Abstract
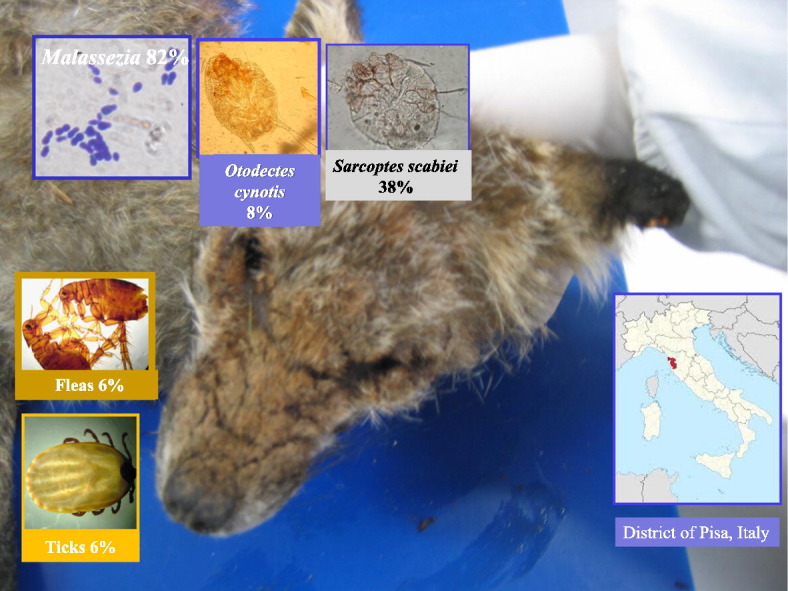
Fifty red foxes (Vulpes vulpes) from the district of Pisa (central Italy) were examined for ectoparasites. Sarcoptic mange was diagnosed on the presence of clearly visible skin lesions with confirmatory demonstration of Sarcoptes scabiei at parasitological and histopathological analysis. Ticks and fleas were collected directly from the carcases during post mortem examination, fixed and identified by morphological examination. For the detection of ear Malassezia and mite infections, cytological and parasitological examinations of ear wax samples were performed. All data were statistically analysed using a χ2 test with the Yates correction. An overall prevalence of 84% for ectoparasitic infections was found in examined subjects. In regard to isolated ectoparasites, 38%, 8%, 82%, 6% and 8% of foxes resulted positive for S. scabiei, Otodectes cynotis, Malassezia spp., fleas (Archaeopsylla erinacei, Pulex irritans, Ctenocephalides canis) and ticks (Ixodes ricinus and Rhipicephalus sanguineus), respectively. Malassezia ear infection was significantly more prevalent in animals older than 1 year (P < 0.01). Prevalence (38%), severity of lesions and poor body conditions observed in most Sarcoptes-infected animals indicate that sarcoptic mange should be considered the most important ectoparasitic infection of red foxes in the examined area.
Keywords: Central Italy, Ectoparasites, Prevalence, Red fox (Vulpes vulpes), Sarcoptic mange
Graphical abstract
Highlights
-
•
An overall prevalence of 84% for ectoparasitic infections was found in 50 Italian red foxes.
-
•
Red foxes were infected by ticks, mites, fleas and Malassezia yeasts.
-
•
Auricular infection with Malassezia was significantly more prevalent in animals older than 1 year.
-
•
Malassezia was almost constantly associated with the presence of Sarcoptes mites and skin lesions.
-
•
Based on prevalence, lesions and body condition, sarcoptic mange was the most relevant disease.
1. Introduction
Red foxes are the most widespread wild carnivores in Italy and the rest of Europe (Magi et al., 2015) and represent a possible reservoir of zoonotic, anthropophilic and domestic animal ectoparasites, such as Ixodes ricinus, Sarcoptes scabiei and Pulex irritans (Márquez et al., 2009, Sréter et al., 2003). In the same way, red foxes may act as reservoirs for vector-borne infections in domestic animals and humans (Márquez et al., 2009, Kaewmongkol et al., 2011, Torina et al., 2013). Among ectoparasitic infections, sarcoptic mange is a well-known cause of severe disease and high mortality rates in red foxes and it is often responsible for a rapid decline in population densities (Sréter et al., 2003, Davidson et al., 2008, Devenish-Nelson et al., 2014, Forchhammer and Asferg, 2000). Available data on ectoparasitic infections of European red fox populations in Italy are very limited. With the exception of a single report on flea species identified in red foxes from southern Italy (Torina et al., 2013) and on sarcoptic mange in red foxes from the western Italian Alps (Balestrieri et al., 2006), informations are almost absent in Italy. The aim of the present study was to investigate ectoparasitic species and prevalence of ectoparasitic infections in a red fox population living in central Italy (Pisa, Tuscany).
2. Materials and methods
2.1. Study area and population
From January to December 2010, 50 red foxes of both genders (20 males and 30 females) and different ages were examined. All examined subjects were shot during the regular hunting season in the Province of Pisa, central Italy (43°N, 10–11°E), and chilled at 4 °C until examination, that was carried out within 24 h. The sampling area is characterised by large woodlands, farmland and hills with an elevation ranging from 4 m to 576 m above the sea level. With an area of 2,448 km2 and a total population of 421,642 inhabitants, the area is densely populated (from about 8 to 865 inhabitants/km2). In this area, red fox population density is of approximately 1.2–1.6 red foxes/km2 (Verin et al., 2010). Higher fox densities (up to 2.6 red foxes/km2) are found in hilly and farmland areas often near urban and peri-urban settings (Mazzarone & Poli, 2012–2015). Age determination of red foxes was achieved based on the weight of dried eye lenses as previously reported (Cavallini & Santini, 1995).
2.2. Parasite sampling
All foxes underwent a complete post mortem examination during which body score was assessed based on the amount of retrobulbar and perirenal fat and the presence or absence of muscular atrophy. More precisely, fox body condition was scored as 1) excellent—excellent amount of fat and no muscle atrophy, 2) average—medium to small amount of fat and no muscle atrophy, 3) poor—no retrobulbar and perirenal fat and no muscle atrophy, and 4) cachectic—no retrobulbar and perirenal fat with muscle atrophy. Ticks and fleas were collected directly from the carcases at post mortem examination. Collected arthropods were fixed in 70% ethanol and identified microscopically with or without Hoyer's solution. For their identification, keys and description reported by Manilla (Manilla, 1998) and Cringoli et al. (Cringoli et al., 2005) for ticks and by Berlinguer (Berlinguer, 1964) for fleas, were used. Cytological and parasitological examination of earwax samples collected by means of cotton swabs were performed in order to detect Malassezia and ear mites' (Demodex spp. and Otodectes cynotis) infections, respectively. Diagnosis of Malassezia otitis was achieved when more than 10 blastospores/microscopic field (mean number of 10 fields) at 400 × were observed in Diff Quick® (Medion Diagnostics AG, Düdingen, Switzerland) stained smears. Sarcoptic mange was diagnosed based on the presence of clearly visible lesions on the skin with confirmatory demonstration of S. scabiei by means of parasitological and histopathological analysis. Skin samples were collected for histopathology at post mortem examinations together with other organ samples (liver, spleen, kidneys, lungs and heart), in order to rule out any concomitant diseases that could have affected the body condition score. These samples were fixed in 10% buffered formalin, processed and paraffin embedded for routine histopathology. Four-μm serial sections were cut and stained with haematoxylin-eosin for general examination. A scoring system of distribution and severity of sarcoptic mange associated lesions was obtained based on a previous published classification (Nimmervoll et al., 2013). At histopathological examination of skin samples from subjects with lesions consistent with sarcoptic mange, the presence of bacteria and yeasts was also evaluated. For parasitological analysis of mange infections, microscopic examination of skin scrapings and ear wax samples at 40 × under a dissecting microscope and at 100 × and 400 × under an optical microscope was performed for the presence of mites and eggs, then digested in boiling 10% NaOH, centrifuged and microscopically examined (at 100 × and 400 ×) according to the method described by Sréter et al. (Sréter et al., 2003). Malassezia yeasts found in skin histological sections and in ear wax samples were identified by their morphology (Griffin et al., 2007, Outerbridge, 2006), while Otodectes and Sarcoptes mites were identified according to Sweatman (Sweatman, 1958) and Fain (Fain, 1968), respectively.
2.3. Statistical analysis
Data of different parasitic species isolated and biological data of red foxes sampled were statistically analysed using the statistical package SPSS Advanced Statistics 13.0 (SPSS Inc., Chicago, IL, USA). A χ2 test with the Yates correction was chosen as reference test. Statistical significance was considered when P < 0.05.
3. Results
According to age determination, 27 out of 50 red foxes examined in this study were classified as juveniles (< 1 year), while the remaining 23 foxes were adults (> 1 year). Overall, 84% (42/50) of examined red foxes were found to be positive for ectoparasites. In particular, 82% (41/50) of animals resulted positive for Malassezia spp., compatible with Malassezia pachydermatis by morphology, from ears and 16% (8/50) from skin lesions, 8% (4/50) of foxes resulted positive for Otodectes cynotis, 6% (3/50) were positive for fleas (Archaeopsylla erinacei erinacei 2%, P. irritans 2% and Ctenocephalides canis 2%), 8% (4/50) resulted positive for ticks (I. ricinus 6% and Rhipicephalus sanguineus 2%), while 38% (19/50) of foxes resulted positive for S. scabiei (Table 1). Lice and Demodex mites were not isolated. Except for S. scabiei, a low number (≤ 10) of parasites per infected fox were counted in regard to all other isolated arthropod species. A significant positive correlation was found between the age of foxes and Malassezia in ears (P < 0.01), with Malassezia ear infections more prevalent in animals older than 1 year old. In the S. scabiei infected red foxes, gross lesions were distributed mainly on the tail and focally on the distal legs (pattern 1), on the back and neck as well as the previously described regions (pattern 2) or diffusely including the head and ears (pattern 3) (Table 1). Sarcoptic lesions ranged from focally extensive moderately hyperkeratotic, proliferative and scaling dermatitis (type A), associate with few mites at histopathology, in 31% (6/19) of the infected subjects, to generalised severe hyperkeratotic lesions with thick crusts and numerous mites (type B) in 47% (9/19) of infected foxes or focal alopecic lesions without crusting and very rare mites observed (type C), in almost 21% (4/19) of the S. scabiei positive animals (Table 1; Fig. 1.1,2). Histopathological lesions were characterised by different degrees of epidermal edema, degeneration and necrosis, and moderate to severe areas of spongiosis, hyperkeratosis, multifocal parakeratosis and sero-cellular crusts (Fig. 1.3). Dermal changes included edema, superficial, often perivascular to interstitial infiltration of eosinophils and mast cells, lymphocytes and macrophages. Focally, cutaneous ulcerations could be observed with increased number of neutrophils and plasma cells around dense bacterial colonies of bacillary elements that were often associated, on the skin, with numerous bottle-shaped yeasts showing broad based budding, consistent with M. pachydermatis by morphology. Malassezia yeasts were constantly associated with hyperkeratotic lesions and the presence of a large number of S. scabiei mites (type B lesions). Large numbers of mites were seen multifocally and mainly associated with more severe hyperkeratotic and proliferative lesions (type B lesions). Mites were found embedded in the epidermal layer or free in the surface of the epidermis (Fig. 1.4). Focally, inside adult female mites, numerous eggs were observed.
Table 1.
Results of the 19 Sarcoptes-positive red foxes (Vulpes vulpes) from the Province of Pisa (Tuscany, central Italy) divided according to age (in year), sex, positivity for Malassezia overgrowth in ears, body score, presence of Malassezia (M) or bacteria (B) in histological sections of skin, gross distribution patterna and gross/histopathological scoreb of Sarcoptes-related cutaneous lesions according to Nimmervoll et al. (Nimmervoll et al., 2013).
| Fox N° | Age | Sex | Malassezia (ear) | Cutaneous gross lesion distribution patterna | Cutaneous gross/histopathological lesion scoreb | Malassezia and/or bacteria at histology | Body score |
|---|---|---|---|---|---|---|---|
| 3 | < 1 | M | Pos | 1 | A | B | 2 |
| 9 | > 1 | F | Pos | 2 | B | M, B | 3 |
| 10 | < 1 | F | Neg | 2 | B | M, B | 3 |
| 14 | > 1 | M | Pos | 2 | A | – | 3 |
| 21 | > 1 | M | Pos | 1 | C | – | 2 |
| 22 | > 1 | F | Pos | 2 | A | – | 3 |
| 24 | < 1 | F | Pos | 1 | A | – | 2 |
| 25 | < 1 | F | Pos | 2 | B | M | 3 |
| 26 | > 1 | F | Pos | 3 | B | – | 3 |
| 27 | > 1 | F | Pos | 3 | C | – | 2 |
| 28 | < 1 | F | Pos | 2 | B | M, B | 3 |
| 31 | > 1 | F | Pos | 1 | C | M | 2 |
| 32 | < 1 | M | Pos | 2 | B | – | 3 |
| 36 | < 1 | M | Pos | 2 | C | – | 4 |
| 37 | < 1 | F | Pos | 3 | A | – | 2 |
| 38 | > 1 | F | Pos | 2 | B | M, B | 4 |
| 42 | < 1 | F | Pos | 2 | B | M, B | 3 |
| 44 | > 1 | M | Pos | 2 | B | M, B | 3 |
| 46 | > 1 | F | Pos | 1 | A | B | 2 |
1: only tail and/or hind limbs; 2: in addition to the aforementioned regions, the back and/or thorax affected; 3: in addition to the aforementioned regions, neck and/or head involved.
A: early stage (thin crusts, focally extensive distribution, few mites; B: fatal form (thick crusts, hyperkeratosis, lichenification, diffuse distribution, numerous mites); C: healing form (alopecia, no crusts, focal distribution, hyperpigmentation, very rare mites).
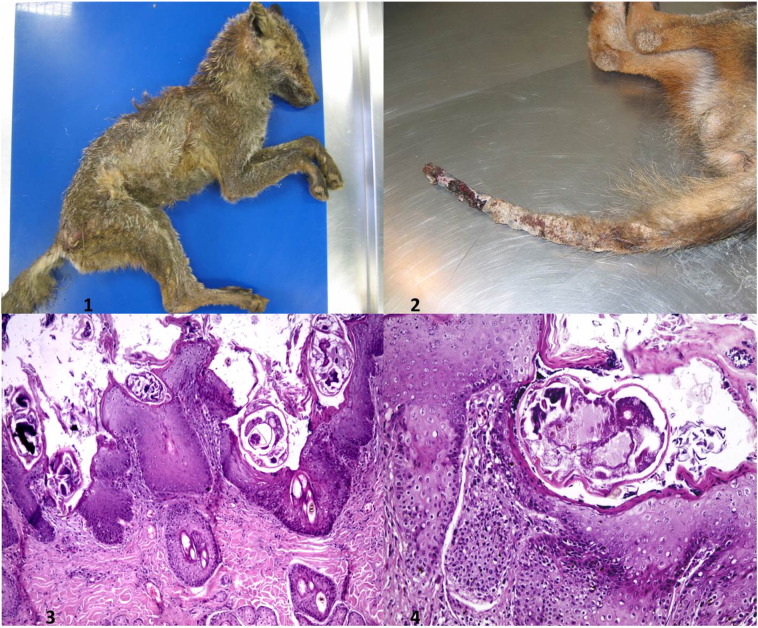
Fig. 1.
Examples of gross and histopathological lesions observed in Sarcoptes- positive (19/50) red foxes (Vulpes vulpes) from central Italy examined for ectoparasitic infections.
1. Red fox (Vulpes vulpes) No 9. Example of pattern 3 distribution of gross lesions associated with sarcoptic mange infection distributed throughout the entire body surface and characterised by diffuse severe hyperkeratotic, proliferative and exudative dermatitis;
2. Red Fox (Vulpes vulpes) No 46. Example of pattern 1 distribution of gross lesions associated with sarcoptic mange infection, confined to the tail and hind limbs characterised by moderate to severe multifocal to coalescing areas of ulceration, alopecia and crusting;
3. Red Fox (Vulpes vulpes) No 38. Section of haired skin including epidermis and superficial dermis with diffuse severe orthokeratotic hyperkeratosis, hyperplasia and multifocal areas of parakeratosis associated with several cross sections of mites embedded in the epidermis or free on the epidermal layer along with numerous bacteria. Dermal changes include moderate multifocal perivascular to interstitial mixed inflammatory infiltrates. (H&E; Ob. 20X);
4. Red Fox (Vulpes vulpes) No 38. Haired skin, epidermis showing severe diffuse orthokeratotic hyperkeratosis, parakeratosis, hyperplasia, degeneration, spongiosis and one embedded cross section of an adult mite. (H&E; Ob. 40 ×).
In a Sarcoptes infected animal, diffusely in the renal glomeruli a focally extensive deposit of acellular, finely fibrillar to waxy, pale eosinophilic material, Congo red positive and consistent with amyloid was also observed. The amyloid was primarily present in the mesangial areas and in the subendothelium of glomerular capillaries, eventually expanding to the interstitium dissecting through surrounding tubules and vessels.
4. Discussion
In Europe, red fox density is highly variable (IUCN, 2015). In the UK, density ranges between 0.98 and 4.7 subjects/km2 with an average of 2.04 individuals/km2 (Harris et al., 1995), but can be as high as 30–58 foxes per km2 in some urban areas where food is superabundant (Devenish-Nelson et al., 2014, IUCN, 2015). Fox density in Switzerland is about 1–3 fox per km2 or 0.3–0.4 family per km2 (IUCN, 2015, Weber et al., 1999). In other European countries, red fox density ranges between 0.1 and 3.2 subjects/km2 (García Peiró et al., 2009). Density of fox population in the studied area is about 1.2–1.6 red foxes/km2 (Verin et al., 2010), but higher densities are found in hilly and farmland areas near urban and peri-urban settings (Mazzarone & Poli, 2012–2015). Thus, red fox density in the examined area should be considered similar or higher than in other European areas.
A high prevalence of ectoparasitic infections was found in the examined red fox population, and most of the isolated species have zoonotic potential (Sréter et al., 2003). However, in the examined red fox population, intensity and prevalence of tick, flea and O. cynotis infestations were low. I. ricinus was the most prevalent (6%) and common tick species encountered among examined animals, while only a single fox was found infected by R. sanguineus. Indeed, the red fox is considered a particularly important host for the maintenance and the geographical distribution of I. ricinus, due to foxes being able to host all three different developmental stages of I. ricinus (Mackenstedt et al., 2015). Foxes can also carry a large number of ticks, may migrate over long distances, and are often attracted by peri-urban and urban areas (Mackenstedt et al., 2015), as in the area examined in this study. Nevertheless, prevalence of I. ricinus found in the examined fox population was lower than that found in other European countries, such as Spain, France, Germany and Hungary (Sréter et al., 2003, Dominguez-Penafiel et al., 2011). Although reported, R. sanguineus has not been frequently observed within European wild fox populations during previous studies (Martínez-Carrasco et al., 2007). All the flea species isolated in this study, i.e. the human flea P. irritans, the dog flea C. canis and the hedgehog flea A. erinacei erinacei, have been previously reported in foxes from other European countries (Sréter et al., 2003, Dominguez-Penafiel et al., 2011), while of these species only C. canis has been previously reported in red foxes from Italy (Torina et al., 2013). According to Sréter et al. (Sréter et al., 2003), the isolation of P. irritans and C. canis may be indicative of the urbanisation of foxes and of closer contact with animals living in the rural and suburban areas. However, the prevalence (6%) of flea infections found in this study was lower when compared with previous data (Sréter et al., 2003, Dominguez-Penafiel et al., 2011). This finding could be explained by the variable time frame between the death of the animals and the post mortem examination in which the foxes were stored refrigerated. On the contrary, the prevalence (8%) of O. cynotis, the ear canker mite of many domestic and wild carnivores (Lohse et al., 2002), found in free-ranging red foxes in this study is consistent with previous data reported for other European red fox populations (Sréter et al., 2003), showing low to medium (2–17%) prevalence rates. S. scabiei is the etiological agent of sarcoptic mange in wild and domestic mammals and of scabies in humans and it is considered as a single species divided into several varieties which show a certain degree of host specificity (Fain, 1978, Zahler et al., 1999). Epidemiological studies have shown the occurrence of cross-transmission episodes between wild and domestic animal species and zoonotic infections of S. scabiei in humans (Arlian, 1989, Alasaad et al., 2013, Reintería-Solís et al., 2014). Evidence of sarcoptic mange transmission from foxes to dogs has been documented (Devenish-Nelson et al., 2014), and this is one of the most frequently diagnosed diseases in free ranging red fox populations in Europe (Sréter et al., 2003, Reintería-Solís et al., 2014). Data from epidemiological studies (Devenish-Nelson et al., 2014) suggest that juveniles are more prone to infection due to less effective immune systems and increased nutritional stress from independent foraging and that they encounter infected individuals more often than adults due to their specific ranging pattern. The prevalence of sarcoptic mange observed in the present study (38%) is higher if compared with the previous data reported in free ranging red foxes in most European countries (14–25%) (Sréter et al., 2003, Davidson et al., 2008) and in northern Italy (25.3%) (Balestrieri et al., 2006) although in some other countries considerably higher prevalence rates (about 45–67%) have been reported (Al-Sabi et al., 2014, Smith, 1978). In regard to the high contagiousness of sarcoptic mange, the parasite can spread in the fox population, particularly among cubs and youngs within the fox family and to other susceptible animals. Considering that density of red fox population examined in the present study is higher in areas near urban and peri-urban settings, the possibility of direct spreading from these foxes to domestic dogs should be considered as a possible event. The spread of sarcoptic mange in the fox population can cause severe episodes of mortality with a sudden population decline, as observed in Bristol during the period 1994–1996 (Baker et al., 2000). Studies performed during this epizootic of sarcoptic mange have demonstrated that in the fox population territorial ranging patterns change rapidly and the home range of a died fox is quickly occupied by the neighbour foxes causing a sudden reduction of population density (Potts et al., 2013). In our study, despite the high prevalence of sarcoptic mange detected, no reduction in fox population density was observed (Mazzarone & Poli, 2012–2015). Approximately one month after exposure, infected foxes commonly develop skin lesions characterised by hyperkeratosis (Bornstein et al., 1995). Severe loss of hair and progressive deterioration of body condition eventually follows and infected foxes may die within two to three months due to starvation (Nimmervoll et al., 2013, Little et al., 1998). In the present study, a body score of 3 and 4, with absence of retrobulbar and perirenal fat without or with muscle atrophy respectively, was mainly associated with severe crusting and the presence of more than 3 mites/high power field at histopathology (type B lesions), suggesting that S. scabiei was primarily involved in the pathogenesis of the generalised wasting observed in examined red foxes. The gross lesions and the histopathological findings described in the examined population are consistent with the previous study from Nimmervoll et al. (Nimmervoll et al., 2013) in which three main presentations, i.e. focally extensive thin crusting, diffuse thick crusting and focal alopecia without crusts, were observed and associated with different degrees of S. scabiei infection intensity. Glomerular amyloidosis observed at histopathology in a severely S. scabiei infected animal included in this study, is a further finding confirming previous data (Arlian et al., 1990, Burgess, 1994) regarding the frequent observation of amyloid deposits in internal organs of chronically S. scabiei-infected subjects. Another data from this study that is consistent with the previous studies (Nimmervoll et al., 2013, Salkin et al., 1980), is the almost constant association of skin yeasts with the presence of thick crusting and numerous mites, while the finding of bacteria on skin lesions was associated only with the presence of ulcers and crusting on the skin and not with the presence of mites. This observation further reinforces the hypothesis (Salkin et al., 1980) according to which mites might act as a carrier for Malassezia yeasts through the exoskeleton, and further suggests a possible secondary pathogenic effect of yeasts contributing to the progression of sarcoptic lesions. Malassezia yeasts are lipophilic organisms which are recognised members of the normal skin flora (Salkin et al., 1980, Boekhout et al., 2010). On the other hand, Malassezia overgrowth is a very well known cause of several skin diseases of humans as well as a wide range of warm-blooded animals, including domestic and wild canids (Salkin et al., 1980, Boekhout et al., 2010). With regard to Malassezia, a very high percentage (82%) of foxes examined in the present study was found also positive for Malassezia overgrowth at cytological analysis of ear cerumen.
In conclusion, the high prevalence of ectoparasitic infections found in the red fox population living in the province of Pisa (Tuscany, central Italy) may suggest that in this area the red fox may act as a reservoir host for several ectoparasites, among which are included potentially zoonotic species and arthropod species that are reservoirs of vector-borne infections. In addition, as previously reported for other European red fox populations (Sréter et al., 2003), based on prevalence (38%), severity of lesions and poor body conditions of most examined S. scabiei-infected animals, sarcoptic mange should be considered as the most important ectoparasitic infection of the red fox also in the area considered in this study.
Acknowledgements
The authors thank the Italian Ministry of University and Research (MIUR) for financing this study (grant number PRIN 2010P7LFW4_004).
References
- Magi M., Guardone L., Prati M.C., Mignone W., Macchioni F. Extraintestinal nematodes of the red fox Vulpes vulpes in north-west Italy. J. Helminthol. 2015;89:506–511. doi: 10.1017/S0022149X1400025X. [DOI] [PubMed] [Google Scholar]
- Márquez F.J., Millán J., Rodríguez-Liébana J.J., García-Egea I., Muniain M.A. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med. Vet. Entomol. 2009;23:393–398. doi: 10.1111/j.1365-2915.2009.00830.x. [DOI] [PubMed] [Google Scholar]
- Sréter T., Széll Z., Varga I. Ectoparasite infestations of red foxes (Vulpes vulpes) in Hungary. Vet. Parasitol. 2003;115:349–354. doi: 10.1016/s0304-4017(03)00216-4. [DOI] [PubMed] [Google Scholar]
- Kaewmongkol G., Kaewmongkol S., Fleming P.A., Adams P.J., Ryan U., Irwin P.J., Fenwick S.G. Zoonotic Bartonella species in fleas and blood from red foxes in Australia. Vector Borne Zoonotic Dis. 2011;11:1549–1553. doi: 10.1089/vbz.2011.0646. [DOI] [PubMed] [Google Scholar]
- Torina A., Blanda V., Antoci F., Scimeca S., D'Agostino R., Scariano E., Piazza A. A molecular survey of Anaplasma spp., Rickettsia spp., Ehrlichia canis and Babesia microti in foxes and fleas from Sicily. Transbound. Emerg. Dis. 2013;60:125–130. doi: 10.1111/tbed.12137. [DOI] [PubMed] [Google Scholar]
- Davidson R.K., Bornstein S., Handeland K. Long-term study of Sarcoptes scabiei infection in Norwegian red foxes (Vulpes vulpes) indicating host/parasite adaptation. Vet. Parasitol. 2008;156:277–283. doi: 10.1016/j.vetpar.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Devenish-Nelson E.S., Richards S.A., Harris S., Soulsbury C., Stephens P.A. Demonstrating frequency-dependent transmission of sarcoptic mange in red foxes. Biol. Letters. 2014;10 doi: 10.1098/rsbl.2014.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forchhammer M.C., Asferg T. Invading parasites cause a structural shift in red fox dynamics. Proc. R. Soc. Lond. 2000;267:779–786. doi: 10.1098/rspb.2000.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrieri A., Remonti L., Ferrari N., Ferrari A., Lo Valvo T., Robetto S., Orusa R. Sarcoptic mange in wild carnivores and its co-occurrence with parasitic helminths in the Western Italian Alps. Eur. J. Wildlife Res. 2006;52:196–201. [Google Scholar]
- Verin R., Poli A., Ariti G., Nardoni S., Bertuccelli Fanucchi M., Mancianti F. Detection of Leishmania infantum DNA in tissues of free-ranging red foxes (Vulpes vulpes) in Central Italy. Eur. J. Wildlife Res. 2010;56:689–692. [Google Scholar]
- V. Mazzarone, A. Poli. Provincia di Pisa. Piano Faunistico Venatorio Provinciale 2012–2015. http://www.provincia.pisa.it/uploads/PianoFaunisticoVenatorioProvinciale.pdf
- Cavallini P., Santini S. Age determination in the red fox in a Mediterranean habitat. Z. Saugetierkd. 1995;60:136–142. [Google Scholar]
- Manilla G. Vol. 36. Edizioni Calderini; Bologna, Italy: 1998. Acari Ixodida Fauna d'Italia. [Google Scholar]
- Cringoli G., Iori A., Rinaldi L., Veneziano V., Genchi C. Rolando Editore; Napoli, Italy: 2005. Zecche Mappe Parassitologiche. [Google Scholar]
- Berlinguer G. 1964. Aphaniptera D'Italia Studio Monografico, Il Pensiero Scientifico. Roma, Italy. [Google Scholar]
- Nimmervoll H., Hoby S., Robert N., Lommano E., Welle M., Ryser-Degiorgis M.P. Pathology of sarcoptic mange in red foxes (Vulpes vulpes): macroscopic and histologic characterization of three disease stages. J. Wildl. Dis. 2013;49:91–102. doi: 10.7589/2010-11-316. [DOI] [PubMed] [Google Scholar]
- Griffin J.W., Scott D.W., Erb H. Malassezia otitis externa in the dog: the effect of heath-fixing otic exudate for cytological analysis. J. Vet. Med. 2007;54:424–427. doi: 10.1111/j.1439-0442.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- Outerbridge C.A. Mycologic disorders of the skin. Clin. Tech. Small Anim. Pract. 2006;21:128–134. doi: 10.1053/j.ctsap.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Sweatman G.K. Biology of Otodectes cynotis, the ear canker mite of carnivores. Can. J. Zool. 1958;36:849–862. [Google Scholar]
- Fain A. Study of the variability of Sarcoptes scabiei with a revision of the sarcoptidae. Acta Zool. Pathol. Antverp. 1968;47:1–196. [Google Scholar]
- IUCN The IUCN Red List of Threatened Species Version 2015.4, Vulpes vulpes. 2015. http://www.iucnredlist.org/details/23062/0 Retrieved March 16, 2016. from.
- Harris S., Morris P., Wray S., Yalden D. Joint Nature Conservation Committee; Peterborough: 1995. A Review of British Mammals: Population Estimates and Conservation Status of British Mammals Other than Cetaceans.http://jncc.defra.gov.uk/pdf/pub03_areviewofbritishmammalsall.pdf [Google Scholar]
- Weber J.M., Meia J.S., Meyer S. Breeding success of the red fox Vulpes vulpes in relation to fluctuating prey in central Europe. Wildlife Biol. 1999;5(4):247–250. [Google Scholar]
- García Peiró I., Robledano Aymerich F., Esteve Selma M.Á. Abundancias y densidades relativas de zorro Vulpes vulpes (Linnaeus, 1758) en un humedal del sudeste ibérico: implicaciones para la conservación de sus poblaciones. Anales de Biología. 2009;31:43–48. [Google Scholar]
- Mackenstedt U., Jenkins D., Romig T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 2015;4:71–79. doi: 10.1016/j.ijppaw.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Penafiel G., Gimenez-Pardo C., Gegundez M.I., Lledo L. Prevalence of ectoparasitic arthropods on wild animals and cattle in the Las Merindades area (Burgos, Spain) Parasite. 2011;18:251–260. doi: 10.1051/parasite/2011183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Carrasco C., Ruız De Ybáñez M.R., Sagarmınaga J.L., Garıjo M.M., Moreno F., Acosta I., Hernandez S. Parasites of the red fox (Vulpes vulpes Linnaeus, 1758) in Murcia, southeast Spain. Rev. Med. Vet. 2007;158:331–335. [Google Scholar]
- Lohse J., Rinder H., Gothe R., Zahler M. Validity of species status of the parasitic mite Otodectes cynotis. Med. Vet. Entomol. 2002;16:133–138. doi: 10.1046/j.1365-2915.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- Fain A. Epidemiological problems of scabies. Int. J. Dermatol. 1978;17:20–30. doi: 10.1111/j.1365-4362.1978.tb06040.x. [DOI] [PubMed] [Google Scholar]
- Zahler M., Essig A., Gothe R., Rinder H. Molecular analyses suggest monospecificity of the genus Sarcoptes (Acari: Sarcoptidae) Int. J. Parasitol. 1999;29:759–766. doi: 10.1016/s0020-7519(99)00034-x. [DOI] [PubMed] [Google Scholar]
- Arlian L.G. Biology, host relations, and epidemiology of Sarcoptes scabiei. Annu. Rev. Entomol. 1989;34:139–161. doi: 10.1146/annurev.en.34.010189.001035. [DOI] [PubMed] [Google Scholar]
- Alasaad S., Rossi L., Heuckelbach J., Pérez J.M., Hamarsheh O., Otiende M., Zhu X.Q. The neglected navigating web of the incomprehensibly emerging and re-emerging Sarcoptes mite. Infect. Genet. Evol. 2013;17:253–259. doi: 10.1016/j.meegid.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Reintería-Solís Z., Min A.M., Alasaad S., Müller K., Michler F.U., Schmäschke R., Wittstat U. Genetic epidemiology and pathology of raccoon-derived Sarcoptes mites from urban areas of Germany. Med. Vet. Entomol. 2014;28:98–103. doi: 10.1111/mve.12079. [DOI] [PubMed] [Google Scholar]
- Al-Sabi M.N., Halasa T., Kapel C.M. Infections with cardiopulmonary and intestinal helminths and sarcoptic mange in red foxes from two different localities in Denmark. Acta Parasitol. 2014;59:98–107. doi: 10.2478/s11686-014-0214-6. [DOI] [PubMed] [Google Scholar]
- Smith H.J. Parasites of red foxes in Brunswick and Nova Scotia. J. Wildl. Dis. 1978;14:366–370. doi: 10.7589/0090-3558-14.3.366. [DOI] [PubMed] [Google Scholar]
- Baker P.S., Funk M., Harris S., White P.C.L. Flexible spatial organization of urban foxes, Vulpes vulpes, before and during an outbreak of sarcoptic mange. Anim. Behav. 2000;59:127–146. doi: 10.1006/anbe.1999.1285. [DOI] [PubMed] [Google Scholar]
- Potts J.R., Harris S., Giuggioli L. Quantifying behavioral changes in territorial animals caused by sudden population declines. The Am. Naturalist. 2013;182:E73–E82. doi: 10.1086/671260. [DOI] [PubMed] [Google Scholar]
- Bornstein S., Zakrisson G., Thebo P. Clinical picture and antibody response to experimental Sarcoptes scabiei var. vulpes infection in red foxes (Vulpes vulpes) Acta Vet. Scand. 1995;36:509–519. doi: 10.1186/BF03547665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S.F., Davidson W.R., Rakich P.M., Nixon T.I., Bounous D.I., Nettles V.E. Response of red foxes to first and second infection with Sarcoptes scabiei. J. Wildl. Dis. 1998;34:600–611. doi: 10.7589/0090-3558-34.3.600. [DOI] [PubMed] [Google Scholar]
- Arlian L.G., Bruner R.H., Stuhlman R.A., Ahmed M., Vyszenski-Moher D.L. Histopathology in hosts parasitized by Sarcoptes scabiei. J. Parasitol. 1990;76:889–894. [PubMed] [Google Scholar]
- Burgess I. Sarcoptes scabiei and scabies. Adv. Parasitol. 1994;33:235–292. doi: 10.1016/s0065-308x(08)60414-5. [DOI] [PubMed] [Google Scholar]
- Salkin I.F., Stone W.B., Gordon A. Association of Malassezia (Pityrosporum) pachydermatis with sarcoptic mange in New York State. J. Wildl. Dis. 1980;16:509–514. doi: 10.7589/0090-3558-16.4.509. [DOI] [PubMed] [Google Scholar]
- Boekhout T., Guého-Kellermann E., Mayser T., Velegraki A. Springer–Verlag; Berlin, Germany: 2010. Malassezia and the Skin: Science and Clinical Practice. [Google Scholar]