Abstract
Aedes aegypti is an important vector for Dengue and Dengue hemorrhagic fever. Considering its medical importance and its relevance as a model system, this study was undertaken to evaluate the impact of different doses of gamma radiation for three generations of A. aegypti. Two to three days old virgin males of A. aegypti were irradiated with 15 doses of gamma radiation, ranging from 1 to 50 Gy and were immediately mass mated with the same aged virgin females. Observations were made for changes on their life history traits, particularly fecundity, hatchability, adult emergence, sex ratio and longevity, for three generations. Adult males exposed 30, 35, 40, 45 and 50 Gy doses showed a significant decrease in fecundity in F0 generations. While hatchability was observed to have decreased with increasing radiation doses from 3 Gy onwards in the F1 generation, samples irradiated with 30, 35, 40, 45 and 50 Gy maintained significant decline in hatchability in their succeeding generations, F2 and F3 also. Similarly, a decline was observed in adult emergence from 3 Gy onwards in all three generations. A male favoring sex ratio distortion was observed at the doses of 35, 40, 45 and 50 Gy in all three generations. Following exposure to 4 Gy, parental males and the resultant progeny showed increased longevity by 10.56 and 8.66 days respectively. Similarly, the F1 generations of samples irradiated with 30, 35 and 40 Gy exhibited an increase in longevity by 7.16, 7.44 and 6.64 days respectively. Dose response curve for fertility among the three generations was drawn and presented. The effect of radiological exposure on the life history traits of A. aegypti varies with dose for the three generations studied. These results have potential implications in mutational studies and risk assessment and also contribute to a better understanding towards employment of the sterile insect technique in A. aegypti, plausibly paving the way to an effective mosquito genetic control program.
Keywords: Radiation, Fecundity, Hatchability, Longevity, Genetic control, Sterile insect technique
1. Introduction
The effects of ionizing radiation on various units of the ecosystem have always been of immense scientific interest. Numerous studies have documented the adverse effects of these radiations on human beings. However, studies of its effects on non-human biota are relatively fewer (EMRAS (Environmental Modeling for RAdiation Safety), 2007; Moller and Mousseau, 2013). While there are studies that suggest that ionizing radiations induce an adaptive or hormetic response in living organisms, especially at low doses, most surveys have revealed its detrimental attributes. Understanding the bigger picture involves discerning the impact of ionizing radiations on all components of the ecosystem (UNSCEAR, 2011). Hence, studying the effect of ionizing radiation on other, non-human yet significant members of the ecosystem is necessary. The interaction between radiation and a wide range of biological species is not very well understood because of their great variation in life cycles, life spans, and exposure pathways (Singhal et al., 2009).
Aedes aegypti is an excellent model organism; because of its ready adaptability to laboratory culture and short life span with high reproductive potential, this species has been used as a test animal for many physiological, developmental, biochemical and behavioral studies (Craig and Hickey, 1967, Clemons et al., 2010). This species is more popularly known as the primary vector for Dengue fever/Dengue hemorrhagic fever (Ahmad et al., 2007). Numerous vector control measures have been initiated to curb its proliferation. Popularly practiced insecticide based control of insect vectors/mosquitoes faces two important challenges, namely — the development of resistance to insecticides and the environmental pollution caused due to the constant application of insecticides. It is desirable to have alternate strategies which do not involve resistance among the vectors and genetic control or autocidal control is one such method (Shetty, 1997). The Sterile Insect Technique (SIT) is one of the genetic control methods, which involves the release of sterile males into the environment in an attempt to check its proliferation (Knipling, 1967). Considering, the relevance of A. aegypti as a model system and its medical importance as a vector species, radiation studies on this organism would be highly beneficial. Understanding the effect of ionizing radiation on this species is not only necessary from an ecological context but also from an anthropical and prophylactic perspective (Hart, 1980). A previous study on gamma irradiation of adult males of A. aegypti has shown reduced productivity or offspring at a high dose of 50 Gy (Rodriguez, 1977).This study however has not delved into the detailed impact of radiation on the life history traits of the said species. Ionizing radiation induced lethal mutations can be expressed at any stage in the insect's life cycle (LaChance and Crystal, 1965). Our prime interest is to understand the variation in effects produced by different doses of radiation on the life history traits of the said vector species. A comprehensive study such as the present one, will give better insights to the effects produced by the different doses, on the physiology of the organism and whether it is carried into the next generation. This could also help identify an optimal dose for radiation induced male sterility in A. aegypti, thus providing useful insights into a genetic control program.
Apart from radiation, various environmental factors have also been shown to induce variations in the life-history traits of several mosquito species (Lyimo et al., 1992, Phelan and Rotiberg, 2013). As with such studies, it is necessary to evaluate the persistence of the induced effects of different doses of radiation across multiple generations, in order to achieve a clear perspective of the consequences of exposure of a population, to ionizing radiation. Within this context, this paper presents a comprehensive study examining the effects of fifteen doses of gamma radiation, ranging from 1 to 50 Gy, on the life history traits of A. aegypti, with specific focus on fecundity, hatchability, adult emergence, sex ratio and longevity for three generations.
2. Materials and methods
2.1. Mosquito culture
A. aegypti used in the present study was originally collected from J.P. Nagar, Bangalore, India and has been colonized since 2011. The larvae and adults were reared in an insectary maintained at 25 ± 1 °C and 75 ± 5% relative humidity under 14 h photoperiod (Shetty, 1983). Adults were maintained in 21 cm cubic cages of iron frames covered by cotton net cloth and fed on 10% sucrose solution in a jar with a cotton wick. Females were provided with the blood meal from restrained mice upon maturation. A plastic cup (7.62 cm diameter) containing clean water lined with filter paper was placed inside the cage for oviposition. The hatched larvae were transferred to enamel trays and reared. Powdered mixture of yeast tablets (Geo Pharmaceuticals, Bangalore) and dog biscuits (Pedigree, Mars Industries, Hyderabad) were provided as larval diet. All mosquitoes used in the experiments were reared at a density of ~ 600 larvae per tray (25 × 30 cm) containing ± 2 l of water (water depth 2 cm).
2.2. Pure line (PL) synthesis
In order to eliminate the recessive lethal or sterile genes, in the laboratory populations, if any, about fifty gravid females were randomly selected from the population cages and were isolated individually in separate vials for egg laying, following the procedure of Shetty (1983). Females producing highest number of viable eggs were selected for further inbreeding. The process of selection and inbreeding was continued for seven generations in order to increase the fertility rate. Stocks with high fertility rate were used for further experimental assays.
2.3. Irradiation study
Experiments were performed in batches for different doses and a control. Overall a total of 1500, 2–3 days old unmated PL males were isolated and irradiated with different doses of gamma radiation from 60Co (Theratron 780-C Telecobalt Unit, AECL, Ontario, Canada) source with a dose rate of 253.56 cGy/min, at the Kidwai Memorial Institute of Oncology, Bangalore. Mosquitoes were irradiated in small 2.5 cm depth plastic square boxes (5 × 4 cm) covered with fine net cap. A dosimetry was used to quantify the dose received by the irradiated insects and it was confirmed that all the doses delivered lay within a 5% error range. Doses of 1, 2, 3, 4, 6, 8, 10, 15, 20, 25, 30, 35, 40, 45 and 50 Gy were chosen for the study. Each batch consisted of 100 adult mosquitoes receiving a specific dose of the radiation. The irradiated males (n = 100) were transferred into five different cages (20 × 20 × 20 cm) and immediately mass mated with virgin females of the same age (n = 100), such that each cage contained 20 irradiated males and 20 virgin females. Each cage was maintained separately to serve as replicates, such that the progeny from each of the five different cages of mosquitoes having received the same dose are not combined at point during the study. Three days were allowed for mating before the females were given blood meal and gravid females were isolated individually in 2 × 9.5 cm shell vials with water and filter paper lining for oviposition.
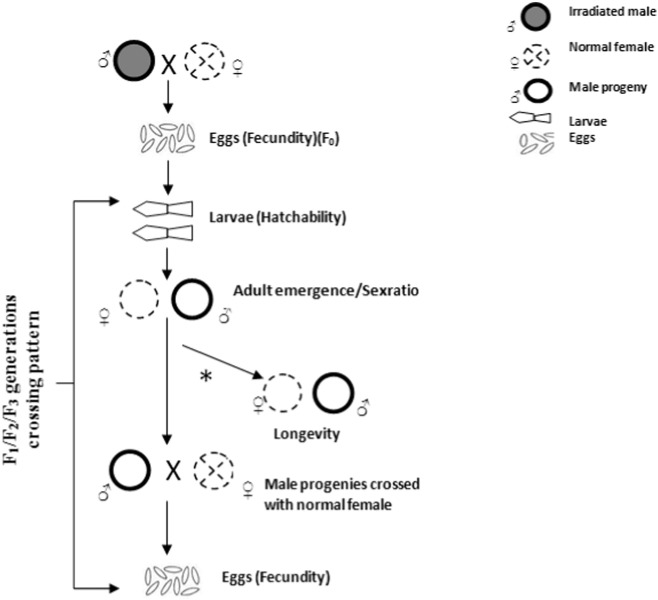
Irradiated males were considered F0. Progeny from the cross between irradiated males and untreated PL females were designated as F1. Progeny resulting from the cross between F1 males and untreated PL females were designated as F2. Similarly progeny resulting from the cross between F2 males and untreated PL females were designated as F3. Using mosquitoes from F0, F1, F2 and F3 observations were made for changes in their life history traits particularly fecundity, hatchability, adult emergence, sex-ratio and longevity. For each dose of radiation and the control set, 20 crosses in 5 replicates (total of 100) were analyzed (Fig. 1).
Fig. 1.
Diagrammatic representation of crossing pattern of irradiated males and its offsprings for three generations.
*The longevity of the twenty emerged males and females were determined separately by the removal and counting of dead mosquitoes at 24 h intervals.
2.4. Parameters measured
2.4.1. Fecundity and hatchability
Eggs, obtained from individual PL females crossed with F0♂, F1♂, F2♂, F3♂ (of particular doses) and PL♂ (control), were incubated for 72–74 h. Newly hatched larvae were counted for seven consecutive days. This count was used to calculate the percentage of hatchability. Subsequently, the number of eggs laid per female was counted and this count was used to calculate fecundity. Fecundity was calculated by the average number of eggs laid per female. Percentage of hatchability was calculated as the ratio of the number of larvae hatched to number of eggs laid per female.
2.4.2. Adult emergence and sex ratio
Larvae obtained from the above procedure were allowed to pupate. Pupae were manually segregated according to sex (females larger than males). Adult mosquitoes emerged from the pupae were scored positively; dead pupae or semi-emerged adults were scored as non-emerged. Emergent pupae were collected in plastic open mouthed containers (2 in. in diameter; 10 ml volume). These containers were placed in respective cages. Adults emerged approximately two days after pupation, and sex was rechecked at this time. Adult emergence was calculated as the total number of adults emerged. Sex ratio was calculated as the ratio of number of males to number of females emerged from a population of a single isolated female (isofemale). Larvae from the PL population served as control.
Following this analysis, 20 adult males (of a particular dose and 5 replicates) were randomly selected and allowed to breed with 20 PL females in order to produce the next generation.
2.4.3. Longevity
The longevity of the twenty emerged males and females (five replicates) was determined separately by the removal and counting of dead mosquitoes at 24 h intervals. Mosquitoes were fed with 10% sucrose solution throughout the study. The average life span of irradiated males (F0), males and females of the succeeding three generations (F1, F2 and F3) and males and females from PL (control) was calculated.
2.4.4. Fertility and dose response curve
Fertility was calculated as a ratio of number of adults emerged to the number of eggs laid in each case. The percentage of fertility of this value was plotted against the respective doses to yield the dose response curve.
2.5. Data analysis
Data collected from the experiments for the above mentioned parameters using different doses of radiation was subjected to various statistical analyses. Data from all mosquitoes of a particular replicate of the same dose were pooled to get an average value. Data values from 5 such replicates were obtained in each case, and its standard error per dose was calculated. The fecundity and hatchability data were log transformed prior to analysis. The arcsine transformation of sex ratio data was performed to attain homogeneity of variances. The variance of collected data was analyzed by repeated measures analysis of variance (rm ANOVA) with ‘generations’ as within subject effects and ‘doses’ as between subject effects. Tests for fecundity, hatchability and sex ratio were found to violate sphericity assumptions as determined by examining Mauchly's W statistic. Hence to correct the sphericity, Huynh–Feldt correction was carried out and found to be significant. In instances where the mean group of adult emergence found to be insignificant for sphericity test, multivariate (Pillai's trace) and univariate tests were carried out and were found to be significant. Post Hoc tests Bonferroni and Duncan were used for pairwise comparison between generations and multiple comparisons between different doses respectively. Further, the longevity of irradiated males and all three generations of both male and female mosquitoes exposed to different doses were assessed by Kaplan Meier analysis using Log Rank test. All statistical analyses were performed using SPSS 15.0 software for Windows.
3. Results
The effects of different doses of gamma radiation on the life history traits of A. aegypti for three generations were studied using the following parameters.
3.1. Fecundity
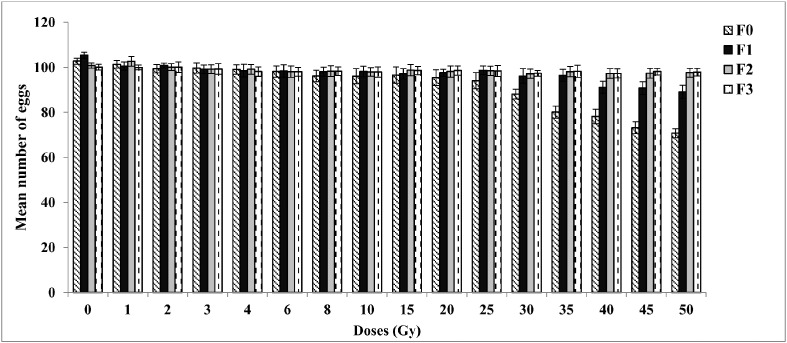
The average numbers of eggs laid per female per dose were counted and results are shown in Fig. 2. Doses of 1, 2, 3, 4, 6, 8, 10, 15, 20 and 25 Gy did not show any significant difference (P > 0.05) when compared to the control. Doses of 30, 35, 40, 45 and 50 Gy however, showed a significant decrease (P < 0.05) in fecundity (Average number of eggs ± SE = 88.14 ± 2.19, 80.16 ± 2.66, 78.17 ± 1.17, 73.21 ± 1.63, and 70.78 ± 1.63 respectively in F0 generations), when compared to control (Average number of eggs ± SE = 102.80 ± 1.36). Similar reductions were not seen in subsequent generations (P > 0.05) F1, F2 and F3. A significant difference (P < 0.05) among the samples exposed to different doses of gamma radiation (Huynh–Feldt test, F = 186.6; df = 15) and subsequent generations (Huynh–Feldt test, F = 586.89; df = 3) was recorded.
Fig. 2.
Mean number of eggs of Aedes aegypti for three generations in response to different irradiation doses (1–50 Gy) on adult males (F0).
The error bars represent standard error of five replicates in each generation (n = 100).
0 — control, F0 — parental generation, F1 — first filial generation, F2 — second filial generation, F3 — third filial generation.
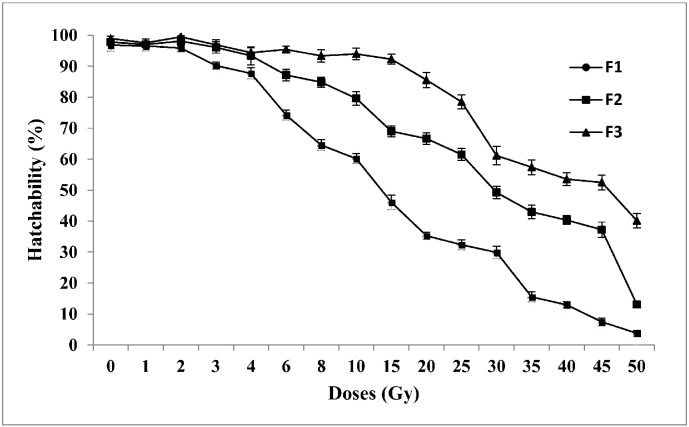
3.2. Hatchability
It was observed that different doses of gamma radiation produced varied levels of effects on hatchability (Fig. 3). A significant reduction (P < 0.05) in hatchability was recorded in the samples irradiated with 3 Gy (Percent of hatchability ± SE = 90.21 ± 1.09) onwards in F1 generation, when compared to the control (Percent of hatchability ± SE = 96.80 ± 0.81). However, doses of 20, 25, 30, 35, 40, 45 and 50 Gy brought about significant reduction (P < 0.05) in hatchability in all three generations F1, F2 and F3. Maximum reduction in the percentage of hatchability was observed at 50 Gy with 93.01% in F1 generation. Moreover this decline was maintained in subsequent generations with 84.72% and 58.82% respectively in F2 and F3 generations. Significant differences (P < 0.05) among different doses of exposure (Huynh–Feldt test, F = 5826.05; df = 15) and between three generations (Huynh–Feldt test, F = 5718.5; df = 2) were recorded.
Fig. 3.
Percent of hatchability of Aedes aegypti for three generations in response to different irradiation doses (1–50 Gy) on adult males (F0). The error bars represent standard error of five replicates in each generation (n = 100).
0 — control, F1 — first filial generation, F2 — second filial generation, F3 — third filial generation.
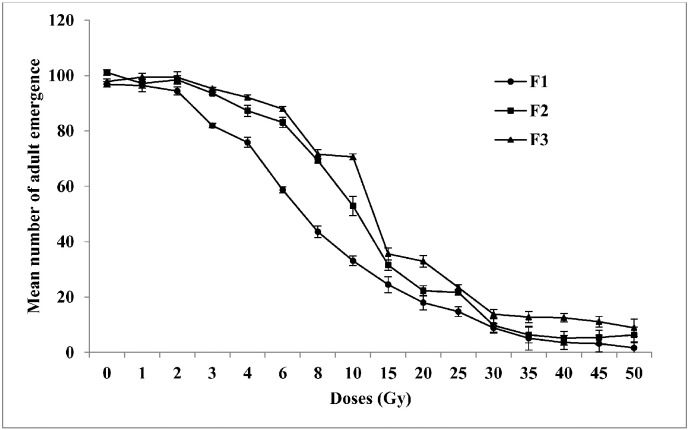
3.3. Adult emergence
Effects of ionizing radiation on the mean number of adult emergence are summarized in Fig. 4. The results indicate that adult emergence decreased remarkably with increasing doses of radiation. A significantly reduced (P < 0.05) mean number of adult emergence was initially observed in the 3 Gy samples in F1 generation (Mean number of adult emergence ± SE = 81.94 ± 1.66), 93.60 ± 1.80 and 95.26 ± 1.82 in F2 and F3 respectively. Exposure to 50 Gy was found to produce maximum reduction in mean number of adult emergence (1.65 ± 0.16, 6.33 ± 0.42, and 8.88 ± 0.54 in F1, F2, and F3 generations respectively). The difference in adult emergence among the samples irradiated was found to be statistically significant (P < 0.05) between doses (Pillai's trace: F = 2373.8; df = 2) and within three generations (Univariate test: F = 11,644.05; df = 15).
Fig. 4.
Mean number of adult emergence of Aedes aegypti for three generations in response to different irradiation doses (1–50 Gy) on adult males (F0). The error bars represent standard error of five replicates in each generation (n = 100).
0 — control, F1 — first filial generation, F2 — second filial generation, F3 — third filial generation.
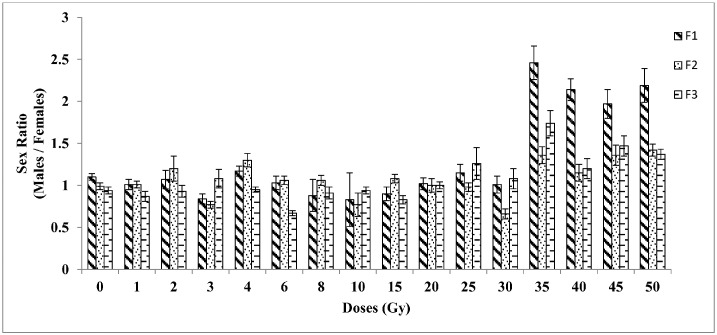
3.4. Sex ratio
The effects of gamma radiation on the sex ratio of adults are shown in Fig. 5. Samples irradiated with doses ranging from 1 to 30 Gy were unaffected when compared to control, whereas, higher doses of 35, 40, 45 and 50 Gy produced a significantly (P < 0.05) higher proportion of male emergence in the F1 and its subsequent generations F2 and F3, when compared to the control. Samples exposed to different doses (Huynh–Feldt test, F = 25.74; df = 15) and their subsequent generations (Huynh–Feldt test, F = 40.4; df = 2) showed a significant difference (P < 0.05) in the sex ratio.
Fig. 5.
Sex ratio of Aedes aegypti for three generations in response to different irradiation doses (1–50 Gy) on adult males (F0). The error bars represent standard error of five replicates in each generation (n = 100).
0 —control, F1 — first filial generation, F2 — second filial generation, F3 — third filial generation.
3.5. Longevity
Table 1 shows the mean longevity of the irradiated males and its progeny in the subsequent three generations. An exposure to 4 Gy of gamma radiation resulted in an increased life span in the parental males (F0) and the F1 males by 10.56 (27.38%) and 8.66 (22.92%) days respectively, when compared to control. However, samples exposed to 1 and 2 Gy did not show any significant changes with respect to longevity (Log Rank test: 1 Gy: χ2 = 3.522, df = 7, P = 0.861; 2 Gy: χ2 = 8.412, df = 7, P = 0.298; 3 Gy: χ2 = 7.274, df = 7, P = 0.401). A decline in longevity was observed in parental males (F0) exposed to the radiation from 6 Gy onwards. Though a decline in longevity in males exposed to higher doses of 30, 35 and 40 Gy was noted in F0 generation, interestingly, life span was increased in F1 generation males by 7.16 (19.73%), 7.44 (20.35%) and 6.64 (18.38%) days in the three doses respectively, when compared to the control (30 Gy: χ2 = 241.495, df = 7, P < 0.0001; 35 Gy: χ2 = 179.591, df = 7, P < 0.0001; 40 Gy: χ2 = 317.683, df = 7, P < 0.0001), whereas in the adult females this effect was merely in F1 generations of 30 Gy by 5.61 (9.4%) days (χ2 = 45.252, df = 7, P < 0.0001). The highest decline in longevity by 19.61 (69.9%) days was observed in F0 (parental males) of 50 Gy. Kaplan Meier analysis of longevity using Log Rank test showed a significant difference (P < 0.0001) among males exposed to different doses (χ2 = 967.12, df = 15).
Table 1.
Mean longevity (days) of males (n = 100) and females (n = 100) in three generations of irradiated A. aegypti.
| Mean longevity (in days) ± SE⁎ | |||||||
|---|---|---|---|---|---|---|---|
| Dosage (Gy) | F0a |
F1 |
F2 |
F3 |
|||
| Male | Male | Female | Male | Female | Male | Female | |
| 0 (Control) | 28.02 ± 0.33 | 29.13 ± 0.48 | 54.1 ± 0.43 | 29.89 ± 0.36 | 54.1 ± 0.31 | 29.07 ± 0.49 | 54.31 ± 0.42 |
| 1 | 28.6 ± 0.58 | 28.49 ± 0.41 | 54.08 ± 0.47 | 29.84 ± 0.56 | 53.93 ± 0.43 | 29.23 ± 0.46 | 54.49 ± 0.44 |
| 2 | 30.16 ± 0.64 | 29.3 ± 0.61 | 53.74 ± 0.62 | 27.66 ± 1.03 | 54.77 ± 0.49 | 29.13 ± 0.54 | 54.26 ± 0.37 |
| 3 | 28.25 ± 052 | 29.01 ± 0.50 | 54.46 ± 0.52 | 28.59 ± 0.58 | 55.22 ± 0.31 | 28.79 ± 0.37 | 54.34 ± 0.39 |
| 4 | 38.58 ± 0.51 | 37.79 ± 0.58 | 56.81 ± 0.39 | 30.63 ± 0.62 | 54.57 ± 0.27 | 28.64 ± 0.51 | 54.49 ± 0.44 |
| 6 | 26.13 ± 0.43 | 27.42 ± 0.56 | 52.29 ± 0.43 | 30.19 ± 0.58 | 53.34 ± 0.25 | 27.31 ± 0.49 | 53.44 ± 0.32 |
| 8 | 23.28 ± 0.52 | 27.61 ± 0.47 | 51.24 ± 0.49 | 27.17 ± 0.51 | 54.56 ± 0.43 | 26.62 ± 0.79 | 53.78 ± 0.58 |
| 10 | 28.33 ± 0.66 | 27.39 ± 0.57 | 52.51 ± 0.46 | 28.19 ± 0.48 | 53.44 ± 0.54 | 29.37 ± 0.43 | 53.35 ± 0.48 |
| 15 | 27.47 ± 0.56 | 29.67 ± 0.52 | 53.55 ± 0.51 | 30.67 ± 0.49 | 54.64 ± 0.54 | 29.54 ± 0.55 | 54.66 ± 0.45 |
| 20 | 25.56 ± 0.55 | 29.62 ± 0.38 | 52.27 ± 0.58 | 30.59 ± 0.44 | 53.75 ± 0.61 | 26.62 ± 0.49 | 54.79 ± 0.57 |
| 25 | 30.71 ± 0.63 | 26.81 ± 0.88 | 55.76 ± 0.38 | 33.75 ± 0.72 | 54.53 ± 0.40 | 30.55 ± 0.71 | 53.71 ± 0.49 |
| 30 | 26.58 ± 0.46 | 36.29 ± 0.54 | 59.71 ± 0.59 | 37.53 ± 0.70 | 53.58 ± 0.61 | 30.47 ± 0.93 | 54.56 ± 0.55 |
| 35 | 26.53 ± 0.41 | 36.57 ± 0.42 | 56.49 ± 0.49 | 36.49 ± 0.49 | 54.69 ± 0.40 | 27.73 ± 0.41 | 53.61 ± 0.43 |
| 40 | 17. 44 ± 0.45 | 35.78 ± 0.44 | 55.64 ± 0.61 | 30.67 ± 0.38 | 53.73 ± 0.57 | 29.49 ± 0.41 | 53.78 ± 0.58 |
| 45 | 14.76 ± 0.49 | 24.64 ± 0.57 | 55.77 ± 0.72 | 29.46 ± 0.40 | 53.47 ± 0.75 | 28.47 ± 0.51 | 53.81 ± 0.53 |
| 50 | 8.41 ± 0.42 | 15.43 ± 0.46 | 45.74 ± 0.76 | 26.65 ± 0.64 | 50.64 ± 0.86 | 28.77 ± 0.59 | 53.66 ± 0.74 |
F0 – Irradiated parental male.
SE – dtandard error.
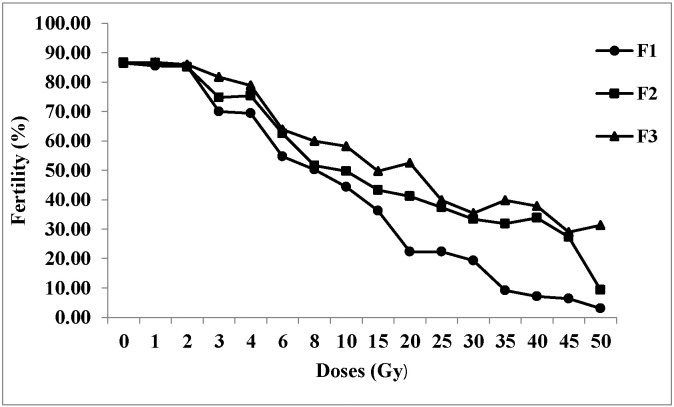
3.6. Dose response curve
The dose response curve for percentage of fertility among three generations was drawn and presented (Fig. 6). It was observed that following irradiation with 3 Gy the mean fertility dropped by 15.12%, 10.35% and 3.41% in the F1, F2 and F3 generations respectively when compared to the control. Also, as the doses increased, the percentage of fertility decreased. The lowest percentage of fertility was observed in samples irradiated with 50 Gy, recording a decline by 82.09%, 75.82% and 53.77% respectively in F1, F2 and F3 generations.
Fig. 6.
Dose response curve for fertility among three generations of irradiated samples.
4. Discussion
The study was attempted to understand the effect of 15 discrete doses of gamma radiation (1–50 Gy) on the life history traits, including fecundity, hatchability, adult emergence, sex ratio and longevity of A. aegypti.
Fecundity was the least affected parameter in this study. Doses ranging from 1 to 25 Gy did not produce any significant reduction in fecundity, and only higher doses such as 30, 35, 40, 45 and 50 Gy brought about a significant decline in the fecundity of the F0 generations, but not the subsequent generations F1, F2 and F3.The results of egg production from the present investigation are in accordance with an earlier study by Terzian and Stahler (1958), that showed normal female A. aegypti continued to lay eggs in considerable numbers in F1 generation despite being mated with males that were irradiated with ranging from 10 to 300 Gy of gamma radiation. In case of Anopheles arabiensis, the fecundity of the females mated with irradiated males was similar for all treatments (0–100 Gy) compared to the control (Helinski et al., 2006). A study on Anopheles pharoensis however has shown a significant rise in fecundity at 10 Gy, followed by no significant effects at doses between 15 and 20 Gy, post which a highly significant decline was observed at 70 Gy (Abdel-Malek et al., 1966).
Fecundity is mostly a female driven trait. Causes for the lack of fecundity may be: (i) the inability to lay eggs (infecundity), (ii) the inability of males to produce sperm (aspermia), or nonfunctional sperms, and (iii) the inability to mate (LaChance, 1967, Lance and McInnis, 2005). In the present study, only male flies were irradiated. Thus an insignificant effect on fecundity primarily implies that the lower doses have no effect, and although higher doses may impact either the ability of males to produce sperm or their mating capability, this effect is not retained in the subsequent generations F1, F2 and F3.
With respect to hatchability, it was observed that males irradiated with doses ranging from 3 to 50 Gy suffered a significant decline when compared to the control, while doses of 1 and 2 Gy did not produce any such effect. Interestingly, samples irradiated with 6 Gy onwards maintained this effect in the subsequent generations F2 and F3. Likewise, exposure of fully embryonated eggs of A. aegypti to 5 Gy gamma radiation was reported to result in retarded development of larvae hatching from them (Asman and Rai, 1972). Also, pupal irradiation ranging from 5 to 70 Gy on An. pharoensis and 25 to 100 Gy on An. arabiensis has also been shown to induce a significant reduction in hatchability (Abdel-Malek et al., 1966, Helinski et al., 2006).
Studies have shown that radiation induces dominant lethal mutations in germ cells and though such mutations may not affect the maturation of the cell into a gamete or the ability of the gamete to form the zygote, it can still cause the death of the developing embryo resulting inviable eggs (LaChance, 1967). Radiation-induced dominant lethal mutations arise mainly as a result of chromosomal damage in the treated cells (Muller, 1940, Lea, 1955, LaChance, 1967). Thus the study shows a probable induction of dominant lethal mutations in the germ line when adult males of A. aegypti are irradiated with gamma rays from 3 Gy onwards.
In the present study, mortality appears to be a highly affected trait, with pronounced consequences on adult emergence. Results show a gradual decline in adult emergence from 3 Gy onwards in all three generations, albeit a slight increase in subsequent generations when compared to F1. In another study, irradiation of pupal males of Anopheles quadrimaculatus, with doses as high as 129 Gy was reported to not affect adult emergence (Weidhaas et al., 1962). Also, the previous studies of Abdel-Malek et al. (1967) and Helinski et al. (2006) have reported no significant effect on adult emergence when An. pharoensis and An. arabiensis are irradiated as pupae even at doses ranging between 5 to 70 Gy and 25–100 Gy respectively. This leads to indicate that adult emergence is more affected when adult males are irradiated against pupal irradiation.
With respect to sex ratio, the present study revealed that gamma radiation exposure on adult males upsets the normal sex ratio 1:1 and results in a larger excess of males in A. aegypti. At lower doses (≤ 30 Gy) the sex ratio was unaffected and at higher doses (≥ 35 Gy) the proportion of male emergence was significantly increased when compared to the control. It should be noted that the present study used normal females which were crossed with either irradiated males or male progeny of the irradiated males. A consistently altered sex ratio favoring males may be the result of a genetic factor as studied by Hickey (1965) that distorts sex ratio in favor of males. This male producing factor was reported to act only in males and passed only to the male offspring. The mechanism appears to be a meiotic drive operating at or near the sex locus thereby causing a selective production of male-determining sperms (Craig, 1967). Such meiotic-drive genes have been described in two species of mosquito, A. aegypti and Culex quinquefasciatus (Roger and Martha, 1991). The present data supports the earlier reports of inheritance of the male producing phenomenon in the progeny of F1 generations and which is inherited further in F2 and F3 generations.
The study on the effects of gamma radiation on longevity does not reveal any remarkable responses. Notable however, is the 10–12 day spike in longevity observed in the irradiated males and its progeny following irradiation with 4 Gy. This effect was neither seen in the females nor the subsequent generation of males. Doses 30, 35 and 40 Gy again produced a stimulatory effect in the males of the F1 generation, though in the females this effect was observed merely in the F1 generation of the 30 Gy irradiated samples. Such hormetic dose responses to gamma radiation have been recorded in many insect species (Dauer, 1965, Vaiserman et al., 2003, Seong et al., 2011). The results are comparable to a study on the adult or pupal stages of An. arabiensis where it was shown that, an overall similar or higher survival was observed in the irradiated samples, when compared to the control (Helinski et al., 2006). For An. pharoensis, a slight increase in longevity of males irradiated with doses ranging from 5–70Gy was reported (Abdel-Malek et al., 1966). In An. quadrimaculatus, the irradiation of young pupae (1–4 h) with 90 Gy resulted in greatly reduced longevity (Davis et al., 1959). Irradiation induced reduction in longevity has been recorded in several anopheline species such as An. stephensi, An. pharoensis and Anopheles gambiae s.s. as dose increases beyond 80 Gy (Abdel-Malek et al., 1967, Curtis, 1976, Sharma et al., 1978). Similar decline in longevity was recorded in the present study following exposure with 45 and 50 Gy in F1 and F2 generations. An earlier work has shown that exposure to low level X-rays on early stages of A. aegypti, including the egg, larval and pupal stages resulted in the prolongation of adult life spans in F1 generation demonstrating a hormetic dose response (Willard, 1965). There have been extensive findings concerning how ionizing radiation administered in the early developmental stages affects adult longevity. The present study however provides data on enhancement of longevity following administration of radiation to adult flies. The short day photoperiod and larger crowded larval breeding conditions of A. aegypti affected considerably by increase and decline of adult longevity respectively (Costanzo et al., 2015, Hawley, 1985). As well irradiation on different mosquito species at various stages of life cycle showed increased or declined effect on adult life span including in its subsequent generations (Helinski et al., 2006, Abdel-Malek et al., 1966). Therefore, it is proved that life span of an adult mosquitoes greatly influenced by environmental changes or treatment of early life stages of mosquitoes. However, radiation induced phenomenon of increased adult longevity compared to the control population, clear indication of a hormetic effect, that is the radiation induced mutation of primary gene action at particular dose may be benefitted in the adult stage by increased longevity.
The dose response curve for fertility illustrated that the fertility rate decreased as the dose increased. A plot of percentage of fertility against the dose, illustrates a reduction in fertility with the increase in dose. Fig. 2 shows a linear dose response curve indicating that the dominant lethal mutations that have arisen are predominantly due to a single event in the germ line. The departure from linearity in case of higher doses suggests a “multi-hit” relationship implying more than one dominant mutation in the germ line (LaChance, 1967, Curtis, 1971). The fertility rate was decreased to 82.09% in F1 generation following exposure with 50 Gy while in male Aedes albopictus irradiated with 35 Gy of gamma radiation, the mean fertility dropped to 7% only (Oliva et al., 2012). However, the 137Cesium irradiation with 40 Gy, pupae of A. albopictus males induced high level of sterility (> 99%) at any male pupal age for all the strains tested (Balestrino et al., 2010) immensely.
Studying a host cellular phenomena using dominant lethal mutation as a tool or an easily measured biological end point in an organism. Any theory proposed to explain dominant lethal mutations must be consistent with the observed shape of the dosage response curve and such curve reveals a great deal about the mode of action of the mutagens (LaChance and Crystal, 1965). A consideration of the implications derived from these curves can be extremely informative to understanding dominant lethal mutations. The hatchability technique of scoring dominant lethal mutations will be accurate and providing most of the lethal are expressed before hatching of the eggs. When the percentages of hatchability were compared with rate of fertility it indicated that the lethality was prolonged into larval or pupal stages in case of higher doses. Dosage curve envisages the dose that produces maximum frequency of dominant lethal mutations without over dosage to the insects (LaChance, 1967).
The experimental results related to inherited sterility in F1, F2 and F3 generations showed a significant reduction in fecundity and hatchability mainly at higher doses ranging from 30 to 50 Gy. Inherited reduction in fertility or semisterility from generation to generation due to radiation induced chromosomal translocations in Anopheles fluviatilis and An. stephensi has been suggested as one of the potential means to control mosquito vector of diseases through genetic manipulations (Shetty, 1983, Gayathri and Shetty, 1992, Madhyastha and Shetty, 2005). Similar studies also observed double translocation heterozygotes in the filarial vector C. quinquefasciatus (Shetty, 1987, Shetty, 1993). Thus, the results from the present study provide collective information on radiation induced effect on life cycle of said species and also plausibly paving the way to an effective mosquito genetic control program.
5. Conclusions
The selected doses of gamma radiation induced varied dose dependent responses on the life history traits of A. aegypti in each of the three generations that were studied. Recording the persistence of radiation induced effects across three subsequent generations was beneficial to understand the inheritance of such effects. Results clearly demonstrate that, impact of radiation on each life history trait of the species varies with dose. It has been determined that a radiological exposure of up to 2 Gy of gamma radiation does not produce any significant effect on A. aegypti with respect to the parameters studied. Of all parameters, fecundity was the least affected, and only so in higher doses ranging from 30 to 50 Gy. Sex ratio also was affected only in higher doses ranging from 35 to 50 Gy. However, changes in hatchability, followed by adult emergence and longevity were more prominently observed with increasing dose.
The results of the present study can be applied in mutational studies, risk assessment and genetic control programs and are of considerable biological, evolutionary and public health interest. The study reveals that radiations induced dominant lethal mutation that caused cessation of development prior to egg hatchability although in some cases mortality was observed in larval or pupal stages. The results will facilitate comparative studies between various organisms of related species and evaluation of the potential harm that radionuclide releases can induce on non-human biota. The study also examines the potential of using male favoring sex ratio distortion as a control agent, probably in combination with selecting appropriate translocations. It was also noted that higher doses of ionizing radiation which induced potentially deleterious effects (e.g.: reproductive parameters) while extending life span, offer an effective method of genetic control of mosquitoes using Sterile Insect Techniques (SIT).
Acknowledgments
This work was supported by the grants of Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE), Bhabha Atomic Research Centre (BARC), Mumbai (No.2009/36/80-BRNS/2394 Dated 9/12/2009). We are grateful to Department of Radiation Physics, Kidwai Memorial Institute of Oncology, Bengaluru, for providing radiation facility.
References
- Abdel-Malek A.A., Tantawy A.O., Wakid A.M. Studies on the eradication of Anopheles pharoensis Theobald by the sterile male technique using cobalt-60. I. Biological effect of gamma radiation on different developmental stages. J. Econ. Entomol. 1966;59:672–678. doi: 10.1093/jee/59.3.672. [DOI] [PubMed] [Google Scholar]
- Abdel-Malek A.A., Tantawy A.O., Wakid A.M. Studies on the eradication of Anopheles pharoensis Theobald by the sterile-male technique using Cobalt-60. III. Determination of the sterile dose and its biological effects on different characters related to “fitness” components. J. Econ. Entomol. 1967;60:20–23. doi: 10.1093/jee/60.1.20. [DOI] [PubMed] [Google Scholar]
- Ahmad I., Astari S., Tan M. Resistance of Aedes aegypti (Diptera:Culicidae) in 2006 to pyrethroid insecticides in Indonesia and its association with oxidase and esterase levels. Pak. J. Biol. Sci. 2007;10:3688–3692. doi: 10.3923/pjbs.2007.3688.3692. [DOI] [PubMed] [Google Scholar]
- Asman S.M., Rai K.S. Developmental effects of ionizing radiation in Aedes aegypti. J. Med. Entomol. 1972;9:468–478. doi: 10.1093/jmedent/9.5.468. [DOI] [PubMed] [Google Scholar]
- Balestrino F., Medici A., Candini G., Carrieri M., Maccagnani B., Calvitti M. Gamma ray dosimetry and mating capacity studies in the laboratory on Aedes albopictus males. J. Med. Entomol. 2010;47:581–591. doi: 10.1093/jmedent/47.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons A., Haugen M., Flannery E., Tomchaney M., Kast K., Jacowski C. Aedes aegypti: an emerging model for vector mosquito development. Cold Spring Harb. Protoc. 2010;2010 doi: 10.1101/pdb.emo141. (pdb.emo141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo K.S., Schelble S., Jerz K., Keenan M. The effect of photoperiod on life history and blood-feeding activity in Aedes albopictus and Aedes aegypti (Diptera:Culicidae) J. Vector Ecol. 2015;40:164–171. doi: 10.1111/jvec.12146. [DOI] [PubMed] [Google Scholar]
- Craig G.B., Jr. Genetic control of mosquitoes. Bull. World Health Organ. 1967;36:628–632. [PMC free article] [PubMed] [Google Scholar]
- Craig G.B., Jr., Hickey W.A. Genetics of Aedes aegypti. In: Wright J.W., Pal R., editors. Genetics of Insect Vectors of Diseases. Elsevier; Amsterdam: 1967. p. 67. [Google Scholar]
- Curtis C.F. Progress Reports on Mosquito Research, No. 31–33. Ross Institute of Tropical Hygiene; London: 1976. Radiation sterilisation. Mosquito studies at the Ross Institute of Tropical Hygiene, London. [Google Scholar]
- Curtis C.F. Induced sterility in insects. Adv. Reprod. Physiol. 1971;5:120–165. [PubMed] [Google Scholar]
- Dauer M. X-irradiation of pupae of the house-fly, Musca domestica L, and male survival. J. Gerontol. 1965;20:219–223. doi: 10.1093/geronj/20.2.219. [DOI] [PubMed] [Google Scholar]
- Davis A.N., Gahan J.B., Weidhaas D.E., Smith C.N. Exploratory studies on gamma radiation for the sterilization and control of Anopholes quadrimaculatus. J. Econ. Entomol. 1959;52:868–870. [Google Scholar]
- EMRAS (Environmental Modeling for RAdiation Safety) Modelling radiation exposure and radionuclide transfer for non-human species (report of the Biota Working Group of EMRAS Theme 3) 2007. http://www-ns.iaea.org/downloads/rw/projects/emras/final-reports/biota-final.pdf
- Gayathri D., Shetty N.J. Chromosomal translocations and inherited semisterility in the malaria vector Anopheles stephensi. J. Commun. Dis. 1992;24:70–74. [PubMed] [Google Scholar]
- Hart D.R. Vol. 6798. Chalk River Publisher, AEC; 1980. A selected bibliography of radiation effects on whole organisms, population and ecosystems; pp. 1–38. (Atomic Energy of Canada). [Google Scholar]
- Hawley W.A. The effect of larval density on adult longevity of a mosquito, Aedes sierrensis: epidemiological consequences. J. Anim. Ecol. 1985;54:955–964. [Google Scholar]
- Helinski M.E.H., Parker A.G., Knols B.G.J. Radiation induced sterility for pupal and adult stages of the malaria mosquito Anopheles arabiensis. Malar. J. 2006 doi: 10.1186/1475-2875-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey W.A. University of Notre Dame; Notre Dame, Indiana: 1965. Distortion of sex ratios in Aedes aegypti (Diptera:Culicidae) Ph.D. thesis. [Google Scholar]
- Knipling E.F. Sterile technique – principles involved, current application, limitations, and future application. In: Wright J.W., Pal R., editors. Genetics of Insect Vectors of Disease. Elsevier; Amsterdam: 1967. p. 587. [Google Scholar]
- LaChance L.E. The induction of dominant lethal mutations in insects by ionizing radiation and chemicals-as related to the sterile male technique of insect control. In: Wright J.W., Pal R., editors. Genetics of Insect Vectors of Disease. Elsevier; Amsterdam: 1967. pp. 617–650. [Google Scholar]
- LaChance L.E., Crystal M.M. Induction of dominant lethal mutations in insect oocytes and sperm by gamma rays and an alkylating agent: dose–reponse and joint action studies. Genetics. 1965;51:699–708. doi: 10.1093/genetics/51.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance D.R., McInnis D.O. Biological basis of the sterile insect technique. In: Dyck V.A., Hendrichs J., Robinson A.S., editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Springer; Dordrecht: 2005. pp. 70–94. [Google Scholar]
- Lea D.E. second ed. The University Press; Cambridge: 1955. Actions of Radiations on Living Cells; p. 416. [Google Scholar]
- Lyimo E.O., Takken W., Koella J.C. Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae. Entomol. Exp. Appl. 1992;63:265–271. [Google Scholar]
- Madhyastha A.D., Shetty N.J. Vol. 48. 2005. Radiation induced chromosomal translocation and inherited semisterility in Anopheles stephensi Liston; pp. 85–89. (A malaria vector, The nucleus). [Google Scholar]
- Moller A.P., Mousseau T.A. The effects of natural variation in background radioactivity on humans, animals and other organisms. Biol. Rev. 2013;88:226–254. doi: 10.1111/j.1469-185X.2012.00249.x. [DOI] [PubMed] [Google Scholar]
- Muller H.J. An analysis of the process of structural change in chromosomes of Drosophila. J. Genet. 1940;40:1–66. [Google Scholar]
- Oliva C.F., Jacquet M., Gilles J., Lemperiere G., Maquart P.-O., Quilici S. The sterile insect technique for controlling populations of Aedes albopictus (Diptera:Culicidae) on Reunion Island: mating vigour of sterilized males. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0049414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan C., Rotiberg B.D. An age-size reaction norm yields insight into environmental interactions affecting life-history traits: a factorial study of larval development in the malaria mosquito Anopheles gambiae sensu stricto. Ecol. Evol. 2013;7:1837–1847. doi: 10.1002/ece3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P.H. Effects on the productivity of irradiated male populations of Aedes aegypti (Diptera:Culicidae) J. Med. Entomol. 1977;14:493–494. doi: 10.1093/jmedent/14.4.493. [DOI] [PubMed] [Google Scholar]
- Roger J.W., Martha E.N. Sex-ratio distortion caused by meiotic drive in mosquitoes, symposium: the genetics and evolutionary biology of meiotic drive. Am. Nat. 1991;137:379–391. [Google Scholar]
- Seong K.M., Kim C.S., Seo S.W., Jeon H.Y., Lee B.S., Nam S.Y., Jin Y.W. Genome-wide analysis of low-dose irradiated male Drosophila melanogaster with extended longevity. Biogerontology. 2011;12:93–107. doi: 10.1007/s10522-010-9295-2. [DOI] [PubMed] [Google Scholar]
- Sharma V.P., Razdan R.K., Ansari M.A. Anopheles stephensi: effect of gamma-radiation and chemosterilants on the fertility and fitness of males for sterile male releases. J. Econ. Entomol. 1978;71:449–452. doi: 10.1093/jee/71.3.449. [DOI] [PubMed] [Google Scholar]
- Shetty N.J. Chromosomal translocations and semisterility in the malaria vector Anopheles fluviatilis James. Indian J. Malariol. 1983;20:45–48. [Google Scholar]
- Shetty N.J. Genetic sexing system for the preferential elimination of females in Culex quinquefasciatus. J. Am. Mosq. Control Assoc. 1987;3:84–86. [PubMed] [Google Scholar]
- Shetty N.J. Genetic control of mosquitoes, chromosomal translocations and inherited semi-sterility in Culex quinquefasciatus - a filarial mosquito. J. Cytol. Genet. 1993;28:181–187. [Google Scholar]
- Shetty N.J. Genetic control of mosquito vectors of diseases. J. Parasit. Dis. 1997;21:113–121. [Google Scholar]
- Singhal R.K., Ajay K., Usha N., Reddy A.V.R. Evaluation of doses from ionizing radiation to non-human species at Trombay, Mumbai, India. Radiat. Prot. Dosim. 2009;133:214–222. doi: 10.1093/rpd/ncp048. [DOI] [PubMed] [Google Scholar]
- Terzian A.L., Stahler N. A study of some effects of gamma radiation on the adults and eggs of Aedes aegypti. Biol. Bull. 1958;115:536–550. [Google Scholar]
- UNSCEAR . vol. II. United Nations; New York: 2011. 2008 Report to the General Assembly with Scientific Annexes. (Sources and Effects of Ionizing Radiation). [Google Scholar]
- Vaiserman A.M., Koshel N.M., Litoshenko A.Y., Mozzhukhina T.G., Voitenko V.P. Effects of X-irradiation in early ontogenesis on the longevity and amount of the S1 nuclease-sensitive DNA sites in adult Drosophila melanogaster. Biogerontology. 2003;4:9–14. doi: 10.1023/a:1022460817227. [DOI] [PubMed] [Google Scholar]
- Weidhaas D.E., Schmidt C.H., Chamberlain W.F. IAEA; Vienn: 1962. Radioisotopes and Radiation Entomology; pp. 257–265. [Google Scholar]
- Willard W.K. Long-term effects of acute low-level X-rays on the population dynamics of the yellow fever mosquito, Aedes aegypti. Health Phys. 1965;11:1577–1583. doi: 10.1097/00004032-196512000-00035. [DOI] [PubMed] [Google Scholar]