance of benefits to harms, patient values and preferences, and resource considerations. Harms incorporate risks and burdens to the patient, which, for example, can include convenience or inconvenience, difficulty of administration, and invasiveness. These in turn affect patient preferences. The resource considerations go beyond economics and should factor in time and other indirect costs. The authors of these recommendations have considered these parameters in determining the strength of the recommendations and associated grades.
Key questions and parameters of eligibility were developed for this topic. Existing guidelines, systematic reviews, and primary studies were assessed for relevance and quality and were used to support the evidence-based graded recommendations or suggestions. A highly structured consensus-based (CB) Delphi approach was used to provide expert advice on all guidance statements. The total number of eligible voters for each guidance statement varied based on the number of managed individuals recused from voting on any particular statements because of their potential conflicts of interest (COIs). For example, C. T. F., A. B. C., S. S. B., and R. S. I. were recused from developing and voting on the recommendations that included mentioning specific QoL instruments. Writing committee member COIs related to the recommendations were identified and are presented in a COI grid (e-Appendix 1). Transparency of process was documented. Further details of the methods have been published elsewhere.7, 8
The Executive Committee of the CHEST Expert Cough Panel convened a subcommittee to formulate recommendations or suggestions that pertain to the assessment of cough frequency and severity. This subcommittee on assessment of cough based its recommendations on a recently published comparative effectiveness review (CER) commissioned by the US Agency for Healthcare Research and Quality (AHRQ) 9 and a corresponding summary. 5 Various members of the Expert Cough Panel provided the stimulus for the AHRQ CER (R. S. I.) and were invited to participate as key informants, technical expert panelists, and peer reviewers. 9 The CER included a comprehensive search of the literature indexed in MEDLINE, Embase, and the Cochrane Database of Systematic Reviews to identify English-language evaluative studies of instruments used to assess the frequency or impact of acute or chronic cough. Included studies had to (1) compare one cough assessment with another or with clinical assessment of cough or (2) evaluate change in response to treatment over time using a given tool. The literature search began with the inception of these databases; the last literature search date for the CER was June 4, 2012. The literature search was subsequently updated by two authors of the CER (R. R. C., D. C. M), who are also members of the subcommittee on assessment of cough, using the same selection criteria used for the original CER project. This updated literature search identified 27 eligible studies10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 published between June 2012 and November 2013, inclusive, that were not included in the CER.
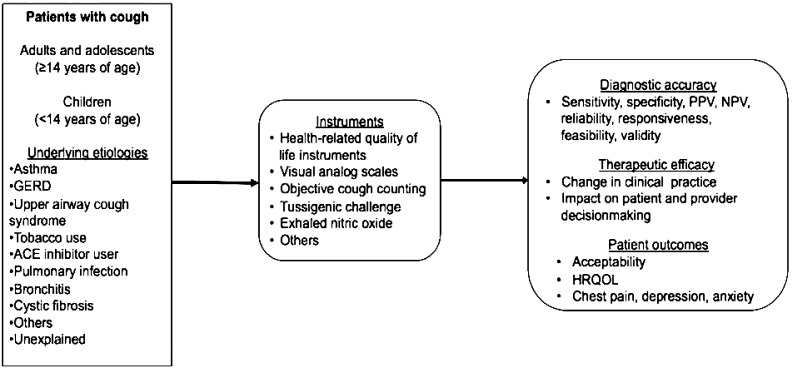
The CER 9 included an analytic framework constructed by using the general approach of specifying the population of interest, interventions, comparators, outcomes, timing of outcomes, and settings (PICOTS) to address the following key question: In adults and adolescents (≥ 14 years of age) and children (< 14 years of age), what is the comparative diagnostic accuracy, therapeutic efficacy, and patient outcome efficacy of instruments used to assess cough? The criteria used to screen articles for inclusion and exclusion at both the title-and-abstract and the full-text screening stages are detailed inTable 1.Figure 1 depicts this key question within the context of the PICOTS framework. The figure shows that the CER compared the diagnostic accuracy, therapeutic efficacy, and patient outcome efficacy of instruments to assess the severity, frequency, and impact of cough on patient outcomes. Subgroups considered included children aged < 14 years and patients with differing underlying cough etiologies. The subcommittee formulated the additional key clinical research questions presented in the Results section.
TABLE 1.
PICOTS Elements Table With Inclusion and Exclusion Criteria
| Study Characteristic | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Populations | • Humans | None |
| • Patients with cough (any duration) | ||
| Interventions | • Qualitative and quantitative instruments used to assess cough (eg, general and cough-specific health-related quality of life instruments, visual analog scales, objective cough counting, tussigenic challenge, exhaled nitric oxide) | None |
| Comparators |
|
None |
| Outcomes | • Study assesses an outcome of interest: | None |
| ∘ Diagnostic accuracy (eg, sensitivity, specificity, positive predictive value, negative predictive value, validity, reliability, responsiveness, feasibility) | ||
| ∘ Therapeutic efficacy (eg, change in clinical practice, impact on patient or provider decision-making) | ||
| ∘ Patient outcome efficacy (eg, acceptability, quality of life, chest pain, depression, anxiety) | ||
| Timing | • Timing of follow-up not limited | None |
| Setting | • Inpatient and outpatient | None |
| Study design | • Evaluation studies | • Not a clinical study (eg, editorial, nonsystematic review, letter to the editor, case series) |
| Publications |
|
• Non-English-language publications |
PICOTS = population of interest, interventions, comparators, outcomes, timing of outcomes, and settings.
Figure 1.
Analytic framework for comparative effectiveness review. ACE = angiotensin-converting enzyme; GERD = gastroesophageal reflux disease; HRQL = health-related quality of life; NPV = negative predictive value; PPV = positive predictive value.
The strength of the evidence for the key question was rated using the general approach described in the Methods Guide for Effectiveness and Comparative Effectiveness Reviews 37 and the Methods Guide for Medical Test Reviews. 38 In brief, the approach required assessment of four domains: risk of bias, consistency, directness, and precision. These domains were considered qualitatively, and a summary rating of high, moderate, or low strength of evidence was assigned after discussion by two reviewers (R. R. C. and D. C. M.) (Table 2).14, 16, 19, 20, 21, 22, 24, 29, 31, 32, 34, 35, 36, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100 In some cases, high, moderate, or low ratings were impossible or imprudent to make. For example, when no evidence was available or when evidence on the outcome was too weak, sparse, or inconsistent to permit any conclusion to be drawn, a grade of insufficient was assigned. Two members of the subcommittee on assessment of cough revised the strength of evidence conclusions reported in the AHRQ report to include additional information reported in the studies identified by the updated literature search. Prior to publication, experts reviewed this guideline and addressed all suggestions and criticisms.
TABLE 2.
Summary of Strength of Evidence and Effect Estimate for Clinical Research Question 1
| Instrument (Dimension Assessed) | Validity (ie, Correlation With Other Measures of Cough) | Reliability | Responsiveness | Citations | |
|---|---|---|---|---|---|
| Internal Consistency (Cronbach α) | Repeatability | ||||
| LCQ (severity/QoL) | High SOE 17 studies; 1,191 subjects Range of r = 0.93-0.93 | High SOE 4 studies; 430 subjects Range of r = 0.77-0.93 | High SOE 2 studies; 256 subjects Range of r = 0.86-0.92 | High SOE 12 studies; 862 subjects Range of ES = 0.84-19.5 | 20, 24, 31, 32, 34, 36, 40, 42-56 |
| CQLQ and ACOSa (severity/QoL) | High SOE 6 studies; 360 subjects Range of r = 0.24-0.79 | Low SOE 2 studies; 208 subjects Range of r = 0.63-0.92 | Low SOE 2 studies; 76 subjects Range of r = 0.75-0.93 | Moderate SOE 8 studies; 484 subjects Range of MID = 10.6-21.9 | 28, 39, 41, 53, 56-59 |
| PC-QOL (severity/QoL) | Moderate SOE 4 studies; 593 subjects Range of r = 0.01-0.70 | Moderate SOE 3 studies; 247 subjects Range of r = 0.56-0.91 | Insufficient SOE 1 study; 43 subjects Range of r = 0.40-0.51 | Moderate SOE 4 studies; 519 subjects Range of ES = 0.32-0.41 | 60-62, 99 |
| Electronic recording devices (frequency) | High SOE 23 studies; 774 subjects Range of r = 0.89-0.99 | NA | Moderate SOE 6 studies; 239 subjects Range of r = 0.8-1.0 | Low SOE 2 studies; 129 subjects Detected change with treatment | 19, 21, 22, 24, 29, 35, 43-45, 48, 49, 59, 62-92, 100 |
| Visual analog scales (severity/QoL) | Insufficient SOE 13 studies; 638 subjects No summary measure | NA | Insufficient SOE 1 study; 54 subjects Range of r = 0.71-0.78 | Moderate SOE 6 studies; 250 subjects Detected change with treatment | 14, 16, 22, 28, 35, 51, 57, 92-98 |
ACOS = Adverse Cough Outcome Survey; CQLQ = Cough-Specific Quality of Life Questionnaire; ES = effect size; LCQ = Leicester Cough Questionnaire; MID = minimal important difference; NA = not applicable; PC-QOL = Parent Cough-Specific Quality of Life Questionnaire; QoL = quality of life; SOE = strength of evidence.
The ACOS has been revised and replaced by the CQLQ.
Results
The original CER report identified 115 articles representing 121 unique studies that underwent full-text review and 78 studies that met inclusion criteria for the review.5, 9 The updated literature search identified an additional 105 studies for full-text review, 27 of which met inclusion criteria, for a total of 105 eligible studies. The updated search did not change the conclusions of the initial report.
Evidence and Recommendations
Clinical Research Question 1: What Is the Most Important Patient-Related Outcome in Assessing Cough Severity and Treatment in Adult and Adolescent Patients (≥ 14 Years of Age) Complaining of Chronic Cough?
Summary of the Evidence and Interpretation
A variety of tools are available to assess cough, capturing different concepts used to evaluate various aspects of the symptom.5, 9 The choice of instruments in studies is determined by the specific research question and study design. Patient-reported outcomes, such as QoL questionnaires and VASs, reflect the patient's experience of the impact and severity of coughing, whereas cough counting provides objective quantification of the symptom, and cough challenge testing offers mechanistic insights. Cough frequency can be measured using electronic audio and video recording devices. Limited data suggest that audio recordings may be marginally more accurate than video recordings. 75 Although both are reliable compared with other methods of assessing cough frequency, the moderate to poor correlations between cough counting and QoL appear to demonstrate a lack of convergent validity between the two constructs being measured.5, 9 As such, all these tools complement one another and are frequently used in combination. Furthermore, although the majority of studies on cough assessment evaluated various aspects of the comparative diagnostic accuracy of the various cough measurement instruments, they did not evaluate their comparative therapeutic or patient outcome efficacies.5, 9
Although multidimensional QoL questionnaires describe only one facet of cough consequences, they appear to be the most comprehensive instruments to evaluate the impact of this symptom on the patient. 9 QoL instruments may vary in content and therefore measure different aspects of QoL so that some may be more useful in certain situations. Furthermore, because they provide a personal subjective evaluation of the effects of cough, which may differ greatly from one patient to another, some assessments may be better for subgroups of patients. Because health-related QoL studies have tended to focus on populations with longer durations of cough, we have specified chronic cough in the present recommendation. In this context, health-related QoL questionnaires have been shown to be valid and reliable in adults and adolescents and in pediatric populations.5, 9 In adults and adolescents, QoL questionnaires are based on self-report. In the pediatric population, the questionnaires are completed by the parents, not the child; therefore, the results reflect the parents' perception of the impact of cough on their child's QoL. Older children (generally aged > 7 years) may be able to report their QoL, but self-reported pediatric cough-specific QoL questionnaires are not yet available.
1. In adult and adolescent patients (≥ 14 years of age) complaining of chronic cough, we recommend that validated and reliable health-related QoL questionnaires be used as the measurement of choice to assess the impact of cough on patients (Grade 1B).
Clinical Research Question 2: in Adults and Adolescents (≥ 14 Years of Age), Which Health-Related QoL Questionnaires Are Validated to Assess Cough Severity, and How Do They Compare in Regard to Their Properties?
Summary of the Evidence and Interpretation
The Cough-Specific Quality-of-Life Questionnaire (CQLQ) and Leicester Cough Questionnaire (LCQ) have content validity for assessing how patients perceive the effect of their cough; this is based on both questionnaires being developed with input from patients with chronic cough.39, 40, 101, 102 Both instruments have been found to be reliable, valid, and responsive measures of the impact of chronic cough on adults and adolescents (Table 2).5, 9 Although there is good responsiveness data for the CQLQ and LCQ, they are limited at this time.5, 9 These two questionnaires have been the most extensively studied, whereas other cough-specific as well as general and disease-focused (eg, cough in patients with lung cancer) health-related QoL instruments have not been extensively studied or used. 9
2. In adults and adolescents with chronic cough, we recommend the CQLQ and LCQ, as they are the most extensively studied and commonly used previously validated and reliable cough-specific health-related QoL questionnaires to assess the impact of cough (Grade 1B).
Clinical Research Question 3: in Children (< 14 Years of Age) With Chronic Cough, Which Is the Measurement of Choice to Assess the Impact of Cough?
Summary of the Evidence and Interpretation
As for adults, QoL questionnaires have been demonstrated to be valid and reliable instruments to assess the impact of cough in children.5, 9 Because of limited and insufficient evidence to determine the reliability or concurrent validity of the various cough diaries, numeric rating scales, VASs, or tussigenic challenges, these are not recommended as primary outcome measures to assess the impact of cough.5, 9
Based on studies performed in children, it appears that electronic audio and video recording devices are valid methods of assessing cough frequency.5, 9 Because cough counting does not lend itself to directly measuring the impact of cough as perceived by the patient, establishing concurrent validity of cough counting may best be assessed by comparing one cough counting method with another cough frequency measure.
3. In children (< 14 years of age) with chronic cough, we recommend that validated and reliable health-related QoL questionnaires be used as the measurement of choice to assess the impact of cough (Grade 1B).
Clinical Research Question 4: in Children (< 14 Years of Age) With Chronic Cough, Which Cough-Specific Health-Related QoL Questionnaire(s) Is/Are Recommended to Assess the Impact of Cough?
Summary of the Evidence and Interpretation
In children, the Parent Cough-Specific Quality of Life Questionnaire (PC-QOL), the most extensively studied QoL instrument in this age-group, has been found to be a reliable and valid instrument of measuring parental perception of the impact of chronic cough on their child (Table 2).5, 9 This questionnaire has content validity based on its development with input from parents of children with chronic cough. 99 Although there is good responsiveness data for the PC-QOL, they are limited at this time (Table 2).5, 9
4. In children (< 14 years of age) with chronic cough, we recommend the PC-QOL, the most extensively studied and commonly used previously validated and reliable health-related QoL questionnaire, as the measurement of choice to assess the impact of cough (Grade 1B).
Clinical Research Question 5: in Adult and Adolescent Patients (≥ 14 Years of Age) and Children (< 14 Years of Age), Does Cough Frequency Monitoring (Objective Cough Counting) Provide an Added Value to Assess Health-Related QoL or Cough Severity?
Summary of the Evidence and Interpretation
The literature draws moderate to poor correlations between cough counting and health-related QoL, suggesting that these outcomes are measuring different concepts related to the phenomenon of coughing.5, 9 Despite the fact that cough frequency monitoring appears to be measuring data reflecting a concept different from health-related QoL or cough severity, its measurement may still be informative based on study design and outcomes (eg, assessment of drug efficacy).
5. To standardize the development, utilization, and reporting of cough-specific QoL questionnaires, we suggest that cough counting alone not be used to establish validity of the questionnaires (CB).
Clinical Research Question 6: How Should VASs or Numeric Rating Scales Be Used to Assess Cough?
Summary of the Evidence and Interpretation
VASs and patient diaries are widely used in both clinical research and practice. Although they have the potential to assess cough severity, few data exist on their accuracy in doing so, and correlations with other cough measurement tools have been inconsistent.5, 9
The panel considered that if a VAS is used as an outcome measure, it must be done so in a standardized manner.103, 104, 105, 106 For example, to establish concurrent validity for a cough-specific QoL scale, it is important to ensure that the phenomenon measured by the VAS is that of cough. Therefore, it is important to use the word “cough” in the descriptor at both ends of the scale, and words such as “symptoms” should not be interchanged with the word “cough.” For the purpose of promoting reliability and validity in the use of the VAS, the ends of the scale should be closed by perpendicular lines, and the descriptions on each end should be outside these lines and not within the scale itself (Fig 2). 103 This design will help to avoid confusion on the part of the subject about where to mark the line.
Figure 2.
Representative 100-mm visual analog scale (VAS) for measuring subject self-reported cough severity. Instruct subject to put an X on the line to indicate the severity of his or her cough during the past week or previously identified referent time period. The point of intersection of the crossed lines of the X should be used as the VAS value.
6. To standardize the development, use, and reporting of cough severity by VASs or numeric rating scales, we suggest that they be used in standard fashion (CB).
Clinical Research Question 7: in All Patients, Can We Improve Our Confidence in the Validity of Cough Study Outcomes by Asking That Cough QoL Questionnaires or Other Subjective Rating Instruments Be Copyrighted so That They Cannot Be Altered or Translated Without Going Through Appropriate Steps?
Summary of the Evidence and Interpretation
Because any change in cough-related QoL questionnaires or any QoL questionnaires in general can potentially lead to different results, these questionnaires should not be modified, regardless of whether they have been copyrighted. If they have been modified, permission should be obtained from the developers or from those who hold their copyright. If and when a QoL questionnaire is modified, the modified version should be retested for validity and reliability. 107
All translations of existing questionnaires into another language should be conducted using an established methodology to preserve content validity and other measurement properties (eg, backward and forward translation steps, cognitive interviews, international harmonization meetings). 108 The results of these procedures must be published.
7. To ensure the integrity of health-related QoL questionnaires and other patient-reported outcomes that have been shown to be valid and reliable, we suggest that a modified version should not be used and reported unless the modified version has been shown to be reliable and valid (CB).
Clinical Research Question 8: in Adults and Adolescents, What Is the Clinical Usefulness of Cough Reflex Testing With Inhalation Cough Challenges?
Summary of the Evidence and Interpretation
Because of limited and insufficient evidence to determine the reliability or concurrent validity of the various types of tussigenic challenges,5, 9 these are not recommended as primary outcome measures for determining cough severity or the impact of cough. However, tussigenic challenges may be useful to investigate the mechanisms of cough. At this time, no valid comparisons exist among the various tussigenic challenges regarding their value in clinical or research settings.
8. In adult and adolescent patients with cough of any duration, we suggest that tussigenic challenges have a role in research settings to understand mechanisms of cough (CB).
Clinical Research Question 9: in Patients of All Ages, How Should Cough Counting Be Done to Assess Cough?
Summary of the Evidence and Interpretation
Electronic recording devices appear to be valid assessments of cough frequency compared with human counts. However, correlation between cough counting and other assessments, such as health-related QoL, is moderate to poor,5, 9 suggesting that cough counting is not a reliable way to assess the impact of coughing on patients. If performed, however, it should be done through objective means. Although electronic audio and video recording devices are reliable compared with other methods of assessing cough frequency,5, 9 the moderate to poor correlations between cough counting and QoL appear to demonstrate a lack of convergent validity between the two constructs being measured.
9. In patients of all ages, we recommend acoustic cough counting to assess cough frequency but not cough severity (Grade 1B).
Areas for Future Research
To advance the field, a number of research endeavors should be undertaken, as follows:
-
•
It should not be assumed that QoL questionnaires would perform equally in all studies, across different cultures, and in different populations. Therefore, to assess the performance of QoL questionnaires, their reliability and validity should be reassessed in all studies going forward, including longitudinal studies. Concurrent validity of QoL questionnaires may be assessed by comparing results of cough severity with QoL or QoL of one questionnaire with another.28, 109
-
•
To standardize the development, use, and reporting of responsiveness of health-related QoL questionnaires, the minimal important difference should be assessed with a prospective (eg, Punum Ladder) 41 as well as a retrospective (eg, global rating of change scale) measure of change to assess whether a consistent difference exists between the two. Based on an empirically supported theory by Streiner and Norman, 110 there is recall bias inherent in retrospective measures. 41 The only study comparing the two types of measures to calculate the minimal important difference found a difference when prospective and retrospective measures of change were used. 41 If future studies confirm this finding and reveal that the difference is consistent, a recommendation should be made about which type of change measure should be routinely used.
-
•
Because there are limited data on the responsiveness of electronic audio and video cough counting devices,5, 9 future research should focus on this gap in knowledge as well as on determining the most useful duration of the monitoring session.
-
•
There is a need to establish reliability and validity of cough-specific VASs and numeric rating scales using appropriate methodology.5, 9
-
•
There is a need to develop self-reported pediatric cough-specific QoL questionnaires for older children.
-
•
When changing an established questionnaire from a hard copy to an electronic format, measurement properties must be confirmed because the change represents instrument modification. 109
-
•
Although QoL questionnaires provide important information in the research setting, future research should be directed to transitioning the use of these questionnaires in an appropriate format for the clinical setting. 111
Conclusions
Since publication of the 2006 CHEST cough guidelines, it is clear that the field of cough assessment has advanced based on the results of the systematic review commissioned by AHRQ and performed by methodologists with no conflicts of interest at the Duke Evidence-based Practice Center. By updating the original review through November 2013 and using the updated results as the basis for the present deliberations, the CHEST Expert Cough Panel has made a series of recommendations and suggestions for carrying out clinical research in assessing cough. This article has also identified gaps in our knowledge and areas for future research.
Acknowledgments
Author contributions: L.-P. B. and R. S. I. were the guarantors and topic editors for this article. L.-P. B., R. R. C., D. C. M., C. T. F, A. B. C., S. S. B., J. S., R. L. D., B. R., and R. S. I. contributed to the development of the key questions using the PICOTS format and review of the data and elaboration of recommendations, including their grading; R. L. D. was the methodologist; and R. R. C. and D. C. M. were among the investigators from the Duke Evidence-based Practice Center who conducted the systematic review that formed the basis for the recommendations.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Drs Coeytaux and McCrory are authors on the AHRQ evidence report on which this guideline is based, and their efforts on this project were supported by a contract from AHRQ. Dr French is a codeveloper and co-copyright holder of the CQLQ. She has not received any financial compensation for CQLQ in > 3 years. Dr Chang is a codeveloper of the PC-QOL, a member of the advisory panel for the AHRQ systematic review on assessing cough severity, and a reviewer of the final AHRQ document. Dr Birring is a developer of the adult QoL and cough counting tools. Dr Smith has a patent for a cough counting device. Dr Irwin is a codeveloper and co-copyright holder of the CQLQ. He has not received any financial compensation for CQLQ in > 3 years. Dr Irwin was also a member of the advisory panel for the AHRQ systematic review on assessing cough severity and a reviewer of the final AHRQ document. Although Dr Irwin is the Editor in Chief of CHEST, all reviews and decisions made concerning this manuscript were made by others, independent of Dr Irwin. Drs Boulet and Rubin and Ms Diekemper have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Endorsements: The following organizations have endorsed this document: American Academy of Otolaryngology – Head and Neck Surgery Foundation, American Thoracic Society, Asian Pacific Society of Respirology, Canadian Thoracic Society, and Irish Thoracic Society.
Collaborators: Members of the CHEST Expert Panel: Todd M. Adams, MD; Kenneth W. Altman, MD, PhD; Alan F. Barker, MD; Surinder S. Birring, MBChB, MD; Fiona Blackhall, MD, PhD; Donald C. Bolser, PhD; Louis-Philippe Boulet, MD, FCCP; Sidney S. Braman, MD, FCCP; Christopher Brightling, MBBS, PhD, FCCP; Priscilla Callahan-Lyon, MD; Anne Bernadette Chang, MBBS, PhD, MPH; Remy Coeytaux, MD, PhD; Terrie Cowley; Paul Davenport, PhD; Rebecca L. Diekemper, MPH; Satoru Ebihara, MD, PhD; Ali A. El Solh, MD, MPH; Patricio Escalante, MD, FCCP; Anthony Feinstein, MPhil, PhD; Stephen K. Field, MD; Dina Fisher, MD; Cynthia T. French, PhD, ANP-BC; Peter Gibson, MBBS; Philip Gold, MD, FCCP; Michael K. Gould, MD, FCCP; Cameron Grant, MBChB, PhD; Susan M. Harding, MD, FCCP; Anthony Harnden, MBChB; Adam T. Hill, MBChB, MD; Richard S. Irwin, MD, Master FCCP; Peter J. Kahrilas, MD; Karina A. Keogh, MD; Andrew P. Lane, MD; Sandra Zelman Lewis, PhD; Kaiser Lim, MD; Mark A. Malesker, PharmD, FCCP; Stuart Mazzone, PhD, FCCP; Douglas C. McCrory, MD, MHS; Lorcan McGarvey, MD; Alex Molasiotis, PhD, RN; M. Hassan Murad, MD, MPH; Peter Newcombe, PhD; Huong Q. Nguyen, PhD, RN; John Oppenheimer, MD; David Prezant, MD; Marcos I. Restrepo, MD, FCCP; Mark Rosen, MD, Master FCCP; Bruce Rubin, MD, MEngr, MBA; Jay H. Ryu, MD, FCCP; Jaclyn Smith, MBChB, PhD; Susan M. Tarlo, MBBS, FCCP; Anne Vertigan, PhD, MBA; Gang Wang, MD, PhD; Miles Weinberger, MD; Kelly Weir, MsPath.
Role of sponsors: CHEST was the sole supporter of these guidelines, this article, and the innovations addressed within.
Other contributions: The authors thank CHEST for supporting this work.
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
Supplementary Material
Footnotes
FUNDING/SUPPORT: Funding was provided by ACCP (CHEST).
DISCLAIMER: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://dx.doi.org/10.1378/chest.1473S1.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Contributor Information
Louis-Philippe Boulet, Email: lpboulet@med.ulaval.ca.
CHEST Expert Cough Panel:
Todd M. Adams, Kenneth W. Altman, Alan F. Barker, Surinder S. Birring, Fiona Blackhall, Donald C. Bolser, Louis-Philippe Boulet, Sidney S. Braman, Christopher Brightling, Priscilla Callahan-Lyon, Anne Bernadette Chang, Remy Coeytaux, Terrie Cowley, Paul Davenport, Rebecca L. Diekemper, Satoru Ebihara, Ali A. El Solh, Patricio Escalante, Anthony Feinstein, Stephen K. Field, Dina Fisher, Cynthia T. French, Peter Gibson, Philip Gold, Michael K. Gould, Cameron Grant, Susan M. Harding, Anthony Harnden, Adam T. Hill, Richard S. Irwin, Peter J. Kahrilas, Karina A. Keogh, Andrew P. Lane, Sandra Zelman Lewis, Kaiser Lim, Mark A. Malesker, Stuart Mazzone, Douglas C. McCrory, Lorcan McGarvey, Alex Molasiotis, M. Hassan Murad, Peter Newcombe, Huong Q. Nguyen, John Oppenheimer, David Prezant, Marcos I. Restrepo, Mark Rosen, Bruce Rubin, Jay H. Ryu, Jaclyn Smith, Susan M. Tarlo, Anne Vertigan, Gang Wang, Miles Weinberger, and Kelly Weir
References
- 1.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey: 2010 Outpatient Department Summary Tables. Atlanta, GA: Centers for Disease Control and Prevention; 2013.
- 2.Irwin RS, Baumann MH, Bolser DC. American College of Chest Physicians (ACCP). Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1_suppl):1S–23S. doi: 10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irwin RS, Boulet LP, Cloutier MM. Managing cough as a defense mechanism and as a symptom: a consensus panel report of the American College of Chest Physicians. Chest. 1998;114(2_suppl):133S–181S. doi: 10.1378/chest.114.2_supplement.133s. [DOI] [PubMed] [Google Scholar]
- 4.Morice AH, McGarvey L, Pavord I. British Thoracic Society Cough Guideline Group. Recommendations for the management of cough in adults. Thorax. 2006;61(suppl 1):i1–i24. doi: 10.1136/thx.2006.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmit KM, Coeytaux RR, Goode AP. Evaluating cough assessment tools: a systematic review. Chest. 2013;144(6):1819–1826. doi: 10.1378/chest.13-0310. [DOI] [PubMed] [Google Scholar]
- 6.Irwin RS. Assessing cough severity and efficacy of therapy in clinical research: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1_suppl):232S–237S. doi: 10.1378/chest.129.1_suppl.232S. [DOI] [PubMed] [Google Scholar]
- 7.Lewis SZ, Diekemper RL, French CT, Gold PM, Irwin RS. CHEST Expert Cough Panel; CHEST Expert Cough Panel. Methodologies for the development of the management of cough: CHEST guideline and expert panel report. Chest. 2014;146(5):1395–1402. doi: 10.1378/chest.14-1484. [DOI] [PubMed] [Google Scholar]
- 8.Lewis SZ, Diekemper R, Ornelas J, Casey KR. Methodologies for the development of CHEST guidelines and expert panel reports. Chest. 2014;146(1):182–192. doi: 10.1378/chest.14-0824. [DOI] [PubMed] [Google Scholar]
- 9.McCrory DC, Coeytaux RR, Yancy WS, et al. Assessment and Management of Chronic Cough. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed]
- 10.Ahmed N, Sutcliffe A, Tipper C. Feasibility study: honey for treatment of cough in children. Pediatr Rep. 2013;5(2):31–34. doi: 10.4081/pr.2013.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albrecht H, Vernon M, Solomon G. Patient-reported outcomes to assess the efficacy of extended-release guaifenesin for the treatment of acute respiratory tract infection symptoms. Respir Res. 2012;13:118. doi: 10.1186/1465-9921-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton A, Gaydecki P, Holt K, Smith JA. Data reduction for cough studies using distribution of audio frequency content. Cough. 2012;8(1):12. doi: 10.1186/1745-9974-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya M, Joshi N, Yadav S. To compare the effect of dextromethorphan, promethazine and placebo on nocturnal cough in children aged 1-12 y with upper respiratory infections: a randomized controlled trial. Indian J Pediatr. 2013;80(11):891–895. doi: 10.1007/s12098-013-1002-2. [DOI] [PubMed] [Google Scholar]
- 14.Cai C, He MZ, Zhong SQ. Add-on montelukast vs double-dose budesonide in nonasthmatic eosinophilic bronchitis: a pilot study. Respir Med. 2012;106(10):1369–1375. doi: 10.1016/j.rmed.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Cohen HA, Rozen J, Kristal H. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics. 2012;130(3):465–471. doi: 10.1542/peds.2011-3075. [DOI] [PubMed] [Google Scholar]
- 16.Faruqi S, Sedman P, Jackson W, Molyneux I, Morice AH. Fundoplication in chronic intractable cough. Cough. 2012;8(1):3. doi: 10.1186/1745-9974-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hareendran A, Palsgrove AC, Mocarski M. The development of a patient-reported outcome measure for assessing nighttime symptoms of chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2013;11:104. doi: 10.1186/1477-7525-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hrisanfow E, Hägglund D. Impact of cough and urinary incontinence on quality of life in women and men with chronic obstructive pulmonary disease. J Clin Nurs. 2013;22(1-2):97–105. doi: 10.1111/j.1365-2702.2012.04143.x. [DOI] [PubMed] [Google Scholar]
- 19.Kavitt RT, Higginbotham T, Slaughter JC. Symptom reports are not reliable during ambulatory reflux monitoring. Am J Gastroenterol. 2012;107(12):1826–1832. doi: 10.1038/ajg.2012.342. [DOI] [PubMed] [Google Scholar]
- 20.Koskela HO, Purokivi MK. Capability of hypertonic saline cough provocation test to predict the response to inhaled corticosteroids in chronic cough: a prospective, open-label study. Cough. 2013;9:15. doi: 10.1186/1745-9974-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson S, Comina G, Gilman RH, Tracey BH, Bravard M, López JW. Validation of an automated cough detection algorithm for tracking recovery of pulmonary tuberculosis patients. PLoS One. 2012;7(10):e46229. doi: 10.1371/journal.pone.0046229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KK, Matos S, Evans DH, White P, Pavord ID, Birring SS. A longitudinal assessment of acute cough. Am J Respir Crit Care Med. 2013;187(9):991–997. doi: 10.1164/rccm.201209-1686OC. [DOI] [PubMed] [Google Scholar]
- 23.Molassiotis A, Ellis J, Wagland R. The Manchester cough in lung cancer scale: the development and preliminary validation of a new assessment tool. J Pain Symptom Manage. 2013;45(2):179–190. doi: 10.1016/j.jpainsymman.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Sumner H, Woodcock A, Kolsum U. Predictors of objective cough frequency in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(9):943–949. doi: 10.1164/rccm.201211-2000OC. [DOI] [PubMed] [Google Scholar]
- 25.Anderson-James S, Marchant JM, Acworth JP, Turner C, Chang AB. Inhaled corticosteroids for subacute cough in children. Cochrane Database Syst Rev. 2013;2:CD008888. doi: 10.1002/14651858.CD008888.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang AB, Robertson CF, van Asperen PP. A cough algorithm for chronic cough in children: a multicenter, randomized controlled study. Pediatrics. 2013;131(5):e1576–e1583. doi: 10.1542/peds.2012-3318. [DOI] [PubMed] [Google Scholar]
- 27.Kahrilas PJ, Howden CW, Hughes N, Molloy-Bland M. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest. 2013;143(3):605–612. doi: 10.1378/chest.12-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lechtzin N, Hilliard ME, Horton MR. Validation of the Cough Quality-of-Life Questionnaire in patients with idiopathic pulmonary fibrosis. Chest. 2013;143(6):1745–1749. doi: 10.1378/chest.12-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KK, Savani A, Matos S, Evans DH, Pavord ID, Birring SS. Four-hour cough frequency monitoring in chronic cough. Chest. 2012;142(5):1237–1243. doi: 10.1378/chest.11-3309. [DOI] [PubMed] [Google Scholar]
- 30.Newcombe PA, Sheffield JK, Chang AB. Parent cough-specific quality of life: development and validation of a short form. J Allergy Clin Immunol. 2013;131(4):1069–1074. doi: 10.1016/j.jaci.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa H, Fujimura M, Takeuchi Y, Makimura K. Clinical experience with low-dose itraconazole in chronic idiopathic cough. Cough. 2013;9(1):1. doi: 10.1186/1745-9974-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet. 2012;380(9853):1583–1589. doi: 10.1016/S0140-6736(12)60776-4. [DOI] [PubMed] [Google Scholar]
- 33.Shembel AC, Rosen CA, Zullo TG, Gartner-Schmidt JL. Development and validation of the cough severity index: a severity index for chronic cough related to the upper airway. Laryngoscope. 2013;123(8):1931–1936. doi: 10.1002/lary.23916. [DOI] [PubMed] [Google Scholar]
- 34.Sundar KM, Daly SE, Willis AM. A longitudinal study of CPAP therapy for patients with chronic cough and obstructive sleep apnoea. Cough. 2013;9(1):19. doi: 10.1186/1745-9974-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunger K, Powley W, Kelsall A, Sumner H, Murdoch R, Smith JA. Objective measurement of cough in otherwise healthy volunteers with acute cough. Eur Respir J. 2013;41(2):277–284. doi: 10.1183/09031936.00190111. [DOI] [PubMed] [Google Scholar]
- 36.Van de Kerkhove C, Goeminne PC, Van Bleyenbergh P, Dupont LJ. A cohort description and analysis of the effect of gabapentin on idiopathic cough. Cough. 2012;8(1):9. doi: 10.1186/1745-9974-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed]
- 38.Agency for Healthcare Research and Quality. Methods Guide for Medical Test Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [PubMed]
- 39.French CT, Irwin RS, Fletcher KE, Adams TM. Evaluation of a cough-specific quality-of-life questionnaire. Chest. 2002;121(4):1123–1131. doi: 10.1378/chest.121.4.1123. [DOI] [PubMed] [Google Scholar]
- 40.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fletcher KE, French CT, Irwin RS, Corapi KM, Norman GR. A prospective global measure, the Punum Ladder, provides more valid assessments of quality of life than a retrospective transition measure. J Clin Epidemiol. 2010;63(10):1123–1131. doi: 10.1016/j.jclinepi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Berkhof FF, Boom LN, ten Hertog NE, Uil SM, Kerstjens HA, van den Berg JW. The validity and precision of the Leicester Cough Questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes. 2012;10:4. doi: 10.1186/1477-7525-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birring SS, Matos S, Patel RB, Prudon B, Evans DH, Pavord ID. Cough frequency, cough sensitivity and health status in patients with chronic cough. Respir Med. 2006;100(6):1105–1109. doi: 10.1016/j.rmed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 44.Decalmer SC, Webster D, Kelsall AA, McGuinness K, Woodcock AA, Smith JA. Chronic cough: how do cough reflex sensitivity and subjective assessments correlate with objective cough counts during ambulatory monitoring? Thorax. 2007;62(4):329–334. doi: 10.1136/thx.2006.067413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faruqi S, Thompson R, Wright C, Sheedy W, Morice AH. Quantifying chronic cough: objective versus subjective measurements. Respirology. 2011;16(2):314–320. doi: 10.1111/j.1440-1843.2010.01893.x. [DOI] [PubMed] [Google Scholar]
- 46.Huisman AN, Wu MZ, Uil SM, van den Berg JW. Reliability and validity of a Dutch version of the Leicester Cough Questionnaire. Cough. 2007;3:3. doi: 10.1186/1745-9974-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones RM, Hilldrup S, Hope-Gill BD, Eccles R, Harrison NK. Mechanical induction of cough in idiopathic pulmonary fibrosis. Cough. 2011;7:2. doi: 10.1186/1745-9974-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelsall A, Decalmer S, Webster D. How to quantify coughing: correlations with quality of life in chronic cough. Eur Respir J. 2008;32(1):175–179. doi: 10.1183/09031936.00101307. [DOI] [PubMed] [Google Scholar]
- 49.Key AL, Holt K, Hamilton A, Smith JA, Earis JE. Objective cough frequency in idiopathic pulmonary fibrosis. Cough. 2010;6:4. doi: 10.1186/1745-9974-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma W, Yu L, Wang Y, Li X, Lü H, Qiu Z. Changes in health-related quality of life and clinical implications in Chinese patients with chronic cough. Cough. 2009;5:7. doi: 10.1186/1745-9974-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morice AH, Menon MS, Mulrennan SA. Opiate therapy in chronic cough. Am J Respir Crit Care Med. 2007;175(4):312–315. doi: 10.1164/rccm.200607-892OC. [DOI] [PubMed] [Google Scholar]
- 52.Murray MP, Turnbull K, MacQuarrie S, Pentland JL, Hill AT. Validation of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J. 2009;34(1):125–131. doi: 10.1183/09031936.00160508. [DOI] [PubMed] [Google Scholar]
- 53.Polley L, Yaman N, Heaney L. Impact of cough across different chronic respiratory diseases: comparison of two cough-specific health-related quality of life questionnaires. Chest. 2008;134(2):295–302. doi: 10.1378/chest.07-0141. [DOI] [PubMed] [Google Scholar]
- 54.Raj AA, Pavord DI, Birring SS. Clinical cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? Handb Exp Pharmacol. 2009;187:311–320. doi: 10.1007/978-3-540-79842-2_16. [DOI] [PubMed] [Google Scholar]
- 55.Singapuri A, McKenna S, Brightling CE. The utility of the mannitol challenge in the assessment of chronic cough: a pilot study. Cough. 2008;4:10. doi: 10.1186/1745-9974-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalpaklioglu AF, Kara T, Kurtipek E, Kocyigit P, Ekici A, Ekici M. Evaluation and impact of chronic cough: comparison of specific vs generic quality-of-life questionnaires. Ann Allergy Asthma Immunol. 2005;94(5):581–585. doi: 10.1016/S1081-1206(10)61137-4. [DOI] [PubMed] [Google Scholar]
- 57.Field SK, Conley DP, Thawer AM, Leigh R, Cowie RL. Effect of the management of patients with chronic cough by pulmonologists and certified respiratory educators on quality of life: a randomized trial. Chest. 2009;136(4):1021–1028. doi: 10.1378/chest.08-2399. [DOI] [PubMed] [Google Scholar]
- 58.Shaheen NJ, Crockett SD, Bright SD. Randomised clinical trial: high-dose acid suppression for chronic cough-a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33(2):225–234. doi: 10.1111/j.1365-2036.2010.04511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith J, Owen E, Earis J, Woodcock A. Cough in COPD: correlation of objective monitoring with cough challenge and subjective assessments. Chest. 2006;130(2):379–385. doi: 10.1378/chest.130.2.379. [DOI] [PubMed] [Google Scholar]
- 60.Chang AB, Robertson CF, Van Asperen PP. A multicenter study on chronic cough in children: burden and etiologies based on a standardized management pathway. Chest. 2012;142(4):943–950. doi: 10.1378/chest.11-2725. [DOI] [PubMed] [Google Scholar]
- 61.Newcombe PA, Sheffield JK, Chang AB. Minimally important change in a Parent-Proxy Quality-of-Life questionnaire for pediatric chronic cough. Chest. 2011;139(3):576–580. doi: 10.1378/chest.10-1476. [DOI] [PubMed] [Google Scholar]
- 62.Newcombe PA, Sheffield JK, Juniper EF, Petsky HL, Willis C, Chang AB. Validation of a Parent-Proxy Quality of Life Questionnaire for Paediatric Chronic Cough (PC-QOL) Thorax. 2010;65(9):819–823. doi: 10.1136/thx.2009.133868. [DOI] [PubMed] [Google Scholar]
- 63.Barry SJ, Dane AD, Morice AH, Walmsley AD. The automatic recognition and counting of cough. Cough. 2006;2:8. doi: 10.1186/1745-9974-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coyle MA, Keenan DB, Henderson LS. Evaluation of an ambulatory system for the quantification of cough frequency in patients with chronic obstructive pulmonary disease. Cough. 2005;1:3. doi: 10.1186/1745-9974-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crawford B, Monz B, Hohlfeld J. Development and validation of a cough and sputum assessment questionnaire. Respir Med. 2008;102(11):1545–1555. doi: 10.1016/j.rmed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 66.Freestone C, Eccles R. Assessment of the antitussive efficacy of codeine in cough associated with common cold. J Pharm Pharmacol. 1997;49(10):1045–1049. doi: 10.1111/j.2042-7158.1997.tb06039.x. [DOI] [PubMed] [Google Scholar]
- 67.Hsu JY, Stone RA, Logan-Sinclair RB, Worsdell M, Busst CM, Chung KF. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. Eur Respir J. 1994;7(7):1246–1253. doi: 10.1183/09031936.94.07071246. [DOI] [PubMed] [Google Scholar]
- 68.Kelsall A, Houghton LA, Jones H, Decalmer S, McGuinness K, Smith JA. A novel approach to studying the relationship between subjective and objective measures of cough. Chest. 2011;139(3):569–575. doi: 10.1378/chest.10-0438. [DOI] [PubMed] [Google Scholar]
- 69.Kelsall A, Decalmer S, McGuinness K, Woodcock A, Smith JA. Sex differences and predictors of objective cough frequency in chronic cough. Thorax. 2009;64(5):393–398. doi: 10.1136/thx.2008.106237. [DOI] [PubMed] [Google Scholar]
- 70.Krahnke J, Gentile D, Angelini B, Danzig M, Skoner D. Comparison of objective and subjective measurements of cough frequency in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;93(3):259–264. doi: 10.1016/S1081-1206(10)61498-6. [DOI] [PubMed] [Google Scholar]
- 71.Krajnik M, Damps-Konstanska I, Gorska L, Jassem E. A portable automatic cough analyser in the ambulatory assessment of cough. Biomed Eng Online. 2010;9:17. doi: 10.1186/1475-925X-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leconte S, Liistro G, Lebecque P, Degryse JM. The objective assessment of cough frequency: accuracy of the LR102 device. Cough. 2011;7(1):11. doi: 10.1186/1745-9974-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marsden PA, Smith JA, Kelsall AA. A comparison of objective and subjective measures of cough in asthma. J Allergy Clin Immunol. 2008;122(5):903–907. doi: 10.1016/j.jaci.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 74.Matos S, Birring SS, Pavord ID, Evans DH. An automated system for 24-h monitoring of cough frequency: the Leicester Cough Monitor. IEEE Trans Biomed Eng. 2007;54(8):1472–1479. doi: 10.1109/TBME.2007.900811. [DOI] [PubMed] [Google Scholar]
- 75.Smith JA, Earis JE, Woodcock AA. Establishing a gold standard for manual cough counting: video versus digital audio recordings. Cough. 2006;2:6. doi: 10.1186/1745-9974-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith JA, Owen EC, Jones AM, Dodd ME, Webb AK, Woodcock A. Objective measurement of cough during pulmonary exacerbations in adults with cystic fibrosis. Thorax. 2006;61(5):425–429. doi: 10.1136/thx.2005.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith J, Owen E, Earis J, Woodcock A. Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2006;117(4):831–835. doi: 10.1016/j.jaci.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 78.Thomas JS, Lyons HA, Shepherd DA. D.A.T.A.—an advanced automated electronic cough counting system for the evaluation of antitussive agents. Curr Ther Res Clin Exp. 1978;23(1):66–77. [Google Scholar]
- 79.Woodcock A, McLeod RL, Sadeh J, Smith JA. The efficacy of a NOP1 agonist (SCH486757) in subacute cough. Lung. 2010;188(suppl 1):S47–S52. doi: 10.1007/s00408-009-9197-8. [DOI] [PubMed] [Google Scholar]
- 80.Hamutcu R, Francis J, Karakoc F, Bush A. Objective monitoring of cough in children with cystic fibrosis. Pediatr Pulmonol. 2002;34(5):331–335. doi: 10.1002/ppul.10174. [DOI] [PubMed] [Google Scholar]
- 81.Paul IM, Wai K, Jewell SJ, Shaffer ML, Varadan VV. Evaluation of a new self-contained, ambulatory, objective cough monitor. Cough. 2006;2:7. doi: 10.1186/1745-9974-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Archer LN, Simpson H. Night cough counts and diary card scores in asthma. Arch Dis Child. 1985;60(5):473–474. doi: 10.1136/adc.60.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang AB, Newman RG, Carlin JB, Phelan PD, Robertson CF. Subjective scoring of cough in children: parent-completed vs child-completed diary cards vs an objective method. Eur Respir J. 1998;11(2):462–466. doi: 10.1183/09031936.98.11020462. [DOI] [PubMed] [Google Scholar]
- 84.Chang AB, Newman RG, Phelan PD, Robertson CF. A new use for an old Holter monitor: an ambulatory cough meter. Eur Respir J. 1997;10(7):1637–1639. doi: 10.1183/09031936.97.10071637. [DOI] [PubMed] [Google Scholar]
- 85.Chang AB, Phelan PD, Robertson CF, Roberts RG, Sawyer SM. Relation between measurements of cough severity. Arch Dis Child. 2003;88(1):57–60. doi: 10.1136/adc.88.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corrigan DL, Paton JY. Pilot study of objective cough monitoring in infants. Pediatr Pulmonol. 2003;35(5):350–357. doi: 10.1002/ppul.10267. [DOI] [PubMed] [Google Scholar]
- 87.Dales RE, White J, Bhumgara C, McMullen E. Parental reporting of childrens' coughing is biased. Eur J Epidemiol. 1997;13(5):541–545. doi: 10.1023/a:1007311912777. [DOI] [PubMed] [Google Scholar]
- 88.Falconer A, Oldman C, Helms P. Poor agreement between reported and recorded nocturnal cough in asthma. Pediatr Pulmonol. 1993;15(4):209–211. doi: 10.1002/ppul.1950150405. [DOI] [PubMed] [Google Scholar]
- 89.Fuller P, Picciotto A, Davies M, McKenzie SA. Cough and sleep in inner-city children. Eur Respir J. 1998;12(2):426–431. doi: 10.1183/09031936.98.12020426. [DOI] [PubMed] [Google Scholar]
- 90.Hoskyns EW, Thomson A, Decker E, Hutchins A, Simpson H. Effect of controlled release salbutamol on nocturnal cough in asthma. Arch Dis Child. 1991;66(10):1209–1212. doi: 10.1136/adc.66.10.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zihlif N, Paraskakis E, Lex C, Van de Pohl LA, Bush A. Correlation between cough frequency and airway inflammation in children with primary ciliary dyskinesia. Pediatr Pulmonol. 2005;39(6):551–557. doi: 10.1002/ppul.20202. [DOI] [PubMed] [Google Scholar]
- 92.Irwin RS, Zawacki JK, Wilson MM, French CT, Callery MP. Chronic cough due to gastroesophageal reflux disease: failure to resolve despite total/near-total elimination of esophageal acid. Chest. 2002;121(4):1132–1140. doi: 10.1378/chest.121.4.1132. [DOI] [PubMed] [Google Scholar]
- 93.Barnabè R, Berni F, Clini V. The efficacy and safety of moguisteine in comparison with codeine phosphate in patients with chronic cough. Monaldi Arch Chest Dis. 1995;50(2):93–97. [PubMed] [Google Scholar]
- 94.Del Donno M, Aversa C, Corsico R. Efficacy and safety of moguisteine in comparison with dextromethorphan in patients with persistent cough. Drug Investigation. 1994;7(2):93–100. [Google Scholar]
- 95.Guyatt GH, Townsend M, Kazim F, Newhouse MT. A controlled trial of ambroxol in chronic bronchitis. Chest. 1987;92(4):618–620. doi: 10.1378/chest.92.4.618. [DOI] [PubMed] [Google Scholar]
- 96.Jackson IM, Barnes J, Cooksey P. Efficacy and tolerability of oral acetylcysteine (Fabrol) in chronic bronchitis: a double-blind placebo controlled study. J Int Med Res. 1984;12(3):198–206. doi: 10.1177/030006058401200312. [DOI] [PubMed] [Google Scholar]
- 97.Ramsay J, Wright C, Thompson R, Hull D, Morice AH. Assessment of antitussive efficacy of dextromethorphan in smoking related cough: objective vs. subjective measures. Br J Clin Pharmacol. 2008;65(5):737–741. doi: 10.1111/j.1365-2125.2008.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sabot G, Bagnato A, Frigerio G. Controlled evaluation of the antitussive activity of viminol p-hydroxybenzoate. Int J Clin Pharmacol Biopharm. 1977;15(4):181–183. [PubMed] [Google Scholar]
- 99.Newcombe PA, Sheffield JK, Juniper EF. Development of a parent-proxy quality-of-life chronic cough-specific questionnaire: clinical impact vs psychometric evaluations. Chest. 2008;133(2):386–395. doi: 10.1378/chest.07-0888. [DOI] [PubMed] [Google Scholar]
- 100.Birring SS, Fleming T, Matos S, Raj AA, Evans DH, Pavord ID. The Leicester Cough Monitor: preliminary validation of an automated cough detection system in chronic cough. Eur Respir J. 2008;31(5):1013–1018. doi: 10.1183/09031936.00057407. [DOI] [PubMed] [Google Scholar]
- 101.French CL, Irwin RS, Curley FJ, Krikorian CJ. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158(15):1657–1661. doi: 10.1001/archinte.158.15.1657. [DOI] [PubMed] [Google Scholar]
- 102.Irwin RS, Curley FJ. The treatment of cough. A comprehensive review. Chest. 1991;99(6):1477–1484. doi: 10.1378/chest.99.6.1477. [DOI] [PubMed] [Google Scholar]
- 103.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 104.Gift AG. Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs. 1989;14(6):323–325. doi: 10.1002/j.2048-7940.1989.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 105.Wewers ME, Rachfal C, Ahijevych K. A psychometric evaluation of a visual analogue scale of craving for cigarettes. West J Nurs Res. 1990;12(5):672–681. doi: 10.1177/019394599001200508. [DOI] [PubMed] [Google Scholar]
- 106.Gift AG. Visual analogue scales: measurement of subjective phenomena. Nurs Res. 1989;38(5):286–288. [PubMed] [Google Scholar]
- 107.Juniper EF. Medical questionnaires are copyrighted to ensure that validity is maintained. Chest. 2009;136(4):951–952. doi: 10.1378/chest.09-1447. [DOI] [PubMed] [Google Scholar]
- 108.Acquadro C, Conway K, Giroudet C, et al. Linguistic Validation Manual for Patient-Reported Outcomes (PRO) Instruments. Lyon, France: MAPI Research Institute; 2004.
- 109.US Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER); Center for Devices and Radiological Health (CDRH). Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Rockville, MD: Food and Drug Administration; 2009.
- 110.Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to Their Development and Use. Oxford, England: Oxford University Press; 2008.
- 111.Cella D, Yount S, Rothrock N. on behalf of the PROMIS Cooperative Group. Med Care. 2007;45(5 suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.