Abstract
Oral transmission of Trypanosoma cruzi is a frequent cause of acute Chagas disease (ChD). In the present cross-sectional study, we report the epidemiological, clinical, serological and molecular outcomes of the second largest outbreak of oral ChD described in the literature. It occurred in March 2009 in Chichiriviche de la Costa, a rural seashore community at the central littoral in Venezuela. The vehicle was an artisanal guava juice prepared at the local school and Panstrongylus geniculatus was the vector involved. TcI genotype was isolated from patients and vector; some showed a mixture of haplotypes. Using molecular markers, parasitic loads were high. Eighty-nine cases were diagnosed, the majority (87.5%) in school children 6–15 years of age. Frequency of symptomatic patients was high (89.9%) with long-standing fever in 87.5%; 82.3% had pericardial effusion detected by echocardiogram and 41% had EKG abnormalities. Three children, a pregnant woman and her stillborn child died (5.6% mortality). The community was addressed by simultaneous determination of specific IgG and IgM, confirmed with indirect hemagglutination and lytic antibodies. Determination of IgG and IgA in saliva had low sensitivity. No individual parasitological or serological technique diagnosed 100% of cases. Culture and PCR detected T. cruzi in 95.5% of examined individuals. Based on the increasing incidence of oral acute cases of ChD, it appears that food is becoming one of the most important modes of transmission in the Amazon, Caribbean and Andes regions of America.
Keywords: Chagas disease, Oral transmission, Saliva, Foodborne, Chichiriviche de la Costa, Venezuela
Graphical abstract

1. Introduction
Oral transmission of Trypanosoma cruzi has become the most frequent cause of acute cases of Chagas disease (ChD) in Brazil (Pinto et al., 2008, Shikanai-Yasuda and Carvalho, 2012, Coura, 2015) and in Venezuela (Alarcón de Noya et al., 2015). Andrade et al. (Andrade et al., 2014) registered 73 reports of acute ChD in Brazil during the past ten years contrasting with 41 cases that were reported in the previous 20 years (1981–2001). In Venezuela, 249 cases were reported since 2007 (Alarcón de Noya et al., 2015), and there were six new cases in 2015 alone, suggesting the progressive increase of oral outbreaks of ChD in the Amazon, Caribbean and Andes regions (Alarcón de Noya & Noya, 2015).
The acute phase of ChD has been traditionally considered difficult to diagnose due to the nonspecific clinical symptoms (Bastos et al., 2010). However, when the outbreaks of orally transmitted ChD occur in families or schools, the discovery of a case leads to the diagnosis of individuals at risk. The severe episode of the oral acute phase of ChD is the result of the host–pathogen interaction (Andrade et al., 2014, Cardillo et al., 2015) in which parasite inoculum, its genetic composition and the host's immune response are involved. The reasons for which the symptoms are so severe and mortality so high in oral acute cases remains unknown and the unexpected appearance of this medical emergency have limited the ability of the immunological studies to explain these pathogenic mechanisms.
The first large oral outbreak of ChD occurred in an urban school in a middle class area of Caracas, Venezuela in 2007. The outbreak resulted in an astonishing 103 infected individuals (Alarcón de Noya et al., 2010). Soon after, in March 2009, a second occurrence of fever and myocarditis arose, mainly in children, again in a school, from Chichiriviche de la Costa, a rural and touristic seashore community located on the central Venezuelan coast (Alarcón de Noya & Martínez, 2009). The aim of the present work is to report the epidemiological and clinic characteristics as well as the diagnostic procedures, the immunoglobulin isotype's response in serum and saliva and the Trypanosoma cruzi molecular characterization of the second largest outbreak of ChD described in the literature, highlighting the importance of this entity as a foodborne disease.
2. Materials and methods
2.1. Study population
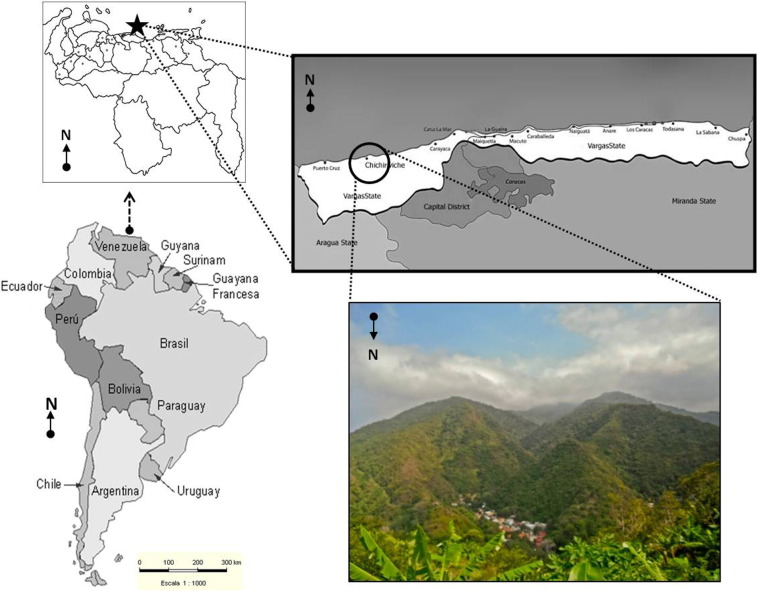
It consisted of students, teachers and administrative personnel from a school located in Chichiriviche de la Costa. Fig. 1 shows the location of the school (10°31′53.97″N 67°15′36.02″W), which belongs to Vargas state at the central-north littoral in Venezuela, a small touristic town with a group of houses on the seashore and the town 1 km away, nestled in the mountain.
Fig. 1.
Geographical location of Chichiriviche de la Costa, Vargas State in Venezuela. A coastal place hidden in the mountains in the north central littoral of Venezuela.
The simultaneous appearance of prolonged febrile syndrome in tens of children called the attention of the epidemiologist who thought about the presence of acute oral ChD (OChD) based on a previous experience in the Venezuelan Municipality of Chacao (Alarcón de Noya et al., 2010, Alarcón de Noya and Martínez, 2009). Once the first 20 serum samples were diagnosed at the “Instituto de Medicina Tropical (IMT), Universidad Central de Venezuela” in Caracas, the health authorities cordoned off the area and sampled the entire population. The National Health Department organized a door-to-door investigation, interviewing the population. Blood samples were sent to three Venezuelan reference centers, including the IMT.
2.2. Interview
Data was recorded from all infected persons and from a group of non-infected persons regarding their link to the school, their ingestion of artisanal juices in the school, their knowledge of triatomines at home and their contact with them, the type and duration of symptoms and whether or not they were hospitalized.
2.3. Clinical management
Patients were seen at various health centers and a specific protocol was not applied to everyone. In general, laboratory tests were completed as well as electrocardiogram (EKG) and echosonography when necessary.
2.4. Laboratory diagnosis
A complete screening was done by taking one blood sample by venipuncture for serologic tests (IgM and IgG) to quickly identify the cases. From positive serological persons, a second blood sample was taken for culture and PCR as well as a saliva sample.
2.4.1. Immunoenzymatic assay
An in-house immunoenzymatic assay (ELISA) was performed with a T. cruzi epimastigotes delipidized antigen and processed using Maxisorp plates (Díaz-Bello et al., 2008). ELISAs involving anti-human IgM and IgG alkaline phosphatase conjugates were carried out simultaneously in all sera from individuals at risk. ELISA for saliva was achieved for the detection of specific IgG and IgA antibodies, as well as for serum IgA.
2.4.2. Fresh and stained blood smears
Direct fresh and Giemsa thin smears were done from some blood samples.
2.4.3. Indirect hemagglutination (IHA)
Previously described methods were used in the IHA procedure (Jacobs & Lunde, 1957) in which the delipidized T. cruzi epimastigotes antigen was adsorbed by fresh sheep red cells.
2.4.4. Lytic antibodies (LA)
They were performed with trypomastigotes from cellular cultures based on Krettli et al. methodology (Krettli, 2009). After standardization in our laboratory, over 22% of lysis was considered a positive test.
2.4.5. Cultures
Aliquots of 2 mL blood samples collected by venipuncture in sodium citrate tubes were added to bacto agar blood base culture medium and observed every 10 days. Once the cultures were found to be positive for epimastigotes, they were transferred to liver infusion tryptose medium with 10% fetal bovine serum (Mourão & Mello, 1975).
2.4.6. Molecular studies
2.4.6.1. Polymerase chain reaction (PCR)
5 mL of patients' blood was mixed with an equal volume of 6 M guanidine HCl/0.2 mM EDTA and processed according to Schijman et al. (Schijman et al., 2003). The PCR amplifications targeted a 330-bp fragment of T. cruzi kinetoplastid minicircle DNA (Sturm et al., 1989).
2.4.6.2. Parasitic load
It was assessed by duplex real time PCR quantification using Satellite DNA (SatDNA) qPCR and kinetoplastid DNA (kDNA) qPCR assays as described (Duffy et al., 2013) and modified (Qvarnstrom et al., 2012). Standard curves were plotted with 1/10 serial dilutions of total DNA obtained from a GEB-seronegative sample that was spiked with 105 parasite equivalents per milliliter of blood (par. eq./mL).
2.4.6.3. Molecular typing of T. cruzi isolates
Genomic DNA from T. cruzi isolates was extracted and genotyped by PCR using three molecular targets: the intergenic region of the mini-exon gene (SL-IR gene), the D7 divergent domain of the 24Sa rRNAas (Souto et al., 1996) and the size-variable domain of the 18S rRNA sequence (Clark & Pung, 1994). To detect the different TcI SL-IR genotypes, the amplified products were excised from the gels, purified and cloned into a pGEM-T easy Vector (Promega, USA). Both strands of at least three clones per sample were sequenced.
2.5. Case definition
A confirmed case of ChD was any individual with parasites in their peripheral blood and all individuals with at least two positive serologic tests based on different methods (WHO, 2002). Consequently, in this study, oral transmission cases included all individuals related to the school, either with or without parasitemia, showing positive results in ELISA (IgG/IgM) and at least one other positive confirmatory test (IHA).
2.6. Statistical analysis
For this cross-sectional study, we used SPSS 19 for Windows (Version 19.0; Copyright SPSS Inc., 2010). In order to determine whether there was an association between variables on study, clinical and epidemiological factors (except duration of fever) were expressed as categorical variables and grouped in contingency tables to analyze prevalence, odds ratio (OR) with its respective 95% confidence interval (95% CI), and the chi-square test (χ2). Continuous variables were analyzed by the t test for independent samples. Statistical significance was considered when p < 0.05.
2.7. Ethics
This study protocol was approved by the Scientific Ethic Committee of the “IMT, Universidad Central de Venezuela”. Each patient or legal representative signed, after reading the term of free and informed consent, agreed the taking of samples and physical and other para-clinical exams.
3. Results
3.1. Demographic characteristics of the population
A serologic screening based on simultaneous determination of specific anti-T. cruzi IgG and IgM antibodies was achieved to 238 females (53.9%) and 203 males (46%). By that time, a 7 year old child had died with high fever and myocarditis. This case is taken into account for the confirmed cases and attack rate but does not count for the laboratory outcomes (Table 1). Of the 89 infected persons (51 females and 38 males), 3 children, an over 20 week pregnant woman and her stillborn child died. The mortality was of 5 people (5.6%). The age distribution of cases was asymmetrically concentrated between 6 and 15 years (87.5%). In the > 25 year old group, two asymptomatic persons not related to the school were found IgG positive and were considered as chronic cases or past infection.
Table 1.
Age distribution of population, IgG and IgM antibodies response, prevalence and mortality attributable to oral Chagas disease, Chichiriviche de la Costa, Venezuela, 2009.
| Age | Population | Antibodies response in examined population |
Confirmed n (%) | Mortality | |
|---|---|---|---|---|---|
| IgG | IgM | ||||
| 0–5 | 39 | 1/38 | 0 | 0 | 1 (stillborn) |
| 6–10 | 102a | 50 | 52 | 59 (58.4) | 2 |
| 11–15 | 72 | 18 | 18 | 19 (26.4) | 1 |
| 16–20 | 48 | 2 | 1 | 2 (4.2) | 0 |
| 21–25 | 19 | 2 | 2 | 2 (10.5) | 1 |
| > 25 | 161 | 9b | 7 | 7 (4.3) | 0 |
| Total | 441 | 82 | 80 | 89 (20.2) | 5 |
One 7 year-old boy died with fever and myocarditis before performing tests.
Two asymptomatic adults, chronic cases with IgG +, not belonging to the school.
3.2. Risk factors
We obtained information on the consumption of artisanal juices in the school from 83 infected persons and 30 non-infected (Table 2). All positive patients consumed any type of juice at school and this fact was statistically associated with T. cruzi acute infection (p < 0.05). When the type of juice consumed is compared between infected and uninfected people, the guava juice was statistically rationed to OChD (p < 0.05); results of OR suggest a direct relation between oral infection by T. cruzi and the habit of drinking artisanal guava juice and no other type of juice.
Table 2.
Risk factors in persons with and without oral Chagas disease, Chichiriviche de la Costa, Venezuela, 2009.
| Risk factor |
T. cruzi infection |
Statistical parameter |
|||||
|---|---|---|---|---|---|---|---|
| Infected (n = 83) |
Uninfected (n = 30) |
χ2 | p | OR | 95% CI | ||
| Consumption and type of juice | Consumption of juice | 83 (100%) | 23 (76.7%) | 20.646 | 0.000 | … | … |
| Guava juice | 61 (73.5%) | 13 (43.3%) | 8.869 | 0.003 | 3.626 | 1.517–8.665 | |
| Melon juice | 41 (49.4%) | 10 (33.3%) | 2.296 | 0.130 | 1.952 | 0.816–4.672 | |
| Passion fruit juice | 44 (53.0%) | 10 (33.3%) | 3.420 | 0.064 | 2.256 | 0.943–5.402 | |
| Tamarind juice | 45 (54.2%) | 11 (36.7%) | 2.715 | 0.099 | 2.045 | 0.866–4.829 | |
| Peach or soursop juice | 36 (43.4%) | 9 (30.0%) | 1.645 | 0.200 | 1.787 | 0.731–4.367 | |
| Pineapple juice | 38 (45.8%) | 10 (33.3%) | 1.398 | 0.237 | 1.689 | 0.705–4.045 | |
| Watermelon juice | 35 (42.2%) | 8 (26.7%) | 2.246 | 0.134 | 2.005 | 0.800–5.027 | |
| Orange juice | 36 (43.4%) | 8 (26.7%) | 2.587 | 0.108 | 2.106 | 0.841–5.276 | |
| Papaya juice | 34 (41.0%) | 7 (23.3%) | 2.963 | 0.085 | 2.280 | 0.880–5.910 | |
| Age | Age ≤ 18 years-old | 80 (89.9%) | 9 (10.1%) | 51.942 | 0.000 | 9.847 | 4.792–20.233 |
| Relation with school | School-related | 88 (98.9%) | 1 (1.1%) | 56.252 | 0.000 | 101.538 | 13.21–780.76 |
χ2 of Pearson calculated with one degree of freedom; OR: odds ratio; 95% CI: 95% confidence interval.
Another risk factor evaluated was the relation with school and acute infection by T. cruzi. From 89 patients, 88 were school-related. The one left was an indigent man who sporadically visited the school. We found statistical significance (p = 0.000) and direct relation between the fact of belonging to the school and the risk to acquire OChD as suggested by OR results (Table 2). Age ≤ 18 years-old also was a risk factor for OChD in Chichiriviche de la Costa, since from 89 infected, 80 (80.9%) were under 18 years of age (p = 0.000) (Table 2). We questioned 94 individuals, 44 referred to have observed sometime triatomines in houses or in the school. Some P. geniculatus were collected by persons of the community.
3.3. Symptomatology
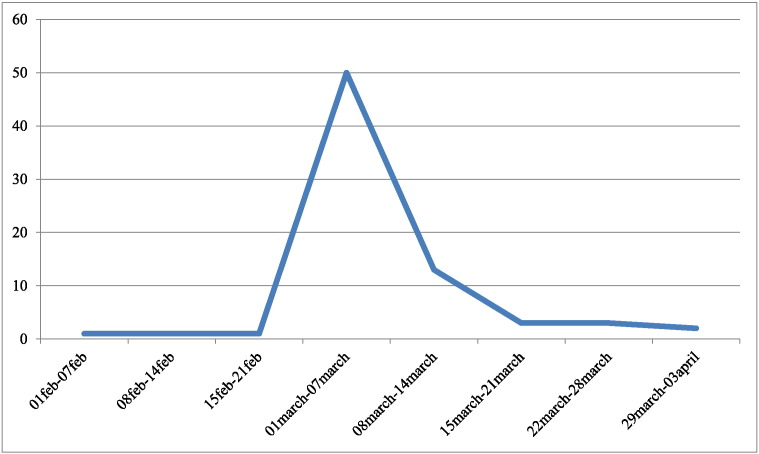
From the 89 patients with OChD, 80 (89.9%) presented any symptomatology. Fever started at the beginning of March 2009, 77 out of 88 patients presented this symptom (Table 3). Results of OR (95% CI) suggest a direct relation between fever and presence of infection. In patients with OChD, the fever had mean duration of 11.4 days (range 2–23 days), while in a serologic negative group (n = 22) that declared fever, it lasted 4.3 days (2–10). The t-test demonstrated statistical difference between duration of fever between infected and non-infected people (p = 0.02). A graphical overview on the timeline of the microepidemic shows the spatial distribution of diagnosed cases (Fig. 2).
Table 3.
Symptomatology in 88 persons with acute oral Chagas disease. Chichiriviche de la Costa, Venezuela, 2009.
| Symptoms | n (%) |
|---|---|
| Fever | 77 (87.5%) |
| Cardiac | 23 (26.1%) |
| Respiratory | 7 (8.0%) |
| Gastro-intestinal | 26 (29.5%) |
| Edema | 25 (28.4%) |
| Adenopathy | 5 (5.7%) |
| Weakness, dizziness, asthenia | 10 (11.4%) |
| Other symptoms | 38 (43.2%) |
Fig. 2.
Timeline of the onset of fever in oral transmitted Chagas disease patients, Chichiriviche de la Costa, Venezuela, 2009.
Cardiac symptoms included dyspnea, precordial pain and palpitations; dry cough, odynophagia and dysphonia among respiratory symptoms; and abdominal pain, nausea, vomiting and diarrhea into the gastro-intestinal symptoms. The frequency of these symptoms in the confirmed cases is shown in Table 3. Twenty-five infected individuals presented edema (Table 3); in 22, the edema committing the entire face; two (one of 11 year-old and one of 21 year-old) had anasarca and one 34 year-old woman presented only edema in limbs.
3.4. Cardiac outcomes
Most of the 80 echocardiograms performed resulted with any alteration (98.8%) with varying degrees of pericardial effusion (82.3%), 14 had left ventricle hypertrophy (15.7%) with or without pericardial effusion (Table 4). There was not statistical significance (p = 0.980) between altered echocardiogram according to age in patients with acute OChD. Fig. 3 shows facial edema, thoracic X Ray and echocardiogram of the pregnant women who died in this outbreak.
Table 4.
Echographical and electrocardiographical findings in 89 patients with acute oral Chagas disease, Chichiriviche de la Costa, Venezuela, 2009.
| Age | Cardiological tests |
|||||
|---|---|---|---|---|---|---|
| Echocardiogram |
Electrocardiogram |
|||||
| Infected | Realized | Pericardial effusion | HLVa | Realized | Abnormal | |
| 6–10 | 59 | 56 | 44 | 10 | 44 | 16 |
| 11–15 | 19 | 18 | 13 | 4 | 11 | 7 |
| 16–20 | 2 | 1 | 1 | 0 | 1 | 0 |
| 21–25 | 2 | 1 | 1 | 0 | 1 | 0 |
| > 25 | 7 | 4 | 4 | 0 | 4 | 2 |
| Total | 89 | 80 | 65 | 14 | 61 | 25 |
Hypertrophy of left ventricle.
Fig. 3.
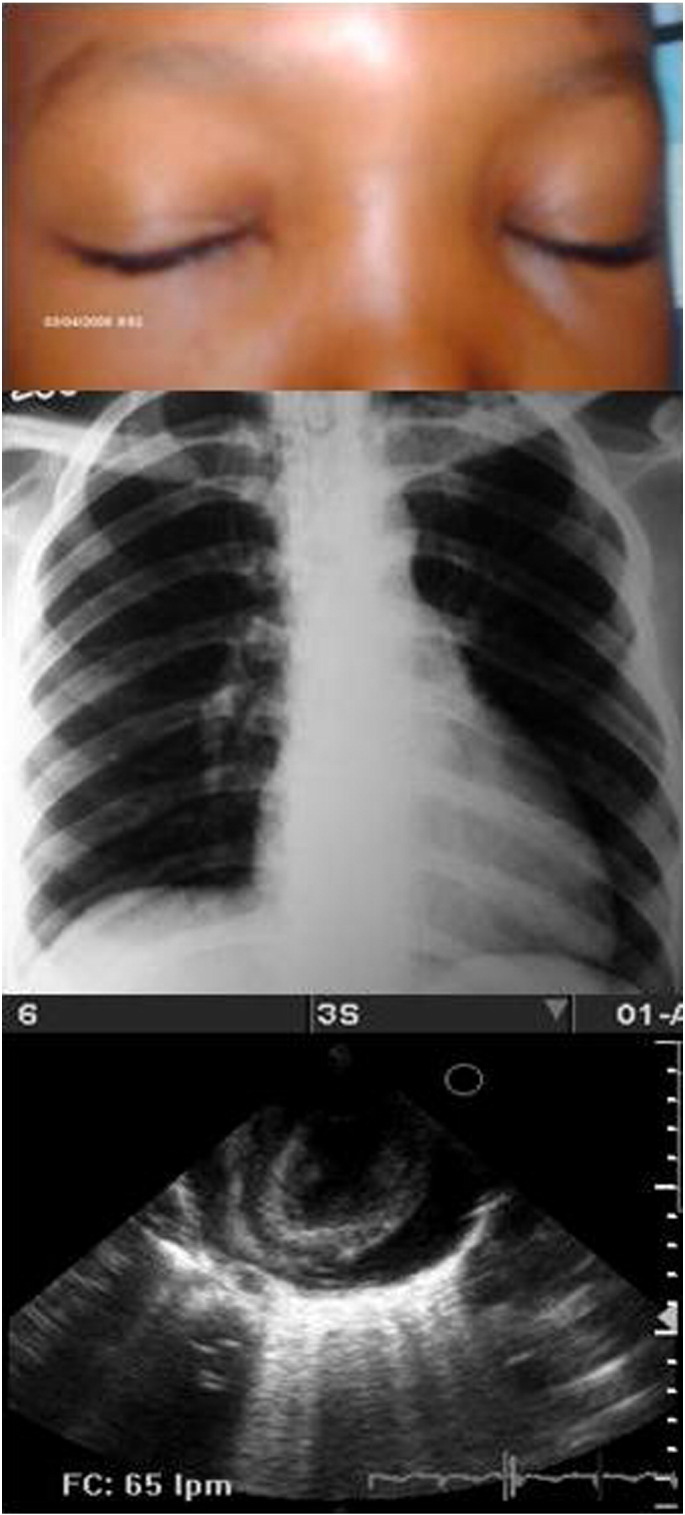
A fatal orally-transmitted Chagas disease case: a pregnant cook lady 24 years old. Noticeable facial edema. X Ray: cardiothoracic relationship > 50% without signs of pulmonary hypertension. Cardiac enlargement due to pericardial effusion. Echosonogram: short axis projection where pericardial effusion and increased left ventricular wall are evidenced by edema.
Of the 61 EKGs, 25 (41%) had some kind of alteration, especially in children (92%). Yet, there was not statistical significance (p = 0.374) with continuous age and altered EKG. Hospitalization occurred in 73 patients and they were spread in at least 5 hospitals.
3.5. Laboratory diagnosis
The first 20 serum samples were received at the IMT (April 6th, 2009), 4–6 weeks after the onset of symptoms one week later the samples of the entire population at risk (n = 440) arrived and were searched for IgG and IgM anti-T. cruzi antibodies. All other tests (IHA, LA, serum IgA, IgG and IgA in saliva, cultures and PCR) were performed on samples from confirmed cases. T. cruzi parasites were seen in fresh and stained thin blood smears of 14 non-treated hospitalized persons. Fresh preparations were examined from all the tubes taken for culture (n = 67), but, in the middle of the emergency these results were not recorded. Table 5 shows the results of laboratory tests on serum, saliva and blood distributed by age group of confirmed cases indicating the denominators in each case. No parasitological or serological test diagnosed 100% of those positive. The IHA and the determination of LA were the tests with highest sensitivity (95.4% and 92.7%, respectively). Sensitivity was 80.9% for both IgM and IgG. Serum IgA was positive in 73 patients out of 83 diagnosed (87.9%). Instead, both IgG and IgA in saliva resulted with very low sensitivity (22.9% and 36.4%, respectively).
Table 5.
Laboratory tests performed in serum, saliva and blood by age range groups in confirmed cases, Chichiriviche de la Costa, Venezuela, 2009.
| Age | Sera |
Saliva |
Blood |
||||||
|---|---|---|---|---|---|---|---|---|---|
| IgM | IgG | IgA | IHA | LA | IgG | IgA | Culture | PCR | |
| 0–5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6–10a | 52/58 | 50/58 | 50/56 | 54/58 | 45/49 | 11/50 | 16/50 | 33/43 | 25/32 |
| 11–15 | 18/19 | 18/19 | 15/18 | 19/19 | 13/13 | 2/17 | 6/17 | 13/16 | 10/12 |
| 16–20 | 1/2 | 2/2 | 2/2 | 2/2 | 1/1 | 0/1 | 1/1 | 1/2 | 0/0 |
| 21–25 | 2/2 | 2/2 | 1/1 | 2/2 | 0/0 | 0/0 | 0/0 | 2/2 | 1/2 |
| > 25 | 7/7 | 7/7 | 5/6 | 7/7 | 5/6 | 4/6 | 4/6 | 2/4 | 4/4 |
| Total | 80/88 | 79/88 | 73/83 | 84/88 | 64/69 | 17/74 | 27/74 | 51/67 | 40/50 |
One 7 year-old boy died before performing tests; IHA: Indirect hemagglutination; LA: lytic antibodies positives/examined.
Blood samples for culture and PCR were taken from the same individuals, but 17 of the PCR were lost. T. cruzi was found in cultures of 51 patients out of 67 (76.1%) taken from the 88 confirmed cases and parasite DNA was detectable in 40 out of 50 patients examined (80%). Positive cultures and positive PCR agreed on 27 people. Direct or indirect demonstration of the parasite by these means was possible in 95.5% of the evaluated individuals.
3.6. Molecular characterization
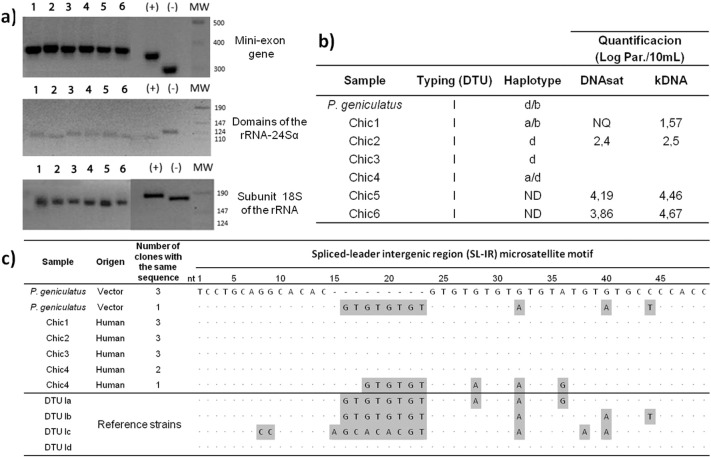
The PCR analysis of the mini-exon gene, domains of the rRNA-24Sα and subunit 18S of the rRNA, revealed that the T. cruzi DTU in this environment is TcI (Fig. 4a and b). Analysis of the SL-IR gene for haplotype identification shows heterogeneity in the circulating populations of T. cruzi: 40% of strains are haplotype TcId and the remaining 60% is identified as multiclonal. Isolate Chic1 is TcIa + TcIb, isolate Chic 4 is TcIa + TcId and isolate from P. geniculatus is TcIb + TcId (Fig. 4b and c). The values of parasitic loads of four random samples of T. cruzi infected individuals in this outbreak, are in the range of the 2.4–4.19 Log Par./10 mL and 1.57–4.67 Log Par./10 mL by the DNAsat and kDNA qPCR methods (clinical sensitivity for acute ChD, 100%) (Fig. 4b).
Fig. 4.
Molecular characterization of Trypanosoma cruzi isolates of the outbreak of Chagas disease by oral route occurred in Chichiriviche de la Costa, Venezuela. a) Schematic representation of the amplified products expected for each of the Trypanosoma cruzi DTUs. b) Description of molecular data of the different Chagas disease patients included in this study, and their parasitic loads measured by SatDNA and kDNA qPCR assays. c) Sequence of the SL-IR microsatellite motif and characterization of haplotypes by each isolate of Trypanosoma cruzi from vector and patients. (+): CQ strain (DTU I); (−): CL-Brener strain (DTU VI); ND: Undetermined; NQ: Unquantifiable; nt: nucleotides. SatDNA qPCR limit of quantification (1.53 par.eq./mL); kDNA qPCR limit of quantification (0.90 par.eq./mL).
4. Discussion
Several biotic and abiotic conditions have favored the urban domiciliation of P. geniculatus (Alarcón de Noya & Noya, 2015). Some cultural customs, such as the preparation of artisanal juices (Alarcón de Noya et al., 2010) and the consumption of contaminated sugar cane and aҫai (Valente et al., 1999), are factors that have contributed to the food contamination by triatomine feces or whole infected bugs (Alarcón de Noya & Noya, 2015). Based on the progressive increase in the number of cases, our view is that foodborne transmission will increase and therefore, oral transmission will become the most important route of ChD infection, more than cutaneous vectorial, congenital and transfusional modes of transmission in this region (Pinto et al., 2008, Alarcón de Noya and Noya, 2015).
After an outbreak occurred in an urban school in Venezuela, we thought that a fortuity episode had occurred and that we would never again see something like Chacao school microepidemic (Alarcón de Noya et al., 2010). Even though the screening was performed on the population present at the time of sampling, the confirmed acute cases had occurred in members of the school (students, teachers, cooks, administrative staff and an indigent individual). During the microepidemic, prolonged fever, being under 18, attending the school and drinking guava juice at school were statistically significant risk factors. The association between those infected and guava juice intake recalls the testimony of the preparation of juice exactly as it occurred in the microepidemic in the Chacao school (Alarcón de Noya et al., 2010). Because Chichiriviche de la Costa is in within a forest, infected triatomines could fall into the guava preparation and contaminate it. For a second time, a contaminated juice was the vehicle of trypomastigotes causing the infection of many children. In endemic countries, widespread consumption of handmade juices in schools should be banned, and replaced by pasteurized ones.
Despite the fact that symptoms started simultaneously, it was not possible to speculate about the infection date or week since guava juice consumption was frequent. High prolonged fever occurred in 87.5% of the infected individuals and it was the predominant symptom. Other symptoms, such as edema, weakness, pre-cordial and abdominal pain were less frequent (26–29. 5%). By the time that the ChD outbreak occurred, there was dengue (short-lasting fever) in the region, confusing the initial diagnosis. In Chichiriviche de la Costa, symptomatology and mortality (5.6%) were much higher than in Chacao, (Alarcón de Noya et al., 2010) possibly because the approach occurred later. When sample processing initiated, people already had symptoms for four to six weeks. This time was critical allowing the deaths of four persons and one stillborn. The only autopsied case demonstrated a disseminated T. cruzi infection in all examined organs in the young pregnant cook (Suárez et al., 2010). Moreover, 73 infected persons were hospitalized in different medical facilities, making the appropriate treatment difficult and sometimes worsening the clinical picture with the use of steroids, masking the presence of an active systemic infection by T. cruzi. Pericardial effusion was present in 81.3% of the infected individuals and this demonstrable sign was not accompanied by related symptoms in the same proportion. While the largest frequency occurred in the 6 to 10-age group, probably children most likely did not complain of dyspnea or precordial pain or adults might not have paid much attention to them. Of 61 EKGs available from infected individuals by the time they started treatment, 41% were abnormal, showing mainly alteration of the repolarization. In the present cohort, pericardial effusion seems to be more frequent than the arrhythmias described in other outbreaks (Shikanai-Yasuda and Carvalho, 2012, Bastos et al., 2010, Marques et al., 2013), probably because the echosonographic studies were systematically performed in all infected individuals since the beginning and we did not examine their early EKGs.
The clinical diagnosis of the acute phase of the ChD is not simple because the symptoms are nonspecific and in the cases of children, the voicing of chest pain, dizziness and weakness, is ambiguous. Once the physician has a suspicion and confirms the first cases, as happened in this occurrence, a panel of tests must be performed for the unequivocal verification of acute cases. In anticipation of the presence of a large outbreak by an increase in number of symptomatic individuals, the most efficient way to address a community is to perform simultaneous determination of specific IgG and IgM. Once the infected population is identified, confirmation of cases is necessary. This confirmation was achieved with IHA and LA, which proved to be more sensitive techniques in this cohort. The antibody kinetics of IgM, IgG, IgA and lytic antibodies depends upon the moment in which the population is studied (Antas et al., 1999). Noteworthy, no individual technique was able to detect 100% of the confirmed cases. These results are similar to those found in the first school's microepidemic (Alarcón de Noya et al., 2012). Serologic studies can be quickly performed and the combination of two or three techniques is enough for the detection of 100% of cases (WHO, 2002). During the acute phase in oral outbreaks, attention should be paid to symptomatic people since initially there may be false negatives. Consequently, febrile patients linked to the outbreak with negative tests or only one serological positive result should be treated (Alarcón de Noya et al., 2010, Alarcón de Noya et al., 2012).
As said above, the incubation period is unknown and all samples were taken at least four weeks after the onset of symptoms. By this time, IgA had probably decreased in blood and saliva. While the determination of serum IgA fared well with a sensitivity of 87.9%, saliva tests were not good. Sampling saliva can be an alternative for people with reluctance to give a blood sample. In chronic ChD patients, Pinho et al. (Pinho et al., 1999) had a sensitivity and specificity of IgG in saliva of 90.4% and 95%, respectively. This was not the case in acute OChD patients whose IgG and IgA sensitivities in saliva were very low.
Although the present results are not comparable to other less numerous outbreaks (Shikanai-Yasuda and Carvalho, 2012, Alarcón de Noya et al., 2015, Rueda et al., 2014, Díaz et al., 2015, Añez et al., 2013), it certainly represents one of the occurrences in which it was possible to demonstrate the presence of the parasite in almost all patients, thus relating in this way the symptoms to the causative agent T. cruzi. The direct or indirect detection of the parasite in this outbreak was higher compared to that in the Chacao microepidemic, since in Chichiriviche combined culture and PCR detected T. cruzi in 95.5% of the confirmed cases.
The difficulty of laboratory diagnosis is based on: a. It is difficult for a single technique to diagnose 100% of cases; b. More than one blood sample should be taken on more than one occasion; c. Hospitals are neither prepared to diagnose OChD nor to offer the determination of IgM antibodies; d. Reference laboratories may be distant from the outbreak location delaying the arrival of samples and therefore the diagnosis. However, the direct fresh visualization of the parasite and the microhematocrit are rapid tests that can be done at any medical level with good sensitivity in the acute phase (Freilij et al., 1983).
The intensity and severity of the acute phase should influence the course of the infection and the establishment of myocardial injury (Andrade et al., 2014). Alarcón de Noya et al. (Alarcón de Noya et al., 2011) warned about the evolution of patients in the two Venezuelan school microepidemics in which, after two and three years post-treatment, about 70% of patients continued to be LA- and PCR-positive even though the treatment had been supervised to ensure appropriate dosages and duration and that it was administered to children in the acute phase, when it is considered to be more effective (Viotti & Vigliano, 2015). Certainly, the longer the patients take to heal, the greater the damage that parasites can generate. All suspected and confirmed cases received benznidazol 7 mg/kg for 60 days.
As expected, parasitic loads in these acute ChD samples were approximately twice as high for both molecular markers than in chronic cases (Ramírez et al., 2015). The two samples with the highest parasitic loads belonged to patient Chic5 and patient Chic6, infected with TcI (haplotype non-determinate). However, in one sample (Chic1), the parasitic load could not be quantified by satDNA qPCR method. This can be explained because the analytical sensitivity is more uniform among the different T. cruzi DTUs for kDNA qPCR than for SatDNA qPCR, indicating lower gene dosage in their genomes (Duffy et al., 2009, Lewis et al., 2009).
The presence of TcIb is very important because this is the second report where this haplotype, originally described by Herrera et al. (Herrera et al., 2007), is linked with wild and peridomestic cycles and vectors of the genus Triatoma and Rhodnius and wild vectors such as Panstrongylus (Falla et al., 2009). Also, this is the first evidence of a mixture of haplotypes in a sylvatic vector, suggesting repeated reinfection (Muñoz-Calderón et al., 2013).
For those T. cruzi isolates from orally-infected individuals and assuming all patients were infected from a common source, T. cruzi exhibited TcI haplotype heterogeneity, suggesting that wild vectors such as P. geniculatus are able to accommodate different TcI haplotypes naturally. On the other hand, Diaz et al. (Díaz et al., 2015) analyzing parasite DTU from 6 Colombian outbreaks found that DTUs are not strictly correlated with clinical forms of the disease. In our results, the finding of at least two TcI haplotypes in persons with severe forms of the disease is motive of histopathogenicity studies of the TcI haplotypes alone or combined.
In a small town of no more than 500 inhabitants, the massive infection of children will impact the community in a few years. It seems that population is not getting cured as expected, probably leaving those who are evolving to chronic ChD with higher cardiac morbidity than those with the natural course of vectorial infection. As a touristic place where submarine activity is a mode of life, lower cardiorespiratory capacity is a serious limitation. Girls will soon start to be pregnant with the possibility of congenital transmission and blood transfusions will be very limited among relatives under emergency situations.
5. Short conclusion
In Chichiriviche de la Costa, Venezuela, a large outbreak of oral transmitted ChD occurred in a rural school in 2009 affecting 89 persons with 5 deaths. The evaluation of risk factors indicated that the ingestion of artisanal guava juice, distributed at school, was the vehicle of T. cruzi. Foodborne transmission allowed the intake of a large inoculum, reflected by the severity of cases, a high parasitic load in patients, the demonstration of the causative agent in the majority of them, and the systemic invasion of T. cruzi in an autopsied case. To prevent these outcomes and knowing that no individual parasitological, serological or molecular technique can diagnose the 100% of acute cases, a set of tests must be timely applied to the population at risk to prevent morbidity and mortality. Chagas disease as a foodborne entity deserves special attention by health authorities because it will not diminish left alone according to the ecological changes in the endemic areas.
Acknowledgments
The authors are deeply grateful to the authorities of the school “Rómulo Monasterio” especially to Judith Romero, the Director. Thanks to the nurse Herenia Bello and the personnel of the local health center “Rafael Martinez”. To Oscar Noya Alarcón, María Teresa Maniscalchi, Víctor Saravia, Néstor Martínez and Alejandro Schijman for their contributions. The authors are grateful to Mariela Losada, Andrés Picon and Noel Mueller for English corrections. The authors gratefully appreciate the support from National Scientific and Technological Ministry with the Project FONACIT 2012001295.
References
- Pinto A.Y.N., Valente S.A.S., Valente V.C., Ferreira Junior A.G., Coura J.R. Fase aguda da doença de Chagas na Amazônia brasileira. Estudo de 233 casos no Pará, Amapá e Maranhão observados entre 1988 e 2005. Rev. Soc. Bras. Med. Trop. 2008;41:602–614. doi: 10.1590/s0037-86822008000600011. [DOI] [PubMed] [Google Scholar]
- Shikanai-Yasuda M.A., Carvalho N.B. Oral transmission of Chagas disease. Clin. Infect. Dis. 2012;54:845–852. doi: 10.1093/cid/cir956. [DOI] [PubMed] [Google Scholar]
- Coura J.R. A Comprehensive Review. Vol. 110. 2015. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions; pp. 277–282. (Mem. Inst. Oswaldo Cruz). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón de Noya B., Díaz-Bello Z., Colmenares C., Ruiz-Guevara R., Mauriello L., Muñoz-Calderón A., Noya O. Update on oral Chagas disease outbreaks in Venezuela: epidemiological, clinical and diagnostic approaches. Mem. Inst. Oswaldo Cruz. 2015;110:377–386. doi: 10.1590/0074-02760140285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade D.V., Gollob K.J., Dutra W.O. Acute Chagas disease: new global challenges for an old neglected disease. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón de Noya B., Noya O. An ecological overview on the factors that drives to Trypanosoma cruzi oral transmission. Acta Trop. 2015;151:94–102. doi: 10.1016/j.actatropica.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Bastos C.J.C., Aras R., Mota G., Reis F., Dias J.P., Jesus R.S. Clinical outcomes of thirteen patients with acute Chagas disease acquired through oral transmission from two urban outbreaks in Northeastern Brazil. PLoS Negl.Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo F., Teixeira de Pinho R., Zuquim-Antas P.R., Mengel J. Immunity and immune modulation in Trypanosoma cruzi infection. Pathog. Dis. 2015;73 doi: 10.1093/femspd/ftv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón de Noya B., Díaz-Bello Z., Colmenares C., Ruiz-Guevara R., Mauriello L., Zavala-Jaspe R. Large urban outbreak of orally-acquired acute Chagas disease, at a school in Caracas, Venezuela. J. Infect. Dis. 2010;201:1308–1315. doi: 10.1086/651608. [DOI] [PubMed] [Google Scholar]
- Alarcón de Noya B., Martínez J. Transmisión oral de la enfermedad de Chagas en Venezuela: un segundo brote escolar. Salus. 2009;13:11–20. [Google Scholar]
- Díaz-Bello Z., Zavala-Jaspe R., Díaz-Villalobos M., Mauriello L., Maekelt A., Alarcón de Noya B. Diagnóstico confirmatorio de anticuerpos anti-Trypanosoma cruzi en donantes referidos por bancos de sangre en Venezuela. Investig. Clin. 2008;49:141–150. [PubMed] [Google Scholar]
- Jacobs L., Lunde M.N. A hemagglutination test for toxoplasmosis. J. Parasitol. 1957;43:308–314. [PubMed] [Google Scholar]
- Krettli A. The utility of anti-trypomastigote lytic antibodies for determining cure of Trypanosoma cruzi infections in treated patients: an overview and perspectives. Mem. Inst. Oswaldo Cruz. 2009;104(Suppl. 1):142–151. doi: 10.1590/s0074-02762009000900020. [DOI] [PubMed] [Google Scholar]
- Mourão O.G., Mello O.C. Hemocultura para o diagnóstico parasitológico na fase crônica da doença de Chagas. Rev. Soc. Bras. Med. Trop. 1975;9:183–188. [Google Scholar]
- Schijman A.G., Altcheh J., Burgos J.M., Biancardi M., Bisio M., Levin M.J. Aetiological treatment of congenital Chagas disease diagnosed and monitored by the polymerase chain reaction. J. Antimicrob. Chemother. 2003;52:441–449. doi: 10.1093/jac/dkg338. [DOI] [PubMed] [Google Scholar]
- Sturm N., Degrave W., Morel C., Simpson L. Sensitive detection and schizodeme classification of T. cruzi cells by amplification of kinetoplastid minicircle DNA sequences: use in diagnosis of Chagas disease. Mol. Biochem. Parasitol. 1989;33:205–214. doi: 10.1016/0166-6851(89)90082-0. [DOI] [PubMed] [Google Scholar]
- Duffy T., Cura C.I., Ramírez J.C., Abate T., Cayo N.M., Parrado R. Analytical performance of a multiplex real-time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. PLoS Negl. Trop. Dis. 2013 doi: 10.1371/journal.pntd.0002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvarnstrom Y., Schijman A.G., Verón V., Aznar C., Steurer F., da Silva A.J. Sensitive and specific detection of Trypanosoma cruzi DNA in clinical specimens using a multi-target real-time PCR approach. PLoS Negl. Trop. Dis. 2012 doi: 10.1371/journal.pntd.0001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto R.P., Fernandes O., Macedo A.M., Campbell D.A., Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- Clark C.G., Pung O.J. Host specificity of ribosomal DNA variation in sylvatic Trypanosoma cruzi from North America. Mol. Biochem. Parasitol. 1994;66:175–179. doi: 10.1016/0166-6851(94)90052-3. [DOI] [PubMed] [Google Scholar]
- WHO. Control of Chagas disease. Second Report of the WHO Expert Committee. World Health Organization. Geneva. WHO Technical Report Series No 905. 2002. [PubMed]
- Valente S.A.S., Valente V.C., Fraiha-Neto H. Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon. Mem. Inst. Oswaldo Cruz. 1999;94(Suppl. 1):395–398. doi: 10.1590/s0074-02761999000700077. [DOI] [PubMed] [Google Scholar]
- Suárez J., de Suárez C.B., Alarcón de Noya B., Espinosa R., Chiurillo M.A., Villaroel P.A. Enfermedad de Chagas sistémico en fase aguda por transmisión oral: diagnóstico integral de un caso autopsiado. Gac. Méd. Caracas. 2010;118:212–222. [Google Scholar]
- Marques J., Mendoza I., Noya B., Acquatella H., Palacios I., Marques-Mejias M. ECG manifestations of the biggest outbreak of Chagas disease due to oral infection in Latin-America. Arq. Bras. Cardiol. 2013;101:249–254. doi: 10.5935/abc.20130144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antas P.R.Z., Mendrano-Mercado N., Torrico F., Ugarte-Fernández R., Gómez F., Correa-Oliveira R. Early, intermediate, and late acute stages in Chagas´ disease: a study combining anti-galaptose IgG, specific serodiagnosis and polymerase chain reaction analysis. Am. J. Trop. Med. Hyg. 1999;61:308–314. doi: 10.4269/ajtmh.1999.61.308. [DOI] [PubMed] [Google Scholar]
- Alarcón de Noya B., Díaz-Bello Z., Colmenares C., Zavala-Jaspe R., Abate T., Contreras R. The performance of laboratory tests in the management of a large outbreak of orally transmitted Chagas disease. Mem. Inst. Oswaldo Cruz. 2012;107:893–898. doi: 10.1590/s0074-02762012000700009. [DOI] [PubMed] [Google Scholar]
- Pinho R., Pedrosa R., Costa P., Castello L. Saliva ELISA: a method for the diagnosis of chronic Chagas disease in endemic areas. Acta Trop. 1999;72:31–38. doi: 10.1016/s0001-706x(98)00075-8. [DOI] [PubMed] [Google Scholar]
- Rueda K., Trujillo J.E., Carranza J.C., Vallejo G.A. Transmisión oral de Trypanosoma cruzi: una nueva situación epidemiológica de la enfermedad de Chagas en Colombia y otros países suramericanos. Biomédica. 2014;34:631–641. doi: 10.1590/S0120-41572014000400017. [DOI] [PubMed] [Google Scholar]
- Díaz M.L., Leal S., Mantilla J.C., Molina-Berríos A., López-Muñoz R., Solari A. Acute Chagas outbreaks: molecular and biological features of Trypanosoma cruzi isolates, and clinical aspects of acute cases in Santander, Colombia. Parasit. Vectors. 2015;8:608–622. doi: 10.1186/s13071-015-1218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Añez N., Crisante G., Rojas A., Dávila D. Brote de enfermedad de Chagas agudo de posible transmisión oral en Mérida. Boll. Mal. Salud Amb. 2013;53:1–11. [Google Scholar]
- Freilij H., Muller L., González-Cappa M. Direct micromethod for diagnosis of acute and congenital Chagas' disease. J. Clin. Microbiol. 1983;18:327–330. doi: 10.1128/jcm.18.2.327-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón de Noya B., Díaz-Bello Z., Colmenares C., Muñoz-Calderón A., Ruiz-Guevara R., Balzano L. Eficacia terapéutica en el primer brote de transmisión oral de la enfermedad de Chagas en Venezuela. Biomédica. 2011;31(Supp. 3):64–65. [Google Scholar]
- Viotti R., Vigliano C. Tratamiento etiológico. In: Viotti R., Vigliano C., editors. Enfermedad de Chagas. Un enfoque Práctico Basado en la Investigación Clínica. Editorial Médica Panamericana; Argentina: 2015. pp. 253–268. [Google Scholar]
- Ramírez J.C., Cura C.I., da Cruz Moreira O., Lages-Silva E., Juiz N., Velázquez E. Analytical validation of quantitative real-time PCR methods for quantification of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. J. Mol. Diagn. 2015;17:605–615. doi: 10.1016/j.jmoldx.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy T., Bisio M., Altcheh J., Burgos J.M., Diez M., Levin M.J. Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in Chagas disease patients. PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.D., Ma J., Yeo M., Carrasco H.J., Llewellyn M.S., Miles M.A. Genotyping of Trypanosoma cruzi: systematic selection of assays allowing rapid and accurate discrimination of all known lineages. Am. J. Trop. Med. Hyg. 2009;81:1041–1049. doi: 10.4269/ajtmh.2009.09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C., Bargues M.D., Fajardo A., Montilla M., Triana O., Vallejo G.A. Identifying four Trypanosoma cruzi I isolate haplotypes from different geographic regions in Colombia. Infect. Genet. Evol. 2007;7:535–539. doi: 10.1016/j.meegid.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Falla A., Herrera C., Fajardo A., Montilla M., Vallejo G.A., Guhl F. Haplotype identification within Trypanosoma cruzi I in Colombian isolates from several reservoirs, vectors and humans. Acta Trop. 2009;110:15–21. doi: 10.1016/j.actatropica.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Muñoz-Calderón A., Díaz-Bello Z., Valladares B., Noya O., López M.C., Alarcón de Noya B. Oral transmission of Chagas disease: typing of Trypanosoma cruzi from five outbreaks occurred in Venezuela shows multiclonal and common infections in patients, vectors and reservoirs. Infect. Genet. Evol. 2013;17:113–122. doi: 10.1016/j.meegid.2013.03.036. [DOI] [PubMed] [Google Scholar]