Abstract
As a part of the lymphatic filariasis (LF) transmission assessment survey (TAS)/soil-transmitted helminths (STH) prevalence survey in Western Division of Fiji, a pilot screen for Strongyloides stercoralis (SS) in school children was undertaken using a combination of the Baermann concentration (BC) method and real-time PCR assays.
Using BC, faecal samples collected from 111 children of 7 schools were examined. A single child was positive for larvae of SS and underwent a clinical examination finding an asymptomatic infection. Other members of this child's household were screened with BC, finding none infected. Aliquots of 173 faecal samples preserved in ethanol originating from all schools were examined by real-time PCR, and the prevalence of SS infection was 3.5%.
Our study confirms the existence of SS infection on Fiji and showed that assessing SS prevalence alongside TAS/STH survey is a convenient access platform, allowing introduction of other surveillance techniques such as BC and real-time PCR.
Abbreviations: NTDs, neglected tropical diseases; LF, lymphatic filariasis; STH, soil-transmitted helminths; SS, Strongyloides stercoralis; TAS, transmission assessment survey; BC, Baermann concentration; PCR, polymerase chain reaction
Keywords: Neglected tropical diseases, Surveillance, Pacific, Real-time PCR
1. Introduction
Oceania is a region of tropical and sub-tropical islands in the Pacific where one quarter of the population is impoverished placing them at increased risk of several neglected tropical diseases (NTDs) (Kline et al., 2013). Among others, lymphatic filariasis (LF) and soil-transmitted helminthiasis (STH) are particularly widespread in the region (WHO, 2015). The epidemiology and importance of Strongyloides stercoralis (SS) infection, however, is not well-known, (Kline et al., 2013, Olsen et al., 2009) outside of Australia (Miller et al., 2014), Papua New Guinea (Viney et al., 1991, Igra-Siegman et al., 1981) and the Solomon Islands (Pattison and Speare, 2008).
With the 4th largest population in Oceania (Fiji Bureau of Statistics, 2015), some 835 K Fijians reside mostly on two major islands, Viti Levu and Vanua Levu, out of the 100 or so currently inhabited. Throughout Fiji there has been a long history of interventions against LF and STH (WHO, 2015). However, typical of other islands in the vicinity, given the warm and humid climate and often-inadequate sanitation, the occurrence of Strongyloides stercoralis (SS) infection is likely (Muller, 2002) but not well known. Previous parasitological surveys have attempted to determine the general prevalence of STH but since insensitive diagnostic methods were used, such as direct faecal smears (Lott, 1980) (Jansen et al., 1991), all geohelminthes infections have likely been under reported, especially SS infections. However, several clinical cases of SS infection in travellers or migrants from Fiji have been reported and point towards existing on-going transmission (Coulter et al., 1992), which needs to be affirmed locally.
To shed light on the occurrence of SS infections on Fiji, our study aimed to pilot epidemiological assessments by taking advantage of the existing national LF and STH programme evaluation activities, rather than conducting a stand-alone initiative. We detailed how a transmission assessment survey (TAS) for LF and STH survey provided a convenient access opportunity to determine the extent of SS infection in school-aged children.
2. Material and methods
The survey was conducted in Western Division, which is a drier western half of the Fiji's Viti Levu (Fig. 1). The areas is further classified ecologically into Lowland dry zone in the western half, and Lowland intermediate zone in the east (FAO, 2016). Annual mass drug administration (MDA) using albendazole and diethylcarbamazine citrate for LF was conducted from 2001 to 2009, resulting in a very low prevalence (< 1%) in the survey conducted in 2007 (unpublished data, Ministry of Health). This warranted the 1st TAS in 2011 revealing one positive child for LF antigen in serum, and subsequently the 2nd TAS was planned for 2014 which forms the access opportunity for this investigation. Since 2010, annual albendazole distribution targeting school-aged children was also started in the surveyed area as part of National Micronutrient Supplementation Programme together with ferrous sulphate tablets administration, but its nationwide coverage was 32% in 2013 and considered low (Vasu, 2015).
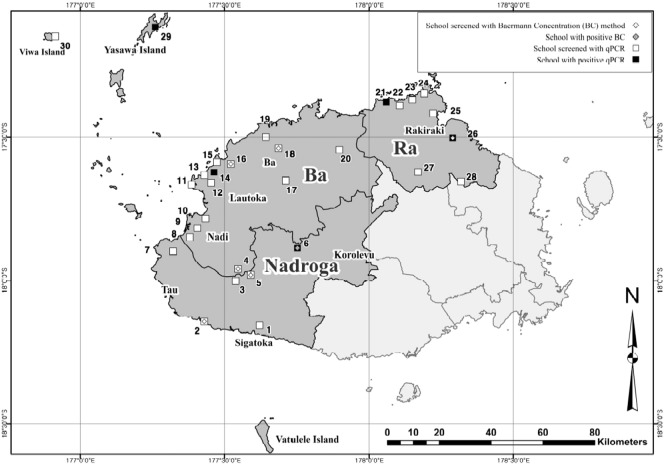
Fig. 1.
Sketch map of the 30 sampled schools and the combination of techniques used [BC and (or) real-time PCR] at each school. SS was found by BC at school 6 only, while by real-time PCR, SS positives were found at 4 more schools: 6, 14, 21, 26, and 29, Western Division, Fiji, March 2014.
There are 249 primary schools registered at the Ministry of Education in the Western Division, and 77 schools were systematically selected for the 2nd TAS, using Survey Sample Builder (The Task Force for Global Health, Georgia, USA). Furthermore 30 schools were sub-sampled for STH, 10 schools per each of 3 ecological zones (10 urban and rural in western dry zone and 10 in intermediate), following the World Health Organization's guidelines (World Health Organization, 2011). The spatial coordinates of each school were recorded by a hand-held GPS unit (e-trex, Garmin Ltd., Kansas, USA) in decimal degrees.
Prior to finger-prick for LF antigen testing, grade 1 and 2 student, who are born between 2007 and 2008, were asked to submit stool containers with their fresh morning stool Then stool samples were transported to the national parasitology reference laboratory, using cooler boxes. The BC method (Stothard et al., 2008) was applied for the detection of SS larvae for all stool samples arrived at the lab during 3 days specifically designated for the SS surveillance. To save BC processing time, between 4 and 8 stools were pooled and examined simultaneously. Seeking a wider appraisal across the Western Division, a selection of samples identified positive for all geohelminthes by coproscopy together with a systematic sub-sample (every 10th negative samples) were filtered through a 212 μm metal sieve and preserved in 95% ethanol, then transported to the Netherlands for molecular diagnostics of SS using real-time PCR, as this was not locally available in Fiji. Parasitic-specific DNA was detected in faecal samples as described previously (Verweij et al., 2009, Verweij and Stensvold, 2014).
3. Results
A total of 111 faecal samples were examined using BC as a set within 19 pooled samples. Multiple SS larvae were found in one sample, out of a pooled batch comprised of 7 stool samples from school 6 (see Fig. 1). When BC was performed with each of 7 samples within that batch, we identified one stool sample positive with the SS larvae (Table 1). The infected child, a 6-year boy, was subsequently followed-up in an effort to ascertain the clinical manifestations. The child did not present with abdominal pain, diarrhoea, cough, urticaria or pruritus nor sign of malnourishment, wheezing or skin abnormality. Also laboratory tests indicated no eosinophilia or anaemia. This child was judged to be asymptomatic for SS infection, and treatment with albendazole was administered at 400 mg, once per day, for 7 days. Parents of the child were interviewed and stool samples from his family members, including 4 siblings, were tested using BC. None was found infected upon these examinations.
Table 1.
Sampling and screening of S. stercoralis infection among 30 primary school children in Western Division of Fiji, March 2014.
| Baermann | PCR | Total | |
|---|---|---|---|
| Number of students screened | 111 | 173 | 262⁎ |
| Number of students infected | 1 | 6 | 6⁎⁎ |
| Prevalence (%) | 0.9 | 3.5 |
NB: ⁎Stool samples of 22 students were screened by both BC and PCR; ⁎⁎Number includes 1 positive and 1 negative by BC.
4. Discussion
To shed light on the geographical distribution of SS infection, altogether 173 faecal samples from children of 30 schools in Western Division were tested by real-time PCR. In total, 6 samples (2 from grade 1 and 4 from grade 2 students) were positive for SS, including one identified by BC, at schools across the Western Division (Fig. 1, school 6, 14, 21, 26, and 29) and overall prevalence of SS infection was 3.5% (Table 1).
Having a full appraisal of SS infection in the Oceania region continues to be problematic due to present diagnostic difficulties in both operational and reference diagnostic settings (Kline et al., 2013). Previously, there were only three studies in Fiji with limited focus on SS during 1960 to 1980 (Lott, 1980), (Jansen et al., 1991). In 1966, a survey in a rural area of Naitasiri, Central Division reported SS prevalence levels of 3% among villagers of 7 villages (Jansen et al., 1991), with differences in ethnic groups. A much higher prevalence level was reported in a survey undertaken in a village near Rewa river, with a SS prevalence level up to 50% among Fijian ethnicity of all ages groups (Jansen et al., 1991). Lastly a study that covered 60 school children (age 5–15) on the island of Ovalau, Eastern Division using unstained smears, reported the SS prevalence of 13%, equally in girls and boys (Lott, 1980).
In this study, we have found that SS infection exists among 1st and 2nd grade school children in 3 different eco-zones of the Western Division using BC and real-time PCR (Fig. 1). General prevalence of SS infection is estimated to be up to 3.5% and we believe the infection is endemic (> 1%) in the areas (Muller, 2002). In light of this, some 1.5 K children are likely to be infected with SS, but it may be possible that the actual burden of the infection is greater, considering a poor diagnostic sensitivity from a single stool sample (Mirdha, 2009).
Whilst the infected child encountered during this survey cannot be considered symptomatic, management of asymptomatic SS is recommended since the infection may persist for many years without appropriate drug treatment (Mirdha, 2009). Considering that treatment with albendazole is lengthy and shows variable results, the preferred option would be single dose administration of ivermectin (Marti et al., 1996). However, most of these potential cases will not have opportunities of being treated, since the country is not endemic for onchocerciasis, ivermectin is not included in the Essential Medicines List. We encourage that the health authorities in Fiji reconsider their Essential Medicine List with the hope of making ivermectin treatment available in future.
Our study has several limitations. The actual SS prevalence could have been higher as we had examined a single stool sample per student rather than consecutive 3 day samples (Mirdha, 2009), and also through pooling of samples rather than being analysed individually may have decreased diagnostic sensitivity. As it was the first time that BC was performed, we mainly opted to secure its maximal feasibility in a local laboratory setting by applying it to several batches of the sample. Furthermore, only samples received during the specifically designated 3 days were examined with BC owing to the logistic constraints in the field, limiting the coverage of the schools surveyed. The SS prevalence could have been more precise if we had covered more number of schools. Regarding the sampling strategy for real-time PCR, we were able to include 20% of the all available stool samples, among which half of them were systematically selected. Inclusion of another half was based on the need of confirmative diagnosis, thus the level of representativeness might have been lower in these samples. This may be less robust, depending on the level of clustering and heterogeneity of geo-helminthes infections in the area (Smith et al., 2015). However, we have included them based on the fact that the prevalence estimates in two groups do not differ statistically.
In summary, we were able to shed new light on the distribution of SS infection across the island by using the LF TAS/STH survey which provided a practical platform to integrate SS surveillance. Using species-specific diagnostic tools such as BC or real time PCR and archiving stool samples could be helpful to find further future synergy within the TAS/STH survey sampling framework (Chu et al., 2014).
Financial support
Sample collection and laboratory analysis in Fiji was supported by J.W. Lee Centre for Global Medicine, Seoul National University College of Medicine as a part of ‘Project for control of soil transmitted helminthiases in Fiji’ and Liverpool School of Tropical Medicine, UK.
Ethical standards, consent and permissions
The study was approved by the Fiji Ministry of Health and Medical Services National Health Research Committee, and the Ethical Review Board of Liverpool School of Tropical Medicine. Parents were requested to sign a consent form provided in the language if they would like their children to take part in the study. The authors assert that all procedures contributing to this work comply with the ethical standards of the national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Competing interests
All authors declare that no commercial other association that might pose a competing interest exists.
Author's contribution
SHK, MR, MRQ, MK, ER, MHC, STH, JRS defined the study design and SHK, MR, MRQ, MK, ER wrote the protocol. SHK, NT, MHC, JRS, JJV carried out laboratory work. SHK carried out case investigations and statistical analysis. SHK, MK, ER, STH, MHC, JJV, LKH, JRS drafted parts of the manuscript. All authors read and approved the final version of the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgements
The authors would like to thank the teams from the Fiji Centre for Communicable Disease Control, Ministry of Health and Medical Service for their assistance, and children and their family for participating in the study. Special thanks to Dr. Padmasiri Aratchige of WHO Division of Pacific Technical Support, Mr. Gimin Bang and Dr. John Lowry from University of South Pacific, and Ms. Seinimere Kato-Bavoro for their support and assistance.
Contributor Information
S.H. Kim, Email: sunghye.kim@post.harvard.edu.
M. Rinamalo, Email: milika.rinamalo@govnet.gov.fj.
M. Rainima-Qaniuci, Email: mary.qaniuci@gmail.com.
N. Talemaitoga, Email: nemtale@yahoo.com.
M. Kama, Email: mike.kama@health.gov.fj.
E. Rafai, Email: eric.rafai@govnet.gov.fj.
M.-H. Choi, Email: mhchoi@snu.ac.kr.
S.T. Hong, Email: hst@snu.ac.kr.
J.J. Verweij, Email: j.verweij@elisabeth.nl.
L. Kelly-Hope, Email: Louise.Kelly-Hope@lstmed.ac.uk.
J.R. Stothard, Email: russell.stothard@lstmed.ac.uk.
References
- Chu B.K., Gass K., Batcho W., Ake M., Dorkenoo A.M., Adjinacou E. Pilot assessment of soil-transmitted helminthiasis in the context of transmission assessment surveys for lymphatic filariasis in Benin and Tonga. PLoS Negl. Trop. Dis. 2014;8(2):e2708. doi: 10.1371/journal.pntd.0002708. Feb 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter C., Walker D.G., Günsberg M., Brown I.G., Bligh J.F., Prociv P. Successful treatment of disseminated strongyloidiasis. Med. J. Aust. 1992;157(5):331–332. Sep 7. [PubMed] [Google Scholar]
- FAO Fiji country pasture/forage resource profiles [internet]. Fiji country pasture/forage resource profiles. 2016. http://www.fao.org/ag/agp/agpc/doc/Counprof/southpacific/fiji.htm [cited Mar 11]. Available from.
- Fiji Bureau of Statistics 2007 census of population [internet]. 2007 census of population. 2015. http://www.statsfiji.gov.fj/index.php/2007-census-of-population [cited Nov 17]. Available from.
- Igra-Siegman Y., Kapila R., Sen P., Kaminski Z.C., Louria D.B. Syndrome of hyperinfection with Strongyloides stercoralis. Rev. Infect. Dis. 1981;3(3):397–407. doi: 10.1093/clinids/3.3.397. Jun. [DOI] [PubMed] [Google Scholar]
- Jansen A.A.J., Parkinson S., Robertson A.F.S. Fiji: Dept of Nutrition and Dietetics. 1991. Food and nutrition in Fiji: nutrition-related diseases and their prevention [Internet] (Fiji School of Medicine and the Institute of Pacific Studies of the University of south Pacific). Available from: https://books.google.co.uk/books?id=dZq8VcalXV0C&pg=PA334&lpg=PA334&dq=strongyloides+fiji&source=bl&ots=hfPBCUP6qy&sig=UVbuv8PVSwn-c3d3NZeki-9nqVc&hl=en&sa=X&ved=0ahUKEwjB0pm_v6fJAhVGshQKHYG9BHoQ6AEINTAD#v=onepage&q=strongyloides%20fiji&f=false. [Google Scholar]
- Kline K., McCarthy J.S., Pearson M., Loukas A., Hotez P.J. Neglected tropical diseases of Oceania: review of their prevalence, distribution, and opportunities for control. PLoS Negl. Trop. Dis. 2013;7(1) doi: 10.1371/journal.pntd.0001755. Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott D.A. A survey of intestinal nematodes in children of a primary school in Ovalau. S. Pac. J. Nat. Sci. 1980;1:22. [Google Scholar]
- Marti H., Haji H.J., Savioli L., Chwaya H.M., Mgeni A.F., Ameir J.S. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. Am.J.Trop. Med. Hyg. 1996;55(5):477–481. doi: 10.4269/ajtmh.1996.55.477. Nov. [DOI] [PubMed] [Google Scholar]
- Miller A., Smith M.L., Judd J.A., Speare R. Strongyloides stercoralis: systematic review of barriers to controlling strongyloidiasis for Australian indigenous communities. PLoS Negl. Trop. Dis. 2014;8(9) doi: 10.1371/journal.pntd.0003141. Sep 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirdha B.R. Human strongyloidiasis: often brushed under the carpet. Trop. Gastroenterol. 2009;30(1):1–4. Mar. [PubMed] [Google Scholar]
- Muller R. 2nd ed. CABI; Wallingford, Oxon, UK: 2002. Worms and human disease. [Google Scholar]
- Olsen A., van Lieshout L., Marti H., Polderman T., Polman K., Steinmann P. Strongyloidiasis–the most neglected of the neglected tropical diseases? Trans. R. Soc. Trop. Med. Hyg. 2009;103(10):967–972. doi: 10.1016/j.trstmh.2009.02.013. Oct. [DOI] [PubMed] [Google Scholar]
- Pattison D.A., Speare R. Strongyloidiasis in personnel of the Regional Assistance Mission to Solomon Islands (RAMSI) Med. J. Aust. 2008;189(4):203–206. doi: 10.5694/j.1326-5377.2008.tb01982.x. Aug 18. [DOI] [PubMed] [Google Scholar]
- Smith J.L., Sturrock H.J.W., Assefa L., Nikolay B., Njenga S.M., Kihara J. Factors associated with the performance and cost-effectiveness of using lymphatic filariasis transmission assessment surveys for monitoring soil-transmitted helminths: a case study in Kenya. Am.J.Trop. Med. Hyg. 2015;92(2):342–353. doi: 10.4269/ajtmh.14-0435. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard J.R., Pleasant J., Oguttu D., Adriko M., Galimaka R., Ruggiana A. Strongyloides stercoralis: a field-based survey of mothers and their preschool children using ELISA, Baermann and Koga plate methods reveals low endemicity in western Uganda. J. Helminthol. 2008 Sep;82(3):263–269. doi: 10.1017/S0022149X08971996. [DOI] [PubMed] [Google Scholar]
- Vasu K. 2015. Nationa Iron and Micronutrient Supplementation Project Annual Report 2014. [Google Scholar]
- Verweij J.J., Stensvold C.R. Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections. Clin. Microbiol. Rev. 2014;27(2):371–418. doi: 10.1128/CMR.00122-13. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J.J., Canales M., Polman K., Ziem J., Brienen E.A.T., Polderman A.M. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans. R. Soc. Trop. Med. Hyg. 2009;103(4):342–346. doi: 10.1016/j.trstmh.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Viney M.E., Ashford R.W., Barnish G. A taxonomic study of Strongyloides Grassi, 1879 (Nematoda) with special reference to Strongyloides fuelleborni von linstow, 1905 in man in Papua New Guinea and the description of a new subspecies. Syst. Parasitol. 1991;18(2):95–109. Feb. [Google Scholar]
- WHO | PCT databank [Internet]. WHO | PCT databank. 2015. http://www.who.int/neglected_diseases/preventive_chemotherapy/lf/en/ [cited Sep 8]. Available from.
- World Health Organization . 2nd ed. World Health Organization; Geneva: 2011. Helminth Control in School-Age Children. [Google Scholar]