Abstract
The up-regulation of immune checkpoint molecules, such as PD-1 and CTLA4 on immune cells occur during acute infections, such as malaria, as well as during chronic persistent viral infections, including HIV and hepatitis B virus (HBV). These pathways are important for preventing immune-driven pathology, but can also limit immune-mediated clearance of the infection. The recent success of immune checkpoint blockade in cancer therapy suggests that targeting these pathways could also be effective for preventing and treating a range of infectious diseases. Here, we review our current understanding of immune checkpoint pathways in the pathogenesis of infectious diseases and discuss the potential for therapeutically targeting these pathways in this setting.
Introduction
Immune checkpoint molecules are inhibitory receptors expressed on immune cells that trigger immunosuppressive signalling pathways. These molecules are crucial for maintaining self-tolerance and for modulating the length and magnitude of effector immune responses in peripheral tissues, in order to minimize collateral tissue damage1,2. Signalling via these molecules can drive effector immune cells (especially T cells), into a state known as ‘exhaustion’. T cell exhaustion is defined by reduced effector function, sustained expression of immune checkpoint molecules (such as PD-1), poor recall responses and a transcriptional state distinct from that of functional effector or memory T cells3. There are numerous types of activating and inhibitory interactions that occur between antigen-presenting cells (APCs) and T cells, and these regulate the nature of immune responses (Figure 1). It is now clear that many pathogens and cancers promote inhibitory interactions between immune cells via immune checkpoint proteins to escape immune control.
Figure 1. Interactions that regulate T cell responses.
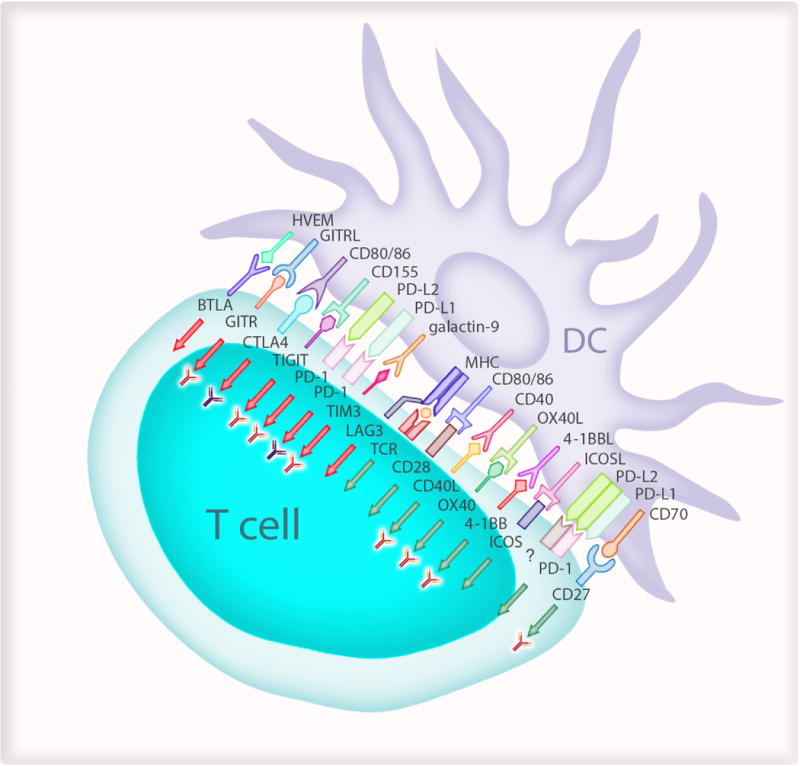
Antigen presenting cells such as dendritic cells (DCs) regulate T cell response to specific pathogens or antigens from malignant cells. The T cell receptors (TCR) on antigen-specific T cells first recognise their cognate antigen via the major histocompatibility complex (MHC) molecules on antigen presenting cells. This step has to be followed by signals to CD28 on T cells from CD80 on the APC and is described as “signal 2”. Several different ligands on DCs then provide signals to T cells which decide the quality and duration of the effector response (green arrows). These include CD40/CD40 ligand (CD40L); OX40/OX40 ligand (OX40L); 4-1BB (CD137)/4-1BB ligand (41BBL; CD137 Ligand); ICOS (Inducible T-cell COStimulator; CD278)/ICOS Ligand (ICOS-L); CD27/CD70. There are also signals to suppress immune responses (red arrows) to maintain self tolerance and limit the duration of immune responses to minimize bystander damage to host tissue. These include LAG3 (lymphocyte activation gene 3); MHC class II; TIM3 (T cell immunoglobulin and mucin-domain containing-3; HAVCR2 in humans)/galectin-9; PD-1 (programmed cell death-1)/PD-L1 (programmed cell death-1-ligand 1) and PD-L2 (programmed cell death-1-ligand 2); TIGIT (T cell immunoreceptor with Ig and ITIM domains)/CD155; CTLA4 (cytotoxic T-lymphocyte-associated protein 4)/CD86 or CD80; GITR (Glucocorticoid-induced TNFR-related protein)/GITR-L (GITR-ligand) and BTLA (B and T lymphocyte attenuator)/HVEM (Herpesvirus entry mediator). Antibody symbol represents pathways being tested in current clinical trials. The “?” refers to an unknown receptor which “activates” T cells. The “red” antibodies indicate pathways undergoing clinical trials for cancer and the “dark coloured” antibodies indicate clinical use.
Investigation of these immunosuppressive interactions has led to the clinical development and licensing of novel efficacious cancer treatments, which use specific antibodies to improve immune responses by blockade of checkpoint protein functions (Box 1). Antibodies targeting PD-1 (Pembrolizumab; Nivolumab), CTLA4 (ipilimumab) and PD-L1 (atezolizumab; avelumab) are currently licensed as monotherapies for various types of cancer (Box 2). In addition, combined therapeutic targeting of PD-1 and CTLA4 was shown to be more effective than either therapy alone for treatment of melanoma4, although such combination therapy also leads to increased toxicity in patients. Therapies targeting several other immune checkpoint pathways have also shown promise for controlling various types of cancer (Table 1 and reviewed in Ref.2). It is also possible to enhance immunity by directly targeting molecules on T cells which improve T cell functions (Box 1), and their clinical utility is currently being assessed in clinical trials. These antibody-mediated treatments use the individual’s own immune system to eliminate or slow the growth of cancer cells and have shown remarkable success in malignancies such as melanoma.
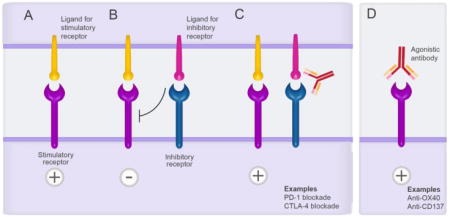
Box 1. Immunotherapy for treating cancer.
Immunotherapy is a type of cancer treatment designed to boost the body’s natural immune response to fight the cancer. There are currently two main types of immunotherapy. (A-C) Antibodies can block immunosuppressive interactions between antigen presenting cells or cancer cells with effector cells (e.g. T cells), to improve immune responses. For example, (A) stimulatory signals to T cells by corresponding ligands are (B) attenuated by when TCR signalling is coincident with inhibitory receptors interacting with their ligands, which decreases the magnitude of responses by T cells. (C) Antibody blockade of the inhibitory receptor/ligand interaction(s) reinstates T cell functions. The two best examples are blockade of PD-1 and CTLA4 which are both expressed on effector T cells and mediate exhaustion when in contact with their ligands, PD-L1 and CD80/CD86 respectively. (B) Antibodies that directly “activate” T cells via activating receptors. Two examples of this type of therapy are targeting OX40 and CD137 on T cells to improve their respective functions. While the development of immunotherapy has been restricted to the treatment of cancer, there is high interest in the role of these antibodies for infectious diseases.
Box 2. Overview of immune checkpoint molecules currently being targeted in cancer therapy.
Below, we describe three checkpoint proteins that are currently targeted for cancer therapy and one being clinically trialled. Therapies targeting several other immune checkpoint pathways have also shown promise for controlling various cancers and some of these drugs have progressed to clinical trials (Table 1, and reviewed in detail in Ref.108).
CTLA-4 is a member of the immunoglobulin superfamily which is expressed by activated T cells along with the T cell co-stimulatory protein, CD28. Both molecules bind to CD80 (B7-1) and CD86 (B7-2), on DCs but CTLA-4 binds with greater affinity and avidity than CD28. While CD28 transmits a stimulatory signal109, CTLA-4 is able to outcompete CD28 for CD80/CD86 binding to inhibit T cell functions110. Of note, CTLA-4 expressed on effector T cells, is increased only after T cell receptor (TCR) and CD28-mediated T cell activation to permit downstream control of immunity.
PD-1 has potent inhibitory effects on immunity. PD-1 is expressed on T cells, B cells, natural killer T cells; DCs, and activated monocytes111,112. PD-1 has two ligands, PD-L1 (also known as B7-H1) and PD-L2 (also known as B7-DC). PD-1 is up-regulated on the surface of T cells, within 24 hours of stimulation and the effects of PD-1 ligation can be seen within a few hours113. Significantly, signalling T cells through the PD-1 inhibitory receptor by PD-L1 expressed on DCs and tumor cells, attenuates TCR signals and inhibits T cell expansion, cytokine production and cytolytic function114. New studies demonstrate that the CD28/B7 co-stimulatory pathway is essential for effective PD-1 therapy in tumor-bearing mice and during chronic viral infection115.
PD-L1, which drives PD-1-mediated immune-inhibition, is constitutively expressed on T cells, B cells, macrophages, DCs116, non-lymphoid tissues such as heart and lung111, parenchymal cells117 as well as the surface of tumor cells118. PD-L1, but not PD-L2, is also detected at low levels on cardiac endothelium, pancreatic islets, and syncyciotrophoblasts in the placenta, highlighting a role for PD-L1 in immunological tolerance119. PD-L1 blockade has also demonstrated efficacy in lung, bladder and other cancers120–122.
PD-L2 is also an immune checkpoint inhibitor, but its function is not as well understood as PD-L1, and thus its clinical utility is still being explored. The engagement of PD-1 by PD-L2 dramatically inhibits TCR-mediated proliferation and cytokine production by CD4+ T cells123. Antigen presenting cells (APC) from PD-L2−/− mice demonstrated an enhanced potential to activate T cells, both in vitro and in vivo124, suggesting that PD-L2 has an inhibitory role similar to PD-L1. However, recent studies showed PD-L2 expressed as an aggregated form on DCs could inhibit PD-L1/PD-1-binding, and increase CD3 and Inducible T-cell COStimulator (ICOS) expression on T cells possibly via a putative second receptor9. Previous studies also showed that PD-L2 could improve T cell function via a PD-1 independent mechanism125–127. Thus PD-L2 has a complex function and PD-L2 proteins are being investigated in clinical trials.
Table 1.
Summary of other major immune checkpoint pathways
| Checkpoint Receptor | Cell type affected | Ligand | Notes | Ref. |
|---|---|---|---|---|
| T cell immunoglobulin and mucin-domain containing-3 (TIM3; HAVCR2 in humans) | Th1-T cells | Galectin-9 on APC | Galectin-9 induces intracellular calcium flux, aggregation and death of Th1 cells in vitro. Co-expression of TIM3 and PD-1 identifies CD8+ T cell in mice with an exhaustion phenotype. Targeting TIM3 and PD-1 pathways can reverse T cell exhaustion and restore anti-tumor immunity. | 140–142. |
| Lymphocyte activation gene 3 (LAG3; CD223) | iTreg, nTreg | MHC Class II on APC | LAG3 enhances the function of regulatory T cells. LAG3 and PD-1 are commonly co-expressed on anergic or exhausted T cells and combined blockade can cure most mice of established tumours that were largely resistant to single antibody treatment. | 143,144. |
| T cell immunoreceptor with Ig and ITIM domains (TIGIT) | T cells, NK | CD155 on DCs | CD96 & TIGIT exert immunosuppressive effects by competing with CD226 for CD155. TIGIT-Fc fusion protein inhibits T cell activation by generating regulatory DCs. Blocking CD96 or TIGIT with mAbs improve tumor control in mice, in particular when used in combination with PD-1/PD-L1 blockade. | 145–148. |
| CD96 | ||||
| B and T lymphocyte attenuator (BTLA; CD272) | Naïve T and B cells and is further upregulated on activation. | Herpesvirus entry mediator (HVEM) expressed by most hematopoietic, endothelial & epithelial cells | Ligation of LIGHT by HVEM can be co-stimulatory, while BTLA-HVEM binding is considered co-inhibitory. BTLA has been linked to T cell dysfunction during cancer and dual blockade of BTLA and PD-1 clearly enhances anti-tumour immunity | 149–152. |
| Tumor necrosis factor superfamily member 14 (TNFSF14; LIGHT) | Innate and adaptive immune cells including T cells | |||
| Glucocorticoid-induced TNFR-related protein (GITR) | Regulatory T cells at high levels, resting conventional T cells at low levels but increases upon activation | GITR-ligand on APC | GITR plays a key role in dominant immunological self-tolerance maintained by CD25+CD4+ regulatory T cells. The ligand for GITR is mainly expressed on APC and antibodies to GITR have been shown to promote an anti-tumor response through loss of Treg lineage stability. | 153–156. |
| V-domain immunoglobulin (Ig)-containing suppressor of T cell activation (VISTA) | Hematopoietic cells | unknown | Preclinical studies with VISTA blockade show promising improvement in anti-tumor T cell responses and improved survival. | 157,158. |
A major challenge in immunotherapy is to understand why treatment responses are variable and thus there is a search for predictive ‘biomarkers” of a favourable clinical response. PD-L1 expression on tumor cells can identify patients who would most benefit from PD-1 or PD-L1 blockade therapy5. There are also more complex “gene signatures” in tumours to identify patients who will show the best responses6. Earlier T cell expansion following anti-PD-1 therapy in small lung cancer has been associated with improved responses7 and a composite biomarker of the T cell proliferative response together with pre-treatment tumor burden can predict response to anti-PD-1 in individuals with metastatic melanoma8. Given the cost and toxicity of immune checkpoint blockade, identifying biomarkers that predict a clinical response are currently a top priority. It is feasible that a composite marker of functional responses by T cells and burden of residual infection may also be relevant for infectious diseases.
Whether immunotherapies can also be effective for treating infectious diseases is less well explored. However, the fact that these inhibitory pathways are also exploited for immune evasion by pathogens suggests that blockade could be used for the prevention and treatment of infectious diseases, in either the acute or chronic phases of infection. Currently, checkpoint blockade is being evaluated for reversing T cell exhaustion that follows on from chronic disease, but there is potential for also treating acute infections to generate long term immunity9. The development of vaccines for a range of infectious diseases, including malaria, HBV and HIV could also potentially be enhanced through checkpoint blockade. Most importantly, given that drug resistance against malaria10 and many other infections are rising, and that control of both HIV and HBV require life-long treatment, new strategies for treatment or potentially cure are now being considered. Furthermore, parallel searches for biomarkers which inform on the best therapy choice as well as indicate if there is a timeframe when the therapy would be most efficacious are also required. In this review, we describe in detail the impact of immune checkpoint signalling during malaria, HIV and HBV infections, as well as in tuberculosis, and we discuss the potential for therapeutically targeting these pathways in these settings.
Immune checkpoint proteins in malaria
Malaria is a mosquito-borne infectious disease of humans caused by parasitic protozoans of the genus Plasmodium. The majority of malaria infections are caused by P. falciparum and P. vivax, and in 2015, there were 212 million new cases of malaria worldwide with 429,000 deaths due to P. falciparum alone11. These parasites have a complex life cycle within the mammalian host, in which a liver stage of infection is followed by asexual and sexual blood stages of infection; the blood stages cause the severe symptoms and high mortality associated with malaria.
Over the past 20 years, more than 100 vaccines have been developed to control malaria and clinically evaluated. Most vaccines were specifically designed to target liver or blood-stage parasites by inducing protective antibodies and CD4+ T cells, although a few vaccines were designed to generate CD8+ T cell responses against the liver-stage parasites. The best candidate vaccine identified to date is the RTS,S/AS01E vaccine, which will soon be administered to children in Africa; however, this vaccines had an efficacy of only 43.6% in the first year of administration and efficacy decreased to 16.8% by the fourth year12. This highlights the significant challenges in developing an effective malaria vaccine and suggests that new strategies that target potential mechanisms of immune evasion by parasites need consideration.
The symptoms of malaria range from asymptomatic to chronic, severe and finally lethal disease. Partial immunity is developed by those living in endemic areas only after repeated attacks of malaria over several years13–15. Protection against malaria is dependent on both cell-mediated and humoral immune responses. Parasites in the liver stage are known to be cleared by cytotoxic CD8+ T cells and possibly CD4+ T cells16. For blood stage malaria, antibodies have been shown to play a key role in protection as demonstrated by transfer of serum from protected adults into children17. Studies in experimental rodent models of malaria have shown that multiple effector responses are required to protect against blood stage malaria. T helper 1 (Th1) cell responses are critical for controlling the bulk of blood-stage parasites and thus preventing severe disease18,19. Antibodies are required to eliminate the remaining patent parasites20. Recent studies have shown that CD8+ T cells are required for sterile immunity that prevents the acute infection from progressing to chronic malaria21. Antibodies and CD8+ T cells are also required for long-term protection against re-infection22. Studies have also shown that malarial infections caused apoptosis of vaccine-specific memory B cells23 because of compromised dendritic cell (DC) functions24. This could explain why vaccines have not been successful in the field. Several other factors also contribute to short-lived immunity against malaria (reviewed in Ref.25), but the role of PD-1 as a major factor in loss of immunity against malaria has risen to the forefront.
Malaria and T cell exhaustion
As vaccines have been the focus of malaria control, the study of immune checkpoint proteins in malaria infections is relatively new. Field studies in malaria-endemic Mali and Kenya, found that individuals recently infected with P. falciparum expressed PD-1 on CD4+ 26,27 and CD8+ T cells27, implicating this molecule in immune evasion. Similarly, an increased proportion of CD4+ T cells from individuals with acute-phase infections with P. vivax, P. falciparum, or both had increased expression of CTLA4, OX40, GITR, and CD6928 suggesting a role for regulatory T cells in suppressing immunity to malaria and indicating potential targets of checkpoint control. Finally, T cell immunoglobulin and mucin-domain containing 3 (TIM3) expression was significantly increased on key populations of lymphocytes in P. falciparum-infected patients29.
There are four mouse models of malaria that display the major symptoms and pathology of human disease and are routinely used to study malarial pathogenesis (Box 3). A definitive role for PD-1 in malarial pathogenesis was established when PD-1-deficient mice were shown to rapidly and completely clear P. chabaudi infections, which usually cause chronic malaria in mice21. Notably, during the acute phase of P. chabaudi infection, PD-1 was shown to mediate a 95% loss in the numbers and functional capacity of parasite-specific CD8+ T cells, which are required to control chronic disease21.
Box 3. Mouse models of malaria.
Mouse models of malaria have been more informative on the extent to which checkpoint proteins inhibit natural immunity. Four of the most commonly used mouse species and strains of Plasmodium show distinct biology and pathogenicity. P. yoelii 17XNL and P. chabaudi blood-stage infections are non-lethal with the latter causing chronic disease with intermittent parasitemia for up to 200 days. In contrast, P. yoelii YM and P. berghei ANKA are severe, lethal infections with the latter sequestering from the blood into deep tissues including the brain, leading to lethal cerebral disease.
Recent studies of malaria using four mouse models revealed a novel regulatory function for PD-L29. It was shown that while PD-L1 expressed by DCs did indeed attenuate immune responses against malaria, PD-L2 protein expressed on the same DCs improved immune responses by inhibiting PD-L1–PD-1 interactions9. These studies also showed that PD-L2 was essential for establishing effective Th1 cell immunity for protection against lethal malaria (Figure 2). This study also examined healthy human volunteers before and after infection with experimental P. falciparum malaria. They found that the expression of PD-L2 but not PD-L1 on blood DCs, decreased significantly within 7 days of infection to levels that inversely correlated with the level of parasitaemia in each individual9. In other words, higher PD-L2 levels correlated with lower parasitaemia indicating that this was not just a feature of mouse malaria. Overall, this study highlighted the importance of PD-L2 expression for malarial immunity.
Figure 2. PD-L2 protects against lethal malaria and has translational potential.
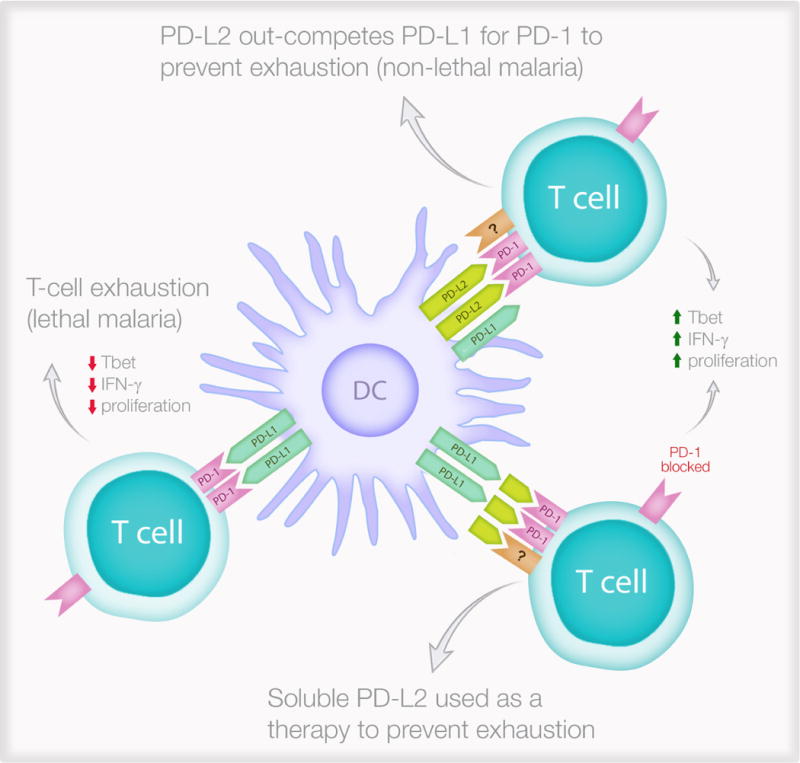
The expression of PD-L2 on dendritic cells determines effector T cell function following a PD-1/PD-L1 interaction. During non-lethal malaria (top right), DCs express PD-L2 which inhibits the immunosuppressive PD-1/PD-L1 interaction while interacting with an unknown receptor (?) to improve T cell functions. This leads to protective immunity characterised by increased T-box transcription factor TBX21 (Tbet) expression, increase interferon-γ (IFN-γ) secretion and better proliferation in response to the parasite. In contrast, during lethal malaria (left), PD-L2 expression is low or absent and this allows the immunosuppressive PD-1/PD-L1 interaction to generate exhausted T cells which do not express Tbet, do not secrete IFN-γ and cannot proliferate in response to the parasite. Soluble PD-L2 administered to mice infected with lethal malaria (lower right) can prevent T cell exhaustion.
Immune checkpoint blockade in malaria
A recent study showed that a multimeric form of PD-L2 fused with the Fc part of immunoglobulin (PD-L2-Fc), given to mice infected with lethal malaria, was sufficient to attenuate the lethal infection and mediate survival following re-infections after several months, without additional PD-L2-Fc9 (Figure 2). Furthermore, combined blockade of the PD-L1 and LAG3 inhibitory molecules with antibodies accelerated the clearance of acute non-lethal blood-stage malaria (P. yoelii) by improving CD4+ T cell function and increasing antibody titres26. Finally, antibody-mediated triggering of OX40 signalling also enhanced helper CD4+ T cell and humoral immunity and thus parasite clearance during non-lethal malarial infections30.
During P. berghei infections in mice resistant to cerebral malaria, antibody-mediated blockade of either CTLA4 or PD-L1, but not PD-L2, resulted in higher levels of T cell activation with enhanced interferon-γ (IFN-γ) production, but increased the incidence of cerebral malaria in these mice31. This was most likely because CTLA4 or PD-L1 blockade did not improve CD4+ T cell functions sufficiently to control systemic parasite growth and sequestration in the brain before improved CD8+ T cell functions could cause bystander pathology in the brain. In contrast, administering soluble multimeric PD-L2-Fc fusion protein reduced the incidence of cerebral malaria by 78%9. Similarly, blocking TIM3 signalling with antibody restored lymphocyte activity in Plasmodium infections resulting in accelerated parasite clearance and reduced symptoms of cerebral disease in P. berghei-infected mice29. The suppressive function of TREG cells in lethal P. yoelii-infected mice was inhibited by GITR blockade indicating another potential target32. BTLA has also been associated with cerebral malaria and blockade significantly reduced the incidence of cerebral malaria compared with control mice33. Overall, several checkpoint proteins contribute to the pathogenesis of malaria, and further investigation of their potential as therapeutic targets is warranted. These therapies may also have the potential to be used to “re-invigorate” immune cells which are suggested to be non-responsive in endemic areas of malaria26,27 to allow vaccines to generate long term immunity. Alternatively, checkpoint blockade could complement malarial drugs to generate long term immunity as seen for PD-L2-Fc9.
Immune checkpoint proteins in HIV
There are 37 million people living with HIV and each year there are 2 million new infections and 1 million deaths34. Antiretroviral therapy (ART) has dramatically reduced HIV-related morbidity and mortality but only 40% of people living with HIV globally are receiving ART34 and there is no vaccine or cure. ART is required lifelong as once treatment is stopped, the virus rapidly rebounds. Given the social and economic impact of lifelong medical care required for people living with HIV, finding a cure has become a major global priority35. Immune checkpoint proteins have been extensively studied during HIV infection, initially in relation to natural history and T cell function, but more recently in relation to complications of HIV infection. In addition, using immune checkpoint blockade could potentially be exploited as a strategy to achieve a cure.
T cell exhaustion and immune checkpoint proteins in HIV infection
T cell exhaustion is a hallmark of many chronic viral infections, including HIV. In untreated HIV infection, there is an up-regulation of multiple immune checkpoint proteins including PD-1, CTLA4, TIM3 and LAG3 on both CD4+ and CD8+ T cells36–38. Following ART, expression of immune checkpoint proteins decline but remain elevated compared to HIV-uninfected controls38. Whether ART is started early (within 6 months of infection) or late (within 2 years of infection), similar levels of expression of immune checkpoint proteins persist39. In HIV infection, expression of immune checkpoint proteins varies on different T cell subsets. Increased expression of PD-1 is predominantly seen on central memory T cells40, while both PD-1 and CTLA4 are expressed by regulatory T cells, and LAG3 is expressed by effector memory T cells41 (Figure 3). In addition, PD-1 is often co-expressed with proteins that help promote T cell activation, such as CD38 and MHC class II molecules42.
Figure 3. Immune checkpoint protein expression in HIV and HBV infection and potential effects of immune checkpoint blockade.
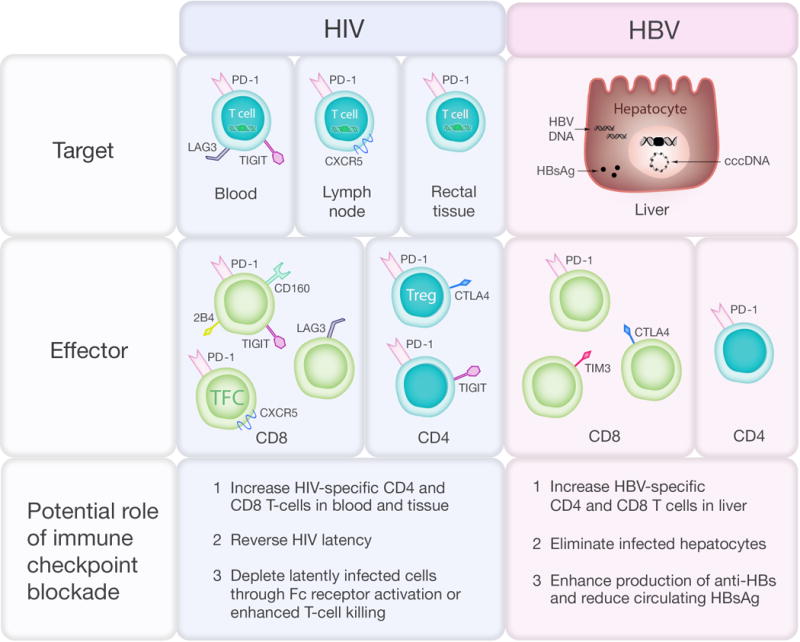
In HIV infection, the virus persists on antiretroviral therapy (ART) in latently infected CD4+ T-cells that contain integrated provirus (green box) and express PD-1 and other immune checkpoint markers in blood, lymph node and rectal tissue. Expression of immune checkpoint markers on total and HIV-specific CD4+ and CD8+ T cell subsets include central memory (CM), effector memory (EM), T follicular cytotoxic (TFC) and T regulatory (Treg) T cells is associated with T-cell exhaustion and reduced T-cell function. In HBV infection, HBV persists on treatment as extrachromosomal closed covalent circular (ccc) DNA and integrated HBV DNA (black box) and there is ongoing production of HBV surface antigen (HBsAg). Increased expression of immune checkpoint markers on CD8+ T cells and increased expression of PD-1 on CD4+ T-cells reduce T cell function.
Elevated levels of PD-1 expression on total and HIV-specific CD8+ T cells in untreated HIV infection was first reported over 10 years ago36,43,44. PD-1 is also highly expressed by cytotoxic CD8+ T cells that migrate into lymphoid follicles; these follicular cytotoxic CD8+ T cells express high levels of CXCR5 and PD-1 but low levels of other immune checkpoint proteins, such as TIM345. There is an inverse association between the frequency of cytotoxic CD8+ T cells and HIV-infected cells in lymphoid follicles and a similar inverse relationship has been recently observed in untreated SIV infection45–47. Finally, ex vivo blockade with anti-PD-1 or anti-PD-L1 resulted in enhanced HIV-specific CD8+ T cell function and killing of infected target cells36,43,44 (Table 2), as described for cancer antigens.
Table 2.
Summary of pre-clinical or ex vivo studies in infectious diseases reporting benefits of targeting inhibitory immune receptors
| Infection | Cell type affected | Inhibitory Receptor | Target Species | Outcomes | Refs |
|---|---|---|---|---|---|
| HIV | CD4+; CD8+ T cells | PD-1; CTLA4 TIGIT; LAG3 | Humans/Mice |
|
37,38,43,64,159,160 |
| SIV | CD8+ T cells | PD-1; PD-L1; CTLA4 | Macaques | PD-1 blockade expands functional virus-specific CD8 T cells and prolongs survival | 38,54,59 |
| HBV | CD4+; CD8+ T cells | PD-1; CTLA4; 2B4; TIM3 | Humans (ex vivo)/mice/woodchuck |
|
84–86,89,97,98 |
| HCV | CD4+; CD8+ T cells | PD-1; PD-L1 | Human |
|
161–164 |
| Influenza | CD8+ T cells | PD-1, TIM3 | Mice |
|
165,166 |
| TB | CD4+; CD8+ T cells | TIM3 | Mice | TIM3 blockade restores T cell function and improves bacterial control, particularly in chronically infected susceptible mice | 106 |
| Listeria | CD8+ T cells | PD-L1 | Mice | PD-L1 plays an important costimulatory role for antigen-specific CD8 T cells | 167 |
| Malaria | CD4+; CD8+T cells; B cells | PD-1; PD-L1; CTLA4; LAG3;TIM3 | Mice |
|
9,26,29,31 |
| Toxoplasma | CD8+ T cells | PD-1 | Mice | Blockade of the PD-1-PD-L1 reinvigorates CD8+T-cell responses with prevention of mortality | 168 |
| Leishmania | CD4+; CD8+ T cells | PD-1, PD-L1 | Mice | PD-1 blockade in vivo restored T cell proliferation and function with complete resolution of chronic lesions. | 169–171 |
Multiple observational studies have demonstrated a clear association between expression of PD-1 on either CD4+ or CD8+ T cells and clinical outcome. In the absence of ART, increased expression of PD-1 was associated with accelerated decline in CD4+ T cells following acute infection48 and untreated chronic infection36. Following ART, PD-1 expression on CD8+ T cells has been associated with impaired CD4+ T cell immune reconstitution49 microvascular disease50, elevated oxidised high and low density lipoproteins51 and a shorter time to viral rebound once ART was stopped52.
In vitro, HIV can establish either productive or latent infection. In latent infection, virus replication is incomplete with the virus integrating in the host genome but not proceeding to transcription, translation or production of virus particles [reviewed in Ref.35]. Following productive infection with HIV or other retroviruses such as feline immunodeficiency virus, PD-L1 expression is up-regulated on infected CD4+ T cells which enhance CD8+ T cell exhaustion and immune escape53. It remains unclear if PD-L1 or PD-L2 expression is increased on CD4+ T cells after ART or on latently infected cells.
Immune checkpoint blockade in vivo for SIV and HIV infection
The administration of anti-PD-1 to simian immunodeficiency virus (SIV)-infected rhesus macaques resulted in rapid expansion of virus-specific CD8+ T cells with improved functional quality as demonstrated by delayed time to death of the macaques and lower SIV RNA in plasma54 (Table 2). Other beneficial effects of anti-PD-1 in the setting of SIV infection have also included reduced interferon signalling and improved gut permeability55. In preliminary work assessing the administration of anti-PD-156 or anti-PD-L1 (avelumab;57) to SIV-infected macaques on ART, there were no adverse effects, but in contrast to the studies in rhesus macaques off ART, there was limited expansion of SIV-specific CD8+ T cells56. It is possible that an effective T cell response to anti-PD-1 requires the presence of antigen, and that given that ART leads to a dramatic reduction in viral antigens, the functional response to immune checkpoint blockade may be limited in this setting. Further work is needed to better understand the effects of immune checkpoint blockade on T cell function following ART.
In HIV-infected individuals who had stopped receiving ART, the up-regulation of CTLA4 expression on HIV-specific CD4+ T cells was also demonstrated over a decade ago and, similarly to up-regulation of PD-1 expression, this was associated with increased HIV disease progression37. When SIV-infected macaques, both on and off ART, were treated with ipilimumab (anti-CTLA4), those that were off ART showed a significant increase in rates of HIV replication, presumably as a result of an increased number of activated CD4+ T cells which would be targets for SIV infection58. In another study of SIV-infected macaques on partially suppressive ART, ipilimumab led to a modest increase in both HIV-specific CD4+ and CD8+ T cells and a significant reduction in cell-associated HIV RNA in lymph node59. These data therefore suggest that anti-CTLA4, may have a significant effect on HIV that persists on ART, through a different mechanism of action to anti-PD-1, leading to a reduction in HIV RNA in lymph node tissue. However, the mechanism of how this is achieved or whether there is antibody activity in HIV-infected subjects on fully suppressive ART remains unknown.
In HIV-infected individuals, LAG3 is also highly expressed on CD4+ and CD8+ T cells in lymph node and blood, and expression is directly related to levels of HIV RNA in plasma but inversely related to CD4+ T cell counts41. Expression of T cell immunoreceptor with Ig and ITIM domains (TIGIT) is also increased on CD8+ T cells in untreated and treated HIV infection compared to HIV-negative controls38 even following early initiation of ART60. HIV-specific CD8+ T cells were almost exclusively TIGIT+, and co-expressed PD-1, CD160 and 2B460. HIV-specific TIGIThi cells were also negatively correlated with polyfunctionality and had reduced expression of the co-stimulatory receptor CD226. Blockade of TIGIT and PD-1 with anti-TIGIT and anti-PD-L1 led to a significant enhancement of HIV-specific function of CD4+ T cells from HIV-infected individuals on ART38. Antibodies to LAG3, TIM3 and TIGIT are all in early clinical development and given their more favourable safety profiles may be more suitable agents to assess in HIV-infected individuals61.
Immune checkpoint proteins and HIV persistence
In contrast to malignant cells, which traditionally express the ligands for immune checkpoint proteins such as PD-L162, in HIV-infected individuals on ART, immune checkpoint proteins (e.g. PD-1) themselves identify cells preferentially infected with HIV that persist on ART (Figure 4)63,64. This observation is of high importance in efforts to eliminate residual virus that persists on ART as these infected cells are a major barrier to a cure. Although HIV can persist in multiple forms in HIV-infected individuals on ART, latently infected cells are the most important. Latency can be established in long-lived and proliferating central and transitional memory T cells as well as other T cell subsets including T follicular helper cells (TFH) and stem cell memory (TSCM) (reviewed in Ref.35).
Figure 4. Proposed role of PD-1 in the establishment and reversal of HIV latency.
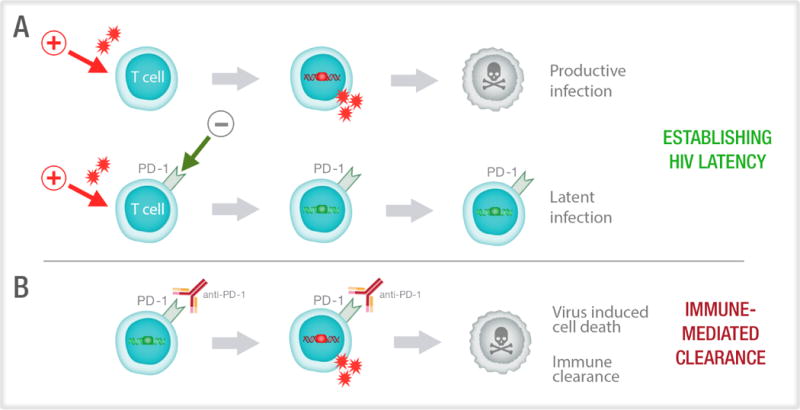
(A) HIV preferentially infects activated CD4+ T cells which have been stimulated via T cell receptor engagement or a mitogen (red arrow). Following HIV integration, the productively infected cell (red box) usually dies by virus mediated cytolysis. Up-regulation of immune checkpoint markers such as PD-1, could potentially limit T-cell activation favouring latent over productive infection, where there is integration (green box) but no virus production. (B) Latently infected cells express immune checkpoint markers, including PD-1. The administration of anti-PD-1, or other immune checkpoint blockers, leads to activation of the T cells and increased expression of transcription factors that can enhance production of virus from latency. This leads to either immune mediated clearance or virus induced cell death.
Many studies have shown a significant correlation between the frequency of PD-1+ CD4+ and CD8+ T cells with HIV persistence on ART in blood63,65,66, lymph node67 and the gastrointestinal tract, which has almost three times the frequency of PD-1+CD4+ T cells compared to lymph node or blood68. However, the most direct evidence of a clear relationship between HIV persistence and PD-1 expression comes from sorting CD4+ T cells from blood where a 10-fold enrichment of HIV in PD-1high compared to PD-1low CD4+ T cells was observed63. Similar findings were also reported from lymph node tissue collected from HIV-infected individuals on ART where HIV was highly enriched in cells expressing PD-1 and CXCR5, which together identify TFH67 (Figure 3). HIV enrichment in PD-1high cells may be due to the inhibitory effects of PD-1 on T cell activation which would limit HIV transcription, RNA export and RNA translation, and therefore favour latent over productive infection (Figure 4).
Immune checkpoint proteins, other than PD-1 may also identify infected cells in individuals on ART. We recently demonstrated that HIV was significantly enriched in sorted cells from HIV-infected individuals on ART that expressed PD-1, TIGIT and LAG3 compared to cells that expressed none of these immune checkpoint proteins64 (Figure 3). The relationship between CTLA4 and virus persistence on ART has been less well studied. In untreated individuals, HIV replicates preferentially in activated CD4+ T cells which express high levels of CTLA4, and therefore virus is enriched in CTLA4+CD4+ T cells69. Rapid internalisation of CTLA4, mediated by the viral protein nef, may potentially play a role in favouring HIV persistence in these cells70. Whether latently infected CTLA4+CD4+ T cells in blood or tissue persist on ART is currently unclear.
These exciting observations are now being exploited by using immune checkpoint blockers to potentially reverse latency, allowing for expression of HIV proteins on the surface of the cell which would lead to immune clearance of virus or virus induced cytolysis (Figure 4). Latency reversal would be attempted in individuals on ART, so that any new virus produced could not go on to infect other cells. Using CD4+ T cells from HIV-infected individuals on ART, the ex vivo administration of anti-PD-1 together with the latency reversing agent bryostatin led to a significant increase in HIV RNA released into supernatant [Dr. Nicolas Chomont, personal communication]. In addition, in an HIV-infected individual on ART with metastatic melanoma, we observed a significant increase in cell associated HIV RNA following anti-CTLA4 (ipilimumab)71 and anti-PD-1 (nivolumab)72 The effects of other immune checkpoint blockers on latency establishment or reversal is unknown and warrants further exploration using antibodies, either alone or in combination.
Clinical trials of immune checkpoint blockade as a strategy for cure for HIV
A phase II dose escalation study of anti-PD-L1 therapy (by Bristol-Myers Squibb) was recently ceased after administration of the lowest dose to 6 individuals with HIV infection on ART73. The study was stopped due to retinal toxicity observed in a simultaneous macaque study. Interestingly, although there were no changes in HIV RNA or DNA, there was a clear increase in Gag-specific CD4+ and CD8+ T cells in 2 of the 6 participants. One of the 6 participants developed hypophysitis many months after receiving anti-PD-L1 therapy. This study remains the only trial of an immune checkpoint blocker in HIV-infected individuals without malignancy and further trials are unlikely to proceed until more safety data of these compounds are available.
To date, few individuals with HIV infection have received even the now licensed immune checkpoint blockers as individuals with HIV infection were excluded from the initial clinical trials of these agents. However, now that these drugs are licensed many individuals with HIV infection are receiving these antibodies as part of clinical care. Several clinical trials in the US and France are currently evaluating the effects of anti-PD-1 and anti-CTLA4 either alone or in combination on HIV-associated malignancies, as well as on proteins of HIV persistence and HIV-specific immunity (reviewed in Ref.74).
In summary, immune checkpoint blockers could have multiple beneficial effects to achieving a cure or allowing individuals to safely stop ART (Figure 4). First, the administration of these drugs could potentially enhance HIV-specific T cell function to eliminate HIV-infected cells. Second, they may lead to direct elimination of infected cells that express the relevant immune checkpoint marker, particularly when using a depleting antibody that activates Fc receptors as described for ipilimumab75 and modified antibodies to PD-176. Third, immune checkpoint blockers could potentially reverse HIV latency. Finally, immune checkpoint blockers can enhance vaccine responsiveness77 and therefore could potentially be combined with other therapeutic vaccines in development.
Immune checkpoint proteins in HBV infection
HBV is a DNA virus which predominantly replicates in the hepatocytes of the liver. Following entry of HBV into a hepatocyte, there is production of intracellular covalently closed circular (ccc) DNA which produces multiple HBV proteins, including hepatitis B surface antigen (HBsAg) as well as production of HBV DNA which is required to form new infectious virions. There are 250 million people living globally with chronic HBV78 and the main complications include end stage cirrhosis and/or hepatocellular carcinoma (HCC)79. Effective treatment of HBV is with self-limited interferon therapy or more commonly with long-term antiviral treatment using nucleotide/nucleoside reverse transcriptase inhibitors (NRTI). Similarly to ART in HIV infected individuals, treatment with NRTI is life-long and there is no cure for HBV80. However, in contrast to HIV, approximately 10-15% of individuals can safely stop NRTI treatment for HBV without viral rebound. Inducing remission for HBV is possible, with the development of antibodies to HBsAg, commonly referred to as seroconversion80.
T cell exhaustion and immune checkpoint proteins in HBV infection
HBV-specific T cells are important in HBV pathogenesis where they play a role in the initial clearance of acute infection, abnormal liver function commonly observed in acute and chronic infection, development of cirrhosis and successful HBsAg seroconversion following antiviral treatment (reviewed in Ref.81). The role and phenotype of HBV-specific T-cells is different at each of these clinical stages as circulating and intrahepatic HBV-specific T cells are infrequent in individuals chronically infected with HBV compared with in individuals who have cleared acute HBV infection82,83.
In untreated chronic HBV infection, total and HBV-specific CD8+ T cells express high levels of PD-1, CTLA4, TIM384–86, and in acute HBV infection circulating and intrahepatic CD8+ T cells express high levels of PD-187 (Figure 3). The up-regulation of PD-1 in acute HBV infection is thought to limit intrahepatic inflammation87. The ligand for PD-1, PD-L1, has also been shown to be elevated on circulating CD14+ monocytes and CD19+ B cells in individuals with chronic HBV infection, liver cirrhosis and HCC and therefore may contribute to ongoing T cell exhaustion88. These exhausted antigen-specific CD8+ T cells are prone to apoptosis through co-expression of the pro-apoptotic protein BIM86. In contrast, HBV-specific CD4+ T cells, defined by MHC Class II tetramers and HBV core peptides, expressed increased levels of PD-1, but no increase in CTLA4, TIM3, KLRG1 and CD24489 (summarised in Figure 3).
A recent genome-wide expression profiling study of HBV-specific CD8+ T cells from individuals with acute and chronic HBV infection revealed extensive down-regulation of multiple pathways, including pathways associated with mitochondrial function, and T cells from these individuals showed functional recovery in the presence of mitochondrial-targeted antioxidants90. These studies demonstrated that defects in T cell function in chronic HBV infection are not limited to increased expression of immune checkpoint proteins, although mitochondrial dysfunction was clearly enriched in PD-1high CD8+ T cells in this study90.
The phenotype of intrahepatic CD4+ and CD8+ T cells has also been extensively characterised in HBV infection. Initial descriptions of intrahepatic T cells in chronic HBV infection showed a high infiltration of total and HBV-specific CD8+ T cells91. Intrahepatic T cells also show up-regulation of PD-187 and TIM384. The ligand of TIM3, galectin-9 was also upregulated on Kupffer cells, perhaps allowing for persisting intrahepatic T cell exhaustion84. These intrahepatic CD8+ T cells also express other proteins of exhaustion including BTLA and can produce IL-10 that further inhibits effective T cell function92.
Immune checkpoint blockade as a strategy for cure for HBV
Multiple ex vivo studies using blood collected from individuals with chronic HBV infection have demonstrated that inhibition of PD-1, CTLA4, 2B4 and TIM3 lead to enhanced HBV-specific CD8+ T cell function83,84,86,93–96. In contrast, only PD-1 blockade partially improved HBV-specific CD4+ T cell functions with production of IFN-γ, IL-2 and tumour necrosis factor-α (TNFα)89. Checkpoint blockade, used either alone or in combination may potentially enhance production of HBV-specific CD8+ T cells and even production of antibody to HBsAg, but there are significant theoretical risks including increased infiltration of reinvigorated T cells into the liver which could trigger inflammation, but this has not been demonstrated in pre-clinical studies or recent clinical trials.
The effects of PD-1 blockade in vivo, during HBV infection, have been evaluated in mouse and woodchuck models. Blockade of the PD-1/PD-L1 or PD-L2 pathways with anti-PD-L1 and anti-PD-L2 antibodies in woodchuck hepatitis virus (WHV)-infected animals partially restored T cell function without hepatotoxicity97. In a separate study of WHV, the combination of antivirals (Entecavir), therapeutic vaccination and anti-PD-L1 blockade, followed by cessation of entecavir, did not result in rebound of WHV in plasma and the development of anti-WHV surface antigen, with complete viral clearance in some animals98. Interestingly, the addition of anti-PD-L1 to the vaccine and Entecavir arm compared to the vaccine and entecavir alone arm, led to a significantly enhanced immunological and clinical response and was not associated with hepatotoxicity98. These studies look very promising for similar interventions to achieve sustained remission off NRTI in human clinical trials.
A recent open label study of Nivolumab (anti PD-1; 3mg/kg), with and without a hepatitis B vaccine, involving 20 participants with virally suppressed chronic HBV infection showed that Nivolumab was safe and well tolerated, and one participant underwent HBsAg serovonversion99. Many other clinical trials of immune checkpoint blockade in individuals with chronic HBV, usually in the setting of HCC, are currently underway. One major study is an open-label, non-comparative study of nivolumab (anti-PD-1) or nivolumab in combination with ipilimumab (anti-CTLA4) in advanced HCC, with or without chronic HBV or hepatitis C virus (HCV) (NCT01658878). Interim results from this trial showed no significant difference in outcomes in individuals infected with HBV. Another 60-month observational study, led by the Taiwan Food and Drug Administration (TFDA), is also in progress100. This is a study of patients with known HBV or HCV infection, regardless of control on antiviral therapy in Taiwan and who are treated with Ipilimumab for advanced (unresectable, recurrent or metastatic) melanoma (NCT NCT02402699). Many other studies of immune checkpoint blockade for HCC, that do not specifically exclude individuals with chronic HBV, are currently enrolling participants.
In summary, immune checkpoint proteins play an important role in the natural history of HBV infection – limiting liver damage in acute infection and potentially facilitating persistent infection in chronic HBV infection. Initial studies indicate that Nivolumab is safe in chronic HBV infection, but further studies are needed to determine whether anti-PD-1 and/or other immune checkpoint blockers can be used to induce HBV remission.
Immune checkpoint proteins in tuberculosis
Mycobacterium tuberculosis, the causal agent of tuberculosis (TB), is among the 10 most fatal diseases worldwide, with 10.4 million symptomatic infections and 1.8 million deaths (including 0.4 million among people with HIV) recorded in 2015. M. tuberculosis characteristically infects the lungs, but can also affect any other organ of the body. TB is treatable and curable if the active, drug-susceptible infection is treated with a standard 6-month course of four antimicrobial drugs. However, the prevalence of multidrug-resistant TB is now increasing and requires longer treatment and more complicated antibiotic regimens.
T cell exhaustion and immune checkpoint proteins in TB infection
CD4+ T cells are required for host resistance to M. tuberculosis. TB-specific CD4+ T cell responses in individuals with active TB infection were found to produce IFN-γ, IL-2 and TNF and express PD-1101. However, PD-1-expression levels on total CD4+ T cells from individuals with active infection were only slightly higher than cells from healthy donors102. Notably, HIV-TB co-infection was consistently and independently associated with a reduced frequency of CD4+ IFN-γ and IL-2-dual secreting T cells and the proportion correlated inversely with HIV RNA in plasma101.
Mouse models used to determine the contribution of PD-1 to TB pathogenesis have had conflicting results. Surprisingly, mice with a PD-1 deletion showed increased pathology and PD-1 deficient CD4+ T cells are sufficient to trigger early mortality103,104. The lungs of the PD-1 deficient mice showed uncontrolled bacterial proliferation and focal necrotic areas with predominantly neutrophilic infiltrates, but a lower number of infiltrating T and B cells105. Pro-inflammatory cytokines, such as TNFα and IL-6 were significantly increased in the lung and sera of these mice, consistent with an aberrant inflammation105. Significantly, TB-specific T cell proliferation was dramatically reduced in PD-1 deficient mice compared with controls due to an increased numbers of TREGS and recruitment of mesenchymal stem cells104. Similarly, functionally exhausted TIM3+ T cells were shown to accumulate during chronic TB, and TIM3-blockade restored T cell functions and improved control, of the bacterial load in chronically infected susceptible mice106.
The treatment of multi-drug-resistant tuberculosis (i.e., the resistance in vitro to at least isoniazid and rifampicin) is complicated. To obtain a clinical and a microbiological cure, patients are treated for long periods because of the lesser effectiveness of the second- and third-line drugs107. The prolonged exposure to these drugs, characterized by a poor safety and tolerability profile, reduces the adherence by patients. The combination of these drugs with checkpoint inhibition may allow immunity to develop when the bacterial burden is under even partial control. For these reasons, checkpoint blockade during chronic TB requires further consideration.
Conclusion
Studies of the interplay between immune activation and suppression have shown an important role for immune checkpoint proteins in the pathogenesis of infectious diseases and malignancies. Notably, immune checkpoint blockade has revolutionized the treatment of cancer with remarkable success. A number of studies have suggested that immune checkpoint blockade may also be highly relevant for treating several infectious diseases, including malaria, HIV infection, HBV infection and TB (Table 2); in these diseases where drug resistance remains a challenge, effective vaccine development has not been possible or lifelong drug treatment is necessary. It should however be recognized, that immune checkpoint blockade may also cause immune-related adverse events as CTLA4, PD-1, LAG3 and TIM3, are also involved in the regulation of peripheral tolerance to prevent autoimmunity (Box 4). Furthermore, whether there will be variable responses to immune checkpoint blockade and clinical outcomes for infectious diseases also remains to be determined. Despite this, immune checkpoint blockade may be an important new strategy for tackling chronic infections for which we are still lacking effective therapies or vaccines.
Box 4. Adverse reactions and limited durability associated with immune checkpoint blockade.
Blocking of checkpoint protein-interactions with antibodies has shown remarkable anti-tumour immunity; however this immunity can be accompanied by immune-related adverse events, resembling autoimmune diseases128. Immune-related adverse events can occur in up to 90% of patients treated with an anti-CTLA4 antibody129 and 70% of patients treated with anti-PD-1/PD-L1 antibodies130,131. These immune-related adverse events typically originate in the skin, gastrointestinal tract, liver, and endocrine system, although other organ systems may also be affected132. While treatment with immunosuppressive drugs such as prednisolone is effective and usually resolves the symptoms, these adverse effects can be fatal. Therefore, significant concerns remain around using these antibodies in otherwise healthy individuals living with HIV or HBV. Autoimmune toxicities such as colitis, myocarditis and pneumonitis occur, more commonly with anti-CTLA4 than anti-PD-1, and whether these can be reduced through modifications of the antibodies and/or reduction in dosage needs to be considered when exploring their use for infectious diseases.
Other factors which could reduce efficacy of immunotherapies include non-responsiveness to treatment and limited durability of restored T cell functions. Blockade of the PD-1/PD-L1 pathway has shown durable benefit in melanoma and other cancers130,131,133 but >50% of patients do not respond or develop resistance to anti-PD-1 therapy. PD-1 also plays a role in the setting of both acute and chronic infections. While PD-1 transcription is rapidly down-regulated in functional antigen-specific CD8+ T cells that develop during acute infection, persistent TCR ligation during chronic viral infections maintains increased levels of PD-1 transcription and generation of a distinct lineage of non-functional “exhausted” antigen-specific CD8+ T cells134,135. These changes in T cell functions are persistent as a result of epigenetic modification, specifically demethylation of the promotor of PD-1. These epigenetic changes persist, even with reduction in antigen, as seen following effective antiviral therapy for HIV infection or following anti-PD-1 therapy136–139. Thus, for infectious diseases, immunotherapy may have optimal effects when used with a vaccine, to minimise immune suppression and thus permit vaccine responses to develop. Alternatively, immunotherapy used in conjunction with antimicrobial agents could allow long term immunity to develop when the immediate threat of a lethal infection had passed.
Acknowledgments
The authors sincerely thank Mrs Madeleine Flynn for the design and implementation of the Figures. The authors acknowledge editorial assistance of Dr Sharon Johnatty from SugarApple Communications in finalizing the manuscript. The authors acknowledge the support of The National Health and Medical Research Council (Australia). SRL is an NHMRC practitioner fellow and is supported by the National Institutes for Health Delaney AIDS Research Enterprise (DARE U19 AI126611), and the American Foundation for AIDS Research.
References
- 1.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016;34:539–573. doi: 10.1146/annurev-immunol-032414-112049. A comprehensive review on inhibitory pathways. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. An excellent review on checkpoint inhibition in the context of cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. A definitive review of T cell exhaustion. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen PL, et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov. 2016;6:827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamphorst AO, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114:4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang AC, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karunarathne DS, et al. Programmed Death-1 Ligand 2-Mediated Regulation of the PD-L1 to PD-1 Axis Is Essential for Establishing CD4(+) T Cell Immunity. Immunity. 2016;45:333–345. doi: 10.1016/j.immuni.2016.07.017. An important study on the mechanism of protective function of PD-L2. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Q, Kyle DE, Gatton ML. Artemisinin resistance in Plasmodium falciparum: A process linked to dormancy? International journal for parasitology Drugs and drug resistance. 2012;2:249–255. doi: 10.1016/j.ijpddr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. World Malaria Report 2016. 2016 http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/
- 12.Olotu A, et al. Four-year efficacy of RTS, S/AS01E and its interaction with malaria exposure. N Engl J Med. 2013;368:1111–1120. doi: 10.1056/NEJMoa1207564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barragan A, Kremsner PG, Weiss W, Wahlgren M, Carlson J. Age-related buildup of humoral immunity against epitopes for rosette formation and agglutination in African areas of malaria endemicity. Infect Immun. 1998;66:4783–4787. doi: 10.1128/iai.66.10.4783-4787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bull PC, et al. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soe S, et al. Premunition against Plasmodium falciparum in a malaria hyperendemic village in Myanmar. Trans R Soc Trop Med Hyg. 2001;95:81–84. doi: 10.1016/s0035-9203(01)90342-6. [DOI] [PubMed] [Google Scholar]
- 16.Mazier D, et al. Hepatic phase of malaria is the target of cellular mechanisms induced by the previous and the subsequent stages. A crucial role for liver nonparenchymal cells. Immunol Lett. 1990;25:65–70. doi: 10.1016/0165-2478(90)90093-6. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, McGregor I, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 18.Langhorne J, Simon-Haarhaus B, Meding SJ. The role of CD4+ T cells in the protective immune response to Plasmodium chabaudi in vivo. Immunol Lett. 1990;25:101–107. doi: 10.1016/0165-2478(90)90099-c. Seminal studies of the role of CD4+ T cells in malarial immunity. [DOI] [PubMed] [Google Scholar]
- 19.Podoba JE, Stevenson MM. CD4+ and CD8+ T lymphocytes both contribute to acquired immunity to blood-stage Plasmodium chabaudi AS. Infect Immun. 1991;59:51–58. doi: 10.1128/iai.59.1.51-58.1991. Seminal studies of the role of T cells in malarial immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von der Weid T, Honarvar N, Langhorne J. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J Immunol. 1996;156:2510–2516. [PubMed] [Google Scholar]
- 21.Horne-Debets JM, et al. PD-1 Dependent Exhaustion of CD8(+) T Cells Drives Chronic Malaria. Cell Reports. 2013;5:1204–1213. doi: 10.1016/j.celrep.2013.11.002. First study to show a role for CD8+ T cell in controlling blood stage malaria. [DOI] [PubMed] [Google Scholar]
- 22.Horne-Debets JM, et al. Mice lacking Programmed cell death-1 show a role for CD8(+) T cells in long-term immunity against blood-stage malaria. Sci Rep. 2016;6:26210. doi: 10.1038/srep26210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wykes MN, Zhou YH, Liu XQ, Good MF. Plasmodium yoelii can ablate vaccine-induced long-term protection in mice. J Immunol. 2005;175:2510–2516. doi: 10.4049/jimmunol.175.4.2510. [DOI] [PubMed] [Google Scholar]
- 24.Liu XQ, et al. Malaria infection alters the expression of B-cell activating factor resulting in diminished memory antibody responses and survival. Eur J Immunol. 2012;42:3291–3301. doi: 10.1002/eji.201242689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce SK, Miller LH. World Malaria Day 2009 what malaria knows about the immune system that immunologists still do not. J Immunol. 2009;182:5171–5177. doi: 10.4049/jimmunol.0804153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler NS, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. First study to show checkpoint blockade during malaria could improve protective immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Illingworth J, et al. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol. 2013;190:1038–1047. doi: 10.4049/jimmunol.1202438. First study to identify an exhausted T cell phenotype during malaria in field studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncalves-Lopes RM, et al. Surface expression of inhibitory (CTLA-4) and stimulatory (OX40) receptors by CD4+ regulatory T cell subsets circulating in human malaria. Microbes Infect. 2016;18:639–648. doi: 10.1016/j.micinf.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou N, et al. T-Cell Immunoglobulin- and Mucin-Domain-Containing Molecule 3 Signaling Blockade Improves Cell-Mediated Immunity Against Malaria. J Infect Dis. 2016;214:1547–1556. doi: 10.1093/infdis/jiw428. [DOI] [PubMed] [Google Scholar]
- 30.Zander RA, et al. PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell Host Microbe. 2015;17:628–641. doi: 10.1016/j.chom.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hafalla JC, et al. The CTLA-4 and PD-1/PD-L1 Inhibitory Pathways Independently Regulate Host Resistance to Plasmodium-induced Acute Immune Pathology. PLoS Pathog. 2012;8:e1002504. doi: 10.1371/journal.ppat.1002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hisaeda H, et al. Resistance of regulatory T cells to glucocorticoid-induced [corrected] TNFR family-related protein (GITR) during Plasmodium yoelii infection. Eur J Immunol. 2005;35:3516–3524. doi: 10.1002/eji.200526073. [DOI] [PubMed] [Google Scholar]
- 33.Lepenies B, et al. Ligation of B and T lymphocyte attenuator prevents the genesis of experimental cerebral malaria. J Immunol. 2007;179:4093–4100. doi: 10.4049/jimmunol.179.6.4093. [DOI] [PubMed] [Google Scholar]
- 34.DALYs GBD, Collaborators, H Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deeks SG, et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med. 2016;22:839–850. doi: 10.1038/nm.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trautmann L, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. One of the first papers to identify PD-1 as a marker of T-cell exhaustion in HIV infection. [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann DE, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 38.Chew GM, et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016;12:e1005349. doi: 10.1371/journal.ppat.1005349. Examination of TIGIT and PD1 in T-cell exhaustion in HIV infection both on and off ART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutishauser R, et al. Early and Delayed Antiretroviral Therapy (ART) Result in Comparable Reductions in CD8+ T Cell Exhaustion Marker Expression. AIDS Res Hum Retroviruses. 2017 doi: 10.1089/AID.2016.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang JY, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 41.Tian X, et al. The upregulation of LAG-3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV-infected subjects. J Immunol. 2015;194:3873–3882. doi: 10.4049/jimmunol.1402176. [DOI] [PubMed] [Google Scholar]
- 42.Sauce D, et al. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. AIDS. 2007;21:2005–2013. doi: 10.1097/QAD.0b013e3282eee548. [DOI] [PubMed] [Google Scholar]
- 43.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 44.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leong YA, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol. 2016;17:1187–1196. doi: 10.1038/ni.3543. The identification of a novel subset of T-cells, T follicular cytotoxic T cells, required for contol of viral infections (e.g.HIV) in lymphoid follicles which also express PD1. [DOI] [PubMed] [Google Scholar]
- 46.Mylvaganam GH, et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci U S A. 2017;114:1976–1981. doi: 10.1073/pnas.1621418114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S, et al. Simian Immunodeficiency Virus-Producing Cells in Follicles Are Partially Suppressed by CD8+ Cells In Vivo. J Virol. 2016;90:11168–11180. doi: 10.1128/JVI.01332-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann M, et al. Exhaustion of Activated CD8 T Cells Predicts Disease Progression in Primary HIV-1 Infection. PLoS Pathog. 2016;12:e1005661. doi: 10.1371/journal.ppat.1005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shive CL, et al. Inflammation Perturbs the IL-7 Axis, Promoting Senescence and Exhaustion that Broadly Characterize Immune Failure in Treated HIV Infection. J Acquir Immune Defic Syndr. 2016;71:483–492. doi: 10.1097/QAI.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinha A, et al. Role of T-Cell Dysfunction, Inflammation, and Coagulation in Microvascular Disease in HIV. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelesidis T, et al. Oxidized lipoproteins are associated with markers of inflammation and immune activation in HIV-1 infection. AIDS. 2016;30:2625–2633. doi: 10.1097/QAD.0000000000001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurst J, et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nature Communications. 2015;6:8495. doi: 10.1038/ncomms9495. The first publication to show a function link between expression of immune checkpoint proteins on T-cells prior to ART, was associate with time to rebound after cessation of ART An important observation in determining the role of immune checkpoint proteins and HIV cure or remission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akhmetzyanova I, et al. PD-L1 Expression on Retrovirus-Infected Cells Mediates Immune Escape from CD8+ T Cell Killing. PLoS Pathog. 2015;11:e1005224. doi: 10.1371/journal.ppat.1005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Velu V, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. doi:nature07662 [pii]10.1038/nature07662. The first demonstration of the effect of PD-1 blockde in vivo in untreated SIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dyavar Shetty R, et al. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest. 2012;122:1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mylvaganam GH, et al. AIDS2016 Vol Abstract 9016. Durban, South Africa: 2016. [Google Scholar]
- 57.Gill AL, et al. Programed death-1/programed death-ligand 1 expression in lymph nodes of HIV infected patients: results of a pilot safety study in rhesus macaques using anti-programed death-ligand 1 (Avelumab) AIDS. 2016;30:2487–2493. doi: 10.1097/QAD.0000000000001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cecchinato V, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hryniewicz A, et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–3842. doi: 10.1182/blood-2006-04-010637. An important paper demonstrating the potential beneficial effects of anti-CTLA4 in treated SIV infection The work has high significance for current strategies on how to use anti-CTLA4 in HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tauriainen J, et al. Perturbed CD8+ T cell TIGIT/CD226/PVR axis despite early initiation of antiretroviral treatment in HIV infected individuals. Sci Rep. 2017;7:40354. doi: 10.1038/srep40354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatterjee S, et al. A humanized antibody for imaging immune checkpoint ligand PD-L1 expression in tumors. Oncotarget. 2016;7:10215–10227. doi: 10.18632/oncotarget.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. The first demonstration of the relationship between PD1 and HIV persistence on ART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fromentin R, et al. CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLoS Pathog. 2016;12:e1005761. doi: 10.1371/journal.ppat.1005761. An important paper demonstrating that multiple immune checkpoint markers are involved in HIV persistence on ART, not just PD-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatano H, et al. A randomized controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infection. J Acquir Immune Defic Syndr. 2012;61:317–325. doi: 10.1097/QAI.0b013e31826e7d0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cockerham LR, et al. Programmed death-1 expression on CD4(+) and CD8(+) T cells in treated and untreated HIV disease. AIDS. 2014;28:1749–1758. doi: 10.1097/QAD.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Banga R, et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med. 2016;22:754–761. doi: 10.1038/nm.4113. The first demonstration that T follicular helper cells, that express high levels of PD-1 are a asignificant reservoir for HIV on ART. [DOI] [PubMed] [Google Scholar]
- 68.Khoury G, et al. HIV persistence and T-cell activation in blood, rectal and lymph node tissue in HIV-infected individuals receiving suppressive ART. J Infect Dis. 2017 doi: 10.1093/infdis/jix039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-Far M, et al. Nef promotes evasion of human immunodeficiency virus type 1-infected cells from the CTLA-4-mediated inhibition of T-cell activation. J Gen Virol. 2015;96:1463–1477. doi: 10.1099/vir.0.000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El-Far M, et al. Down-regulation of CTLA-4 by HIV-1 Nef protein. PLoS One. 2013;8:e54295. doi: 10.1371/journal.pone.0054295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wightman F, et al. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS. 2015;29:504–506. doi: 10.1097/QAD.0000000000000562. Case report showing the effects of anti CTLA4 on an HIV-infected individuals on ART in vivo in reversing HIV latency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van der Sluis RM, et al. Anti-PD-1 disrupts HIV latency in non-proliferating but not in proliferating T-cells. International AIDS Society Towards a Cure workshop. 2017 [Google Scholar]
- 73.Gay CL, et al. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J Infect Dis. 2017 doi: 10.1093/infdis/jix191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rasmussen TA, Anderson JL, Wightman F, Lewin SR. Cancer therapies in HIV cure research. Curr Opin HIV AIDS. 2017;12:96–104. doi: 10.1097/COH.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romano E, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dahan R, et al. FcgammaRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer cell. 2015;28:285–295. doi: 10.1016/j.ccell.2015.08.004. An interesting paper exploring the future direction of immune checkpoint blockers with modifications to the Fc tail so that the antibody activates Fc recptors. [DOI] [PubMed] [Google Scholar]
- 77.Zhou J, et al. PD1-based DNA vaccine amplifies HIV-1 GAG-specific CD8+ T cells in mice. J Clin Invest. 2013;123:2629–2642. doi: 10.1172/JCI64704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 79.Stanaway JD, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Revill P, Testoni B, Locarnini S, Zoulim F. Global strategies are required to cure and eliminate HBV infection. Nat Rev Gastroenterol Hepatol. 2016;13:239–248. doi: 10.1038/nrgastro.2016.7. [DOI] [PubMed] [Google Scholar]
- 81.Guidotti LG, Isogawa M, Chisari FV. Host-virus interactions in hepatitis B virus infection. Curr Opin Immunol. 2015;36:61–66. doi: 10.1016/j.coi.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang JJ, et al. The phenotype of hepatitis B virus-specific T cells differ in the liver and blood in chronic hepatitis B virus infection. Hepatology. 2007;46:1332–1340. doi: 10.1002/hep.21844. [DOI] [PubMed] [Google Scholar]
- 83.Boni C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nebbia G, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS ONE. 2012;7:e47648. doi: 10.1371/journal.pone.0047648PONE-D-12-12853. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol. 2014;61:1212–1219. doi: 10.1016/j.jhep.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 86.Schurich A, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53:1494–1503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Z, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938–1949. 1949 e1931–1933. doi: 10.1053/j.gastro.2008.03.037. Demonstration of the importance of PD-1 expression in acute HBV infection. [DOI] [PubMed] [Google Scholar]
- 88.Huang ZY, et al. Clinical Significance of Dynamics of Programmed Death Ligand-1 Expression on Circulating CD14+ Monocytes and CD19+ B Cells with the Progression of Hepatitis B Virus Infection. Viral Immunol. 2017;30:224–231. doi: 10.1089/vim.2016.0122. [DOI] [PubMed] [Google Scholar]
- 89.Raziorrouh B, et al. Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One. 2014;9:e105703. doi: 10.1371/journal.pone.0105703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fisicaro P, et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat Med. 2017;23:327–336. doi: 10.1038/nm.4275. [DOI] [PubMed] [Google Scholar]
- 91.Maini MK, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H, et al. Hepatic expansion of virus-specific CD8+BTLA+ T cells with regulatory properties in chronic hepatitis B virus infection. Cell Immunol. 2017;311:36–45. doi: 10.1016/j.cellimm.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 93.Fisicaro P, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682–693. 693 e681–684. doi: 10.1053/j.gastro.2009.09.052. [DOI] [PubMed] [Google Scholar]
- 94.Raziorrouh B, et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology. 2010;52:1934–1947. doi: 10.1002/hep.23936. [DOI] [PubMed] [Google Scholar]
- 95.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. doi:S1074-7613(05)00198-6 [pii]10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 96.Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol. 2007;178:2714–2720. doi: 10.4049/jimmunol.178.5.2714. [DOI] [PubMed] [Google Scholar]
- 97.Zhang E, et al. The expression of PD-1 ligands and their involvement in regulation of T cell functions in acute and chronic woodchuck hepatitis virus infection. PLoS One. 2011;6:e26196. doi: 10.1371/journal.pone.0026196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10:e1003856. doi: 10.1371/journal.ppat.1003856. Effect of anti PD-L1 in an animal model of HBV leading to reduced viral rebound after cessation of antiviral therapy and increased antibodies to surface antigen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gane EJ, et al. A Phase 1 Study Evaluating Anti-PD-1 Treatment With or Without GS-4774 in HBeAg Negative Chronic Hepatitis B Patients. European Association for the Study of Liver Disease; 2017. [Google Scholar]
- 100.Sangro B, Melero I, Yau T, et al. 10th Annual International Liver Cancer Association Conference. Vancouver, Canada: 2016. [Google Scholar]
- 101.Pollock KM, et al. PD-1 Expression and Cytokine Secretion Profiles of Mycobacterium tuberculosis-Specific CD4+ T-Cell Subsets; Potential Correlates of Containment in HIV-TB Co-Infection. PLoS One. 2016;11:e0146905. doi: 10.1371/journal.pone.0146905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boer MC, et al. KLRG1 and PD-1 expression are increased on T-cells following tuberculosis-treatment and identify cells with different proliferative capacities in BCG-vaccinated adults. Tuberculosis. 2016;97:163–171. doi: 10.1016/j.tube.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 103.Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol. 2011;186:1598–1607. doi: 10.4049/jimmunol.1003304. doi:jimmunol.1003304 [pii]10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tousif S, et al. T cells from Programmed Death-1 deficient mice respond poorly to Mycobacterium tuberculosis infection. PLoS One. 2011;6:e19864. doi: 10.1371/journal.pone.0019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lazar-Molnar E, et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jayaraman P, et al. TIM3 Mediates T Cell Exhaustion during Mycobacterium tuberculosis Infection. PLoS Pathog. 2016;12:e1005490. doi: 10.1371/journal.ppat.1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sotgiu G, Centis R, D’Ambrosio L, Migliori GB. Tuberculosis treatment and drug regimens. Cold Spring Harb Perspect Med. 2015;5:a017822. doi: 10.1101/cshperspect.a017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Attanasio J, Wherry EJ. Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease. Immunity. 2016;44:1052–1068. doi: 10.1016/j.immuni.2016.04.022. Excellent review of the biology of immune checkpoint pathways in infectious diseases with a focus on small animal models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 110.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. A seminal study of CTLA-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. A seminal study of the PD-1/PD-L1 interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 114.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. doi:jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kamphorst AO, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017 doi: 10.1126/science.aaf0683. An important study highlighting the balance between immune activation and immune suppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamazaki T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 117.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown JA, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 119.Liang SC, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 120.Balar AV, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi: 10.1016/S0140-673616)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rittmeyer A, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Homet Moreno B, Mok S, Comin-Anduix B, Hu-Lieskovan S, Ribas A. Combined treatment with dabrafenib and trametinib with immune-stimulating antibodies for BRAF mutant melanoma. Oncoimmunology. 2016;5:e1052212. doi: 10.1080/2162402X.2015.1052212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci U S A. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tseng SY, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. First study to highlight a protective role for PD-L2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shin T, et al. Cooperative B71/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shin T, et al. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. doi:jem.2005.0072[pii]10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Michot JM, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. European journal of cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 129.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weber JS, Postow M, Lao CD, Schadendorf D. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. The oncologist. 2016;21:1230–1240. doi: 10.1634/theoncologist.2016-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 135.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. S1074-7613(07)00454-2[pii] [DOI] [PubMed] [Google Scholar]
- 136.Youngblood B, et al. Cutting edge: Prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol. 2013;191:540–544. doi: 10.4049/jimmunol.1203161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ahn E, et al. Demethylation of the PD-1 Promoter Is Imprinted during the Effector Phase of CD8 T Cell Exhaustion. J Virol. 2016;90:8934–8946. doi: 10.1128/JVI.00798-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pauken KE, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sen DR, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 141.Zhou Q, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sakuishi K, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 144.Woo SR, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dougall WC, Kurtulus S, Smyth MJ, Anderson AC. TIGIT CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev. 2017;276:112–120. doi: 10.1111/imr.12518. [DOI] [PubMed] [Google Scholar]
- 146.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188:3869–3875. doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu X, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 148.Blake SJ, Dougall WC, Miles JJ, Teng MW, Smyth MJ. Molecular Pathways: Targeting CD96 and TIGIT for Cancer Immunotherapy. Clin Cancer Res. 2016;22:5183–5188. doi: 10.1158/1078-0432.CCR-16-0933. [DOI] [PubMed] [Google Scholar]
- 149.Gonzalez LC, et al. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A. 2005;102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sedy JR, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 151.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 152.Fourcade J, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ephrem A, et al. Modulation of Treg cells/T effector function by GITR signaling is context-dependent. Eur J Immunol. 2013;43:2421–2429. doi: 10.1002/eji.201343451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 155.Schaer DA, Cohen AD, Wolchok JD. Anti-GITR antibodies–potential clinical applications for tumor immunotherapy. Curr Opin Investig Drugs. 2010;11:1378–1386. [PubMed] [Google Scholar]
- 156.Mitsui J, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16:2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 157.Deng J, Le Mercier I, Kuta A, Noelle RJ. A New VISTA on combination therapy for negative checkpoint regulator blockade. Journal for immunotherapy of cancer. 2016;4:86. doi: 10.1186/s40425-016-0190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gao J, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017 doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. doi:jem.20061800 [pii]10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Golden-Mason L, et al. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nakamoto N, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 1937;2008;134(1927–1937):e1921–1922. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lukens JR, Cruise MW, Lassen MG, Hahn YS. Blockade of PD-1/B7-H1 interaction restores effector CD8+ T cell responses in a hepatitis C virus core murine model. J Immunol. 2008;180:4875–4884. doi: 10.4049/jimmunol.180.7.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Urbani S, et al. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: effect of viremia levels and antiviral treatment. J Hepatol. 2008;48:548–558. doi: 10.1016/j.jhep.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 165.Rutigliano JA, et al. Highly pathological influenza A virus infection is associated with augmented expression of PD-1 by functionally compromised virus-specific CD8+ T cells. J Virol. 2014;88:1636–1651. doi: 10.1128/JVI.02851-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sharma S, et al. T cell immunoglobulin and mucin protein-3 (Tim-3)/Galectin-9 interaction regulates influenza A virus-specific humoral and CD8 T-cell responses. Proc Natl Acad Sci U S A. 2011;108:19001–19006. doi: 10.1073/pnas.1107087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu D, et al. A potential new pathway for PD-L1 costimulation of the CD8-T cell response to Listeria monocytogenes infection. PLoS One. 2013;8:e56539. doi: 10.1371/journal.pone.0056539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci U S A. 2011;108:9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mou Z, et al. Parasite-derived arginase influences secondary anti-Leishmania immunity by regulating programmed cell death-1-mediated CD4+ T cell exhaustion. J Immunol. 2013;190:3380–3389. doi: 10.4049/jimmunol.1202537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Esch KJ, Juelsgaard R, Martinez PA, Jones DE, Petersen CA. Programmed death 1-mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function. J Immunol. 2013;191:5542–5550. doi: 10.4049/jimmunol.1301810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Joshi T, Rodriguez S, Perovic V, Cockburn IA, Stager S. B7-H1 blockade increases survival of dysfunctional CD8(+) T cells and confers protection against Leishmania donovani infections. PLoS Pathog. 2009;5:e1000431. doi: 10.1371/journal.ppat.1000431. [DOI] [PMC free article] [PubMed] [Google Scholar]


