Abstract
Introduction
Production of isoprostanes (IsoPs) is enhanced after acute, intense, and prolonged exercise, in untrained subjects. This effect is greater in older subjects. The present study aims to delineate the profile of acute-exercise-induced IsoPs levels in young and older endurance-trained subjects.
Methods
All included subjects were male, young (n = 6; 29 yrs ± 5.7) or older (n = 6; 63.7 yrs ± 2.3), and competitors. The kinetics of F2-IsoPs in blood-sera was assessed at rest, for the maximal aerobic exercise power (MAP) corresponding to the cardio-respiratory fitness index and after a 30-min recovery period.
Results
No significant time effect on F2-IsoPs kinetics was identified in young subjects. However, in older athletes, F2-IsoPs blood-concentrations at the MAP were higher than at rest, whereas these blood-concentrations did not differ between rest and after the 30-min recovery period.
Conclusion
Because plasma glutathione (GSH) promotes the formation of some F2-IsoPs, we suggest that the surprising decrease in F2-IsoPs levels in older subjects would be caused by decreased GSH under major ROS production in older subjects. We argue that the assessment F2-IsoPs in plasma as biomarkers of the aging process should be challenged by exercise to improve the assessment of the functional response against reactive oxygen species in older subjects.
Keywords: Isoprostanes, Aging, Exercise, Training
Abbreviations: ROS, reactive-oxygen species; IsoP, Isoprostane; , Maximal oxygen uptake; MAP, Maximal aerobic power; Lamax, Venous blood-lactate concentration at ; La30, Venous blood-lactate concentration at 30 min after exercise; BHT, Butylated hydroxytoluene; MS, Mass spectrometry; HPLC, High-performance liquid chromatography; FSHD, Facioscapulohumeral dystrophy; Nrf2, Erythroid 2-like factor 2; GSH, Glutathione
Highlights
-
•
Acute exercise promotes an increase in F2-IsoPs plasma level in older athletes.
-
•
The F2-IsoPs plasma level significantly decreased after recovery in older athletes.
-
•
This kinetic of F2-IsoPs could reflect a decrease of glutathione (GSH).
-
•
Oxidative stress status determination should be challenged by exercise.
-
•
Assessment of F2-IsoPs plasma level should be paired to GSH assessment.
1. Introduction
The number of people aged >60 years is expected to double by 2050 (more than 1 in 5 people will be aged >60 years), according to a new report released by the World Health Organization [1]. The phenotypes of the aging process are heterogeneous: some older people will have a level of functionality similar to middle-aged people whereas others will require assistance for daily tasks. Loss of skeletal mass and function, termed “sarcopenia”, is one of the most notable changes during aging and can greatly affect physical performance [2].
In a recent review for the SPRINTT (Sarcopenia & Physical fRailty IN older people: multi-component Treatment strategies) consortium, Calvani et al. reminded us that impaired physical performance, when related to sarcopenia, is associated with a physically frail phenotype, and is a predictor for major negative outcomes [3]. This review supports the need to develop biomarkers to detect and help prevent frailty and sarcopenia. This strategy needs to assess the physiopathological processes and their corresponding biomarkers associated with an impaired muscular function in older subjects. These biomarkers should then be added to a clinical and usual assessment process of frailty in elderly patients.
Oxidative stress is a key biological mechanism that contributes to functional decline during aging [4]. More reactive-oxygen species (ROS) are produced from the mitochondrial respiratory chain, which is known to functionally decline with age [5], but of interest is the associated level of antioxidant activity that may not match age-related oxidant activity. An imbalance between antioxidant and oxidant activity can lead to oxidative molecular damage, defined as “oxidative stress”, and is associated with an impaired functional phenotype. This association has been recently supported in an epidemiological study that reported the results from stepwise models fitted from the Framingham Offspring Study. According to these models, biomarkers of oxidative stress were associated with greater frailty and slower gait speeds amongst patients aged ≥60 years [6]. Oxidative stress forms the central dogma for “the free-radical theory of aging” [7]. New strategies are needed to assess oxidative stress and to implement multivariate methodologies to screen older subjects and reduce aged-related functional impairment, sarcopenia, and frailty [3], [8]. As previously suggested, oxidative stress the defined as a biomarker of the aging process could then be a part of the clinical and usual assessment process of the functional status of elderly patients.
Isoprostanes (IsoPs) are a class of oxidation products. Most IsoPs are produced by ROS that catalyze the peroxidation of polyunsaturated fatty acids. Measurement of IsoPs is considered an accurate way to assess oxidative stress in vivo and can be correlated with numerous diseases [9], [10], [11]. In healthy and young subjects, acute, intense, and prolonged exercise increases plasma IsoPs levels, which then negatively influences the properties of the skeletal contractile muscles, whereas chronic exercise is associated with a decrease in plasma IsoPs [12], [13], [14]. The effect of acute exercise on plasma IsoPs is greater among older adults compared to young subjects [15], [16], but fit older subjects can also reduce the generation of oxidative stress compared to unfit older subjects [17]. However, whether endurance-trained older subjects are less prone than endurance-trained younger subjects to counteract the acute-exercise-induced production of IsoPs remains to be determined. This question addresses the physiology of longevity, i.e., whether endurance training in older subjects can restore a “young anti-oxidative” performance level or simply counteracts the effects of aging on ROS production and oxidative stress? The present study aims to delineate the profile of acute-exercise-induced IsoPs levels in young and older endurance-trained subjects (i.e., master athletes aged ≥60 years).
2. Methods
2.1. Study design
This study was defined as being exploratory and aimed to delineate data that could support a comparative designed study. This biochemical study was designed to assess the interest of the addition of a biological biomarker assessment during a daily medical-care routines exercise testing feature. Time points for biological assessment were the defined in respect to the plan of this daily clinical-care plan.
2.2. Subjects
As a part of our daily medical-care routines regarding testing of exercise functionality, young and older subjects involved in sport often request a maximal-exercise test to delineate their physiological adaptations to endurance exercise. This request is voluntary.
All the subjects included within this study were sporting competitors. Whilst most young competitors daily tested in our laboratory included both males and females, most of the included subgroup of older athletes (aged ≥60 years) were male. All competitors in this study were involved in endurance exercise, with the majority being cyclists. In order to match young and older trained subjects, we prospectively selected young and older male athletes involved in endurance cycling training for competitions. None of the subjects suffered from any medical disease that could have excluded them from intense exercise.
2.3. Clinical examination, exercise testing and blood sampling
All the subjects were assessed for weight and height to define a body mass index (BMI, kg/m2). Percentage body fat was estimated using the skin-fold method (8 skin folds were measured).
Maximal exercise testing was requested by the subjects to implement their exercise-training program. As a part of the physiological indicators for metabolic responses to endurance exercise, the following were assessed: maximal oxygen uptake (, L.min−1, mL.min−1.kg−1), its corresponding power (maximal aerobic power, MAP, watts), and venous blood-lactate concentration during exercise (one sample was measured at , Lamax) and at 30 min after (La30).
According to the usual protocols for exercise testing, a maximal graded exercise test was conducted in our laboratory. Subjects used their own bicycle and equipment. Power output was assessed using a Power Tap mobile cycling ergometer® (Cycle Ops, Madison, WI, USA). During the test, oxygen consumption () was assessed using an Oxycon Pro-ergospirometer® (Erich Jaeger, Viasys Healthcare, Germany). and its MAP were expressed in L.min−1 and watts, respectively. These functional parameters were also expressed as percentages of their corresponding predicted values, calculated according to age, using Wasserman's prediction equation [18]. This was done to ensure that young and older athletes were matched with respect to their corresponding age-category fitness level. was also expressed in mL.min−1.kg−1 to better appreciate the endurance performance level for competition practice.
In addition to assessing gas exchange, some athletes voluntarily requested blood-lactate concentration to be assessed to implement their training plan. Data from gas exchanges and blood-lactate concentrations can be used to define a metabolic approach to improve training. To assess blood-lactate, 2 mL of blood was taken using an antecubital venous catheter. The volume of 2 mL was fixed according to that specified by the ABL 800 Flex Apparatus (Radiometer Copenhagen). Blood sampling to assess lactate concentration was requested by the subjects. All the subjects gave their written informed consent to use some of the sampled blood to assess plasma IsoPs concentrations. After completing assessment of blood-lactate concentrations (i.e., at MAP and at 30 min after exercise), the remainder of the sample was centrifuged and the plasma aliquoted and frozen at −80 °C until assessment of IsoPs. Samples were stored and declared for medical research (National Institute for Health and Medical Research, biological collection n° DC2014-2039). Per-exercise tubes, usually sampled to study lactate kinetics and to adjust training programs, were not included in the IsoPs analyses as the objective of our present study was to delineate the effect of maximal aerobic exercise on IsoPs concentration that included the recovery period, and to not delineate the kinetics of IsoPs levels during exercise.
2.4. Assessment of IsoPs
For each sampling time, plasma (1 mL) was collected, supplemented with butylated hydroxytoluene (BHT, 1% in ethanol), and stored at −80 °C. Samples were spiked with 5 ng of each internal standard. A volume of 985 μL of hydrolysis solution (KOH 1 M in MeOH) was added. The resulting mixture was vortexed and incubated at 40 °C for 30 min. After cooling at room temperature, 2 mL of 40 mM formic acid (pH 4.5 adjusted with 1 M NaOH) was added. Thereafter, the samples were cleaned and extracted by solid-phase extraction to obtain a low matrix effect and a good yield of extraction before analysis using mass spectrometry (MS).
The lipid portion of the tissues or cells was extracted using Folch solution (CHCl3: MeOH, 2:1, v/v) in the presence of antioxidant (0.005% BHT). Solid–phase extraction was performed on a 96-well plate OASIS MAX 60 mg (Waters, USA), using the modified method of Lee et al. [19]. Briefly, the wells were cleaned with 2 mL of MeOH and conditioned with 2 mL of 40 mM formic acid (pH 4.5). After loading the samples, the wells were washed with 2 mL of 2% NH4OH followed by 2 mL of MeOH/20 mM formic acid (20:80 v/v) and 2 mL of hexane. The IsoPs were eluted with 2 mL of hexane/ethanol/acetic acid (70:29.4:0.6 v/v/v). After drying under nitrogen gas, the samples were re-dissolved with 20 μL of MeOH. Some of the sample (5 μL) was taken for liquid chromatography coupled to tandem MS.
High-performance liquid chromatography (HPLC) was performed using an Agilent 1290 Infinity equipped with an autosampler and a thermostat, a binary pump, and a column oven. The analytical column was a Zorbax SB-C18 Rapid Resolution HD (2.1 × 100 mm; 1.8 μm) (Agilent Technologies, USA), which was maintained at 25 °C. The mobile phases consisted of water: formic acid (99.9:0.1; v/v) and acetonitrile: formic acid (99.9:0.1, v/v).
The flow rate was set at 0.3 mL/min. The autosampler was set at 5 °C and 5 μL was injected per analysis. The HPLC system was coupled on-line to an Agilent 6460 triple quadrupole MS (Agilent Technologies, USA) equipped with electrospray ionization which was performed in negative-ion mode.
The MS source parameters were set as follows: source temperature 325 °C, nebulizer gas (nitrogen) flow rate was 10 L/min, sheath gas temperature 350 °C, sheath gas (nitrogen) flow rate 12 L/min, and spray voltage was adjusted to −3000 V. The dwell time used was 10 ms. The analysis was performed using Selected Reaction Monitoring detection mode with nitrogen as the collision gas. The mode for each compound was pre-determined by MS/MS analysis. Peak detection, integration and quantitative analyses were performed using Mass Hunter Quantitative analysis software (Agilent Technologies, USA).
Several IsoPs stereoisomers have been derived from lipids, but the F2-IsoPs isomers originating from arachidonic acid (AA, 20:4 n-6) are recognized as the reference biomarker for lipid peroxidation and oxidative stress [10], [20]. Although some F2-IsoPs isomers are much more abundant than others, we tested several isomers to provide an integrated picture of lipid peroxidation (as recommended) [13]. We focused on detecting IsoPs production that involved 5-F2t-IsoP, 5-epi-5-F2t-IsoP, 15-F2t-IsoP and 15-epi-F2t-IsoP isomer concentrations within the plasma.
2.5. Assessment of blood lactate
Blood lactate was assessed using an ABL 800 Flex analyzer (Radiometer Medical ApS, Brønshøj, Denmark). Blood was measured on an amperometric electrode using the enzyme, lactate oxidase, which converts lactate to pyruvate and hydrogen peroxide. The released hydrogen peroxide is oxidized at a platinum anode, resulting in a release of electrons, which are proportional to the concentration of the sampled lactate [21].
2.6. Statistical analysis
This study aimed to delineate data to support a further comparative study. The number of subjects selected for this study was limited due to financial costs of the biological-assessment procedures.
Continuous measures are reported as means ± standard deviations (SD). For continuous variables independent of time, the unpaired Student's t-test or the Kruskal-Wallis test were used to examine mean differences in related variables between age categories. To examine the effect of age category and time of blood sampling (independent variables) on F2-IsoPs concentration (dependent variable), a two-way ANOVA with repeated measurements for the time factor was performed for each F2-IsoPs. Post-hoc comparisons were processed when a significant time effect was identified.
A probability of P < .05, with a two-sided level of significance, was used to delineate statistical significance in all comparisons. Statistical analyses were computed using Stata 6.0 software (Stata, College Station, TX).
3. Results
3.1. Anthropometric data and functional performance of the subjects
Data linked to anthropometric and functional-performance statuses are listed in Table 1. According to these parameters, young and older athletes were characterized according to fitness status. The anthropometrics were lower than those usually reported for the age-paired general population. Young and older athletes were paired for their age-related fitness levels, according to a cardiorespiratory fitness () level that was 150% of the predicted value for the corresponding age category. Relative age-category fitness did not differ between the young and older athletes. The corresponding power (MAP) was also higher than predicted and older athletes had a relative-to-age MAP that was higher than the young athletes. (in mL.min−1.kg−1) and the relative MAP levels at the 1st and 2nd ventilatory thresholds showed very good endurance performance levels for competition in both young and older athletes.
Table 1.
Anthropometric data and exercise-related data from the athletes. All values are means ± SDs.
| Young athletes | Athletes aged >60 years | p-value | |
|---|---|---|---|
| Age (years) | 29.3 (5.7) | 63.7 (2.6) | |
| Body-mass index (kg/m2) | 21.6 (1.4) | 24.7 (2.0) | .01 |
| Body fat (%) | 14.5 (5.5) | 24.5 (1.4) | .04 |
| (L.min−1) | 4.0 (0.3) | 3.1 (0.3) | .0006 |
| % predicted value | 149 (21) | 157 (28) | .61 |
| (mL.min−1.kg−1) | 59.5 (9.9) | 40.5 (7.2) | .003 |
| Maximal aerobic power (MAP; watts) | 301 (35) | 243 (36) | .02 |
| % predicted value | 133 (22) | 157 (33) | .0001 |
| Maximal cardiac frequency (beats.min−1) | 189 (10) | 169 (16) | .03 |
| % predicted value | 99 (5.6) | 100 (9.2) | .80 |
| Power at the 1st ventilatory threshold % MAP | 75 (13) | 64 (10) | .13 |
| Power at the 2nd ventilatory threshold % MAP | 90 (4.6) | 84 (5.4) | .12 |
| Blood lactate concentration (mmol.L−1) | |||
| at rest | 1.3 (0.3) | 1.1 (0.08) | 0.10 |
| at MAP | 10.7 (2.9) | 78 (0.7) | 0.05 |
3.2. F2-IsoPs isomers kinetic during exercise and recovery
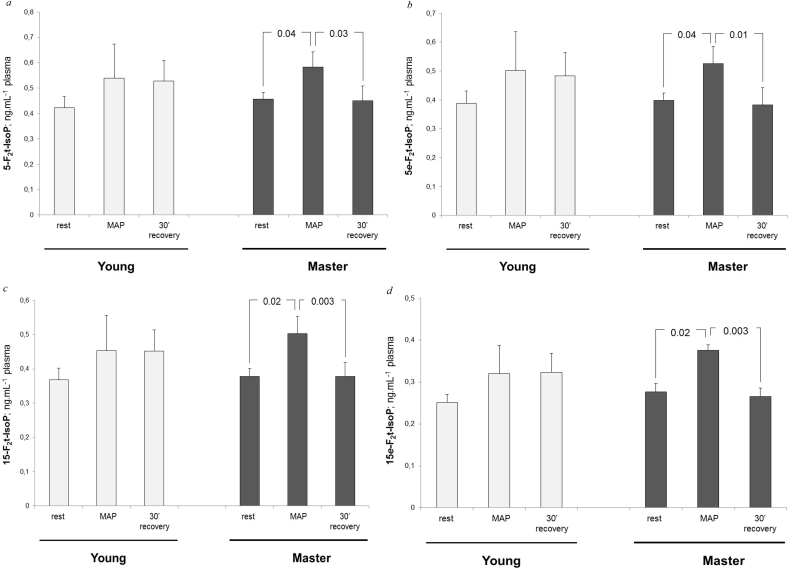
The kinetics of the four F2-IsoPs stereoisomers in blood-sera at rest, at the corresponding MAP intensity, and after 30 min of recovery, are presented in Fig. 1. A time effect was identified for the blood-concentration kinetics of each F2-IsoPs stereoisomer (p = .04, p = .04, p = .02, and p = .04 for 5-F2t-IsoP, 5-epi-5-F2t-IsoP, 5-F2t-IsoP and 15-epi-F2t-IsoP blood-concentration kinetics, respectively). No significant interaction between group and time was detected, but the present study was not designed to detect this interaction. However, to consider the significance of time effect, we stratified the kinetics for blood F2-IsoPs concentrations according to the age-group variable. No significant time effect was identified in young athletes, despite that the mean values of F2-IsoPs blood concentrations at MAP were 21.2%, 24.4%, 22.4%, and 18.1% higher compared to the values at rest for 5-F2t-IsoP, 5-epi-5-F2t-IsoP, 15-F2t-IsoP and 15-epi-F2t-IsoP, respectively. In the older athletes, blood-concentration values of F2-IsoPs at MAP were higher than those at rest and the 30-min recovery values. In older athletes, F2-IsoPs blood-concentrations at MAP were 27.7%, 31.9%, 39.7% and 35.5% higher than those at rest for 5-F2t-IsoPs, 5-epi-5-F2t-IsoP, 15-F2t-IsoP and 15-epi-F2t-IsoP, respectively. In addition, blood-concentrations at rest and after the 30-min recovery period did not significantly differ for any of the F2-IsoPs isomers.
Fig. 1.
Variation in F2-IsoP stereoisomer concentrations in plasma from rest to maximal aerobic power (MAP), and after 30-min of recovery in young and older (master) athletes. a. Variation in plasma 5-F2-IsoP concentrations. b. Variation in 5e-F2-IsoPs concentrations. c. Variation in 15-F2-IsoP concentrations. d. Variation in 15e-F2-IsoP concentrations.
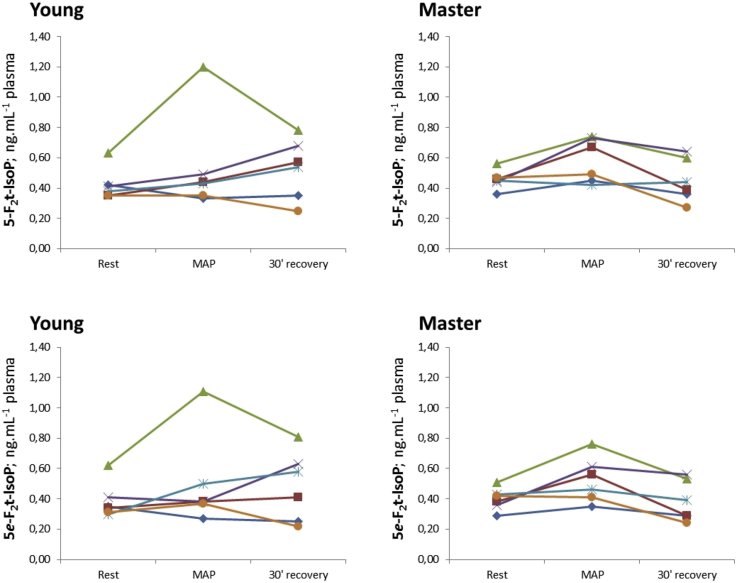
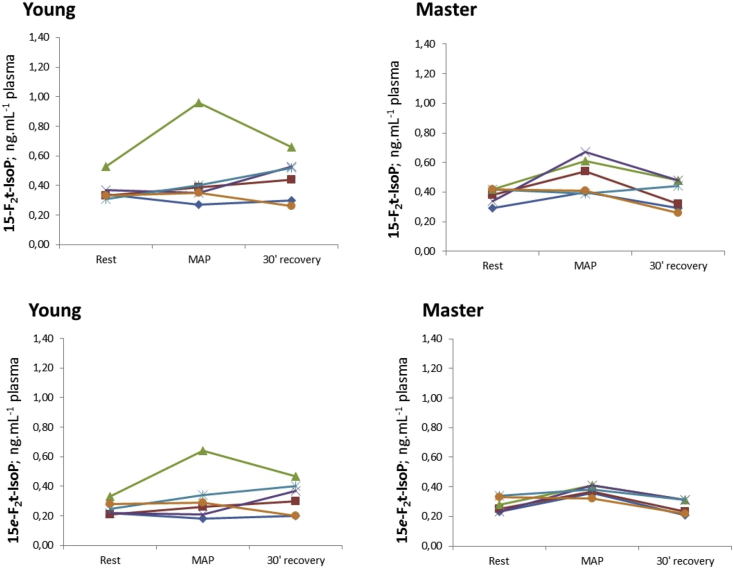
The kinetics of blood concentrations of the four F2-IsoPs stereoisomers are presented in Fig. 2, Fig. 3. These figures show the individual variabilities in the distributions of each F2-IsoPs stereoisomer. The kinetics of each F2-IsoPs stereoisomer was homogeneous in the older athletes whereas the corresponding kinetics were more heterogeneous in the young athletes group.
Fig. 2.
Variations in 5-F2 and 5e-F2-IsoP stereoisomers at rest, at MAP, and after a 30-min recovery period. The data are stratified according to age category: young or aged >60 years (master). For each F2-IsoPs stereoisomer, each line describes the time kinetics for one subject.
Fig. 3.
Variation in 15-F2 and 15e-F2-IsoPs stereoisomers at rest, at MAP, and after a 30-min recovery period. The data are stratified according to age category: young or aged>60 years (master). For each F2-IsoPs stereoisomer, each line describes the time kinetics for one subject.
4. Discussion
This study indicates better recovery kinetics of plasma F2-IsoPs after exhaustive exercise in older athletes compared to young athletes. Considering that F2-IsoPs are common biomarkers for oxidative stress and that oxidative stress increases during the aging process, these results for older adults are surprising.
It is commonly assumed that acute exercise is associated with higher blood concentrations of F2-IsoPs among older adults compared to young subjects [15 16]. However, the study by Traustadottir et al. [17] also showed that fit older subjects tended to reduce oxidative stress more effectively than unfit older subjects after an acute pro-oxidative challenge. To our knowledge, no study has compared the combined effects of training status and aging on the generation of oxidative stress. Despite its exploratory design, our study addresses the question of exercise training and the consequent oxidative stress produced and its correlation with the aging process. As a second original and methodological issue, we would like to underline that we designed our study under actual medical-care conditions.
To investigate the influence of training status on the generation of oxidative stress in older subjects (aged >65 years), Traustadottir et al. [17] assessed the countermeasures against ROS production under a pro-oxidative “forearm ischemia-reperfusion model”. With the same general objective, to assess the countermeasures against acute ROS production in older subjects, we selected an “acute exercise-induced oxidative stress model”. We thought our model would fit better into “real” medical practice than the experimental “forearm ischemia-reperfusion model”. We thought it would be useful to define a biomarker for the generation of oxidative stress that could be used as one of the physiological responses in a clinical, usual and integrative functional testing approach to assess the functional reserves of individuals. We are aware that oxidative stress assessment should have been controlled after a longer recovery period in a biochemical feature but we focused to validate our exploratory hypothesis in actual-care conditions, i.e. using a standardized maximal exercise challenge with a short recovery time.
We designed an acute exercise-testing protocol because we consider the acute exercise challenged response to assess functional reserve of biological systems. Based on such a functional assessment model conducted under daily clinical practice, we hypothesized that the aging process would negatively alter the balance between ROS production and antioxidant responses, despite weekly exercise training. We hypothesized that young trained subjects would have lower levels of oxidative-stress biomarkers after an incremental exhaustive exercise compared to older adults paired according to training status. Under this hypothesis, we stated that regular and significant doses of endurance training would not be sufficient to control the upgraded oxidative stress that is commonly assumed to be a phenotypic characteristic in older subjects. Thus, we expected higher levels of serum F2-IsoPs levels in response to acute and exhaustive exercise in older athletes compared to young athletes. In contrast, although the two groups did not differ in blood F2-IsoPs concentrations at MAP, we found more rapid decrement of blood F2-IsoPs concentration after exhaustive exercise in older compared to younger subjects.
As an explanation for this “acute protective” and surprising variation in plasma F2-IsoPs concentration in older subjects, we could argue that the decrease in the plasma F2-IsoPs levels was caused by decreased plasma glutathione (GSH) level. This explanation is supported by the fact that GSH promotes cyclooxygenase-dependent formation of F2-IsoPs [22]. GSH is central to the antioxidant system [23]. If GSH is depleted, depletion of this cyclooxygenase substrate could, “surprisingly”, be associated with reduced synthesis of F2-IsoPs, despite the major production of ROS. In contrast, maintaining a sufficient plasma GSH concentration would promote formation of F2-IsoPs.
We have previously reported this paradigm when discussing oxidative stress in patients suffering from the facioscapulohumeral dystrophy (FSHD) [24], an inherited muscular disease that we have previously described to be associated with major oxidative stress [25]. As with the older subjects in this present study, some FSHD patients with low plasma F2-IsoPs levels were also characterized by having lower plasma levels of GSH compared to patients with higher plasma levels of F2-IsoPs [24]. The influence of GSH level on F2-IsoPs production could also be relevant in older people because it has been well-described that GSH level is positively controlled by erythroid 2-like factor 2 (Nrf2) transcription, which has a central controlling pathway against the deleterious effects of ROS and is negatively altered during the aging process [26], [27], [28]. A second factor that could alter the positive correlation between ROS production and F2-IsoPs production, as a consequence of reduced GSH plasma level, could be “GSH redox individuality”. Indeed, it is described that although exercise induces oxidative stress in the majority of individuals, it can also induce reductive stress or negligible stress [29], [30]. This dissociation between ROS production and F2-IsoPs synthesis could support the variability of the post-exercise F2-IsoPs synthesis that we observed in young subjects whereas redox individuality could be masked in older subjects by greater production of ROS. The direction of the F2-IsoPs variation was not expected and we did not plan to assess the GSH as a part of a routine medical-care plan. Obviously, a paired assessment of F2-IsoPs and GSH plasma level should have to be of performed as a methodological part of a next comparative study.
Our results and physiological hypothesis are derived from trained subjects. It would be of interest to determine if our results could be observed in untrained older subjects because such a dissociation between pro-ROS production and F2-IsoPs synthesis could aid detection of subjects with an impaired major antioxidant GSH pathway. This could improve our ability to delay or prevent sarcopenia, which is associated with major oxidative stress in aging populations.
5. Conclusion
In conclusion, we assessed the F2-IsoPs plasma kinetic in elderly subjects during a functional assessment procedure to evaluate the functional reserve of this population, in actual-care conditions, which meant using a standardized maximal exercise-challenged procedure. We have shown that plasma F2-IsoPs, assessed at 30 min after an exhaustive endurance exercise, was lower in older compared to young athletes. We speculate that this surprising effect of the aging process on oxidative stress could be a biomarker for an aged-related major ROS production. Because F2-IsoPs are involved in the cyclooxygenase pathway, we argue that plasma F2-IsoPs level may not be a good biomarker for oxidative stress when the level of ROS production is not balanced by insufficient levels of plasma GSH to scavenge ROS. Based on these exploratory results, we argue that assessment of plasma IsoPs could be proposed as a biomarker to assess the functional response against ROS synthesis in older subjects, while also considering the exercise-challenged kinetics of F2-IsoPs in plasma. This original result should be confirmed by a further comparative study that could support a new paradigm to consider that the “F2-IsoPs plasma level” biomarker should be not only assessed at rest but also in a stressed functional status and paired to the assessment of the GSH anti-oxidant pathway.
Conflict of interest
All the authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgment
The study was funded by the Joint research unit 1048 INSERM AdipOlab unit (Institute of Metabolic and Cardiovascular Diseases, Toulouse, France). The clinical studies were conducted at the Sport Medicine department of the Toulouse University Hospital (France). The in vitro studies were conducted at the AdipOlab unit. Fabien Pillard and Cédric Dray designed the study. Claire Vinel and Ophélie Pereira conducted the biological analysis. Claire Vinel conducted data collection. Claire Vinel and Fabien Pillard analyzed the data. All the authors contributed to wrote the paper.
References
- 1.Organization WH . World Health Organization; 2015. World Report on Ageing and Health; p. 246. [Google Scholar]
- 2.Cruz-Jentoft A.J., Landi F., Schneider S.M. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43(6):748–759. doi: 10.1093/ageing/afu115. [published Online First: 2014/09/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvani R., Marini F., Cesari M. Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J. Cachexia Sarcopenia Muscle. 2015;6(4):278–286. doi: 10.1002/jcsm.12051. [published Online First: 2015/12/18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anton S.D., Woods A.J., Ashizawa T. Successful aging: advancing the science of physical independence in older adults. Ageing Res. Rev. 2015;24(Pt B):304–327. doi: 10.1016/j.arr.2015.09.005. [published Online First: 2015/10/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marzetti E., Calvani R., Cesari M. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013;45(10):2288–2301. doi: 10.1016/j.biocel.2013.06.024. [published Online First: 2013/07/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C.K., Lyass A., Larson M.G. Biomarkers of oxidative stress are associated with frailty: the Framingham Offspring Study. Age. 2016;38(1):1. doi: 10.1007/s11357-015-9864-z. [published Online First: 2015/12/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadenas E., Davies K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29(3–4):222–230. doi: 10.1016/s0891-5849(00)00317-8. [published Online First: 2000/10/18] [DOI] [PubMed] [Google Scholar]
- 8.Jacob K.D., Noren Hooten N., Trzeciak A.R. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech. Ageing Dev. 2013;134(3–4):139–157. doi: 10.1016/j.mad.2013.02.008. [published Online First: 2013/02/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadiiska M.B., Gladen B.C., Baird D.D. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005;38(6):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [published Online First: 2005/02/22] [DOI] [PubMed] [Google Scholar]
- 10.Milne G.L., Dai Q., Roberts L.J., 2nd The isoprostanes–25 years later. Biochim. Biophys. Acta. 2015;1851(4):433–445. doi: 10.1016/j.bbalip.2014.10.007. [published Online First: 2014/12/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montuschi P., Barnes P.J., Roberts L.J., 2nd Isoprostanes: markers and mediators of oxidative stress. Faseb. J. 2004;18(15):1791–1800. doi: 10.1096/fj.04-2330rev. [published Online First: 2004/12/04] [DOI] [PubMed] [Google Scholar]
- 12.Mrakic-Sposta S., Gussoni M., Porcelli S. Training effects on ROS production determined by electron paramagnetic resonance in master swimmers. Oxid. Med. Cell Longev. 2015;2015:804794. doi: 10.1155/2015/804794. [published Online First: 2015/04/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikolaidis M.G., Kyparos A., Vrabas I.S. F(2)-isoprostane formation, measurement and interpretation: the role of exercise. Prog. Lipid Res. 2011;50(1):89–103. doi: 10.1016/j.plipres.2010.10.002. [published Online First: 2010/10/19] [DOI] [PubMed] [Google Scholar]
- 14.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Res. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [published Online First: 2008/10/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouzid M.A., Hammouda O., Matran R. Changes in oxidative stress markers and biological markers of muscle injury with aging at rest and in response to an exhaustive exercise. PLos One. 2014;9(3):e90420. doi: 10.1371/journal.pone.0090420. [published Online First: 2014/03/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordin T.C., Done A.J., Traustadottir T. Acute exercise increases resistance to oxidative stress in young but not older adults. Age. 2014;36(6):9727. doi: 10.1007/s11357-014-9727-z. [published Online First: 2014/11/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traustadottir T., Davies S.S., Su Y. Oxidative stress in older adults: effects of physical fitness. Age. 2012;34(4):969–982. doi: 10.1007/s11357-011-9277-6. [published Online First: 2011/06/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wasserman K., Hansen J.E., Sue D.Y. fifth ed. Lippincott Williams and Wilkins; Baltimore, Maryland: 2005. Principles of Exercise Testing and Interpretation Including Pathophysiology and Clinical Applications. [Google Scholar]
- 19.Lee C.Y., Jenner A.M., Halliwell B. Rapid preparation of human urine and plasma samples for analysis of F2-isoprostanes by gas chromatography-mass spectrometry. Biochem. Biophys. Res. Commun. 2004;320(3):696–702. doi: 10.1016/j.bbrc.2004.06.015. [published Online First: 2004/07/09] [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B., Lee C.Y. Using isoprostanes as biomarkers of oxidative stress: some rarely considered issues. Antioxid. Redox Signal. 2010;13(2):145–156. doi: 10.1089/ars.2009.2934. [published Online First: 2009/12/17] [DOI] [PubMed] [Google Scholar]
- 21.Grenache D.G., Parker C. Integrated and automatic mixing of whole blood: an evaluation of a novel blood gas analyzer. Clin. Chim. Acta. 2007;375(1–2):153–157. doi: 10.1016/j.cca.2006.07.002. [published Online First: 2006/08/15] [DOI] [PubMed] [Google Scholar]
- 22.Tsikas D., Suchy M.T., Niemann J. Glutathione promotes prostaglandin H synthase (cyclooxygenase)-dependent formation of malondialdehyde and 15(S)-8-iso-prostaglandin F2alpha. FEBS Lett. 2012;586(20):3723–3730. doi: 10.1016/j.febslet.2012.09.001. [published Online First: 2012/09/18] [DOI] [PubMed] [Google Scholar]
- 23.Jones D.P., Go Y.M. Redox compartmentalization and cellular stress. Diabetes Obes. Metabol. 2010;12(Suppl. 2):116–125. doi: 10.1111/j.1463-1326.2010.01266.x. [published Online First: 2010/11/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Passerieux E., Hayot M., Jaussent A. Effects of vitamin C, vitamin E, zinc gluconate, and selenomethionine supplementation on muscle function and oxidative stress biomarkers in patients with facioscapulohumeral dystrophy: a double-blind randomized controlled clinical trial. Free Radic. Biol. Med. 2015;81:158–169. doi: 10.1016/j.freeradbiomed.2014.09.014. [published Online First: 2014/09/24] [DOI] [PubMed] [Google Scholar]
- 25.Turki A., Hayot M., Carnac G. Functional muscle impairment in facioscapulohumeral muscular dystrophy is correlated with oxidative stress and mitochondrial dysfunction. Free Radic. Biol. Med. 2012;53(5):1068–1079. doi: 10.1016/j.freeradbiomed.2012.06.041. [published Online First: 2012/07/17] [DOI] [PubMed] [Google Scholar]
- 26.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12(12):931–947. doi: 10.1038/nrd4002. [published Online First: 2013/11/30] [DOI] [PubMed] [Google Scholar]
- 27.Kubben N., Zhang W., Wang L. Repression of the antioxidant NRF2 pathway in premature aging. Cell. 2016;165(6):1361–1374. doi: 10.1016/j.cell.2016.05.017. [published Online First: 2016/06/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H., Davies K.J., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88(Pt B):314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [published Online First: 2015/06/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margaritelis N.V., Kyparos A., Paschalis V. Reductive stress after exercise: the issue of redox individuality. Redoxid. Biol. 2014;2:520–528. doi: 10.1016/j.redox.2014.02.003. [published Online First: 2014/03/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullins A.L., van Rosendal S.P., Briskey D.R. Variability in oxidative stress biomarkers following a maximal exercise test. Biomarkers. 2013;18(5):446–454. doi: 10.3109/1354750X.2013.810668. [published Online First: 2013/07/19] [DOI] [PubMed] [Google Scholar]