Abstract
In order to determine the prevalence and risk factors for shedding of Cryptosporidium spp. in dairy calves, a cross-sectional study was carried out in the northeastern region of Buenos Aires Province, Argentina. Fecal samples from a total of 552 calves from 27 dairy herds were collected, along with a questionnaire about management factors. Cryptosporidium spp. oocysts were detected by light microscopy using Kinyoun staining. Putative risk factors were tested for association using generalized linear mixed models (GLMMs). Oocyst shedding calves were found in 67% (CI95% = 49–84) of herds (corresponding to a true herd prevalence of 98%) and 16% (CI95% = 13–19) of calves (corresponding to a true calve prevalence of 8%). Within-herd prevalence ranged from 0 to 60%, with a median of 8%. Cryptosporidium spp. excretion was not associated with the type of liquid diet, gender, time the calf stayed with the dam after birth, use of antibiotics, blood presence in feces, and calving season. However, important highly significant risk factors of oocyst shedding of calves was an age of less or equal than 20 days (OR = 7.4; 95% CI95% = 3–16; P < 0.0001) and occurrence of diarrhea (OR = 5.5; 95% CI95% = 2–11; P < 0.0001). The observed association with young age strongly suggests an early exposure of neonatal calves to Cryptosporidium spp. oocysts in maternity pens and/or an age-related susceptibility. Association with diarrhea suggests that Cryptosporidium spp. is an important enteropathogen primarily responsible for the cause of the observed diarrheal syndrome. Results demonstrate that Cryptosporidium spp. infection is widespread in the study region. Monitoring and control of this parasitic protozoan infection in dairy herds is recommended.
Keywords: Prevalence, Risk factors, Cross-sectional study, Cryptosporidium, Excretion, Oocysts
1. Introduction
Cryptosporidiosis is a parasitic protozoan disease caused by several species of the genus Cryptosporidium. The parasite infects the gastrointestinal tract in a large number of vertebrate species, including man. A cattle is mostly susceptible to infection by two species of the genus: Cryptosporidium parvum, infecting the distal small intestine and Cryptosporidium andersoni infecting the abomasum (de Graaf et al., 1999). The overwhelming number of studies report on infection of calves by C. parvum while other species, in particular C. andersoni, Cryptosporidium ryanae, and Cryptosporidium bovis, commonly infect adult cattle (Santín et al., 2008, Delafosse et al., 2015). So far, C. parvum has been exclusively identified in dairy calves in Argentina based on two independent molecular studies (Tomazic et al., 2013, Del Coco et al., 2014).
An infected calf may excrete up to 6 × 106 oocysts per gram of feces (Fayer et al., 1998) that are immediately infective, thus heavily contaminating the environment. At favorable conditions of temperature and humidity, oocysts may survive in the environment for months (Fayer et al., 2000). Economic loss associated with Cryptosporidium spp. infection are mainly related to diarrhea in calves. Interestingly, diarrhea seems to be a highly variable clinical sign of the infection as it has been observed in 15% (Silverlas et al., 2009) to 100% (Fayer et al., 1998) of oocyst-shedding calves. A conventional method to detect oocysts in stool is Kinyoun's acid-fast stain and microscopical examination. It has been estimated that the sensitivity and specificity of this method is 66.6% and 88.2%, respectively (Elsafi et al., 2014).
In Argentina, depending on the methodology used and the area of study, the prevalence in dairy calves has been reported between 17 and 29% (Bellinzoni et al., 1990, Del Coco et al., 2008, Modini et al., 2011, Tiranti et al., 2011). Although these results seem to agree, they cannot be extrapolated to other dairy calves' populations. An important quantity of dairy activity is located in the northeastern region of the Buenos Aires Province but knowledge of the cryptosporidiosis prevalence in this region is lacking. On the other hand, several studies have identified risk factors for shedding Cryptosporidium spp. oocysts, yet the results have been discordant (Silverlas et al., 2009, Trotz-Williams et al., 2007, Maldonado-Camargo et al., 1998). Risk factors provide important information to establish control strategies allowing to diminish and to prevent the spread of calf infection thus minimizing environmental contamination with parasite oocysts. As efficient drugs and vaccines against cryptosporidiosis are not currently available, knowledge of risk factors is paramount to confine the infection.
Based on this rational, the objective of this study was to estimate the prevalence and determine the relevant risk factors for shedding of Cryptosporidium spp. in calves of dairy farms in the northeast region of Buenos Aires Province, Argentine.
2. Materials and methods
2.1. Sampling frame
There are about 874 dairy herds in the northeast region of Buenos Aires Province, representing about 33% of all dairy farms of this province (M.A.A., 2010). Dairy herds included in this study are situated in the district of Exaltación de la Cruz, General Belgrano, Lobos, Luján, Marcos Paz, Monte, Navarro, and San Miguel del Monte. The common practice in this area is that cows enter maternity pens approximately four weeks before parturition. Between 6 and 36 h after birth, calves are separated from their mother and are then individually tied to stakes fixed in the ground and raised with the use of feed buckets.
2.2. Study design and data collection
A cross-sectional study was carried out between August 2013 and December 2014. Based on data from a previous report (Bellinzoni et al., 1990), the minimum number of dairy farms to be included in this study should be 27 to accept an error of 15% in the estimated prevalence with a confidence level of 95%. Furthermore, it was estimated that a minimum number of 12 calves should be examined per dairy herd (de Blas, 2016); this calculation was based on the assumption of a within-herd prevalence of 20% (Del Coco et al., 2008) and an expected population of 40 dairy calves. Within each farm, calves were randomly selected. Due to limitations in operational capacity, a maximum number of 30 calves were examined per herd.
A questionnaire was used to collect information about management factors filled by a single member of the study team on all farms. The questions were designed in order to gather information about potential factors associated with Cryptosporidium spp. shedding. Physical appearance of feces was evaluated at the time of collection using the scheme to categorize the fluidity of stools proposed by Larson et al. (1977). The occurrence of diarrhea was assigned to feces of score 3 and 4 (liquid or semi-liquid stool) while no diarrhea was assigned to feces that scored 1 and 2 (firm or slightly deformed stool).
2.3. Sampling and detection of oocysts
A single fresh feces sample per calf was collected in a clean polyethylene bag directly from the rectum after anal massage or immediately after deposition. Samples were refrigerated at 4 °C until they were further processed in the laboratory within 48 h. Fecal smears on slides were allowed to air dry and fixed with methanol. Subsequently, Kinyoun staining was performed as previously described (Elsafi et al., 2014, Henriksen and Pohlenz, 1981) and preparations examined with the aid of an optical microscope using oil immersion at 1000 × magnification. Oocysts of Cryptosporidium spp. were considered those whose morphology, optical properties, internal structure, and size matched those described by Trotz-Williams et al. (2005) and Fayer et al. (2008). At least 40 randomly selected fields were observed until the result was determined as positive or negative.
2.4. Data analysis
The analysis was based on a dichotomous outcome (calf positive or negative for shedding of oocysts). An animal was considered positive when a single oocyst was detected after microscopical examination. The studied factors represent categorical variables of the individual animal: age of calf, type of liquid diet, occurrence of diarrhea, length of time the calf stayed with the dam after birth, gender, blood in feces, and calving seasons (spring–summer months: October to April; autumn–winter months: May to September). The age and length of the time the calf stayed with the dam were categorized by the median. Spearman rank correlation was used to assess collinearity, and if > 0.30, the decision for inclusion in the multivariable analysis was based on biological knowledge. Bivariate and multivariate analyses were conducted using generalized logistic mixed models using the package “lme4” (Bates et al., 2015) for R© v. 3.0.2 (R Foundation for Statistical Computing), with logit link and fitted by maximum likelihood (Gauss–Hermite Quadrature). The farm identifier was used as random effect. Model parameters were dropped using AIC (Akaike Information Criterion): when AIC differed by 2 or more units the simpler model was retained. Since P-values are based on asymptotic Wald tests, marginal values must be looked with care; while this is standard practice for generalized linear models, these tests make assumptions both about the shape of the log-likelihood surface and about the accuracy of a chi-squared approximation to differences in log-likelihoods. An odds ratio (OR) and confidence interval of 95% (CI95%) were calculated. Variables significant in the final multivariable model were checked for interaction.
3. Results
3.1. Descriptive data
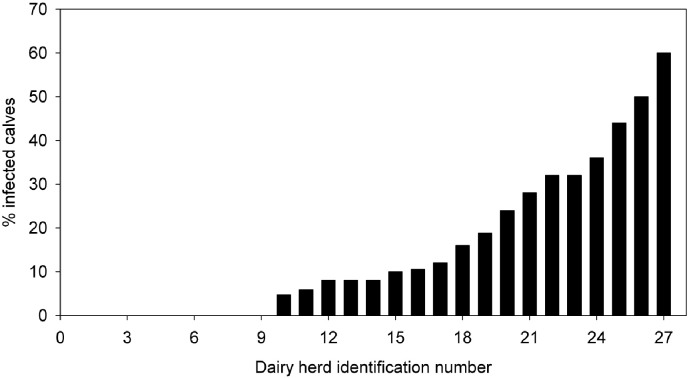
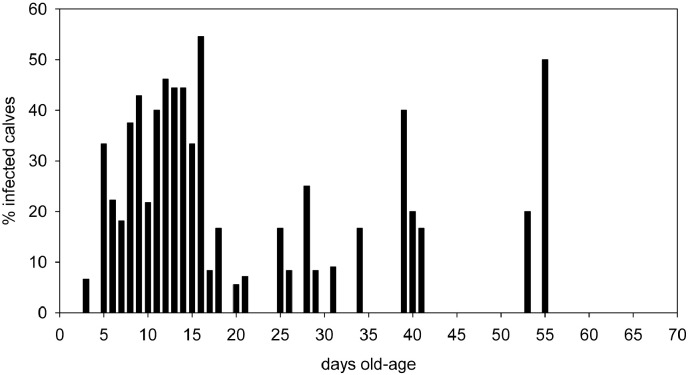
Fecal samples were collected from 552 calves from 27 dairy herds. The number of milking cows per herd ranged from 150 to 2100, with a median of 315. The number of calves examined per herd ranged from 8 to 25, with a median of 23. Sixty-seven out of 100 dairy herds were found positive (CI95% = 49–84). The within-herd prevalence ranged from 0% to 60%, with a median of 8% (Fig. 1). Altogether, 16% (CI95% = 13–19) of calves were positive for oocyst shedding. Based on a recently estimated sensitivity (66.6%) and specificity (88.2%) of the used methodology (Elsafi et al., 2014), this translates into a true herd prevalence of 98% and a true calve prevalence of 8% (Dohoo et al., 2003). The minimum and maximum age of calves found to shed oocysts was 3 and 55 days, respectively (Fig. 2).The median age of shedding calves was 12 days (inter-quartile range 8–16 days), whereas the median age of non-shedding calves was 22 days (inter-quartile range 10 to 37 days).
Fig. 1.
Percentage of infected calves in a herd. Herds are ordered by the size of determined prevalence. Between 8 and 25 calves per farms were analyzed, with a median of 23 calves.
Fig. 2.
Percentage of calves infected with Cryptosporidium spp. (n = 552). Calves between 1 and 70 days of age were examined.
3.2. Bivariate and multivariate analysis
In order to identify most appropriate factors for inclusion in the multivariate analysis a preliminary bivariate analysis was carried out (Table 1). On one hand, animal age at sampling, occurrence of diarrhea, observation of blood in feces, and use of antibiotics was highly significantly associated with the occurrence of shedding of oocysts. On the other hand, the length of time a calf stayed with the dam (> 1 days) was found to be highly significantly associated with protection against oocyst shedding. In contrast, no significant differences were observed for factors calving birth season, type of liquid diet, and gender leading to their exclusion from the following multivariate analysis. As treatment with antibiotics, presence of blood in feces, and occurrence of diarrhea were found to be correlated (r > 0.30; p < 0.0001), the former two were removed from the analysis as rational considerations allow concluding they represent a consequence of the latter. Furthermore, time of separation of calves from dams did not contribute to a smaller AIC in the multivariate model and were removed. Based on this rational, the final multivariate model determined two risk factors for oocysts shedding: a young age of the animal (≤ 20 days) and the occurrence of diarrhea (Table 2). No significant interactions were found between these factors (p > 0.05).
Table 1.
Bivariate analysis of factors significantly associated with oocyst shedding in dairy calves including farm identifier modeled as random effect.
| Variable | Levels | Infected:exposeda | Odds ratio | Confidence interval (95%) | P-value for factorc |
|---|---|---|---|---|---|
| Calf ageb | > 20 days | 14:266 | 1 | ||
| ≤ 20 days | 76:286 | 7.9 | 3.7–17 | < 0.0001 | |
| Type of liquid diet | Whole milk | 67:433 | 1 | ||
| Milk replacer | 23:119 | 0.89 | 0.2–4.01 | 0.88932 | |
| Occurrence of diarrhead | No | 56:437 | 1 | ||
| Yes | 34:115 | 6.3 | 3.3–12.9 | < 0.0001 | |
| Calf age at separation from damb | ≤ 1 days | 31:119 | 1 | ||
| > 1 days | 59:433 | 0.28 | 0.06–1.2 | 0.0014 | |
| Gender | Female | 72:444 | 1 | ||
| Male | 18:108 | 0.9 | 0.4–1.9 | 0.796 | |
| Blood in fecesd | No | 79:519 | 1 | ||
| Yes | 11:33 | 4 | 1.3–12.2 | 0.0149 | |
| Birth season | Autumn–winter | 50:410 | 1 | ||
| Spring–summer | 40:132 | 2.4 | 0.9–6.5 | 0.07771 | |
| Antibioticsd | No | 43:433 | 1 | ||
| Yes | 47:119 | 8.02 | 3.9–16.3 | < 0.0001 |
90 dairy calves were found to shed Cryptosporidium spp. oocysts; 552 dairy calves were studied.
Factor was kept in the multivariate model.
Chi-square test.
Factors found to be correlated with > 30%.
Table 2.
Risk factors for oocyst shedding as identified by multivariate analysis including a farm identifier modeled as random effect.
| Predictor | Regression coefficient | P-value for factor | Adjusted odds ratio | 95% confidence interval |
|---|---|---|---|---|
| Intercept | 0.4088335 | < 0.0001 | – | |
| Age (≤ 20 days) | 2.0112775 | < 0.0001 | 7.4 | 3.3–16.5 |
| Occurrence of diarrhea | 1.7223529 | < 0.0001 | 5.5 | 2.6–11.6 |
4. Discussion
The main objectives of this study were, on one hand, to assess the prevalence, and, on the other hand, the risk factors of oocyst shedding in calves from dairy herds in the northeast region of Buenos Aires Province, Argentina. We observed at least one oocyst-shedding calf in 67% of the dairy herds included in this study. The determined dairy herd prevalence is consistent with that reported in a central region of Argentina by Bellinzoni et al. (1990) and Tiranti et al. (2011). In contrast, in Mexico (Maldonado-Camargo et al., 1998) and Sweden (Silverlas et al., 2009) much higher herd level prevalences have been reported (93.5% and 96% respectively). This discrepancy may be attributed to a different sensitivity of the diagnostic tests used. Interestingly, within-herd variability of the prevalence reported in our study is similar to results reported by Trotz-Williams et al. (2005) suggesting the existence of factors that determine transmission at the herd level independent of geographic region. Our results, confirm previous reports (Bellinzoni et al., 1990, Del Coco et al., 2008, Tiranti et al., 2011) demonstrating that Cryptosporidium spp. infection in dairy calves is endemic in all studied regions of Argentina calling for a further evaluation of its impact on animal health and its zoonotic importance.
The individual level prevalence of oocysts shedding in this study was determined as 16.3% and thus slightly lower than 17.1%, 19.3%, and 24% reported by Del Coco et al. (2008) Tiranti et al. (2011) and Modini et al. (2011), respectively. The slightly higher prevalence determined in their studies compared to our study is likely due to the younger strata of calves analyzed. In another study (Bellinzoni et al., 1990), the reported higher prevalence of 29.6% may be attributed to the fact that calves with history of diarrhea had been investigated, possibly overestimating the true prevalence in population.
In our study, some animals started as early as three days of age to disseminate oocysts. This result suggests that infection with Cryptosporidium spp. occurs at the first hours after birth since the minimum prepatent period reported was between 2 (Silverlas et al., 2009) and 3 days (Fayer et al., 1998). In contrast to the study of Del Coco et al. (2008), who reported excretion of oocysts in calves up to the age of 14, in the present study oocyst dissemination was observed until the age of 53 days. This finding suggests a prolonged period of oocyst shedding in dairy calves, as has been also suggested by other studies (Fayer et al., 1998, Castro-Hermida et al., 2002), which additionally contributes to environmental contamination.
We report here a strong association between oocyst shedding and calf age. The highest frequency of oocyst shedding calves was observed in calves under 20 days of age confirming other studies that report a high prevalence in similar strata (Delafosse et al., 2015, Trotz-Williams et al., 2005). In our study, the frequency of oocyst shedding in calves declined after 20 days of age and other authors (Silverlas et al., 2009) have suggested that this may be due to an age-related declining susceptibility or an acquired immunity after first exposure. Noteworthy, passive transfer of colostral immunoglobulins and/or active immune cells at newborn, have little role in preventing Cryptosporidium spp. infection (Trotz-Williams et al., 2007, Quigley et al., 1994, Harp and Goff, 1998). Possibly, maternity pens turn into an important source of infection for the newborn calf as they accumulate cow feces contributing to their oocyst contamination (Trotz-Williams et al., 2007, Almeida Castro et al., 2009). Based on these observations, early separation of the calf from maternity pens may reduce the risk of fecal–oral contact avoiding neonatal infection. Furthermore, as young calves represent a potential source of zoonotic infection, biosecurity measures in the routine feeding of calves (washing boots, utensils, etc.) may limit zoonotic risk and avoid parasite transmission by fomites.
The strong association with diarrhea suggests that Cryptosporidium spp. is in the study region the predominant enteropathogen responsible for this clinical sign, an observation corroborated by others authors in similar epidemiological situations (Delafosse et al., 2015, Del Coco et al., 2008, Trotz-Williams et al., 2005). Dairy calves seem to be particularly susceptible to Cryptosporidium spp. infection and subsequent development of diarrhea, possibly due to the stress caused by the conditions of artificial rearing. Interestingly, occurrence of diarrhea was not found to be associated with Cryptosporidium spp. infection in beef cattle (Araujo et al., 2011), a finding which may be attributed to the different genetic background of beef breeds and/or their different rearing.
5. Conclusions
Our results indicate that Cryptosporidium spp. are widespread in dairy calves of the northeast region of Buenos Aires Province, Argentina. We show that oocyst excretion can be detected in calves as early as three days of age. Risk factors for shedding of Cryptosporidium spp. oocysts were an age of calves under 20 days and occurrence of diarrhea. Knowledge of the prevalence and risk factors for oocyst shedding are a prerequisite to efficiently monitor the infection and to implement control measures thus potentially limiting parasite transmission and environmental contamination with oocysts.
Acknowledgments
We thank A. Venzano, D. Funes, C. Moreno, M. Bok, C. Vega, and V. Parreño for their collaboration. The authors wish to thank the dairy farmers, veterinaries, and operators who participated in this study. This work was funded by the neonatal diarrhea module of the National Animal Health Project No. 1115053 of the National Agricultural Technology Institute (INTA) of Argentina, the Agencia Nacional de la Promoción Científica y Tecnológica (ANPCyT) (PICT 2013-1708 and PICT 2012-0695), and the Fundación Universidad de Morón (PID 8-2015).
References
- Almeida Castro P.A. Cryptosporidiosis: caracterización de la infección en rodeos lecheros. Livest. Res. Rural. Dev. 2009;21(10) [Google Scholar]
- Araujo A.V., Gómez Muñoz M.Á., Milano A.F. Prevalencia de la infección por Cryptosporidium spp. en bovinos de dos establecimientos del Nordeste Argentino. REDVET Rev. electrón. vet. 2011;12(10) [Google Scholar]
- Bates D. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67(1):1–48. [Google Scholar]
- Bellinzoni R.C. Microbiology of diarrhea in young beef and dairy calves in Argentina. Rev. Argent. Microbiol. 1990;22(3):130–136. [PubMed] [Google Scholar]
- Castro-Hermida J.A., Gonzalez-Losada Y.A., Ares-Mazas E.A. Prevalence of and risk factors involved in the spread of neonatal bovine cryptosporidiosis in Galacia (NW Spain) Vet. Parasitol. 2002;106:1–10. doi: 10.1016/S0304-4017(02)00036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Blas, I., Working in epidemiology [online], accessed 21/03/2016. in URL: http://www.winepi.net, 2016.
- de Graaf D.C. A review of the importance of cryptosporidiosis in farm animals. Int. J. Parasitol. 1999;29(8):1269–1287. doi: 10.1016/S0020-7519(99)00076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Coco V.F., Cordoba M.A., Basualdo J.A. Cryptosporidium infection in calves from a rural area of Buenos Aires, Argentina. Vet. Parasitol. 2008;158(1–2):31–35. doi: 10.1016/j.vetpar.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Del Coco V.F. Cryptosporidium parvum GP60 subtypes in dairy cattle from Buenos Aires, Argentina. Res. Vet. Sci. 2014;96(2):311–314. doi: 10.1016/j.rvsc.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Delafosse A. Cryptosporidium parvum infection and associated risk factors in dairy calves in Western France. Prev. Vet. Med. 2015;118(4):406–412. doi: 10.1016/j.prevetmed.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohoo I., Martin W., Stryhn H. National Library of Canada Cataloguing in Publication; 2003. Veterinary Epidemiologic Research; pp. 1–705. [Google Scholar]
- Elsafi S.H. Comparison of Kinyoun's acid-fast and immunofluorescent methods detected an unprecedented occurrence of Cryptosporidium in the Eastern Region of Saudi Arabia. J. Taibah Univ. Med. Sci. 2014;9(4):263–267. [Google Scholar]
- Fayer R. Cryptosporidium parvum infection in bovine neonates: dynamic clinical, parasitic and immunologic patterns. Int. J. Parasitol. 1998;28(1):49–56. doi: 10.1016/s0020-7519(97)00170-7. [DOI] [PubMed] [Google Scholar]
- Fayer R., Morgan U., Upton S.J. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 2000;30(12–13):1305–1322. doi: 10.1016/s0020-7519(00)00135-1. [DOI] [PubMed] [Google Scholar]
- Fayer, R., C.A. Speer, and J.P. Dubey, The general biology of Cryptosporidium. Editado por Fayer R. y Xiao L. in “Cryptosporidium and Cryptosporidiosis”, 2008. 2nd Ed.: p. 1–41.
- Harp J.A., Goff J.P. Strategies for the control of Cryptosporidium parvum infection in calves1,2. J. Dairy Sci. 1998;81(1):289–294. doi: 10.3168/jds.S0022-0302(98)75578-X. [DOI] [PubMed] [Google Scholar]
- Henriksen S.A., Pohlenz J.F.L. Staining of Cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet. Scand. 1981;22:594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson L.L. Guidelines toward more uniformity in measuring and reporting calf experimental data1. J. Dairy Sci. 1977;60(6):989–991. [Google Scholar]
- M.A.A. 2010. Resumen estadistico de la cadena lactea de provincia de Buenos Aires; pp. 1–43. [Google Scholar]
- Maldonado-Camargo S. Prevalence of and risk factors for shedding of Cryptosporidium parvum in Holstein Freisian dairy calves in central Mexico. Prev. Vet. Med. 1998;36(2):95–107. doi: 10.1016/s0167-5877(98)00084-1. [DOI] [PubMed] [Google Scholar]
- Modini L.B. Infección por Cryptosporidium spp. en ganado vacuno de la cuenca lechera de la provincia de Santa Fe (Argentina) Revista FABICIB. 2011;15:97–107. [Google Scholar]
- Quigley J.D., 3rd Effects of housing and colostrum feeding on the prevalence of selected infectious organisms in feces of Jersey calves. J. Dairy Sci. 1994;77(10):3124–3131. doi: 10.3168/jds.S0022-0302(94)77255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santín M., Trout J.M., Fayer R. A longitudinal study of cryptosporidiosis in dairy cattle from birth to 2 years of age. Vet. Parasitol. 2008;155(1–2):15–23. doi: 10.1016/j.vetpar.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Silverlas C. Prevalence and associated management factors of Cryptosporidium shedding in 50 Swedish dairy herds. Prev. Vet. Med. 2009;90(3–4):242–253. doi: 10.1016/j.prevetmed.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Tiranti K. Prevalence of Cryptosporidium spp. and Giardia spp., spatial clustering and patterns of shedding in dairy calves from Córdoba, Argentina. Rev. Bras. Parasitol. Vet. 2011;20(2):140–147. doi: 10.1590/s1984-29612011000200009. [DOI] [PubMed] [Google Scholar]
- Tomazic M.L. Molecular characterization of Cryptosporidium isolates from calves in Argentina. Vet. Parasitol. 2013;198(3–4):382–386. doi: 10.1016/j.vetpar.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Trotz-Williams L.A. Prevalence of Cryptosporidium parvum infection in Southwestern Ontario and its association with diarrhea in neonatal dairy calves. Can. Vet. J. 2005;46(4):349–351. [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams L.A. Calf-level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Prev. Vet. Med. 2007;82(1–2):12–28. doi: 10.1016/j.prevetmed.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]