Abstract
The prevalence study of Leishmania spp. in hematophagous insects captured from the environment in bat roosts and pigeon nests, or feeding their hosts (cattle, pigs, horses, dogs and humans) in urban, peri-urban and rural areas, between 2012 and 2014. For this study, the amastigotes present in these insects were detected by histochemical and PCR techniques. Positive gene amplification for Leishmania was found in two horseflies of the species Tabanus importunus collected in the environment, and amastigote forms of Leishmania spp., as well as erythrocytes and leukocytes, were histochemically detected in one of that insect. The other analyzed insects were not positive by PCR our by direct parasitological examination. Only horseflies captured in urban and peri-urban areas were positive. During the collection, no phlebotomine sand flies were captured in rural areas far from the city limits. It can be concluded that the discovery of horseflies positive for Leishmania spp. in urban and peri-urban areas indicates the likelihood that urban areas and their surroundings provide vector parasites with an environment suitable for the spread and consequent perpetuation of the biological cycle of this protozoan.
Keywords: Culex spp., Leishmaniasis, Simulium spp., Triatoma sordida, Vectors
1. Introduction
Leishmaniasis is a zoonotic disease widely distributed throughout Brazil, and phlebotomine sand flies act as its main vector (Savani et al., 2009, Silva et al., 2008). This disease, whose epidemiological characteristics are predominantly rural, finds ideal conditions to urbanize its cycle in the precarious sanitary conditions of urban and peri-urban environments (Bevilacqua et al., 2001, Da Silva and Cunha, 2007, Góes et al., 2013).
The northwest region of the state of São Paulo is an endemic area for the occurrence of this protozoan, and natural infection in cats (Coelho et al., 2010, Coelho et al., 2011) as well as coinfection by Leishmania chagasi and Trypanosoma evansi in a dog from this same region have been described. The presence of amastigote forms of Leishmania spp. in a horsefly in this area was also reported for the first time (Coelho and Bresciani, 2013).
In view of the existence of new forms of transmission (Da Silva et al., 2009, De Freitas et al., 2006) and the possible action of new vectors (Coutinho and Linardi, 2007, Coutinho et al., 2005, Otranto and Dantas-Torres, 2010, Paz et al., 2010a, Paz et al., 2010b), the purpose of this study was to use histochemical and polymerase chain reaction (PCR) techniques to evaluate the prevalence of Leishmania spp. in hematophagous dipterans captured in the environment in bat roosts and pigeon nests, or parasitizing cattle, pigs, horses, dogs and humans.
2. Material and methods
2.1. Study sites
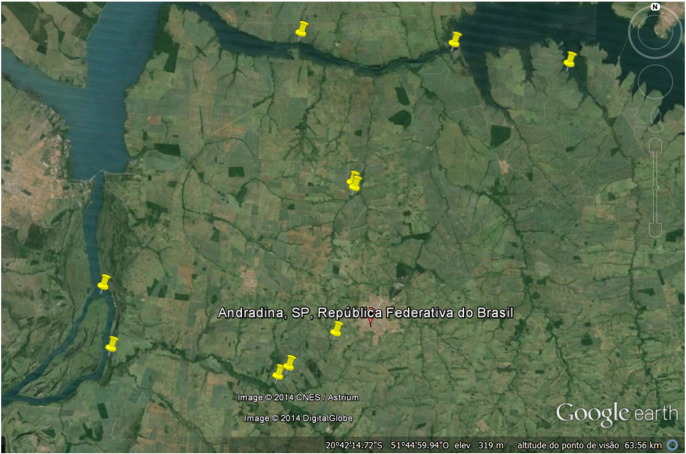
In the period of October 2012 to October 2014, hematophagous insects were captured in urban, peri-urban and rural areas in the municipality of Andradina (20.8961°, 51.37944°, altitude 405 m) (Figs. 1 and 2), in areas close to the Tietê River in the municipalities of Pereira Barreto (20.3818°, 51.0633°, altitude 347 m) and Itapura (20.3846°, 51.3032°, altitude 318 m), and in areas close to the Paraná River in the municipality of Castilho (20.5220°, 51.2915°, altitude 365 m), all in the state of São Paulo, Brazil (Fig. 2).
Fig. 1.
Collection sites of hematophagous insects (yellow markers) in the urban and peri-urban perimeters of the municipality of Andradina, SP, from Oct. 2012 to Oct. 2014.
Legend: yellow markers (collection sites); red arrow (pool of horseflies with gene amplification positive for Leishmania spp.); blue arrow (horsefly with amastigote form of Leishmania spp.).
Fig. 2.
Collection sites of hematophagous insects (yellow markers) in rural areas in the municipalities of Andradina, Pereira Barreto and Castilho, state of Sao Paulo, Brazil, from Oct. 2012 to Oct. 2014.
2.2. Insect capture
A total of 187 horseflies were collected, 55.61% (104/187) of the genus Tabanus spp., 31.5% (59/187) of Chrysops spp., 2.13% (4/187) of Chlorotabanus spp., 4.27% (8/187) of Dicladocera spp. and 6.41% (12/187) of Lepiselaga spp. The insects were captured manually and with entomological nets, during the daytime until twilight, directly from the environment in the proximities of bat roosts and pigeon nests, or on cattle, horses, pigs, dogs and humans. The tabanid flies were classified by consulting taxonomic articles (Benchimol and Sá, 2005, Coscarón and Papavero, 2009).
The 83 black flies (Simulium spp.) that were collected were found only in the vicinity of forests and areas of pastureland close to rural homes as they are parasites of humans during the day and at night.
A total of 77 stable flies (Stomoxys calcitans) were collected from the environment in residential urban, peri-urban and rural areas, and on horses and humans.
To capture phlebotomine sand flies, light traps and carbon dioxide traps were placed in rural areas in the proximities of permanent preservation areas, forests, horse stables, cattle sheds, pigsties, chicken coops and pastures. Collection efforts were carried out at least twice a week. However, no insect was captured in these areas during the period of this study.
A total of 47 mosquitoes (Culex quinquefasciatus and Aedes aegypt) were collected in residential areas within the urban perimeter. A total of 56 culicid flies were also caught in forest areas.
Five specimens of Triatoma sordida and two of Panstrongylus megistus were captured near trash piles, pigsties and chicken coops.
Half of the insects (50%) of each genus were separated into groups, macerated, and sent for PCR tests in a pool of aliquoted samples. The aliquots were subjected to molecular analysis by PCR, using oligonucleotides that amplify the conserved region of the kinetoplast (kDNA) minicircle, using the primers 13A (5′-GTG GGG GAG GGG CGT TCT-3′) and 13B (5′-ATT TTA CAC CAA CCC CCA GTT-3′) (Rodgers et al., 1990). Histological sections were prepared with the remaining 50% of each group of insects, placed on slides and subjected to the standard histological technique, stained with hematoxylin and eosin (HE), and examined under a light microscope with 40 and 100 × magnification.
3. Results
Gene amplification positive for Leishmania was found in the pool of samples of horseflies of the species Tabanus importunus from the peri-urban perimeter (Fig. 1). The histochemical analysis revealed amastigote forms of Leishmania spp. in this species of arthropod, together with erythrocytes and leukocytes (Fig. 3).
Fig. 3.

Microscopic view of an evolutionary amastigote form of Leishmania spp. in a histological section (HE), of a horsefly of the species Tabanus importunus (1000 × magnification).
The other analyzed arthropods showed no positive reaction for PCR, nor for histological examination of tissue sections of insects. Only the T. importunus specimens captured in urban and peri-urban areas tested positive for Leishmania spp. by PCR and histochemistry.
4. Discussion
In this study, DNA of Leishmania spp. was detected in horseflies from urban and peri-urban areas. Based on scientific evidence, it is necessary to consider the possibility of the multiplication of this protozoan in these mechanical vectors under suitable conditions, with perpetuation of the lifecycle of the parasite, which has a significant and diversified number of hosts.
Although the positive tabanid flies in this study were collected directly in the peri-urban environment, amastigote forms of Leishmania spp. were detected in the same municipality in 2013, in a T. importunus horsefly that was captured on an oligosymptomatic dog positive for this protozoan (Coelho and Bresciani, 2013).
Other studies have shown that leishmaniasis may not be transmitted only by the blood meal taken by phlebotomine sand flies (Teichmann et al., 2011). This can also be confirmed by the occurrence of Leishmania in locations where the genus Lutzomyia is not found (Dantas-Torres et al., 2010), suggesting the possibility that there are other vectors (Dantas-Torres, 2006), such as fleas (Paz et al., 2010b) and ticks (Dantas-Torres et al., 2010, Paz et al., 2010a).
In the municipality of Araçatuba, Feitosa et al. (2012) found 4.08% (19/466) of horses seropositive for Leishmania infantum, suggesting that this animal species may be parasitized by phlebotomine sand flies. However, in rural areas far from the urban perimeter, no phlebotomine sand flies were caught in the traps placed near forests, pastures, cattle sheds and pigsties during the two-year period of this study. Conversely, the capture of horse flies, black flies, stable flies and mosquitoes in these places was intense, even from animals and humans.
This fact is noteworthy, given the fact that, in another study by Coelho et al. (Coelho and Bresciani, 2013) in the same municipality, the occurrence of coinfection by T. evansi and Leishmania was observed in a dog from the rural area. No phlebotomine sand flies were captured in this region, but it is an area with a high occurrence of horseflies, stable flies, mosquitoes and black flies.
The absence of phlebotomine sand flies in the rural environments that we found in our study is consistent with the study of Maia-Elkhoury et al. (2008), who reported that the cycle of visceral leishmaniasis has become urbanized due to the environmental changes promoted by humans. These changes include the migration process, the mobilization of wild reservoirs from rural to peri-urban environments, which favors their interaction with infected dogs, and adaptation of the vector Lutzomyia longipalpis to the peri-domicile environment.
5. Conclusions
The discovery of horseflies positive for Leishmania spp. in urban and peri-urban areas augments the possibility that urban areas and their surroundings provide vector parasites with an environment suitable for the spread and consequent perpetuation of the biological cycle of this protozoan.
References
- Benchimol J.L., Sá M.R. 2005. Entomology — Tabanidae. Rio de Janeiro, Fiocruz. [Google Scholar]
- Bevilacqua P.D., Paixão H.H., Modena C.M., Castro M.C.P.S. Urbanização da leishmaniose visceral em Belo Horizonte. Arq. Bras. Med. Vet. Zootec. 2001;53:1–8. [Google Scholar]
- Coelho W.M.D., Bresciani K.D.S. Molecular and parasitological detection of Leishmania spp. in a dipteran of the species Tabanus importunus. Rev. Bras. Parasitol. Vet. 2013;22:605–607. doi: 10.1590/S1984-29612013000400025. [DOI] [PubMed] [Google Scholar]
- Coelho W.M.D., Lima V.M.F., Amarante A.F.T., Langoni H., Pereira V.B.R., Abdelnour A., Bresciani K.D.S. Occurrence of Leishmania (Leishmania) chagasi in a domestic cat (Felis catus) in Andradina, São Paulo, Brazil: case report. Rev. Bras. Parasitol. Vet. 2010;19:256–258. doi: 10.1590/s1984-29612010000400013. [DOI] [PubMed] [Google Scholar]
- Coelho W.M.D., Richini-Pereira V.B., Langoni H., Bresciani K.D.S. Molecular detection of Leishmania sp. in cats (Felis catus) from Andradina municipality, São Paulo state, Brazil. Vet. Parasitol. 2011;10:281–282. doi: 10.1016/j.vetpar.2010.10.052. [DOI] [PubMed] [Google Scholar]
- Coscarón S., Papavero N. Manual of neotropical diptera. Tabanidae. Neotrop. Diptera. 2009;6:1–137. [Google Scholar]
- Coutinho M.T.Z., Linardi P.M. Can fleas from dogs infected with canine visceral leishmaniasis transfer the infection to other mammals? Vet. Parasitol. 2007;147:320–325. doi: 10.1016/j.vetpar.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Coutinho M.T.Z., Bueno L.L., Sterzik A., Fujiwara R.T., Botelho J.R., De Maria M., Genaro O., Linardi P.M. Participation of Rhipicephalus sanguineus (Acari: Ixodidae) in the epidemiology of canine visceral leishmaniasis. Vet. Parasitol. 2005;128:149–155. doi: 10.1016/j.vetpar.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Da Silva L.M.R., Cunha P.R. A urbanização da leishmaniose tegumentar americana no município de Campinas-São Paulo (SP) e região: magnitude do problema e desafios. An. Bras. Dermatol. 2007;82:515–519. [Google Scholar]
- Da Silva S.M., Ribeiro V.M., Ribeiro R.R., Tafuri W.L., Melo M.N., Michalick M.S.M. First report of vertical transmission of Leishmania (Leishmania) infantum in a naturally infected bitch from Brazil. Vet. Parasitol. 2009;166:159–162. doi: 10.1016/j.vetpar.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. Do any insects other than phlebotomine sand flies (Diptera: Psychodidae) transmit Leishmania infantum (Kinetoplastida: Trypanosomatidae) from dog to dog? Vet. Parasitol. 2006;136:379–380. doi: 10.1016/j.vetpar.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Lorusso V., Testini G., De Paiva-Cavalcanti M., Figueredo L.A., Stanneck D., Mencke N., Brandão-Filho S.P., Alves L.C., Otranto D. Detection of Leishmania infantum in Rhipicephalus sanguineus ticks from Brazil and Italy. Parasitol. Res. 2010;106:857–860. doi: 10.1007/s00436-010-1722-4. [DOI] [PubMed] [Google Scholar]
- De Freitas E., Melo M.N., Da Costa-Val A.P., Michalick M.S. Transmission of Leishmania infantum via blood transfusion in dogs: potential for infection and importance of clinical factors. Vet. Parasitol. 2006;137:159–167. doi: 10.1016/j.vetpar.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Feitosa F.L.F., Leal J., Mendes L.C.N., Peiró J.L., Perri S.H.V., Lima V.M.F., Marcondes M. Estudo soroepidemiológico de leishmaniose em equinos na região de Araçatuba-SP, Brasil, área endemic para leishmaniose visceral. 2012. Braz. J. Vet. Res. Anim. Sci. 2012;49:500–502. [Google Scholar]
- Góes M.A.O., Jeraldo V.L.S., Oliveira A.S. Urbanização da leishmaniose visceral: aspectos clínicos e epidemiológicos em Aracajú, Sergipe, Brasil. Rev. Bras. Med. Fam. Comunidade. 2013;9:119–126. [Google Scholar]
- Maia-Elkhoury A.N.S., Alves W.A., Sousa-Gomes M.L., Sena J.M., Luna E. Visceral leishmaniasis in Brazil. Trends and challenges. Cad. Saúde Pública. 2008;24:2941–2947. doi: 10.1590/s0102-311x2008001200024. [DOI] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F. Fleas and ticks as vectors of Leishmania spp. to dogs: caution is needed. Vet. Parasitol. 2010;168:173–174. doi: 10.1016/j.vetpar.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Paz G.F., Ribeiro M.F.B., Michlasky E.M., Da Rocha Lima A.C.V.M., França-Silva J.C., Barata R.A., Fortes-Dias C.L., Dias E.S. Evaluation of the vectorial capacity of Rhipicephalus sanguineus (Acari: Ixodidae) in the transmission of canine visceral leishmaniasis. Parasitol. Res. 2010;106:523–528. doi: 10.1007/s00436-009-1697-1. [DOI] [PubMed] [Google Scholar]
- Paz G.F., Ribeiro M.F.B., De Magalhães D.F., Sathler K.P.B., Morais M.H.F., Fiúza V.O.P., Brandão S.T., Werneck G.L., Fortes-Dias C.L., Dias E.S. Association between the prevalence of infestation by Rhipicephalus sanguineus and Ctenocephalides felis felis and the presence of anti-Leishmania antibodies: a case–control study in dogs from a Brazilian endemic area. Prev. Vet. Med. 2010;97:131–133. doi: 10.1016/j.prevetmed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Rodgers M.R., Popper S.J., Wirth D.F. Amplification of kinetoplast DNA as tool in the detection and diagnosis of Leishmania. Exp. Parasitol. 1990;71:267–275. doi: 10.1016/0014-4894(90)90031-7. [DOI] [PubMed] [Google Scholar]
- Savani E.S.M.M., Nunes V.L.B., Galati E.A.B., Castilho T.M., Zampieri R.A., Floeter-Winter L.M. The finding of Lutzomyia almerioi and Lutzomyia longipalpis naturally infected by Leishmania spp. in a cutaneous and canine visceral leishmaniases focus in Serra da Bodoquena, Brazil. Vet. Parasitol. 2009;160:18–24. doi: 10.1016/j.vetpar.2008.10.090. [DOI] [PubMed] [Google Scholar]
- Silva A.M., Camargo N.J., Santos D.R., Massafera R., Ferreira A.C., Postai C., Cristóvão E.C., Konolsaien J.F., Bisseto A., Jr., Perinazo R., Teodoro U., Galati E.A.B. Diversidade, distribuição e abundância de flebotomíneos (Diptera: Psycodidae) in Paraná State, Southern Brazil. Neotrop. Entomol. 2008;37:209–225. doi: 10.1590/s1519-566x2008000200017. [DOI] [PubMed] [Google Scholar]
- Teichmann C.E., Da Silva A.S., Monteiro S.G., Barbosa C.F., Barcelos R. Evidence of venereal and transplacental transmission of canine visceral leishmaniasis in southern Brazil. Acta Sci. Vet. 2011;39:1–4. [Google Scholar]