Introduction
Numerous reports document cases of iatrogenic sarcoidosis or sarcoidlike granulomatosis in the setting of biologic therapy with interferon (IFN)-α, tumor necrosis factor (TNF)-α inhibitors, and, most recently, immune checkpoint inhibitors. Anakinra, an interleukin (IL)-1 receptor antagonist (IL-1Ra), has only been reported once in the literature to induce sarcoidlike granulomatosis in a patient with TNF receptor–associated periodic syndrome.1 In this report, IL-1Ra–induced sarcoidal granulomas are postulated to be caused by the upregulation of type 1 IFN and IL-1 cytokine pathway inflammation.
Case report
A 48-year-old woman with long-standing pathology-confirmed chronic hidradenitis suppurativa (Hurley stage III), sequentially treated with doxycycline, minocycline, clindamycin-rifampin, cyclosporine, and adalimumab, was taking anakinra monotherapy (100 mg/d subcutaneous injection for 9 months followed by 200 mg/d subcutaneous injection for 15 months) when she presented with acute onset of a tender, warm, erythematous, plaquelike eruption on the bilateral buttocks (Fig 1). The patient was admitted to the hospital for a presumed soft tissue infection from chronic immunosuppression. Blood cultures and IFN-γ release assay were negative. A skin biopsy found an exuberant granulomatous process comprised predominantly of naked noncaseating granulomas throughout the dermis (Fig 2). Fite, acid-fast bacilli, fungal, and bacterial stains were negative. A workup for sarcoidosis was initiated. The patient's complete blood count with differential, basic metabolic panel, serum Ca2+, and thyroid function tests were all within normal limits. Electrocardiogram, transthoracic echocardiogram, chest radiography, and dilated fundal examinations found nothing consistent with sarcoidosis. The patient deferred colonoscopy, as she had no signs or symptoms of inflammatory bowel disease. Anakinra was discontinued, as the treatment was only moderately successful and because of new literature on anti–IL-17 effectiveness in hidradenitis suppurativa. The buttock eruption improved over 4 months with no therapy until secukinumab was approved. The eruption showed no residual activity at the last clinical evaluation performed 11 months after the cessation of anakinra.
Fig 1.
Anakinra-induced sarcoidosis. Buttock erythematous plaque eruption while on anakinra for hidradenitis suppurativa.
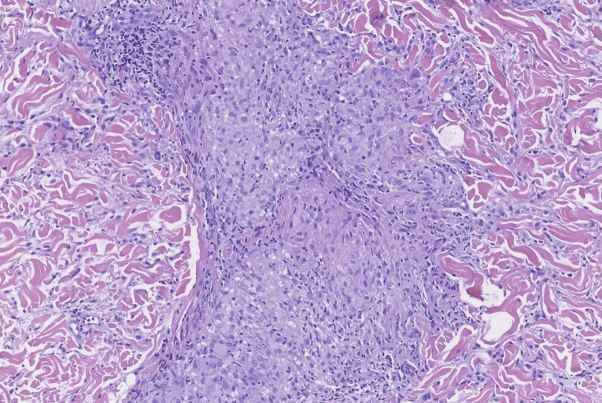
Fig 2.
Anakinra-induced sarcoidosis. Histologic confirmation on noncaseating epithelioid granulomas. (Hematoxylin-eosin stain; original magnification: ×10.)
Courtesy of Jonathan Ho, MD UPMC Dermatopathology.
Discussion
Granulomatous drug eruptions consist of drug-induced reactive granulomatous disease, accelerated rheumatoid nodulosis, drug-induced granuloma annulare, and drug-induced sarcoidosis.2 Drug-induced sarcoidosis (Table I) may cause polymorphic skin lesions and possible systemic involvement weeks to months after drug initiation.1, 20 Diagnosis of isolated single-organ sarcoidosis or sarcoidlike granulomatosis depends on the evolving definition of sarcoidosis and acknowledgement of a single organ variant.20 The most frequently cited cause of drug-induced sarcoidosis is IFN-α, a type I IFN thought to induce sarcoid granuloma formation via induction of a predominant T helper cell type 1 (Th1) cytokine response.2 Granuloma formation is predominantly Th1, with IFN and TNF critical cytokines; however, TNF inhibitor–induced granulomas are more confusing. The formation of anti-TNF drug-induced psoriasis and sarcoid granulomas, theoretically results from imbalances in TNF receptor 2–mediated activation of regulatory T cells and eventual Th1 T cells or enhancement of local IFN-γ.4, 5 Immune checkpoint inhibitors can induce sarcoidosis by modifying cytotoxic, Th1/17 and regulatory T-cell ratios.10, 21
Table I.
Drugs that induce cutaneous sarcoidosis and proposed biologic mechanisms of induction
| Drug | Biologic mechanism |
|---|---|
| IL-1Ra: anakinra1 |
|
| Interferon-α3 |
|
| anti-TNF agents4, 5: entanercept,6 infliximab,7 adalimumab8 |
|
| PD-1 inhibitors: pembrolizumab,9 nivolumab10 |
|
| BRAF inhibitor: vemurafenib11 |
|
| anti-CTLA4 mAb: ipilimumab12 |
|
| anti-IgE mAb: omalizumab13 |
|
| Fillers for aesthetic procedures: hyaluronic acid14 |
|
| Insulin15, 16 |
|
| Botulinum neurotoxin A17 |
|
| Desensitization injections18 |
|
| Ophthalmic drops with sodiumbisulfate19 Leuprorelin injections20 |
|
|
A similar mechanism may underlie the induction of sarcoidosis in the setting of anakinra, a recombinant IL-1 receptor antagonist that competitively blocks IL-1. Studies support a strong counter-regulation effect between IL-1 and type I IFN cytokine pathway, with elevated levels of IL-1b potently antagonizing type I IFN.22 Thus, anakinra therapy may mitigate regulatory mechanisms on type I IFN leading to a paradoxical increase in granulomatous inflammation and a predominant Th1 cytokine response.
In this case, resolution of cutaneous symptoms after cessation of anakinra therapy suggests anakinra-induced sarcoidosis. As such, this report supports expansion of the classes of drugs associated with drug-induced sarcoidosis to include IL-1 receptor antagonists. The diagnosis of drug-induced sarcoidosis is complicated both by the variable time lapse between drug initiation and lesion presentation and the heterogeneous clinical presentation of the disease.19 Thus, it is important to maintain a high index of suspicion for drug-induced sarcoidosis in patients on biologic therapies including anakinra.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Sacre K., Pasqualoni E., Descamps V. Sarcoid-like granulomatosis in a patient treated by interleukin-1 receptor antagonist for TNF-receptor-associated periodic syndrome. Rheumatology (Oxford) 2013;52(7):1338–1340. doi: 10.1093/rheumatology/kes377. [DOI] [PubMed] [Google Scholar]
- 2.Dodiuk-Gad R.P., Shear N.H. Granulomatous drug eruptions. Dermatol Clin. 2015;33(3):525–539. doi: 10.1016/j.det.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Ramos-Casals M., Mañá J., Nardi N. Sarcoidosis in patients with chronic hepatitis C virus infection: analysis of 68 cases. Medicine (Baltimore) 2005;84(2):69–80. doi: 10.1097/01.md.0000157577.69729.e6. [DOI] [PubMed] [Google Scholar]
- 4.Toussirot É., Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2(2):e000239. doi: 10.1136/rmdopen-2015-000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amber K.T., Bloom R., Hertl M. TNF- α: a treatment target or cause of sarcoidosis? J Eur Acad Dermatol Venereol. 2015;29(11):2104–2111. doi: 10.1111/jdv.13246. [DOI] [PubMed] [Google Scholar]
- 6.Vieira M.A., Saraiva M.I., Silva L.K. Development of exclusively cutaneous sarcoidosis in patient with rheumatoid arthritis during treatment with etanercept. Rev Assoc Med Bras. 2016;62(8):718–720. doi: 10.1590/1806-9282.62.08.718. [DOI] [PubMed] [Google Scholar]
- 7.Numakura T., Tamada T., Nara M. Simultaneous development of sarcoidosis and cutaneous vasculitis in a patient with refractory Crohn's disease during infliximab therapy. BMC Pulm Med. 2016;16:30. doi: 10.1186/s12890-016-0193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos G., Sousa L.E., Joao A.M. Exacerbation of recalcitrant cutaneous sarcoidosis with adalimumab—a paradoxical effect? A case report. An Bras Dermatol. 2013;88(6 Suppl 1):26–28. doi: 10.1590/abd1806-4841.20132487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotliar J., Querfeld C., Boswell W.J. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016;2(4):290–293. doi: 10.1016/j.jdcr.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum M.R., Ma M.W., Fleisig S. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3(3):208–211. doi: 10.1016/j.jdcr.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lheure C., Kramkimel N., Franck N. Sarcoidosis in patients treated with Vemurafenib for metastatic melanoma: a paradoxical autoimmune activation. Dermatology. 2015;231:378–384. doi: 10.1159/000439400. [DOI] [PubMed] [Google Scholar]
- 12.Eckert A., Schoeffler A., Dalle S. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology. 2009;218(1):69–70. doi: 10.1159/000161122. [DOI] [PubMed] [Google Scholar]
- 13.Yung S., Han D., Lee J. Cutaneous sarcoidosis in a patient with severe asthma treated with omalizumab. Can Respir J. 2015;22(6):315–316. doi: 10.1155/2015/265734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izikson L. Cutaneous sarcoidosis associated with cosmetic fillers. J Eur Acad Dermatol Venereol. 2009;23(12):1455–1456. doi: 10.1111/j.1468-3083.2009.03249.x. [DOI] [PubMed] [Google Scholar]
- 15.Zargham H., O'Brien E. Cutaneous sarcoidosis at insulin injection sites. CMAJ. 2016;188:674. doi: 10.1503/cmaj.150572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordaan H.F., Sandler M. Zinc-induced granuloma—a unique complication of insulin therapy. Clin Exp Dermatol. 1989;14(3):227–229. doi: 10.1111/j.1365-2230.1989.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 17.Herbert V.G., Blodorn-Schlicht N., Boer-Auer A. Cutaneous granulomatous reactions at botulinum neurotoxin A injection sites: first manifestation of systemic sarcoidosis. Hautarzt. 2015;66:863–866. doi: 10.1007/s00105-015-3651-8. [DOI] [PubMed] [Google Scholar]
- 18.Marcoval J., Moreno A., Mana J. Subcutaneous sarcoidosis localized to sites of previous desensitizing injections. Clin Exp Dermatol. 2008;33(2):132–134. doi: 10.1111/j.1365-2230.2007.02571.x. [DOI] [PubMed] [Google Scholar]
- 19.Carlson J.A., Schutzer P., Pattison T. Sarcoidal foreign-body granulomatous dermatitis associated with ophthalmic drops. Am J Dermatopathol. 1998;20(2):175–178. doi: 10.1097/00000372-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Abbot J., Taylor L.A., Wanat K.A. Isolated subcutaneous sarcoid-like granulomatous inflammation occurring at injection sites: 3 patients treated successfully with minocycline. JAAD Case Rep. 2017;3(1):74–77. doi: 10.1016/j.jdcr.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danlos F.X., Pages C., Baroudjian B. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:e133–e136. doi: 10.1016/j.chest.2015.10.082. [DOI] [PubMed] [Google Scholar]
- 22.Mayer-Barber K.D., Yan B. Clash of the cytokine titans: counter-regulation of interleukin-1 and type I interferon-mediated inflammatory responses. Cell Mol Immunol. 2017;14(1):22–35. doi: 10.1038/cmi.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]