Abstract
Background
Soil-transmitted helminthiases (STHs) are among the most prevalent afflictions of the developing world, with approximately 2 billion people infected worldwide. Heavily infected individuals suffer from severe morbidity that can result in death. These parasitic diseases also impair physical and mental growth in childhood, thwart educational advancement, and hinder economic development. Periodic deworming with Albendazole or Mebendazole of high-risk groups (school-age children, preschool children, and pregnant women) can significantly lower the levels of infections below the threshold associated with morbidity. However, an important proportion of the population (adults) is excluded from this high-risk group treatment based-strategy, and might lead to the persistence of these diseases in endemic areas despite the repeated treatments. The main objective of this study was to evaluate the contribution of this neglected at-risk group in the spread and persistence of STH in Cameroon.
Methods
A cross sectional survey was conducted in the Akonolinga health district (Centre Region, Cameroon) to assess the prevalence and intensity of these helminth infections. Stool samples were collected from males and females, aged 18 years and over, and analyzed using the Kato-Katz technique.
Results
A total of 334 patients, among which 181 (54.2%) females and 153 (45.8%) males, were examined. The STH of major concern was found in this group of individuals, with overall prevalence equal to 18.0% (95% CI: 14.2–22.4) for Ascaris lumbricoides, 43.7% (95% CI: 38.5–49.1) for Trichuris trichiura, and 7.5% (95% CI: 5.1–10.8) for Necator americanus.
Conclusion
This study reveals that STH infections are prevalent in adults in the Akonolinga health district, with moderate to high risk and light intensity of infection. These infected adults might constitute a potential parasite reservoir and a source of dissemination and persistence of these infections, highlighting the need to really take into account this neglected group of individuals in the mass treatment policy.
Keywords: Akonolinga, Cameroon, Kato-Katz, Soil transmitted helminthiasis, Treatment policy
1. Introduction
Soil-transmitted helminth (STH) infections refer to a group of parasitic diseases caused by nematode worms that are transmitted to humans by fecally-contaminated soil. The STH of major concern to humans are the roundworm (Ascaris lumbricoides), the whipworm (Trichuris trichiura), and the hookworms (Necator americanus and Ancylostoma duodenale). They are among the most common infections worldwide which heavily affect the poorest and most deprived communities where the sanitation is inadequate and water supplies unsafe (WHO, 2012a). Latest estimates indicate that approximately 2 billion people (24% of the world's population) are infected with STH infections worldwide (WHO, 2015). Infections are widely distributed in tropical and subtropical areas, with the greatest numbers occurring in sub-Saharan Africa, the Americas, China and East Asia (WHO, 2015).
Although soil-transmitted helminthiases inflict tremendous disability and suffering, they can be controlled or eliminated. The control of STH is based on the periodic single-dose Albendazole (400 mg) or Mebendazole (500 mg) deworming of at-risk population living in endemic areas. At-risk groups are preschool-age children (aged 1–4 years), school-age children (aged 5–14 years), women of reproductive age (including pregnant women in the second and third trimesters and lactating mothers), and adults particularly exposed to STH infections (for example, tea-pickers and miners) (WHO, 2012a, WHO, 2015).The WHO global target is to eliminate morbidity due to STH in children by 2020, through regular treatments of at least 75% of the 873 million children living in endemic areas (WHO, 2015). To be completely successful, this strategy needs to be supported by the improvement in sanitation and health education. Indeed, healthy behaviors may help reducing transmission and reinfection; provision of adequate sanitation is also important but very challenging in resource-constrained settings (WHO, 2008). As schools provide an important entry point for an appropriate deployment of control activities (periodic deworming, health education, sanitation), efforts are mostly focused on school aged children, the other high risk groups being somehow neglected. This situation appears obvious since anthelminthic drug donations are only available for the treatment of school-age children (Hotez, 2009). However, it was recently demonstrated that age groups other than school-age children can have similar exposure risk, by eating similar food as sibling after weaning, and displaying even greater propensity to have dirty hands in mouth (Addiss, 2015, Hollingsworth, 2015). Mathematical modeling further revealed that it would be better to broaden treatments across all age classes instead of treating school-age children twice a year if one needs to interrupt transmission (Anderson et al., 2015). Furthermore, recent survey mappings conducted in Cameroon after more than 10 years of MDA have shown that the disease still persist with relatively high prevalence and intensities of A. lumbricoides and T. trichiura infections (Tchuem Tchuenté et al., 2012, Tchuem Tchuenté et al., 2013).
The present study then aimed to assess the prevalence and intensity of soil-transmitted helminth infections in adults in the Akonolinga health district (Centre Region, Cameroon), in a context where these infections still persist after more than a decade of intervention.
2. Materials and methods
2.1. Study area and population
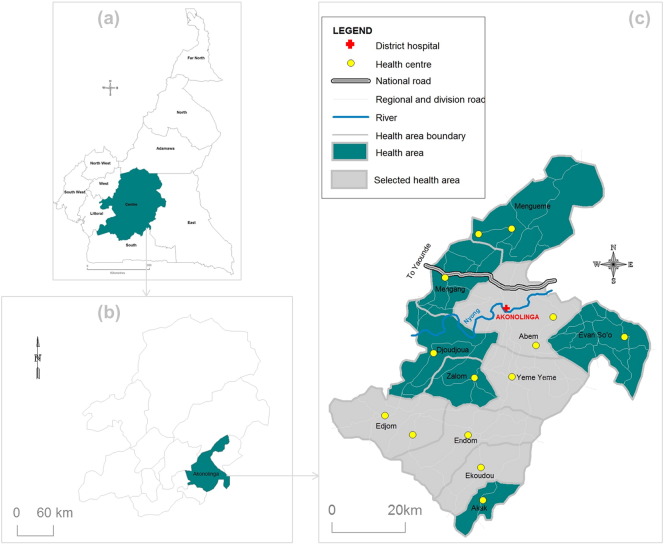
The present study was conducted in the Akonolinga health district (Nyong-et-Mfoumou division, Centre Region, Cameroon). This health district belongs to a forested environment and covers an area of about 4300 km2. The climate is of equatorial type divided into four seasons, with mean temperatures equal to 24.2 °C and mean precipitations of around 1572 mm. The Akonolinga health district covers three subdivisions (Akonolinga, Endom and Mengang) divided into 11 health areas among which five (Abem, Edjom, Ekoudou, Endom and Yeme Yeme) were semi-randomly chosen for the purpose of our survey (Fig. 1). The selection of these health areas was made so that the entire health district was covered spatially. The population of this health district is typically rural, and their main activities are farming (coffee, cocoa, food crops) and fishing (Okalla, 2001). Individuals eligible for this study were both males and females, aged 18 years and over, residing in the selected villages for at least five years.
Fig. 1.
Map showing health areas surveyed (gray zones) in the Akonolinga health district (Centre Region, Cameroon). (a) Location of the Centre Region in Cameroon; (b) location of the Akonolinga health district in the Centre Region of Cameroon; (c) spatial distribution of health areas of the Akonolinga health district.
2.2. Sample collection and processing
In each selected health area, eligible individuals of the selected communities were invited at the health facility and those who agree to participate systematically underwent sample collection. Stool samples were collected to check whether eligible individuals were infected with intestinal helminthes. A 60 mL plastic screw-cap vial was given to each enrollee who returned it to the investigators with stool. These stool samples were examined using the Kato-Katz technique, consisting in a single thick smear technique using a 41.7 mg template (WHO, 1991). The preparations (Kato-Katz slides) were examined by qualified lab technicians, within one hour after slide preparation for identification of hookworm eggs, and subsequently (later the same day or the following day) for other STH eggs or larval (Strongyloides stercoralis) detection. All eggs (or larvae) found in the preparations were identified and counted using bright field microscopy (magnification × 100 or × 400) and the results expressed as eggs or larvae per gram of feces (epg or lpg) for the intensity of infection.
2.3. Data analysis
The data collected were recorded into a purpose-built Microsoft Excel spread sheet and subsequently exported into PASW Statistics version 18 (SPSS Inc., Chicago, IL, USA) for statistical analysis. PASW Statistics “Crosstabs” procedure was used to calculate the proportion of individuals infected by a given parasite species (single infection) or by two or more helminth species (multiple infection). The Wilson score method without the correction for continuity was used to compute the 95% confidence intervals (CIs) (Wilson, 1927), and the Chi square test used to compare these proportions according to sex, age groups as well as health area. PASW Statistics “Descriptives” procedure was used to compute the intensity of infections, when the egg or larval counts were available as arithmetic means; sampling fluctuations were estimated using the standard deviation (sd). Taking into account the over-dispersed nature of such counting data, the non-parametric Mann–Whitney and Kruskal–Wallis tests were used to compare the differences in intensities of single or multiple infections according to sex, age group and health area. The threshold for significance was set at 5% for all statistical analyses.
2.4. Ethics statement
This study was approved by the Cameroon National Ethics Committee for Human Health Research (N° 044/CNE/MP/08). Before enrolment, the objectives and schedule of the study were explained to the eligible population and individuals willing to participate signed an inform consent form. All participants harboring any STH infection received a 400 mg single-dose Albendazole in the framework of this study. Each enrollee was assigned a code and his data analyzed anonymously.
3. Results
In the Akonolinga health district, five health areas were visited during the present survey. A total of 334 individuals aged 18–91 years old (median: 55 years old; Interquartile range, IQR: 41–66) were registered and enrolled in the study. Among these enrollees, 153 (45.8%) were males and 181 (54.2%) were females. All the participants provided stool samples for further analyses.
3.1. Prevalence
Five species of soil transmitted helminthes (A. lumbricoides, T. trichiura, N. americanus, S. stercoralis and Hymenolepis nana) were found in stool samples collected as part of this study. Of the 334 individuals who provided stool samples, 172 (51.5%; 95% CI: 46.2–56.8) harbored at least one of these STH species. Among these infected individuals, 116 (67.4%; 95% CI: 60.1–74.0) harbored a single infection, 46 (26.7%; 95% CI: 20.7–33.8) a double infection, and 10 (5.8%; 95% CI: 3.2–10.4) a triple infection. None of these individuals harbored all the four STH species found in the health district. The overall prevalence in the Akonolinga health district was 18.0% (95% CI: 14.2–22.4) for A. lumbricoides, 43.7% (95% CI: 38.5–49.1) for T. trichiura, 7.5% (95% CI: 5.1–10.8) for N. americanus, and 2.1% (95% CI: 1.0–4.3) for S. stercoralis; only two individuals were infected with H. nana. Table 1 displays the prevalence for each STH species according to the different covariates included in the analysis. The distribution of these parasite species was in general similar in the health district, except for T. trichiura which was more frequent in Abem and Edjom health areas (p < 0.0001) or in men as compared to women (p = 0.004). Also, the prevalence was significantly higher in Edjom and Endom health areas (p = 0.038) for N. americanus, and in Ekoudou and Endom health areas (p = 0.0001) for S. stercoralis.
Table 1.
Prevalence of STHa according to health area, sex and age group.
| Number of individuals examined |
A. lumbricoides (95% CI) | T. trichiura (95% CI) | N. americanus (95% CI) | S. stercoralis (95% CI) | |
|---|---|---|---|---|---|
| Health area | |||||
| Abem | 104 | 23.1% (16.0–32.0) | 67.3% (57.8–75.6) | 1.9% (0.5–6.7) | 0.0% (0.0–3.6) |
| Edjom | 100 | 20.0% (13.3–28.9) | 41.0% (31.9–50.8) | 11.0% (6.2–18.6) | 0.0% (0.0–3.7) |
| Ekoudou | 15 | 13.3% (3.7–37.9) | 26.7% (10.9–52.0) | 0.0% (0.0–0.2) | 13.3% (3.7–37.9) |
| Endom | 73 | 6.8% (3.0–15.0) | 28.8% (19.6–40.0) | 12.3% (6.6–2.2) | 5.5% (2.2–13.3) |
| Yeme Yeme | 42 | 21.4% (11.7–36.0) | 23.8% (13.5–38.5) | 7.1% (2.5–19.0) | 2.4% (0.4–12.3) |
| Sex | |||||
| Male | 153 | 14.4% (9.7–20.8) | 52.3% (44.4–60.1) | 7.2% (4.0–12.4) | 0.7% (0.1–3.6) |
| Female | 181 | 21.0% (15.7–27.5) | 36.5% (29.8–43.7) | 7.7% (4.7–12.6) | 3.3% (1.5–7.0) |
| Age group | |||||
| 18–34 | 53 | 18.9% (10.6–31.4) | 45.3% (32.6–58.5) | 9.4% (4.1–20.2) | 1.9% (0.3–10.0) |
| 35–49 | 67 | 23.9% (15.3–35.3) | 41.8% (30.7–53.7) | 1.5% (0.3–8.0) | 1.5% (0.3–8.0) |
| 50–64 | 108 | 15.7% (10.1–23.8) | 37.0% (28.5–46.5) | 11.1% (6.5–18.4) | 3.7% (1.5–9.1) |
| 65–91 | 106 | 16.0% (10.3–24.2) | 50.9% (41.6–60.3) | 6.6% (3.2–13.0) | 0.9% (0.2–5.2) |
| Overall | 334 | 18.0% (14.2–22.4) | 43.7% (38.5–49.1) | 7.5% (5.1–10.8) | 2.1% (1.0–4.3) |
CI: confidence interval.
The data for H. nana are not presented in this table as only 2 individuals were found to be infected.
3.2. Intensities of infection
The intensity of infection was over-dispersed, ranged between 0 and 148,320 epg (mean: 2277.77; sd: 12,577.30) for A. lumbricoides, 0 and 25,992 epg (mean: 523.33; sd: 2266.82) for T. trichiura, 0 and 4272 epg (mean: 29.82; sd: 260.54) for N. americanus, and 0 and 72 lpg (mean: 0.79; sd: 5.98) for S. stercoralis. Table 2 shows the arithmetic mean (with standard deviation) intensity of infection for each parasite species and according to the different covariates included in the analysis. The mean egg or larval count was quite variable between health areas for T. trichiura, N. americanus and S. stercoralis (p < 0.033), but similar for A. lumbricoides (p = 0.052). According to gender, the intensity of infection was similar between men and women (p > 0.087), except for T. trichiura whose mean egg count was higher in males than in females (p = 0.006). As regards to age group, the intensity of infection was similar for all the STH species (p > 0.109).
Table 2.
Intensity of STHa infection according to health area, sex and age group.
| Number of individuals examined | Mean A. lumbricoides epg (sd) |
Mean T. trichiura epg (sd) |
Mean N. americanus epg (sd) |
Mean S. stercoralis lpg (sd) |
|
|---|---|---|---|---|---|
| Health area | |||||
| Abem | 104 | 4417.15 (19,635.82) | 884.08 (2960.09) | 0.46 (3.31) | 0.00 (0.00) |
| Edjom | 100 | 2580.48 (10,870.33) | 695.52 (2708.69) | 27.12 (120.78) | 0.00 (0.00) |
| Ekoudou | 15 | 323.20 (945.57) | 27.20 (55.08) | 0.00 (0.00) | 3.20 (8.45) |
| Endom | 73 | 271.89 (1692.35) | 167.01 (762.63) | 39.45 (204.72) | 1.97 (8.72) |
| Yeme Yeme | 42 | 444.00 (1298.23) | 16.57 (46.06) | 102.86 (659.02) | 1.71 (11.11) |
| Sex | |||||
| Male | 153 | 794.67 (3438.13) | 627.76 (2608.25) | 36.39 (347.97) | 0.16 (1.94) |
| Female | 181 | 3531.45 (16,709.77) | 435.05 (1935.04) | 24.27 (152.53) | 1.33 (7.89) |
| Age group | |||||
| 18–34 | 53 | 2510.49 (11,682.48) | 674.72 (2284.64) | 12.68 (56.60) | 0.45 (3.304) |
| 35–49 | 67 | 5367.76 (24,460.06) | 813.49 (3505.14) | 0.36 (2.93) | 0.36 (2.93) |
| 50–64 | 108 | 1011.33 (3684.55) | 503.78 (2262.34) | 61.78 (423.85) | 1.56 (8.85) |
| 65–91 | 106 | 1498.64 (5983.74) | 284.15 (806.03) | 24.45 (169.80) | 0.45 (4.66) |
| Overall | 334 | 2277.77 (12,557.30) | 523.33 (2266.82) | 29.82 (260.54) | 0.79 (5.98) |
epg: egg per gram of stool; lpg: larvae per gram of stool; sd: standard deviation.
The data for H. nana are not presented in this table as only 2 individuals were found to be infected.
4. Discussion
During the present study, the STH of major concern have been found in adults living in the Akonolinga health district. Although the overall prevalence of these different STH species follow the same trends as previously described in Cameroon (Tchuem Tchuenté et al., 2012, Tchuem Tchuenté et al., 2013, Nkengazong et al., 2010) and elsewhere (WHO, 2012a, Hotez et al., 2006), the values recorded in this study are relatively high. The results of a recent STH mapping in Cameroon have revealed lower prevalence of these different parasite species in school-aged children of the Centre Region as compared to the prevalence we have observed in our study. This situation might be a consequence of previous mass treatments which may have contributed to reduce the burden of the disease in this high risk group. However, the authors have observed a high heterogeneity across the Region, some sites presenting with prevalence higher than 50% (Tchuem Tchuenté et al., 2012).
We observed that the prevalence were in general similar between gender and age groups, most likely due to the fact that the groups compared are less susceptible and evenly exposed to the parasites. However, significant differences were found between health areas for most of these parasites, Abem and Edjom seemed to be the most affected. Although all these health areas are rural zones where STH infections predominantly occur as previously demonstrated (Hotez et al., 2006), Abem, Edjom and Endom are crossroads town in this rural setting with unplanned slums and squatter settlements which are also ideal for the persistence of STH, especially A. lumbricoides (Crompton and Savioli, 1993).
Regarding the intensity of infections, the values recorded in our study were in general from light intensity following the WHO classification (WHO, 2012a). This was not surprising since individuals included in our study were all adults (or young adults). It was shown that the age-intensity of infection profile is typically convex in form, especially for A. lumbricoides and T. trichiura, with the highest intensities in children aged 5 to 15 years old (Bundy, 1995). Therefore, all the enrollees of our study were on the descending slope of the age-intensity profile curve. Our results show a progressive decrease in intensity of infection for A. lumbricoides and T. trichiura, although the difference in this trend was not significant. This suggests that the accumulation of these STH infections is lower in adults. At the contrary, a progressive increase in N. americanus intensity of infection was observed among age groups, likely due to its mode of transmission which renders adults most exposed to this parasite (Bethony et al., 2001, Brooker et al., 2004). As also observed with the prevalence, the intensity of infection was quite different between health areas. Although the poorly urbanized areas (Abem, Edjom and Endom) are more favorable to the dissemination of A. lumbricoides and T. trichiura, rural areas (Yeme Yeme for example) where agriculture is the main activity seem to be more propitious for hookworms' spread.
The prevalence recorded in this study shows that the surveyed areas are at moderate to high-risk (≥ 20%), and are therefore eligible to preventive chemotherapy (WHO, 2006). In fact, WHO recommends periodic mass deworming to all at-risk people living in endemic areas, administered once a year when the prevalence of STH infections in the community is over 20% (but less than 50%), and twice a year when the prevalence of STH infections in the community is over 50% (WHO, 2015). Unfortunately, due to some logistic constraints in Cameroon and probably elsewhere, only school-aged children are regularly treated (once yearly) (Tchuem Tchuenté et al., 2012, Tchuem Tchuenté et al., 2013). Since a considerable proportion of adults infected with different STH species was observed during our survey in the Akonolinga health district, this might lead to the establishment of an important refuge which may constitute a potential source of dissemination of the parasites in the general population. It was shown that age classes other than school-aged children can have similar exposure risk, by eating similar food as sibling after weaning, and displaying even greater propensity to have dirty hands in mouth (Addiss, 2015, Hollingsworth, 2015). Therefore, if one needs to reduce more efficiently the morbidity of these STH infections, there is a need to take into account the other at risk groups in the mass treatments. Although the complete elimination of STH is not yet among WHO priorities for a nearer future (WHO, 2012b), this cannot be envisioned without taking these adult hosts into consideration. It has been demonstrated using mathematical models that treatment levels and frequency must be much higher, and the breadth of coverage across age classes broader than is typically the current practice, especially if transmission is to be interrupted by mass chemotherapy alone (Truscott et al., 2014). The availability of drugs may appear as a limitation to this strategy since drugs are only donated free of charge for children. However, such study showing very high prevalence in adult hosts might be used to advocate near pharmaceutical company to also donate Albendazole and/or Mebendazole for adult treatment. Also, switching from school-based to community-directed treatments would constitute an opportunity to integrate STH control in the package of other NTDs targeted for control or elimination. Indeed, in areas where onchocerciasis and lymphatic filariasis are endemic, mass treatments with ivermectin (against onchocerciasis) or the combination of ivermectin and Albendazole (against lymphatic filariasis) are given free of charge to individuals aged 5 years and over. This generous drug donation by Merck and Co and GlaxoSmithKline (Hotez, 2009, Hotez et al., 2008) narrows the proportion of the pre-school children and adult population needing deworming treatments, and might facilitate the advocacy near Johnson & Johnson and MedPharm for Albendazole or Mebendazole donation for adults. Finally, the results of a recent mathematical modeling advocate for new treatment guidelines and strategy against STH, indicating that when planning to reduce or eliminate transmission, rather than simply reduce the morbidity, current school-based interventions are unlikely to be enough to achieve the desired results (Truscott et al., 2014).
The present study has revealed that the prevalence of STH infections is relatively high in adult hosts of the Akonolinga health district in Cameroon. Moderate to high risk of STH infection have been observed, the adults sampled harboring light intensity of infection. These infected adult hosts might constitute a potential parasite reservoir and a source of dissemination and persistence of these infections despite the important up to date control efforts deployed. There is an urgent need to really take into account this neglected group of individuals in the mass treatment policy, as has been pointed out by mathematical models.
5. Limitations of the study
The present study was conducted only in one of the 189 health districts of Cameroon. This study then seems to be of local interest although the findings have important implications for the control of STH infections and the design of MDA programs. Also, we didn't attempt to evaluate the contribution of these infected adult in the spread and persistence of STH in the surveyed areas, but these observations are worrying and deserve further investigations. Finally, we didn't record previous individual deworming treatment that might have influence the current figures observed in the present study.
Acknowledgments
The present study was funded by the Centre for Research on Filariasis and other Tropical Diseases (CRFilMT) as part of the Mectizan Donation Program (MDP) support to CRFilMT research activities. We are grateful to the Akonolinga health district population for their participation in this study.
Contributor Information
Jean Bopda, Email: bopda@crfilmt.org.
Hugues Nana-Djeunga, Email: nanadjeunga@crfilmt.org.
Jean Tenaguem, Email: tenaguem@gmail.com.
Joseph Kamtchum-Tatuene, Email: jkamtchum@yahoo.fr.
Raceline Gounoue-Kamkumo, Email: gounoue@crfilmt.org.
Clément Assob-Nguedia, Email: julesclement@yahoo.fr.
Joseph Kamgno, Email: kamgno@crfilmt.org.
References
- Addiss D. Introduction: current strategies and challenges to STH control. Am.J.Trop. Med. Hyg. 2015;93(4):92. (Supplement) [Google Scholar]
- Anderson R.M., Turner H.C., Truscott J.E., Hollingsworth T.D., Brooker S.J. Should the goal for the treatment of Soil Transmitted Helminth (STH) infections be changed from morbidity control in children to community-wide transmission elimination? PLoS Negl. Trop. Dis. 2015;9(8) doi: 10.1371/journal.pntd.0003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethony J., Williams J.T., Kloos H., Blangero J., Alves-Fraga L., Buck G. Exposure to Schistosoma mansoni infection in a rural area in Brazil II. Household risk factors. Tropical Med. Int. Health. 2001;6:136–145. doi: 10.1046/j.1365-3156.2001.00685.x. [DOI] [PubMed] [Google Scholar]
- Brooker S., Bethony J., Hotez P.J. Human hookworm infection in the 21st century. Adv. Parasitol. 2004;58:197–288. doi: 10.1016/S0065-308X(04)58004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy D.A. Epidemiology and transmission of intestinal helminths. In: Farthing M.J.G., Keusch G.T., Wakelin D., editors. Enteric Infection 2, Intestinal Helminths. Chapman & Hall Medical; London: 1995. pp. 5–24. [Google Scholar]
- Crompton D.W., Savioli L. Intestinal parasitic infections and urbanization. Bull. World Health Organ. 1993;71:1–7. [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth D. Implication of epidemiological modelling for STH control programs. Am.J.Trop. Med. Hyg. 2015;93(4):92. (Supplement) [Google Scholar]
- Hotez P.J. Mass drug administration and integrated control for the World's high-prevalence neglected tropical diseases. Clin. Pharmacol. Ther. 2009;85:659–664. doi: 10.1038/clpt.2009.16. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Bundy D.A.P., Beegle K., Brooker S., Drake L., de Silva N. Helminth infections: soil-transmitted helminth infections and schistosomiasis. In: Jamison D.T., Breman J.G., Measham A.R., Alleyne G., Claeson M., Evans D.B., editors. Disease Control Priorities in Developing Countries. The World Bank and Oxford University Press; New York: 2006. pp. 467–482. [Google Scholar]
- Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobson J. Helminths infections: the great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkengazong L., Njiokou F., Wanji S., Teukeng F., Enyong P., Asonganyi T. Prevalence of soil transmitted helminths and impact of albendazole on parasitic indices in Kotto Barombi and Marumba II villages (South-West Cameroon) Afr. J. Environ. Sci. Technol. 2010;4:115–121. [Google Scholar]
- Okalla R. Bulletin de l' Association euro-africaine pour l'anthropologie du changement social et du développement (APAD) 2001. Le district de santé d'Akonolinga. ( http://apad.revues.org/33. Accessed 11 May 2015) [Google Scholar]
- Tchuem Tchuenté L.A., Kamwa Ngassam R.I., Sumo L., Ngassam P., Dongmo-Noumedem C., Luogbou Nzu D.D. Mapping of schistosomiasis and soil-transmitted helminthiasis in the regions of Centre, east and West Cameroon. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchuem Tchuenté L.A., Dongmo-Noumedem C., Ngassam P., Kenfack C.M., Feussom Gipwe N., Dankoni E. Mapping of schistosomiasis and soil-transmitted helminthiasis in the regions of Littoral, North-West, South and South-West Cameroon and recommendations for treatment. BMC Infect. dis. 2013;13:602. doi: 10.1186/1471-2334-13-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott J.E., Hollingsworth T.D., Brooker S.J., Anderson R.M. Can chemotherapy alone eliminate the transmission of soil transmitted helminths? Parasite. Vector. 2014;7:266. doi: 10.1186/1756-3305-7-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO Press; Geneva: 1991. Basic Laboratory Methods in Medical Parasitology. (World Health Organisation) [Google Scholar]
- WHO . WHO Press; Geneva: 2006. Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. (World Health Organization) [Google Scholar]
- WHO Soil-transmitted helminthiasis. Progress report on number of children treated with anthelminthic drugs: an update towards the 2010 global target. Wkly Epidemiol. Rec. 2008;83:237–252. [PubMed] [Google Scholar]
- WHO . WHO Press; Geneva: 2012. Eliminating soil-transmitted helminthiases as a public health problem in children: progress report 2001–2010 and strategic plan 2011–2020. (World Health Organisation) [Google Scholar]
- WHO . WHO Press; Geneva: 2012. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for Implementation. (World Health Organisation) [Google Scholar]
- WHO . WHO Fact Sheet N°366. 2015. Soil-transmitted helminth infections. (Updated May) [Google Scholar]
- Wilson E.B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 1927;22:209–212. [Google Scholar]