Abstract
Crimean-Congo hemorrhagic fever (CCHF) is a viral disease transmitted to humans by bite of infected ticks or by direct contact with blood or tissues of viremic patients or livestock. The present cross-sectional meta-analysis study is based on previous data which have shown that the human CCHFV seroprevalence in specific regions of Greece is high (> 5%). In the absence of human cases, it has been suggested that a non- or low-pathogenic strain is circulating in the country causing asymptomatic infections. A spatial cluster analysis was performed to assess the geographical variations in CCHFV seropositivity and to identify the risk factors. The overall CCHFV seroprevalence is 3.8%, with significant rate difference between the eastern and western part of the country. Apart the risk factors described in previous studies (age, sex, tick bite, agropastoral activities), the altitude, the land cover type and the transitional woodland/shrub land per person, as well as the number of livestock per person, and specifically the number of goats, sheep and cattle per person, were shown to affect significantly the seroprevalence. Tick studies are needed to identify the circulating strains and unravel the mystery of CCHF epidemiology in Greece.
Keywords: Crimean-Congo hemorrhagic fever, Spatial analysis, Seroprevalence, Greece
1. Introduction
Crimean-Congo hemorrhagic fever (CCHF) is a viral disease characterized by fever and hemorrhagic manifestations with fatality rate up to 30% (Papa et al., 2014a). It is transmitted to humans by bite of infected ticks or by direct contact with blood or tissues of viremic patients or livestock. Endemic foci are present in central Asia, the Middle East, southeast Europe and Africa. The distribution of CCHF coincides with that of Hyalomma ticks, the primary competent vectors of CCHF virus (CCHFV) (Ergonul, 2006). Based on the S RNA segment sequences of the virus, CCHFV strains can be grouped into 7 genetic lineages (Papa et al., 2015). Two lineages are present in Greece: Europe 1, which includes pathogenic strains from Balkans, Russia and Turkey, and Europe 2, which contains the strain AP92, isolated in 1975 from Rhipicephalus bursa ticks collected from goats in Greece (Papadopoulos & Koptopoulos, 1980). AP92-like strains have been detected recently in Turkey (in ticks and in few mild human cases), Kosovo and Greece (Midilli et al., 2009, Elevli et al., 2009, Papa et al., 2014b, Sherifi et al., 2014). Since no severe disease has been associated so far with this specific strain, it is considered of low pathogenicity (Papa et al., 2014b). A single (and fatal) case observed in 2008 in northeastern Greece was caused by a strain (Rodopi strain) belonging to lineage Europe 1 (Papa et al., 2008a, Papa et al., 2009). The seroprevalence in the region is 3.14% (Papa et al., 2011). Unexpected high seroprevalence rates (> 5%) have been observed in a country-wide study, especially in the mountainous areas of the central and western part of Greece; living or working in close proximity to livestock (especially goats), slaughtering and history of tick bite were identified as risk factors (Sidira et al., 2012). A study focused in the prefecture with the highest seroprevalence (14.4%, Thesprotia, western Greece) showed similar risk factors; however, tick bites were not significantly associated with seropositivity, and sheep instead of goat was the animal species with significant association (Papa et al., 2013). In a later study conducted in a region not studied previously showed that living at an altitude of ≥ 400 m above sea level and specific land cover types (non-irrigated arable land and land principally occupied by agriculture with significant areas of natural vegetation) play also a role in seropositivity (Sargianou et al., 2013).
Climatic and environmental variables play a crucial role in the distribution, incidence and emergence of vector-borne diseases. The CCHF eco-epidemiology is complicated since many biotic (like tick and domestic and wild animal abundance) and abiotic (like temperature, precipitation, humidity, land cover type) parameters have an impact on the virus life cycle. Previous studies showed that climate or vegetation variables or seasonal components do not account for the delineation of areas of disease in Turkey; in contrary, habitat fragmentation was found to be the key factor driving the spread of CCHF (Estrada-Pena et al., 2010). It has been shown that the transmission and spread of CCHFV is highly dependent upon suitable hosts for adult ticks, like large domestic and wild ungulates (Estrada-Pena et al., 2013). A recent study in Kastamonu in Turkey showed that the cumulative incidence of CCHF was significantly positively correlated with the number of domestic animals and area of agricultural land per person (Aker et al., 2015).
In an effort to obtain a comprehensive insight into the factors related with variations in CCHFV seropositivity in Greece and to portray the variability in space we conducted a meta-analysis of recent seroprevalence studies together with a spatial cluster analysis at prefecture level.
2. Materials and methods
2.1. Seroprevalence dataset
All 13 geographical districts (peripheries) of Greece were represented in a cross-sectional study to describe the variations in CCHFV seroprevalence and to identify potential factors associated with the seropositivity. Data were extracted from 4 seroprevalence studies conducted during 2010–2012 (Papa et al., 2011, Sidira et al., 2012, Papa et al., 2013, Sidira et al., 2013). In total, data from 3152 Greek residents (median age 58 years, range 1–97 years) in 34 of the 54 regions (prefectures) of Greece were analyzed. Data included place of residence, age, sex, occupation, living or working in close proximity with domestic livestock (cattle, sheep, and goats), history of tick bite and other activities or factors related with increased risk for CCHF infection. All sera had been tested for the detection of CCHFV IgG antibodies using the same commercial ELISA (Vektor Best, Koltsovo, Novosibirsk, Russia).
2.2. Ethics statement
All subjects gave their informed consent for inclusion before they participated in the study. The study was approved by the Ethics Committee of the Medical School of Aristotle University of Thessaloniki.
2.3. Statistical data and analysis
The number of human population together with the number of cattle, sheep and goat populations per prefecture, as well as the areas with arable land and transitional woodland/shrub land were extracted from the Statistical Year Book of Greece 2009 & 2010 (ELSTAT, 2011). The number of cattle, sheep and goat per person per prefecture and the area of arable land and transitional woodland/shrub land per person were estimated.
Statistical analysis was carried out using the IBM SPSS 22.0 statistical package. A descriptive analysis was performed. The association between seropositivity and categorical variables was estimated using chi-square test or Fisher's exact test (when the expected count of cells was < 5). Statistical significance was defined as a two-tailed p-value of < 0.05. Odds ratio (OR) and 95% confidence intervals (95% CIs) were calculated using univariate logistic regression analysis and possible risk factors associated with CCHFV seropositivity were identified for inclusion in the final multivariate analysis. The final multivariable model was built with a stepwise backward selection for variables with univariate p < 0.05. Pearson correlation coefficient was conducted to evaluate the relationship between seroprevalence and animal density (goats, sheep, cattle) within the prefectures/regions.
2.4. Mapping
In order to map the sites where seropositive persons were detected, data were imported into ArcGIS v.9.3.1 (Esri, Redlands, CA, USA) (Mitchell, 2005). The following layers were implemented: (i) the location of the sites by transfer of the information from Google Earth; (ii) the digital elevation model using corresponding information from the Greek Databank of Hydrological and Meteorological Information (http://www.hydroscope.gr) that represents the relief of the Greek State at a scale of 1:50,000 (cell size 25 m × 25 m); (iii) the land cover of the area of focus using the CORINE Land Cover Program (http://www.eea.europa.eu/publications/COR0-land- cover); and (iv) the administrative division (NUTS 3) with the corresponding annotation of the areas.
2.5. Spatial cluster analysis
To ensure the existence and measure the spatial autocorrelation (the tendency for pathogen distribution to be clustered in space), Moran's I statistic was applied through ArcGIS based on feature locations and feature values simultaneously. Given a set of features and an associated attribute, Moran's I evaluates whether the expressed pattern is clustered, dispersed, or random. Moran's I statistic uses z-score (standard deviations) and p-value (probability) as indices to express the randomness of spatial features. Both z-scores and p-values are associated with the standard normal distribution. A layer represented the polygons of Greek prefectures was used. Every polygon attributed with fields containing values of CCHFV seroprevalence, cattle, sheep and goat numbers per person. The numeric field used in assessing spatial autocorrelation was the “CCHFV seroprevalence”. Each feature was analyzed within the context of neighboring features. Neighboring features inside the specified critical distance received a weight of 1, and exert influence on computations for the target feature. Neighboring features outside the critical distance receive a weight of zero and have no influence on a target feature's computations. Distance herein is considered the straight line distance between two points.
Since Moran's I statistic showed that the pattern was clustered, a local form of linear regression (Geographically Weighted Regression, GWR) was used to model spatially varying relationships (Fotheringham et al., 2002). GWR provides a local model of the variable of the prediction process by fitting a regression equation to every feature in the dataset. GWR constructs these separate equations by incorporating the dependent and explanatory variables of features falling within the bandwidth of each target feature. “CCHF seroprevalence” was the depended variable while “cattle per head”, “sheep per head” and “goats per head” were the explanatory variables.
The coefficient of each animal species (cattle, sheep, and goat) with the CCHF seropositivity was measured by conducting an Ordinal Least Square (OLS) correlation analysis at prefecture level (through ArcGIS). OLS or linear least squares is a method for estimating the unknown parameters in a linear regression model, with the goal of minimizing the differences between the observed responses in some arbitrary dataset and the responses predicted by the linear approximation of the data.
3. Results
3.1. Risk factors
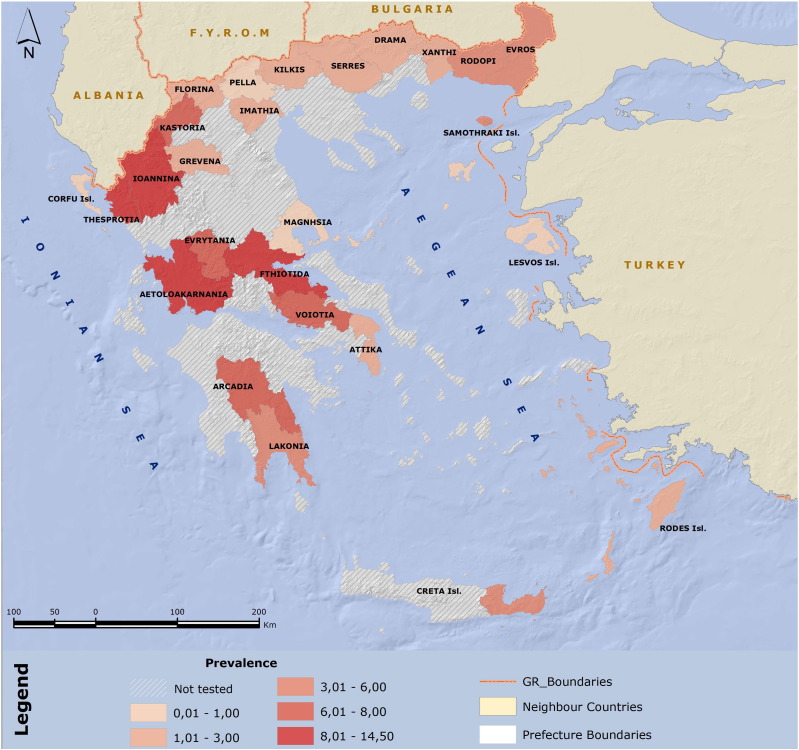
The overall CCHFV seroprevalence is 3.8% (119/3152) ranging from 0% to 14.2% depending on the district and the geographic region (Table 1; Fig. 1). Among participants, 1364 (43.3%) were males and 1788 (56.7%) females; CCHFV seroprevalence was significantly higher among males (68/1364, 4.9%) than females (51/1788, 2.8%) (p = 0.002). The median age of seropositive persons was significantly higher than that of the seronegative persons: 74 years (range 26–90) versus 57 years (range 1–97) (p < 0.001). The mean OR for CCHFV seropositivity was 1.08 for each additional year of age.
Table 1.
Distribution of seropositive and seronegative samples among districts and geographical regions of Greece.
| District | Positive (%) | Negative (%) | Total in district | Region | Positive (%) | Negative (%) | Total in region |
|---|---|---|---|---|---|---|---|
| East Macedonia-Thrace | 35 (3.0) | 1141 (97.0) | 1176 | Drama | 2 (1.4) | 145 (98.6) | 147 |
| Evros | 15 (4.5) | 320 (95.5) | 335 | ||||
| Kavala | 0 (0.0) | 149 (100.0) | 149 | ||||
| Xanthi | 2 (1.1) | 182 (98.9) | 184 | ||||
| Rodopi | 16 (4.4) | 345 (95.6) | 361 | ||||
| Central Macedonia | 11 (2.2) | 499 (97.8) | 510 | Imathia | 3 (1.7) | 171 (98.3) | 174 |
| Thessaloniki | 0 (0.0) | 53 (100.0) | 53 | ||||
| Kilkis | 1 (2.4) | 41 (97.6) | 42 | ||||
| Pella | 3 (3.0) | 98 (97.0) | 101 | ||||
| Serres | 4 (2.9) | 136 (97.1) | 140 | ||||
| West Macedonia | 14 (7.4) | 176 (92.6) | 190 | Grevena | 6 (14.0) | 37 (86.0) | 43 |
| Kastoria | 7 (7.1) | 92 (92.9) | 99 | ||||
| Florina | 1 (2.1) | 47 (97.9) | 48 | ||||
| Epirus | 28 (13.1) | 186 (86.9) | 214 | Thesprotia | 24 (14.5) | 142 (85.5) | 166 |
| Ioannina | 4 (8.3) | 44 (91.7) | 48 | ||||
| Thessaly | 1 (2.0) | 49 (98.0) | 50 | Magnesia | 1 (2.0) | 49 (98.0) | 50 |
| Ionian Islands | 1 (1.1) | 89 (98.9) | 90 | Corfu | 1 (2.0) | 49 (98.0) | 50 |
| Zakynthos | 0 (0.0) | 40 (100.0) | 40 | ||||
| Western Greece | 4 (4.2) | 92 (95.8) | 96 | Aitol/nia | 4 (8.2) | 45 (91.8) | 49 |
| Ilea | 0 (0.0) | 47 (100.0) | 47 | ||||
| Central Greece | 14 (6.5) | 202 (93.5) | 216 | Viotia | 3 (7.1) | 39 (92.9) | 42 |
| Evia | 0 (0.0) | 60 (100.0) | 60 | ||||
| Evritania | 3 (7.5) | 37 (92.5) | 40 | ||||
| Fthiotida | 8 (10.8) | 66 (89.2) | 74 | ||||
| Attica | 1 (1.1) | 90 (98.9) | 91 | Attiki | 1 (1.1) | 90 (98.9) | 91 |
| Peloponnese | 6 (3.8) | 152 (96.2) | 158 | Arcadia | 3 (6.3) | 45 (93.8) | 48 |
| Lakonia | 3 (5.9) | 48 (94.1) | 51 | ||||
| Messenia | 0 (0.0) | 59 (100.0) | 59 | ||||
| N. Aegean | 1 (0.7) | 145 (99.3) | 146 | Lesvos | 1 (1.0) | 100 (99.0) | 101 |
| Samos | 0 (0.0) | 45 (100.0) | 45 | ||||
| S. Aegean | 1 (0.9) | 106 (99.1) | 107 | Kyklades | 0 (0.0) | 41 (100.0) | 41 |
| Dodekanisa | 1 (1.5) | 65 (98.5) | 66 | ||||
| Crete | 2 (1.9) | 106 (98.1) | 108 | Lassithi | 2 (4.2) | 46 (95.8) | 48 |
| Chania | 0 (0.0) | 60 (100.0) | 60 | ||||
| Total | 119 (3.8) | 3033 (96.2) | 3152 | Total | 119 (3.8) | 3033 (96.2) | 3152 |
Fig. 1.
Prevalence of Crimean-Congo hemorrhagic fever virus antibodies in human population, Greece.
Univariate logistic regression analysis showed that apart from the increasing age and the male gender, former tick bite (p < 0.001, OR 3.62), slaughtering (p < 0.001, OR 3.15), agro-pastoral activities (p < 0.001, OR 3.50), living or working in close proximity with livestock (sheep, goat, cattle) (p < 0.001, OR 4.62), and specifically with sheep (p < 0.001, OR 4.72), goats (p < 0.001, OR 3.73), and cattle (p = 0.006, OR 2.14), were significantly associated with CCHFV seropositivity. The median altitude of seropositive and seronegative persons' residence differed significantly (142 m and 83.35 m, respectively, p = 0.001). Multivariable logistic regression analysis showed that age, sex, former tick bite, agricultural activities, and working or living in close proximity with livestock (sheep, goat, cattle) were significantly associated with seropositivity (Table 2). Seropositivity was significantly associated with the land cover type (p < 0.001). Most seropositive persons were living in non-irrigated arable land, in land with complex cultivation patterns, in land principally occupied by agriculture with significant areas of natural vegetation, and in transitional woodland-shrub land. The seroprevalence was significantly correlated with the transitional woodland/shrub land per person (r = 0.575, p < 0.001), and the number of livestock per person (r = 0.538, p = 0.001), and specifically with the number of goats, sheep and cattle per person (r = 0.493, p = 0.003, r = 0.467, p = 0.005, and r = 0.348, p = 0.044, respectively). The arable land per person was not a significant factor (p > 0.05).
Table 2.
Multivariate logistic regression analysis of CCHFV seropositivity in Greece.
| Risk factor | Positive (%) n = 119 |
Negative (%) n = 3033 |
p | OR | 95% CI |
|---|---|---|---|---|---|
| Median age (range) | 74 (26–90) |
57 (1–97) |
< 0.001 | 1.08 | 1.06, 1.10 |
| Sex | 0.021 | 1.63 | 1.08, 2.45 | ||
| Male | 68 (5.0) | 1296 (95.0) | |||
| Female (reference) | 51 (2.9) | 1737 (97.1) | |||
| Tick bite | 0.023 | 1.67 | 1.07, 2.59 | ||
| Yes | 43 (8.1) | 491 (91.9) | |||
| No (reference) | 73 (3.1) | 2306 (96.9) | |||
| Farming | 0.024 | 1.79 | 1.08, 2.96 | ||
| Yes | 85 (6.3) | 1264 (93.7) | |||
| No (reference) | 24 (1.9) | 1247 (98.1) | |||
| Proximity with livestock | < 0.001 | 2.44 | 1.57, 3.79 | ||
| Yes | 62 (10.0) | 559 (90.0) | |||
| No (reference) | 52 (2.3) | 2164 (97.7) |
Among the 13 districts of Greece, Epirus (in the western part of the country) presented the highest seroprevalence (13.1%); specifically, the geographic region of Thesprotia, was the one with the highest rate (14.5%). In general, the seroprevalence differed significantly between eastern (5 regions of East Macedonia and Thrace) and western regions (5 regions of West Macedonia and Epirus) with mean seroprevalence rates being 2.3% and 9.2%, respectively (p = 0.028). The eastern regions are mostly low land, and the main activity of the population is agriculture (tobacco and cotton cultivation), while the western region is mountainous, with poor, arid, calcareous soil, and the main activity is the animal husbandry. These regions differ significantly in the arable land per person (0.29 and 0.13 thousand of stremmas per person in east and west, respectively, p = 0.028), and the number of sheep per person (0.91 in east and 2.2 in west, p = 0.016). The mean altitude of the location where the CCHFV seropositive persons are living in the east is 71.38 m, and it is 369.41 m in the west (p < 0.001). Visit to woods was a significant factor associated with seropositivity only in the west (p = 0.046, OR 3.20, 95% CI 1.02–10.03).
3.2. Cluster analysis
The results of the Moran's I statistic are shown in Fig. 2, with Moran's Index being 0.155, expected Index − 0.018, variance 0.010, z-score 1.691, and p-value 0.090. Given that the z-score was 1.691, there was < 10% likelihood that the pattern could be the result of random chance. The results and the diagnostics of the OLS analysis are presented in Table 3. It was seen that: a. the stronger coefficient with CCHFV seropositivity was seen in the “cattle per person” variable; b. a statistically significance at 0.05 level was seen in all p-values; and c. there was not redundancy in the explanatory variables. Consequently, the model using the above mentioned variables appeared as a well fitted model.
Fig. 2.
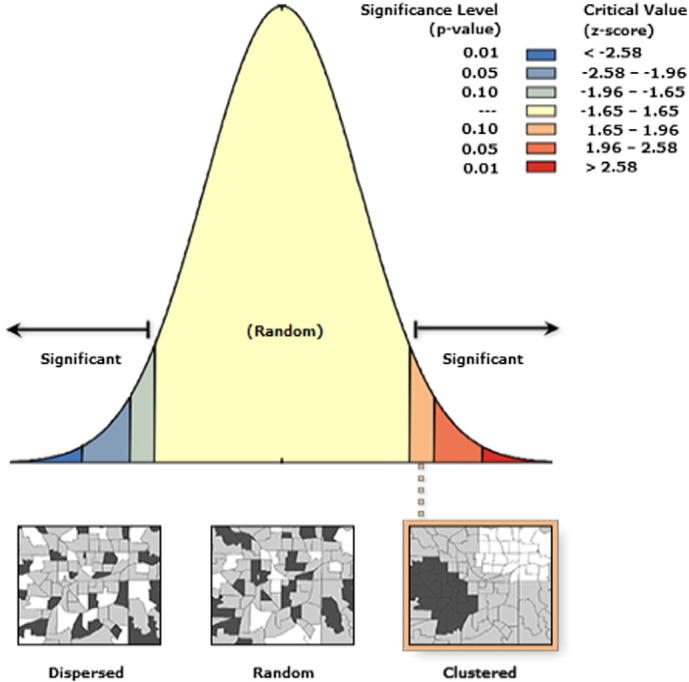
Report of the Moran's I (Spatial autocorrelation) statistic showing that the pattern was clustered. p-Value: probability; z-score: standard deviation.
Table 3.
Results (A) and diagnostics (B) from the OLS analysis regarding the CCHFV seropositivity. The asterisk indicates significance at 0.05 level.
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient | Std error | t-Statistic | Probability | Robust_SE | Robust_t | Robust_Pr | VIF |
| Intercept | − 0.073637 | 0.448572 | − 0.164160 | 0.870255 | 0.292431 | − 0.251811 | 0.802201 | – |
| Cattle per person | 4.880110 | 3.306545 | 1.475894 | 0.146122 | 4.179563 | 1.167613 | 0.248392 | 1.629527 |
| Sheep per person | 0.649600 | 0.462024 | 1.405987 | 0.165793 | 0.623966 | 1.041083 | 0.302745 | 2.389693 |
| Goats per person | 2.050899 | 0.908979 | 2.256267 | 0.028370⁎ | 0.880973 | 2.327993 | 0.023916⁎ | 2.404790 |
| B. | |||
|---|---|---|---|
| Number of Observations | 55 | Number of variables | 4 |
| Degrees of freedom | 51 | Akaike's Information Criterion (AIC) | 259.407792 |
| Multiple R-squared | 0.464206 | Adjusted R-squared | 0.432688 |
| Joint F-statistic | 14.728588 | Prob (> F) | 0.000000⁎ |
| Joint Wald statistic | 44.202973 | Prob of Robust_SE (> chi-squared) | 0.000000⁎ |
| Koenker (BP) statistic | 8.177099 | Prob of Robust_t (> chi-squared) | 0.042490⁎ |
| Jarque-Bera statistic | 30.532400 | Prob of Robust_Pr (> chi-squared) | 0.000000⁎ |
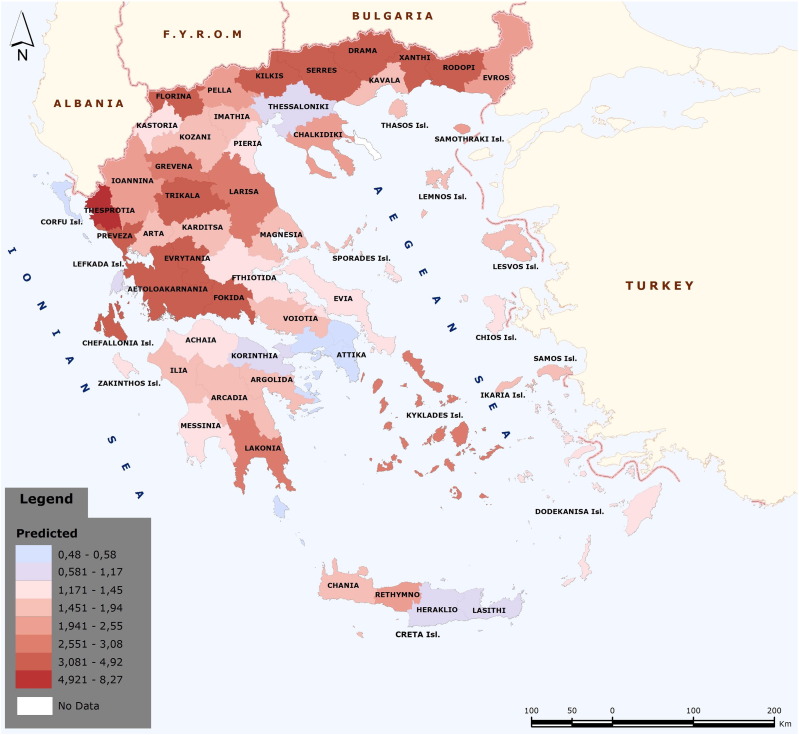
The results of the GWR analysis are shown in the Supplementary Table S1. The GWR analysis provided a spatial prediction model represented with the risk map seen in Fig. 3. As the number of livestock increased and the seropositivity showed a variation in space, the values of the explanatory variables presented a variation that differentiates the prediction model in the prefectures of Greece. The higher the value of the prediction model, the darker red color is displayed in the polygons of the prefectures.
Fig. 3.
Predicted risk map for Crimean-Congo hemorrhagic fever virus seropositivity in Greece based on seroprevalence and number of cattle, sheep and goats per head. Geographically Weighted Regression analysis was applied. The higher the value of the prediction model, the darker red color is displayed in the prefecture polygon.
4. Discussion
CCHF is endemic in Balkan peninsula, and sporadic cases or outbreaks occur every year (Sherifi et al., 2014, Christova et al., 2009, Fajs et al., 2014, Papa et al., 2002a, Papa et al., 2008b, Papa et al., 2002b, Papa et al., 2004). The CCHF epidemiology in Greece is unique, since previous seroprevalence studies in human population showed increased rates (> 5%), however without reports of clinical cases, apart one fatal case in 2008 (Papa et al., 2008a, Papa et al., 2010). In the present study the overall CCHFV seroprevalence in Greece was found to be 3.8% reaching 14.5% in one region in the western part of the country. The association of increasing age with the seropositivity is explained by the fact that older persons have more chances in their life to acquire the infection and CCHFV IgG antibodies persist for several years. Males are more often exposed to the virus than females due to occupation with agricultural activities and animal husbandry. Since ticks are implicated in the life cycle of the virus, former tick bite is an expected risk factor for seropositivity. The land cover type, the density of vegetation and the land use are greatly related with the tick density. The fact that both H. marginatum and Rhipicephalus spp. ticks (vectors of CCHFV) prefer dry climate and low vegetation can explain the finding that most of the seropositive persons were residents in areas with natural vegetation and transitional woodland-shrub land. The number of livestock per person, and especially that of goats and sheep, was significantly associated with seropositivity. Sheep and goats are usually kept under semi-free range conditions, facilitating their infestation with ticks when in pasture.
The interpretation of risk factors is more complicated in the seroprevalence than in clinical studies since the location and the time of acquisition of the infection cannot be identified precisely. For this reason, the mapping analysis was performed at NUTS 3 level. Depending on the administrative region, the CCHF seropositivity in Greece ranges from 0% to 14.5% (mean 3.8%). The highest rates are observed in the northwest part of the country, in the regions of Thesprotia and Grevena (14.5% and 14.0%, respectively). The significant seroprevalence differences between eastern and western regions of the country could be attributed to the differences in landscape, land use, climate, type of cultivation, main occupation of the population and number of livestock per person.
The statistical analysis showed that close proximity with goats, sheep, and cattle were significantly associated with CCHFV seropositivity. The OLS regression diagnostics showed the significant role of the number of goats per person. A significant role of cattle as CCHFV reservoir and driving to prevalence patterns within endemic areas has been reported recently (Messina et al., 2015). In the present study the variable “cattle_per_head” had the stronger coefficient to the seropositivity than the two others, but did not reach significant association with seropositivity (p > 0.05). This may be due to the small population of cattle in Greece in relation to that of goats and sheep.
In general, the model used appeared as well fitted, despite the weak association between independent and dependent values (intercept coefficient near zero). This can be explained by the fact that human infection by CCHFV is a comparatively rare event, but also because additional ecological, climatic and demographic parameters play a role in the virus life cycle. The produced risk map identified areas with increased risk for CCHFV seropositivity. It has to be mentioned that it is not possible to identify the CCHFV lineage to which the antibodies were produced. Based on the circulation of AP92-like strains in Greece, together with the absence of clinical cases, we can suggest that at least part of (if not all) the seropositive persons had a contact with non- or low pathogenic CCHFV strains. The present study showed that the CCHFV seroprevalence in Greece is high, and is related with several biotic and abiotic factors. Apart the factors identified in previous studies (e.g. age, sex, tick bite, agropastoral activities), additional parameters were shown to have an impact on the rate, like the altitude, the land cover type, the transitional woodland/shrub land per person, the number of livestock per person, and specifically the number of goats, sheep and cattle per person. These factors affect the level of human-tick-animal exposure, thus the interaction of humans with CCHFV natural vectors and reservoirs. Given that human disease reports are absent in Greece (apart the single case in 2008), extended tick studies are needed, especially in the regions with the highest seroprevalence, to assess the prevalence of CCHFV competent species and their infection rate, while the molecular characterization of the circulating strain(s) will unravel the mystery of CCHF epidemiology in Greece. Spatial cluster analyses in endemic countries taking into account the occurrence of human cases, the seroprevalence rates in humans and animals and the CCHFV prevalence in ticks will enable the understanding of the CCHF epidemiology globally providing the tools for disease prevention and control.
The following is the supplementary data related to this article.
Parameter estimates of the application of the Geographically Weighted Regression model.
Conflicts of interest
The authors declare no conflict of interests.
Acknowledgement
The present study was financially supported by the European Commission Seventh Framework Programme ANTIGONE (project number 278976).
References
- Aker S., Akinci H., Kilicoglu C., Leblebicioglu H. The geographic distribution of cases of Crimean-Congo hemorrhagic fever: Kastamonu, Turkey. Ticks Tick Borne Dis. 2015 doi: 10.1016/j.ttbdis.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Christova I., Di Caro A., Papa A., Castilletti C., Andonova L., Kalvatchev N., Papadimitriou E., Carletti F., Mohareb E., Capobianchi M.R., Ippolito G., Rezza G. Crimean-Congo hemorrhagic fever, southwestern Bulgaria. Emerg. Infect. Dis. 2009;15:983–985. doi: 10.3201/eid1506.081567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elevli M., Ozkul A.A., Civilibal M., Midilli K., Gargili A., Duru N.S. A newly identified Crimean-Congo hemorrhagic fever virus strain in Turkey. Int. J. Infect. Dis. 2009;14(Suppl. 3):e213–e216. doi: 10.1016/j.ijid.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Ergonul O. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 2006;6:203–214. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Pena A., Vatansever Z., Gargili A., Ergonul O. The trend towards habitat fragmentation is the key factor driving the spread of Crimean-Congo haemorrhagic fever. Epidemiol. Infect. 2010;138:1194–1203. doi: 10.1017/S0950268809991026. [DOI] [PubMed] [Google Scholar]
- Estrada-Pena A., Ruiz-Fons F., Acevedo P., Gortazar C., de la Fuente J. Factors driving the circulation and possible expansion of Crimean-Congo haemorrhagic fever virus in the western Palearctic. J. Appl. Microbiol. 2013;114:278–286. doi: 10.1111/jam.12039. [DOI] [PubMed] [Google Scholar]
- Fajs L., Jakupi X., Ahmeti S., Humolli I., Dedushaj I., Avsic-Zupanc T. Molecular epidemiology of Crimean-Congo hemorrhagic fever virus in Kosovo. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotheringham A.S., Brunsdon C., Charlton M. John Wiley & Sons; 2002. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships. [Google Scholar]
- ELSTAT H. Statistical Year Book of Greece 2009 & 2010. 2011. Statistical Authority. (Piraeus, Greece) [Google Scholar]
- Messina J.P., Pigott D.M., Golding N., Duda K.A., Brownstein J.S., Weiss D.J., Gibson H., Robinson T.P., Gilbert M., William Wint G.R., Nuttall P.A., Gething P.W., Myers M.F., George D.B., Hay S.I. The global distribution of Crimean-Congo hemorrhagic fever. Trans. R. Soc. Trop. Med. Hyg. 2015;109:503–513. doi: 10.1093/trstmh/trv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midilli K., Gargili A., Ergonul O., Elevli M., Ergin S., Turan N., Sengoz G., Ozturk R., Bakar M. The first clinical case due to AP92 like strain of Crimean-Congo hemorrhagic fever virus and a field survey. BMC Infect. Dis. 2009;9:90. doi: 10.1186/1471-2334-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. ESRI Press; 2005. The ESRI Guide to GIS Analysis. [Google Scholar]
- Papa A., Bino S., Llagami A., Brahimaj B., Papadimitriou E., Pavlidou V., Velo E., Cahani G., Hajdini M., Pilaca A., Harxhi A., Antoniadis A. Crimean-Congo hemorrhagic fever in Albania, 2001. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2002;21:603–606. doi: 10.1007/s10096-002-0770-9. [DOI] [PubMed] [Google Scholar]
- Papa A., Bozovi B., Pavlidou V., Papadimitriou E., Pelemis M., Antoniadis A. Genetic detection and isolation of Crimean-Congo hemorrhagic fever virus, Kosovo, Yugoslavia. Emerg. Infect. Dis. 2002;8:852–854. doi: 10.3201/eid0808.010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa A., Christova I., Papadimitriou E., Antoniadis A. Crimean-Congo hemorrhagic fever in Bulgaria. Emerg. Infect. Dis. 2004;10:1465–1467. doi: 10.3201/eid1008.040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa A., Maltezou H.C., Tsiodras S., Dalla V.G., Papadimitriou T., Pierroutsakos I., Kartalis G.N., Antoniadis A. A case of Crimean-Congo haemorrhagic fever in Greece, June 2008. Euro Surveill. Bull. Eur. Sur Les Maladies Transmissibles Eur. Commun. Dis. Bull. 2008;13 doi: 10.2807/ese.13.33.18952-en. [DOI] [PubMed] [Google Scholar]
- Papa A., Bino S., Papadimitriou E., Velo E., Dhimolea M., Antoniadis A. Suspected Crimean Congo haemorrhagic fever cases in Albania. Scand. J. Infect. Dis. 2008;40:978–980. doi: 10.1080/00365540802144125. [DOI] [PubMed] [Google Scholar]
- Papa A., Dalla V., Papadimitriou E., Kartalis G.N., Antoniadis A. Emergence of Crimean-Congo haemorrhagic fever in Greece. Clin. Microbiol. Infect. 2009;16:843–847. doi: 10.1111/j.1469-0691.2009.02996.x. [DOI] [PubMed] [Google Scholar]
- Papa A., Dalla V., Papadimitriou E., Kartalis G.N., Antoniadis A. Emergence of Crimean-Congo haemorrhagic fever in Greece. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2010;16:843–847. doi: 10.1111/j.1469-0691.2009.02996.x. [DOI] [PubMed] [Google Scholar]
- Papa A., Tzala E., Maltezou H.C. Crimean-Congo hemorrhagic fever virus, northeastern Greece. Emerg. Infect. Dis. 2011;17:141–143. doi: 10.3201/eid1701.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa A., Sidira P., Kallia S., Ntouska M., Zotos N., Doumbali E., Maltezou H.C., Demiris N., Tsatsaris A. Factors associated with IgG positivity to Crimean-Congo hemorrhagic fever virus in the area with the highest seroprevalence in Greece. Ticks Tick Borne Dis. 2013;4:417–420. doi: 10.1016/j.ttbdis.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Papa A., Mirazimi A., Koksal I., Estrada-Pena A., Feldmann H. Recent advances in research on Crimean-Congo hemorrhagic fever. J. Clin. Virol. 2014;64:137–143. doi: 10.1016/j.jcv.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa A., Chaligiannis I., Kontana N., Sourba T., Tsioka K., Tsatsaris A., Sotiraki S. A novel AP92-like Crimean-Congo hemorrhagic fever virus strain, Greece. Ticks Tick Borne Dis. 2014;5:590–593. doi: 10.1016/j.ttbdis.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Papa A., Mirazimi A., Koksal I., Estrada-Pena A., Feldmann H. Recent advances in research on Crimean-Congo hemorrhagic fever. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2015;64:137–143. doi: 10.1016/j.jcv.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos O., Koptopoulos G. Crimean-Congo hemorrhagic fever (CCHF) in Greece: isolation of the virus from Rhipicephalus bursa ticks and a preliminary serological survey. In: Vesenjak-Hirjan J., editor. Arboviruses in the Mediterranean Countries. Gustav Fisher Verlag; Stuttgart (Germany): 1980. pp. 117–121. [Google Scholar]
- Sargianou M., Panos G., Tsatsaris A., Gogos C., Papa A. Crimean-Congo hemorrhagic fever: seroprevalence and risk factors among humans in Achaia, western Greece. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2013;17:e1160–e1165. doi: 10.1016/j.ijid.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Sherifi K., Cadar D., Muji S., Robaj A., Ahmeti S., Jakupi X., Emmerich P., Kruger A. Crimean-Congo hemorrhagic fever virus clades V and VI (Europe 1 and 2) in ticks in Kosovo, 2012. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidira P., Maltezou H.C., Haidich A.B., Papa A. Seroepidemiological study of Crimean-Congo haemorrhagic fever in Greece, 2009–2010. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012;18:E16–E19. doi: 10.1111/j.1469-0691.2011.03718.x. [DOI] [PubMed] [Google Scholar]
- Sidira P., Nikza P., Danis K., Panagiotopoulos T., Samara D., Maltezou H., Papa A. Prevalence of Crimean-Congo hemorrhagic fever virus antibodies in Greek residents in the area where the AP92 strain was isolated. Hippokratia. 2013;17:322–325. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parameter estimates of the application of the Geographically Weighted Regression model.