Introduction
Pembrolizumab is an anti–programmed cell death receptor protein-1 (PD-1) monoclonal antibody first approved in 2014 by the US Food and Drug Administration for the treatment of metastatic melanoma. Pembrolizumab targets the PD-1, blocking the immune checkpoint and subsequently boosting the immune response against cancerous cells. Although effective against systemic disease, anti–PD-1 therapy is also associated with adverse effects such as vitiligo and pruritus caused by overstimulation and hyperproliferation of the immune system.1, 2 A recent small study found efficacy of this medication for treatment of systemic natural killer/T-cell lymphoma,3 although, to our knowledge, studies of this medication for treatment of cutaneous T-cell lymphoma (CTCL) have not yet been published.
Case report
We report on a 61-year-old man who had melanoma of 0.35 mm in thickness on the posterior scalp in 2009 (Table I). The melanoma was removed with wide margins.
Table I.
Patient information
| Age at initial melanoma diagnosis, 2009 | 54 |
| Age at lymphoma diagnosis, April 2017 | 61 |
| Sex | Male |
| Initial diagnoses | Melanoma of the scalp—Clark level III, metastatic melanoma to the brain and lung |
| Initial treatments | Ipilimumab (4 mo), pembrolizumab (20 mo) |
| Secondary diagnosis | CD8+/CD3+/CD43+/CD7+/CD56+ epidermotropic peripheral T cell lymphoma |
| Secondary treatments | Local radiation, pralatrexate |
In January 2015, a recurrence of 0.75 mm in thickness was excised. A computerized tomography (CT) scan in February 2015 found 3 lung and 2 liver lesions. A fludeoxyglucose positron emission tomography scan confirmed intense uptake to the 3 pulmonary nodules, suggestive of metastatic melanoma. A subsequent biopsy of the left lung confirmed metastatic melanoma (BRAF wild type).
In April 2015, the patient began treatment with anti–cytotoxic T-lymphocyte–associated protein-4 (CTLA-4) immunotherapy (ipilimumab) but was switched to pembrolizumab because of disease progression in August 2015. A CT scan 2 months later showed significant reductions in size of pulmonary and liver metastases. Subsequent CT scans and magnetic resonance imaging performed between July 2016 and January 2017 indicated stable disease.
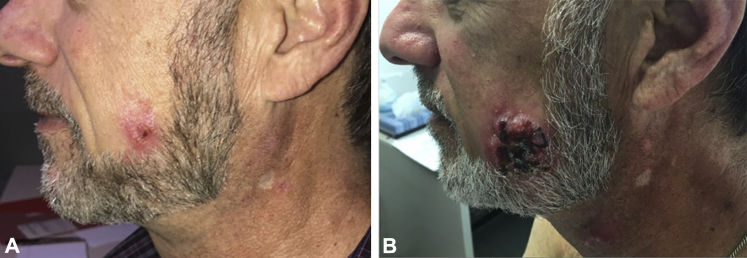
Although the melanoma was under control, adverse effects from immunotherapy began to develop. The patient first observed small papules on his head and neck area in July 2016, clinically assumed to represent an acneiform eruption. The patient also had vitiligo in his neck and chest area in October 2016. Over the course of several weeks in February to March 2017, a papule on his left cheek developed into an ulcerated nodule of 6 cm (Fig 1, A and B). In addition, new papules and nodules developed on his neck, and erythematous patches developed on the upper trunk (Fig 1, B). Biopsies from the left cheek ulcerated nodule, a neck papule, and an erythematous patch on the upper trunk all found an epidermotropic T-cell lymphoma that was CD56+/TIA-1+/CD3+/βF1+/CD7+. There were numerous CD4+ and CD8+ T cells with a slight predominance of CD8+ T cells. A CD30 stain labeled only a few scattered small lymphocytes. In situ hybridization for Epstein-Barr viral mRNA was negative, as was staining for GM3 and CD123. The atypical lymphocytes did not have significant labeling with PD-1.
Fig 1.
Clinical presentation of lymphoma. A, Lesion on left cheek of patient; picture taken February 27, 2017. B, Lesion on left cheek of patient; picture taken 12 weeks later on May 16, 2017. A developing papule on the upper neck can be seen at the bottom of the image.
A fludeoxyglucose positron emission tomography scan in May 2017 found intense abnormal uptake in papules and nodules on the left cheek and upper neck, consistent with the patient's epidermotropic T-cell lymphoma, but no signs of systemic involvement.
Histopathologic and clinical differential diagnosis for this epidermotropic T-cell lymphoma included primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma, CD56+ tumor stage mycosis fungoides, and an unusual CD56+ lymphoma developing in the setting of pembrolizumab-mediated immunomodulation. Because primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma does not typically express CD56, given the CD56 positivity in this case, we thought that this condition could be excluded. Furthermore, because the nodules did not develop within pre-existent patches and plaques, CD56+ tumor-stage mycosis fungoides was ruled out. Therefore, because the patient's lymphoma did not fit neatly into any one known category type of cutaneous lymphoma, we think that it is most likely that his lymphoma represents an unusual CD56+ lymphoma developing in the setting of immunotherapy used to treat the patient's melanoma.
Pembrolizumab treatment was discontinued as of April 2017 given the development of this cutaneous lymphoma. Because an initial staging evaluation did not show systemic disease, and only the facial and neck lesions were symptomatic, the patient was initially treated with radiation therapy to avoid immunosuppressive medications: 37 Gy local to symptomatic areas over 3 weeks. However, the lesions did not resolve, and the patient is currently taking low-dose pralatrexate (15 mg/m2) weekly every 3 of 4 weeks.
Discussion
Given that pembrolizumab influences immune activity by blocking PD-1 signaling, it likely had a role in the development of lymphoma in this patient. Physiologically, strong PD-1 signaling inhibits T-cell proliferation, and a PD-1 blockade can increase immune function via an increase in T-cell proliferative capacity.4 Hyperproliferation of T cells is associated with adverse events such as vitiligo,2 sarcoidosis,5 and autoimmune limbic encephalitis.6 Treatment with pembrolizumab is reported to be associated with exacerbation of existing autoimmune diseases,7 most likely caused by overactive T cells. Hypopigmentation seen clinically in this patient probably indicates CD8+ T-cell destruction of melanocytes, the reputed cause of vitiligo.2
We recognize that the development of the patient's lymphoma cannot be fully attributed to treatment with pembrolizumab. It is possible that the 2 cancers developed coincidentally, although de novo formation of the lymphoma is unlikely, as it had an unusual immunophenotype not characteristic of any common lymphoma. It is alternatively possible that the 4 cycles of ipilimumab, which the patient received before starting pembrolizumab, played a role in leading to T-cell hyperproliferation. Experiments in mice have shown that germline deletion of CTLA-4 leads to severe disorders related to increases in immune proliferation and activity,8 and, more importantly, clinical observations of immune related adverse events caused by anti–CTLA-4, including vitiligo and sarcoidosis, are well established.9 However, because this patient's lymphoma developed a year after discontinuation of ipilimumab, it is unlikely that ipilimumab induced the lymphoma. Therefore, we believe that the patient's lymphoma was most likely induced by CD56+ T-cell hyperproliferation caused by pembrolizumab.
To our knowledge, there are no published studies of PD-1 blockade for treatment of CTCL, although an abstract regarding this was presented at the 2016 World Congress of Cutaneous Lymphoma. In this preliminary study, it was reported that PD-1 blockade was effective in treating about 30% of CTCLs studied.10
We report a case of CTCL during melanoma treatment with the PD-1 inhibitor pembrolizumab. Although a definitive causal relationship is hard to establish, we believe that pembrolizumab has facilitated CTCL because of its effects on the immune system, the chronology, and the lymphoma's atypical histopathology. Clinicians should be aware that atypical cutaneous eruptions can indicate development of cutaneous lymphoma in patients receiving pembrolizumab.
Footnotes
Funding sources: This article is funded in part by the University of California, Berkeley Summer Undergraduate Research Fellowship Program.
Conflicts of interest: None disclosed.
References
- 1.Sanlorenzo M., Vujic I., Daud A. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151(11):1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammed G.F., Gomaa A.H., Al-Dhubaibi M.S. Highlights in pathogenesis of vitiligo. World J Clin Cases WJCC. 2015;3(3):221–230. doi: 10.12998/wjcc.v3.i3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwong Y.-L., Chan T.S.Y., Tan D. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing L-asparaginase. Blood. 2017;129(17):2437–2442. doi: 10.1182/blood-2016-12-756841. [DOI] [PubMed] [Google Scholar]
- 4.Riley J.L. PD-1 signaling in primary T cells. Immunol Rev. 2009;229(1):114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotliar J., Querfeld C., Boswell W.J., Raja N., Raz D., Chen R. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016;2(4):290–293. doi: 10.1016/j.jdcr.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M.P., Hissaria P., Hsieh A.H., Kneebone C., Vallat W. Autoimmune limbic encephalitis with anti-contactin-associated protein-like 2 antibody secondary to pembrolizumab therapy. J Neuroimmunol. 2017;305:16–18. doi: 10.1016/j.jneuroim.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Phadke S.D., Ghabour R., Swick B.L., Swenson A., Milhem M., Zakharia Y. Pembrolizumab therapy triggering an exacerbation of preexisting autoimmune disease. J Investig Med High Impact Case Rep. 2016;4(4) doi: 10.1177/2324709616674316. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5084516/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Intlekofer A.M., Thompson C.B. At the Bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94(1):25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertrand A., Kostine M., Barnetche T., Truchetet M.-E., Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4559965/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khodadoust M., Rook A., Porcu P., Foss F. 3rd World Congress of Cutaneous Lymphomas. Columbia University; New York: 2016. Pembrolizumab for treatment of relapsed/refractory mycosis fungoides and Sezary syndrome: clinical efficacy in a CITN multicenter Phase 2 study. [Google Scholar]