Abstract
Study Objectives:
Obesity is a major risk factor for obstructive sleep apnea (OSA). Patients who are not obese and who have OSA usually present with a low apnea-hypopnea index (AHI) in the lateral sleeping position. Hence, sleep-disordered breathing (SDB) seems more dependent on body mass index (BMI) in the lateral sleeping position than the supine sleep position. This makes obesity a better predictor of SDB in the lateral sleeping position. The objective of this study was to find a negative predictive value of normal BMI for SDB in relation to sleep positions, thus defining a group of patients who could be treated by positional intervention, and prioritizing the use of polysomnography diagnostics.
Methods:
This study comprises a retrospective and prospective part run on groups of 1,181 and 821 consecutive patients, respectively. All had been referred to the university-based sleep laboratory because of suspected OSA and underwent polysomnography.
Results:
In the retrospective study, areas under the receiver operating characteristic curves for normal BMI at AHI ≥ 5 and AHI ≥ 15 events/h were found to be larger in the lateral sleeping positing than supine: 0.79 versus 0.69 and 0.80 versus 0.68, respectively (P < .05). Comparable results were obtained in the prospective study. For normal BMI, the negative predictive value for AHI < 15 events/h in the lateral sleep position was 97.5% and 97.1% in the retrospective and prospective study, respectively.
Conclusions:
Normal BMI offers a high negative predictive value for moderate or severe OSA in the lateral sleeping position.
Citation:
Mokros Ł, Kuczynski W, Gabryelska A, Franczak Ł, Spałka J, Białasiewicz P. High negative predictive value of normal body mass index for obstructive sleep apnea in the lateral sleeping position. J Clin Sleep Med. 2018;14(6):985–990.
Keywords: BMI, polysomnography, positional obstructive sleep apnea, predictive values
BRIEF SUMMARY
Current Knowledge/Study Rationale: Patients who are not obese and who have obstructive sleep apnea generally present with a low apneahypopnea index in the lateral sleeping position. Hence, sleep-disordered breathing seems more dependent on body mass index in the lateral sleeping position than the supine sleep position. We suspect that obesity is a better predictor of sleep-disordered breathing in the lateral sleeping position.
Study Impact: Normal body mass index offers a high negative predictive value for moderate or severe obstructive sleep apnea in the lateral sleeping position. For normal body mass index, the negative predictive value for an apnea-hypopnea index < 15 events/h in the lateral sleeping position reached 97.5% and 97.1% in our retrospective and prospective studies, respectively.
INTRODUCTION
The reported prevalence of obstructive sleep apnea (OSA) in the adult population ranges from 4% to 7% in men and 2% to 5% in women.1,2 Recent data suggest that OSA affects up to 50% of men and 23% of women.3,4 It is a serious health problem considering its high prevalence and deleterious consequences, including traffic accidents and related cardiovascular morbidity and mortality.5–7
Polysomnography is the acknowledged “gold standard” in OSA diagnostics.8 It is a costly and still not widely accessible procedure. Therefore, considering the high prevalence of OSA in the adult population and the limited diagnostic resources, simple clinical measures should be categorized to prioritize the use of polysomnography by identifying low-risk subjects. Obesity, with body mass index (BMI) as its surrogate marker, is one of the strongest risk factors for OSA and an increment in BMI leads to more severe disease.9 Numerous studies have reported the diagnostic value of individual clinical variables or their combinations, which usually include BMI.10–14 Nevertheless, none has shown high enough sensitivity to yield a high negative predictive value (NPV) in OSA diagnostics.
Furthermore, some patients display a difference in OSA severity (reflected as apnea-hypopnea index [AHI]) between sleeping positions, with lower values being found in the lateral position. This has led to the recognition of two OSA phenotypes by somewhat arbitrary criteria: a positional disease, ie, one characterized by sleep-disordered breathing (SDB) restricted to, or predominant in the supine sleeping position, and a position independent of disease characterized by similar AHI in the supine and lateral positions.15,16 High BMI is usually related to the latter, whereas patients with positional disease are generally less obese or even present with the normal body habitus.17 Therefore, BMI may be a more sensitive clinical variable yielding high NPV for SDB in the lateral position as compared to the supine position. This may also have some practical implications, as patients with low AHI in the lateral position may be advised to avoid the supine position, as a simple treatment option.18,19 As continuous positive airway pressure is generally indicated for treatment of symptomatic moderate to severe OSA, in patients with AHI < 15 events/h in the lateral position, the positional treatment is worth trying.8,20 Although multicenter, randomized clinical trials on the efficacy of positional treatment are scarce, some studies suggest that it is a practical option at least in some patients with the positional disease.21 Moreover, being simple and safe, the positional treatment may be recommended before a final, polysomnography-based diagnosis, if only SDB in the lateral position can be excluded with high probability.
Therefore, the current study compares BMI as a predictor of SDB separately in supine and lateral sleeping positions and assesses the NPV of a normal range BMI ≤ 25 kg/m2 in the diagnosis of OSA in both sleeping positions. Our hypothesis is that normal BMI may be used as a marker to exclude OSA with high probability, at least in the lateral sleep position, which can help to prioritize the use of polysomnography and indicate candidates for the positional treatment before polysomnography-based diagnosis.
METHODS
Study Design and Subjects
This study comprises retrospective and prospective parts to evaluate the NPV of BMI in OSA diagnostics. All patients were referred for suspected OSA, based on typical, not mutually exclusive complaints, including snoring, witnessed apneas, excessive daytime sleepiness, or unrefreshing sleep. The retrospective study group consisted of 1,181 consecutive patients of the Sleep and Respiratory Disorders Centre who underwent diagnostic polysomnography from the beginning of 2009 to the end of 2011. In total, 46 patients were excluded based on the following exclusion criteria: less than 3 hours of total sleep time (n = 31), central sleep apnea (n = 3), pure obesity hypoventilation syndrome (n = 4), poor signal quality of recorded channels (n = 8). After exclusion, 1,135 patients (75.4% male) remained who were eligible for analysis.
The prospective study was undertaken to validate the retro spective model. This study group consisted of 821 consecutive patients who underwent diagnostic polysomnography from the beginning of 2014 to the end of 2016. A total of 43 patients were excluded based on the following exclusion criteria: less than 3 hours of total sleep time (n = 41), central sleep apnea (n = 1), poor signal quality of recorded channels (n = 1). Following exclusion, 778 patients (68.9% male) remained who were eligible for analysis.
All patients gave written informed consent for diagnostic polysomnography. This study was conducted in accordance with the amended Declaration of Helsinki, and the Ethics Committee of the Medical University of Lodz approved the study protocol (RNN/23/15/KE).
Polysomnography
Patients were admitted to the sleep laboratory at 9:00 pm (± 0.5 hour) and underwent physical examination (measurement of body mass, height, heart rate, and blood pressure). A standard nocturnal polysomnography was performed by recording the following channels: electroencephalography (C4\A1, C3\A2), chin muscles and anterior tibialis electromyography, electrooculography, measurements of oronasal air flow (a thermistor gauge), snoring, body position (a gravitational gauge placed on the sternum), respiratory movements of chest and abdomen (piezoelectric gauges), unipolar electrocardiogram, and hemoglobin oxygen saturation (SaO2) (Sleep Lab, Jaeger - Viasys, Hoechberg, Germany). Sleep stages were scored according to the criteria based on 30-second epoch standard.22,23 Apnea was attained with the reduction of airflow to less than 10% of the baseline for at least 10 seconds. Hypopnea was defined as at least 30% reduction of airflow for at least 10 seconds, accompanied by 4% or greater decrease in SaO2 or an arousal. Electroencephalography arousals were scored according to American Academy of Sleep Medicine guidelines.24
Study Variables
The evaluation of polysomnography yielded variables related to sleep (eg, total sleep time, sleep duration in the supine and lateral positions) and to SDB, ie, AHI calculated for the whole sleep (AHI-total) and separately for the sleep in the lateral (AHI-side) and supine (AHI-back) positions for every patient. This approach was different from dividing patients into positional and nonpositional disease; the aim of the study was to determine to what extent BMI can predict SDB in different sleeping positions independently of classifying patients as a positional or nonpositional phenotype, which is usually based on the ratio of AHI-back and AHI-side.15,16 An arbitrary 0.5-hour threshold was chosen for the sleep duration in the supine or lateral position in order to calculate a credible AHI. Thus, patients with sleep duration of less than 0.5 hour in either position were excluded, leaving 1,030 patients and 977 for AHI analysis in the retrospective study, and 673 patients and 657 for AHI analysis in the prospective study, respectively. The standard cutoffs for AHI were applied, ie, ≥ 5 and ≥ 15 events/h for diagnosis of any OSA or at least moderate OSA, respectively.25 The NPV of BMI was analyzed at the level of 25 kg/m2 (an arbitrary border between normal and overweight body habitus).
Statistics
The data were analyzed with Statistica 12 (TIBCO Software Inc, Palo Alto, California, United States) with the medical pack. Data distribution was tested with the Shapiro-Wilk test. Receiver operating characteristic curves were created and area under the curve (AUC) was calculated for AHI ≥ 5 and ≥ 15 events/h separately for sleep in the supine and lateral positions using BMI as a predictor variable. To compare AUC, the Z test was calculated with the continuity correction. The predictive values of BMI < 25 kg/m2 for AHI ≥ 5 and ≥ 15 events/h; were calculated separately for sleep in supine and lateral positions. The ratios of sensitivity, specificity, positive predictive value (PPV), and NPV were calculated by creating 2 × 2 contingency tables; chi square test with Yates correction were used to compare the corresponding values calculated for sleep in lateral and supine positions. A value of P < .05 was considered significant.
RESULTS
Clinical Variables
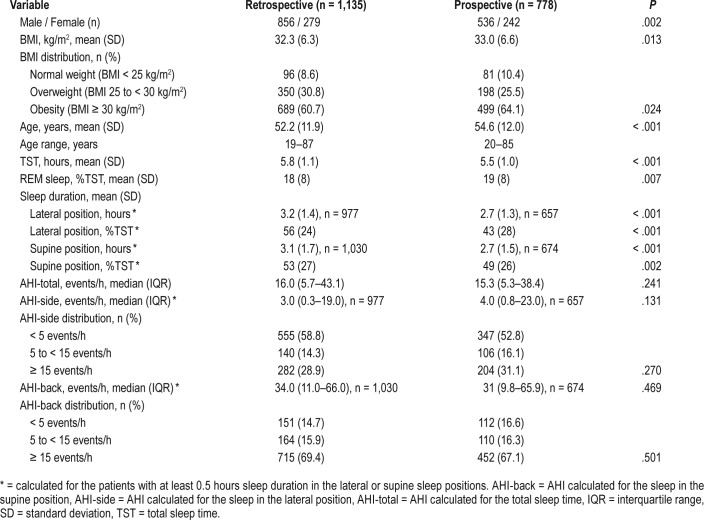
All relevant clinical and sleep study variables are summarized in Table 1. Although there were some statistically significant differences in clinical variables between the retrospective and prospective study populations, they seem clinically irrelevant. No differences were noted in BMI distribution, AHI-total, AHI-side, and AHI-back and their distributions between study populations.
Table 1.
Summary of clinical and sleep study variables.
Receiver Operating Characteristic Curves
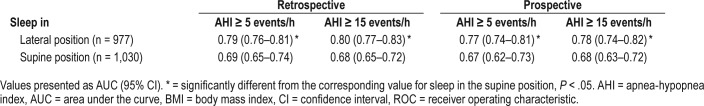
For AHI cutoff of 5 and 15 events/h, an AUC for BMI as a predictor variable was higher for sleep in the lateral position than in the supine position in the retrospective as well as prospective study. Furthermore, no differences were observed in the corresponding AUC between the retrospective and the prospective study (Table 2).
Table 2.
Comparison of AUC of ROC curves at standard AHI cutoffs for BMI as a predictor variable in different sleeping positions.
NPV of Normal BMI for Sleep in Supine and Lateral Position in Retrospective Study
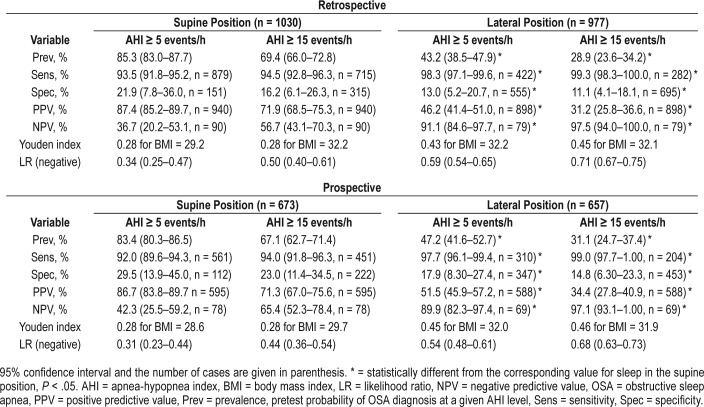
SDB in the supine position was two times more prevalent than in the lateral position. Normal BMI revealed high sensitivity > 90% for both AHI cutoffs and body positions during sleep; the highest one was calculated for AHI ≥ 15 events/h for sleep in the lateral position and reached 99.3% (95% confidence interval [CI] 98.3–100.0%). Specificity was low and the highest one was found for AHI ≥ 5 events/h in the supine position: 21.9% (95% CI 7.8–36.0%). Therefore, NPV changed pretest probability of the negative OSA diagnosis to far greater extent than did PPV for the diagnosis of OSA. Namely, the highest NPV was reached for AHI ≥ 15 events/h in the lateral position: 97.5% (95% CI 94.0–100.0%). However, only 79 of 977 eligible patients (8.1%) for the analysis in the lateral position revealed normal BMI and only 2 from this group presented with AHI ≥ 15 events/h (false negatives). All relevant Bayesian variables for normal BMI are presented in Table 3.
Table 3.
The Bayesian variables for normal BMI (< 25 kg/m2) at the standard AHI cutoffs calculated for sleep in the supine and lateral position.
Prospective Evaluation of the Results of Retrospective Study
Similar results were obtained in the prospective model. The sensitivity and specificity of normal-range BMI as a marker in supine and lateral positions did not differ from those obtained in the retrospective study (Table 3). Similarly, the highest NPV was calculated for AHI ≥ 15 events/h in the lateral position, namely 97.1% (95% CI 93.0–100.0%) and did not differ from the one of the retrospective study. Only 69 patients of 657 (10.5%) who were eligible for the analysis in the lateral position presented with normal BMI and only two from this group presented with AHI ≥ 15 events/h (false negatives).
DISCUSSION
The most significant finding of our study is that normal BMI revealed very high NPV in the lateral position, virtually excluding moderate or severe OSA in this sleeping position. Moreover, the diagnostic value of BMI was better in the lateral than the supine position, as indicated by the larger AUC value for the corresponding AHI cutoffs.
The typical complaints related to OSA must have had good diagnostic value, as the prevalence of OSA in the group of the referred patients was 76.6%. Furthermore, almost half of the referred patients suffered from at least moderate OSA, ie, AHI-total ≥ 15 events/h, yet only about 30% presented with AHI-side ≥ 15 events/h. Mild, moderate, and severe OSA were found to have comparable prevalence in a study conducted on a similar population: the results of an eight-item STOP-BANG questionnaire revealed a NPV of 96% for AHI ≥ 5 events/h, which was close to our 91% based only on BMI, but this was only found for lateral position.14 A low STOP-BANG score was also found to have a similar high NPV in another study on a pediatric population.25 In summary, the sensitivity, and hence the NPV, of BMI was comparable to that of STOP-BANG, but only in the lateral position; in the supine position, NPV was significantly lower.
It is difficult to compare our results with those of other studies on the diagnostic value of various questionnaires because their scores were usually arbitrarily dichotomized.26 Such comparison is further complicated by the fact that a value of the predictor variable can be manipulated to yield either high NPV or PPV, provided that there is a sufficient number of patients above or below a chosen level to have any clinical merit. For instance, in our study BMI ≤ 21 kg/m2 was found to have an NPV of 100% at AHI-total ≥ 5 (data not shown), but only 9 patients (0.08%) were found to have a BMI this low; this score clearly has limited clinical value.
Overall, BMI as a diagnostic tool is not sensitive or specific enough to yield high respective NPV or PPV in a population with high prevalence of OSA due to the high number of false negatives (nonobese with AHI ≥ 5 events/h) and false positives (obese with AHI < 5 events/h), indicating that other risk factors do contribute substantially. One such factor is an unfavorable ratio of the volume of pharyngeal soft tissues to the size of the surrounding bony girdle, which translates into less negative or even positive pharyngeal critical pressure at which soft tissues collapse. Patients with positional OSA had larger isthmus of the fauces and smaller volume of lateral pharyngeal soft tissues, and tended to have lower BMI than patients with nonpositional disease.27,28 Seemingly, when not complicated by obesity, this positional effect is mostly due to the gravitational pull on soft tissues, translocating the base of the tongue toward the back wall of the pharynx. This can explain why the phenomenon of positional OSA in patients with normal BMI is relatively frequent, whereas nonpositional, moderate, or severe OSA in the nonobese is relatively rare. Conversely, high BMI is correlated with the pharyngeal soft tissues being large enough to close the patency of the airways due to sleep-related muscle relaxation alone, independent of sleeping position. This is in accordance with the finding that indices of the upper airway cross-sectional area in normal subjects correlated inversely with BMI.29 Hence, BMI is a better predictor of OSA in the lateral position with the gravitational effect reduced; therefore, the NPV of BMI gains power in the lateral position. Another plausible confounding factor is the phenomenon of SDB occurring predominantly in REM sleep (defined arbitrarily as an AHI in REM sleep at least twice as large as in NREM sleep). REM sleep-related SDB could be responsible for the false-negative cases, ie, yielding high AHI in the lateral sleeping position despite normal BMI, but this proved only partially true in our study. Namely, in the retrospective study there were 2 false negatives at AHI ≥ 15 events/h and none of them had AHI-REM/ AHI-NREM ratio > 2; at AHI ≥ 5 events/h there were 7 false negatives and only 2 had AHI-REM/AHI-NREM ratio > 2. Similarly, in the prospective study there were 2 false negatives at AHI ≥ 15 events/h and one of them had AHI-REM/ AHI-NREM ratio > 2; at AHI ≥ 5 events/h there were 7 false negatives and only 3 had AHI-REM/AHI-NREM ratio > 2. It can be concluded that REM-predominant SDB can be a factor weakening NPV of BMI in the lateral sleeping position.
Two of the key strengths of the current study are the size of the study group and validation of the model by the prospective study; few similar studies have included more than 1,000 patients who underwent nocturnal polysomnography. For instance, although another study assessed the predictive value of anthropometric variables, BMI included, on a population of over 2,000 subjects, the diagnosis was not confirmed by polysomnography.12 One of the weaknesses of the current study is its selection bias, as our results apply to preselected subjects, ie, the referred group of white patients with high pretest probability of disease, not a representative sample of the general adult population. However, this does reflect everyday experience in clinical practice, as a patient's complaints of snoring, witnessed apneas, nonrefreshing sleep, and excessive daytime sleepiness are what have lead to a referral. Assessing BMI in these individuals can further aid in assessing their probability for having OSA and the probability that the OSA is position dependent. Ultimately, this could help to prioritize evaluation by polysomnography. Another limitation related to the clinical relevance of the results is that our attribution of a high NPV to normal BMI can be applied to a limited number of referred patients, as most (approximately 90%) are overweight or obese. Moreover, our study group was racially homogenous, ie, 100% white; this is another limitation, as the results cannot be extrapolated to other populations with mixed racial composition.
In conclusion, normal-range BMI does not necessarily indicate that a symptomatic subject has a low probability of OSA, but the chance of the moderate or severe disease in the lateral sleeping position is low. Our most valuable finding relevant to clinical practice is that a symptomatic patient with BMI lower than 25.0 kg/m2 has a very low chance (< 3%) of AHI ≥ 15 events/h in the lateral position. Hence, although still a subject of ongoing debate regarding the method, feasibility criteria, long-term efficacy and compliance, positional treatment can be an option in nonobese symptomatic patients even prior to polysomnography-based diagnosis.
DISCLOSURE STATEMENT
The study was funded by an institutional grant of the Medical University of Lodz 503/0-079-06/503-01, 564/1-000-00/564-20-021 and 503/1-079-006/503-16-001-002. The authors report no conflicts of interest and have seen and approved the manuscript.
ACKNOWLEDGMENTS
The authors express their gratitude to Aneta Gruchala, Aleksandra Debicka, Magdalena Oset, Karolina Wisniewska, and Kacper Reszka for their help in data collection. Author contributions: all authors were involved in data collection and creation of a database. The contribution of the first two authors is equivalent and accounts for 80% of the contribution to this research. PB conceived the idea of the study. ŁM and PB were involved in statistical analysis. ŁM, WK, and PB wrote the manuscript. All authors edited, revised, and approved the final version of the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AUC
area under the curve
- BMI
body mass index
- NPV
negative predictive value
- OSA
obstructive sleep apnea
- PPV
positive predictive value
- ROC
receiver operating characteristic
- SaO2
hemoglobin oxygen saturation
- SDB
sleep-disordered breathing
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, Gislason T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 2016;47(1):194–202. doi: 10.1183/13993003.01148-2015. [DOI] [PubMed] [Google Scholar]
- 4.Heinzer R, Marti-Soler H, Haba-Rubio J. Prevalence of sleep apnoea syndrome in the middle to old age general population. Lancet Respir Med. 2016;4(2):e5–e6. doi: 10.1016/S2213-2600(16)00006-0. [DOI] [PubMed] [Google Scholar]
- 5.Somers VK, White DP, Abraham WT, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 7.Terán-Santos J, Jiménez-Gómez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340(11):847–851. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 8.Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 9.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 10.Nakano H, Ikeda T, Hayashi M, et al. Effect of body mass index on overnight oximetry for the diagnosis of sleep apnea. Respir Med. 2004;98(5):421–427. doi: 10.1016/j.rmed.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Pataka A, Daskalopoulou E, Kalamaras G, Fekete Passa K, Argyropoulou P. Evaluation of five different questionnaires for assessing sleep apnea syndrome in a sleep clinic. Sleep Med. 2014;15(7):776–781. doi: 10.1016/j.sleep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Bouloukaki I, Kapsimalis F, Mermigkis C, et al. Prediction of obstructive sleep apnea syndrome in a large Greek population. Sleep Breath. 2011;15(4):657–664. doi: 10.1007/s11325-010-0416-6. [DOI] [PubMed] [Google Scholar]
- 13.Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108(5):768–775. doi: 10.1093/bja/aes022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boynton G, Vahabzadeh A, Hammoud S, Ruzicka DL, Chervin RD. Validation of the STOP-BANG Questionnaire among patients referred for suspected obstructive sleep apnea. J Sleep Disord Treat Care. 2013;2(4) doi: 10.4172/2325-9639.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7(2):110–114. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 16.Ravesloot MJL, Frank MH, van Maanen JP, Verhagen EA, de Lange J, de Vries N. Positional OSA part 2: retrospective cohort analysis with a new classification system (APOC) Sleep Breath. 2016;20(2):881–888. doi: 10.1007/s11325-015-1206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112(3):629–639. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 18.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6(3):238–243. [PMC free article] [PubMed] [Google Scholar]
- 19.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115(3):771–781. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 20.Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8):1031–1035. [PubMed] [Google Scholar]
- 21.de Vries GE, Hoekema A, Doff MH, et al. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med. 2015;11(2):131–137. doi: 10.5664/jcsm.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kales A, Rechtschaffen A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: University of California, Los Angeles, Brain Information Service, National Institute of Neurological Diseases; 1968. [Google Scholar]
- 23.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 25.Combs D, Goodwin JL, Quan SF, Morgan WJ, Parthasarathy S. Modified STOP-Bang tool for stratifying obstructive sleep apnea risk in adolescent children. PloS One. 2015;10(11):e0142242. doi: 10.1371/journal.pone.0142242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadi N, Chung SA, Gibbs A, Shapiro CM. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12(1):39–45. doi: 10.1007/s11325-007-0125-y. [DOI] [PubMed] [Google Scholar]
- 27.Soga T, Nakata S, Yasuma F, et al. Upper airway morphology in patients with obstructive sleep apnea syndrome: effects of lateral positioning. Auris Nasus Larynx. 2009;36(3):305–309. doi: 10.1016/j.anl.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Saigusa H, Suzuki M, Higurashi N, Kodera K. Three-dimensional morphological analyses of positional dependence in patients with obstructive sleep apnea syndrome. Anesthesiology. 2009;110(4):885–890. doi: 10.1097/ALN.0b013e31819b5d57. [DOI] [PubMed] [Google Scholar]
- 29.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10(9):2087–2090. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]