Abstract
Objective
To evaluate the association between dry eye symptoms and neuropathic-like ocular pain features, chronic pain conditions, depression, and anxiety in patients presenting for routine ophthalmic examinations.
Methods
233 consecutive patients ≥18 years of age presenting to a comprehensive eye clinic between January and August 2016 were included in this study. Information on demographics, chronic pain conditions, medication use, DE symptoms (dry eye questionnaire, DEQ5), NOP complaints (burning; wind, light, and temperature sensitivity), depression and anxiety indices (patient health questionnaire 9, PHQ9 and symptom checklist 90-revised, SCL-90-R) were collected for each individual. Pearson’s correlation was used to evaluate strengths of association. Logistic regression analysis examined risk factors for any (DEQ5≥6) and severe (DEQ5≥12) DE symptoms.
Results
The mean age of the population was 46.3 years (±13.0); 67.8% (n=158) were female. Per the DEQ5, 40.3% (n=94) had mild or greater DE symptoms and 12% (n=24) had severe symptoms. Severity of DE symptoms correlated with NOP complaints: burning (Pearson r=0.37, p<0.001); sensitivity to wind (r=0.37, p<0.001), sensitivity to light (r=0.34, p<0.001), and sensitivity to temperature (r=0.30, p<0.001). Gender, race, and ethnicity were not significant risk factors for DE symptoms. Risk factors for mild or greater DE symptoms included a greater number of chronic non-ocular pain conditions (odds ratio (OR)= 1.38, p<0.001), arthritic pain (OR=6.34, p<0.001), back pain (OR= 2.47, p= 0.004), headaches (OR= 2.14, p= 0.02), depression (OR= 1.17, p<0.001) and anxiety (OR=1.13, p=0.02).
Conclusion
DE severity positively associated with neuropathic-like ocular pain complaints, comorbid chronic pain conditions, and symptoms of depression and anxiety.
Keywords: Dry Eye, Neuropathic Pain, Chronic Pain
Dry eye (DE) is a significant public health burden with DE symptoms affecting approximately 15% of Americans 50 years or older.1–4 Similar or higher estimates have been found globally.5, 6 Symptoms of DE, such as irritation, burning, foreign body sensation, photophobia, tearing, and blurred vision can interfere with the ability to work and carry out activities of daily living. Thus, DE may significantly decrease a patient’s quality of life.7, 8
DE is a multifactorial diagnosis that is diverse in clinical presentation (e.g. elevated tear osmolarity, ocular surface inflammation, decreased tear production, increased evaporation, and/or corneal and conjunctival staining). To add to the complexity, there is often a discrepancy between clinical signs and symptoms of DE.9 In fact, dry eye symptoms appear to align more closely with factors other than tear film parameters, such as depression, posttraumatic stress disorder, and non-ocular pain measures in studies.10, 11 This discordance hints at factors beyond tear film and ocular surface disturbances that may underlie at least a subset of ocular symptoms that have been broadly categorized as DE.
Neuropathic pain has received increasing recognition as a factor in DE.12, 13 Neuropathic pain is defined as pain caused by a lesion or disease of the somatosensory nervous system.14 Applied to ocular pain, it includes generation of spontaneous pain signals or amplification of noxious or innocuous evoked responses secondary to dysfunction of primary trigeminal afferents (peripheral sensitization) and/or of second and third order neurons (central sensitization). Features of neuropathic pain include spontaneous pain, dysesthesias (unpleasant sensations), and evoked pain (allodynia, hyperalgesia). There is biologic plausibility for the generation of neuropathic pain signaling within the trigeminal system. Terminal nerve endings on the ocular surface may encounter disturbances such as hyperosmolarity, air pollution, and trauma, all of which can cause corneal nerve damage and subsequently alter corneal somatosensory pathway function.14, 15
In support of the contribution of neuropathic pain to DE symptoms, neuropathic-like ocular pain (NOP) symptoms, such as spontaneous burning pain and evoked pain to wind and light, was found to associate with a more severe and chronic DE course16 that responded less favorably to artificial tears in a predominantly male population in studies conducted at a Veterans Affairs eye clinic.17 In the same population, we also found that DE and ocular pain symptoms were more prevalent in patients with comorbid chronic centralized pain conditions18, and this relationship was more robust in patients with NOP complaints.15 Furthermore, these specific metrics were associated with increased corneal19 and cutaneous sensitivity.20 Incomplete responses to therapies targeting peripheral tissues and hypersensitivity to stimuli in areas outside the eye not only imply the presence of central sensitization but also suggest that the differentiation of DE patients by somatosensory status may have significant therapeutic implications. DE has also been found to co-exist with several non-ocular pain conditions and mood disorders.21, 22
A knowledge gap exists, however, on whether these associations remains true in a more generalizable dry eye patient population that is predominantly female.23 We hypothesized that NOP complaints would correlate with DE severity and that eye symptoms would be co-morbid with non-ocular pain conditions in a more typically representative dry eye population presenting for routine ophthalmic care at a tertiary ophthalmic care center.
METHODS
Study Population
All patients over 18 years of age presenting for the first time to a comprehensive eye clinic at Bascom Palmer Eye Institute between January and August 2016 for an initial evaluation were sequentially invited to complete a single intake form. All forms were completed prior to clinical examination. The study was conducted in accordance with the principles of the Declaration of Helsinki, and the University of Miami Institutional Review Board/ Ethics committee approval was obtained to retrospectively review the surveys and to analyze the results.
Data Collected
For each individual, demographics, ocular and non-ocular conditions, medication use, dry eyes symptoms, ocular pain complaints, depression and anxiety indices were collected via standardized questionnaires, using a single intake form. The main outcome measures were the associations between DE and ocular pain symptoms, comorbid non-ocular pain conditions, and symptoms of depression and anxiety.
Dry eye symptoms
Patients completed the dry eye questionnaire 5 (DEQ5) (score 0–22).24 The DEQ5 is a validated, five-item questionnaire that combines patient responses regarding ‘eye discomfort’ (frequency and intensity), ‘eye dryness’ (frequency and intensity), and ‘watery eyes (frequency) during the past month. Higher scores indicate greater severity of symptoms. Mild or greater DE symptoms was defined as a DEQ5≥6.24 Patients with a DEQ5 ≥12 were considered to have severe DE symptoms.
NOP complaints
Based on our prior data,16 NOP complaints were assessed using four questions: (1) presence of spontaneous burning ocular pain, presence of evoked pain, including ocular pain caused or increased by (2) wind, (3) light, or (4) heat/cold with all responses rated on a scale anchored at “0”, no pain sensation, to “10”, the worst pain imaginable. These descriptors were developed through criterion validation and found to be associated with symptoms suggestive of neuropathic ocular pain (i.e. more chronic course, decreased response to topical therapy, and association with local and systemic somatosensory alterations)16, 17, 19, 20, 25
Non-ocular pain conditions
Patients were asked about the presence of chronic non-ocular pain conditions (>3 months in duration). We used the classification proposed by Yunus26 to divide chronic pain conditions into those not known to have an underlying structural basis (e.g. headaches, trigeminal neuralgia, temporomandibular joint pain, fibromyalgia, migraines, tendonitis, abdominal pain, pelvic pain, back pain, muscle pain, and central pain syndrome), and those classically associated with structural abnormalities (e.g. arthritis, chronic postsurgical pain, diabetic neuropathy, sciatica, burn pain, post-herpetic neuralgia, and cancer associated pain).
Non-ocular pain severity
Concurrent non-ocular pain was assessed using a numerical rating scale questionnaire (“how would you describe the overall intensity of your pain, on average, during the last week?” and “how would you describe the overall intensity of your pain, at its worse, during the last week”?). Responses were rated on a scale anchored at “0”, for no pain sensation, to “10”, the worst pain imaginable.
Mental Health Indices
Symptoms of depression were assessed via the patient health questionnaire 9 (PHQ-9), a questionnaire with a score ranging from 0 (no depressive symptoms) to 27 (the most severe symptoms).27, 28 The PHQ-9 was used to assess for patients’ mood, physical symptoms, and cognitive disturbances consistent with depression. The Symptom Checklist 90-revised (SCL-90R) is a well-validated, widely applied assessment tool for a broad range of mental disorders.29 The anxiety dimension of the SCL-90R, consisting of 10 items, was used to assess for symptoms of anxiety. Scores ranged from 0, indicating no anxiety symptoms to 4, indicating maximal symptoms.
Statistical Analysis
All statistical analyses were performed using SPSS statistical package version 22 (SPSS, Chicago, Illinois, USA). Descriptive statistics were used to summarize patient demographic and clinical information. Fisher exact, Chi square, and Student independent t test were used, as appropriate, to compare variables of interest in patients with mild and severe DE symptoms. Pearson’s correlation was used to evaluate the strengths of association between measures. Logistic regression analyses examined risk factors for any (DEQ5≥6) and severe (DEQ5≥12) DE symptoms. A P value <0.05 was considered statistically significant.
RESULTS
Study population
A total of 244 patients presented to the comprehensive clinic for an initial evaluation between January and August 2016. Of those, 233 patients (95.5%) completed the questionnaire. The mean age of the population was 46.3 years (range, 19–77 years) (Figure 1), with a standard deviation (SD) of 13 years; 67.8% (n=158) of the patients were female, 75.5% (n= 176) self-identified as white, and 51.5% (n=120) self-identified as Hispanic. The large majority of patients, 89.3% (n=208), were non-smokers. The most common non-ocular conditions reported were allergies (27.9%, n= 65), followed by hypertension (17.5%, n=41) and arthritis (11.6%, n=27).
Figure 1.
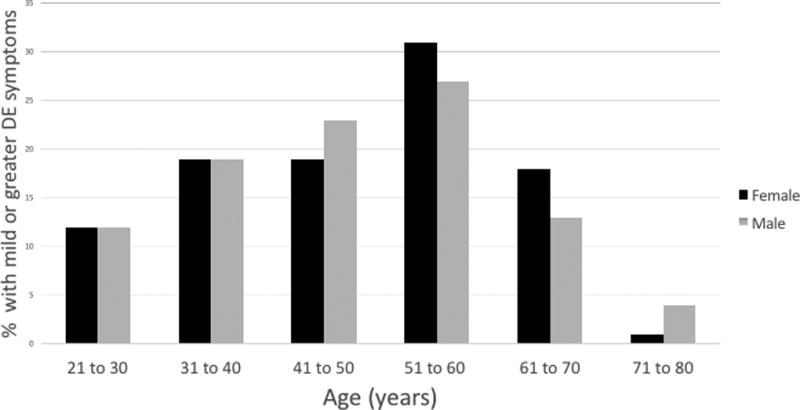
Percentage of patients with mild or greater dry eye (DE) symptoms (dry eye questionnaire 5 (DEQ5) ≥ 6) in a comprehensive eye clinic by gender and age.
Dry eye symptoms and neuropathic-like ocular pain
Regarding DE symptoms, 25.8% (n=60) of patients complained of DE symptoms as a chief complaint on presentation. According to the DEQ5, 40.3% (n=94) of our population had mild or greater DE symptoms and 10.3% (n=24) had severe symptoms. Regarding NOP, 11.2% (n=26) reported mild ocular burning pain (NRS 1 – 3), 2.1% (n=5) reported moderate burning pain (NRS 4 – 6), and 0.4% (n=1) severe burning pain (NRS 7 – 10). In a similar manner, 9.4% (n=22) reported mild sensitivity to wind (NRS 1 – 3) and 3.0% (n=7) reported moderate sensitivity (NRS 4 – 6). Similar proportions were found for sensitivity to light, with 12.4% (n=29) and 4.3% (n=10) having mild and moderate sensitivity, respectively; 10.3% (n=24) and 2.1% (n=5) reported mild and moderate ocular sensitivity to a change in temperature. All features of NOP were positively and significantly associated with DE symptoms (via DEQ5) (r=0.37 for burning (Figure 2); r=0.37 for sensitivity to wind; r=0.34 for sensitivity to light, r=0.30 for sensitivity to temperature (p<0.001).
Figure 2.
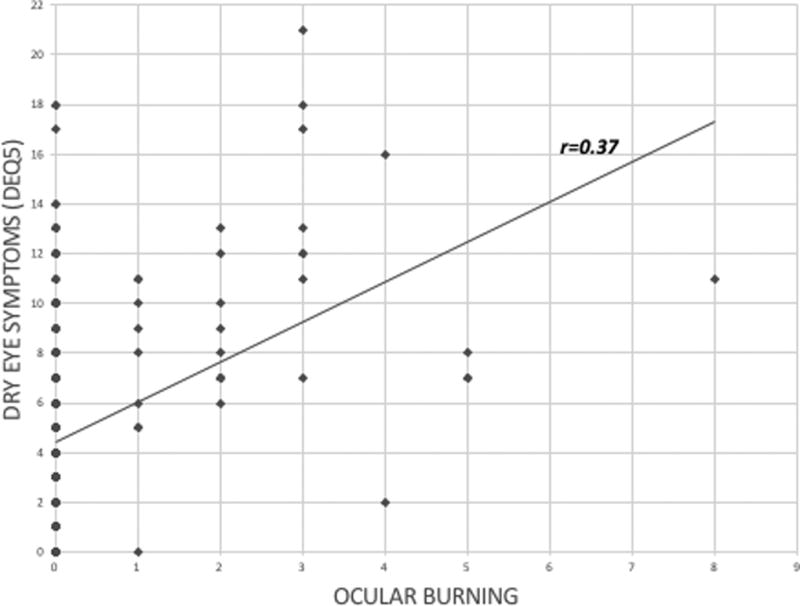
Correlation between dry eye symptoms (dry eye questionnaire 5, DEQ5) and ocular burning. (r= Pearson’s correlation)
Ocular and non-ocular association with DE symptoms
Mild and severe DE symptoms were both significantly associated with allergic conjunctivitis as shown in Tables 1 and 2. Mild DE symptoms (DEQ5≥6) were associated with soft contact lens wear (Table 1) while severe DE symptoms (DEQ5≥12) were associated with diabetes (Table 2). Medications did not confer an increased risk of DE symptoms with the exception of antihistamines.
Table 1.
Patient demographics and comorbidities by dry eye (DE) status (mild or greater DE symptoms, DEQ5≥6, versus no symptoms,DEQ5<6)
| No DE symptoms (n=139) |
Mild or greater DE symptoms (n=94) |
OR* | 95% CI | p value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean±SD | 45.2±12.8 | 48.0±13.3 | 1.02 | 0.97–1.04 | 0.11 |
| Gender, female, n (%) | 90 (65%) | 68 (72%) | 1.42 | 0.80–2.52 | 0.23 |
| Race | |||||
| White, n (%) | 101 (73%) | 75 (80%) | 0.83 | 0.42–1.65 | 0.59 |
| Black, n (%) | 26 (18%) | 16 (17%) | 0.34 | 0.42–1.65 | 0.10 |
| Ethnicity, Hispanic, n (%) | 70 (50%) | 50 (53%) | 0.89 | 0.53–1.51 | 0.67 |
| Comorbidities | |||||
| Diabetes, n (%) | 7 (5%) | 7 (7%) | 1.52 | 0.51–4.48 | 0.45 |
| Hypertension, n (%) | 21 (15%) | 20 (21%) | 1.52 | 0.77–2.99 | 0.23 |
| Sleep apnea, n (%) | 13 (9%) | 12 (13%) | 1.42 | 0.62–3.26 | 0.41 |
| Non-ocular allergies, n (%) | 33 (24%) | 32 (34%) | 1.66 | 0.93–2.96 | 0.09 |
| Allergic conjunctivitis, n (%) | 8 (6%) | 15 (16%) | 3.11 | 1.26–7.67 | 0.01 |
| Thyroid disease, n (%) | 8 (6%) | 9 (10%) | 1.73 | 0.64–4.67 | 0.28 |
| Soft contact lens use, n (%) | 20 (14%) | 24 (26%) | 2.04 | 1.05–3.96 | 0.04 |
| Rosacea, n (%) | 6 (4.3%) | 1 (1%) | 0.24 | 0.03–2.01 | 0.19 |
| Refractive surgery, n (%) | 4 (3%) | 6 (6%) | 2.30 | 0.63–8.39 | 0.21 |
| Glaucoma medications, n (%) | 3 (2%) | 1 (1%) | 0.49 | 0.05–4.76 | 0.54 |
| Pterygium, n (%) | 0 (0%) | 1 (1%) | -- | -- | -- |
| Smoker, n (%) | 0 (0%) | 4 (4%) | -- | -- | -- |
| Medications | |||||
| Anxiolytic, n (%) | 8 (6%) | 9 (10%) | 1.73 | 0.64–4.67 | 0.28 |
| Antidepressant, n (%) | 7 (5%) | 8 (9%) | 1.75 | 0.61–5.01 | 0.29 |
| Antihistamine, n (%) | 14 (10%) | 16 (17%) | 1.83 | 0.85–3.96 | 0.12 |
| Analgesics, n (%) | 14 (10%) | 15 (16%) | 1.70 | 0.78–3.70 | 0.19 |
DEQ5, dry eye questionnaire 5; n, number in each group; SD, standard deviation; OR, odds ratio; CI, confidence interval
A odds ratio greater than 1 represents an increased likelihood of association; -- refers to an odds ratio with unstable estimates
Table 2.
Patient demographics and comorbidities by dry eye (DE) status (severe DE symptoms, DEQ5≥12, versus no, mild or moderate symptoms,DEQ5<12)
| No severe DE symptoms (n=209) |
Severe DE symptoms (n=24) |
OR* | 95% CI | p Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean±SD | 45.76±13.12 | 51.34±11.30 | 1.04 | 1.00–1.07 | 0.05 |
| Gender, female, n (%) | 139 (67%) | 19 (79%) | 1.91 | 0.69–5.34 | 0.22 |
| Race | |||||
| White, n (%) | 155 (74%) | 21 (88%) | 0.57 | 0.16–2.00 | 0.57 |
| Black, n (%) | 39 (19%) | 3 (13%) | 0.00 | 0.00-NA | 1.00 |
| Ethnicity, Hispanic, n (%) | 108 (52%) | 12 (50%) | 1.07 | 0.46–2.59 | 0.88 |
| Comorbidities | |||||
| Diabetes, n (%) | 10 (5%) | 4 (17%) | 3.98 | 1.14–13.86 | 0.03 |
| Hypertension, n (%) | 36 (17%) | 5 (21%) | 1.27 | 0.44–3.61 | 0.66 |
| Sleep apnea, n (%) | 20 (10%) | 5 (21%) | 2.49 | 0.84–7.38 | 0.10 |
| Non-ocular allergies, n (%) | 53 (25%) | 12 (50%) | 2.94 | 1.25–6.95 | 0.01 |
| Allergic conjunctivitis, n (%) | 17 (8%) | 6 (25%) | 3.77 | 1.32–10.74 | 0.01 |
| Thyroid disease, n (%) | 14 (7%) | 3 (13%) | 1.99 | 0.53–7.49 | 0.31 |
| Soft contact lens use, n (%) | 37 (17%) | 7 (29%) | 1.91 | 0.74–4.95 | 0.18 |
| Rosacea, n (%) | 7 (3%) | 0 (0%) | 0.00 | -- | 1.00 |
| Refractive surgery, n (%) | 8 (4%) | 2 (8%) | 2.28 | 0.46–11.44 | 0.32 |
| Glaucoma medications, n (%) | 4 (2%) | 0 (0%) | 0.00 | -- | 1.00 |
| Pterygium, n (%) | 1 (1%) | 0 (0%) | 0.00 | -- | 1.00 |
| Smoker, n (%) | 4 (2%) | 0 (0%) | -- | -- | 1.00 |
| Medications | |||||
| Anxiolytic, n (%) | 15 (7%) | 2 (8%) | 1.17 | 0.25–5.48 | 0.84 |
| Antidepressant, n (%) | 13 (6%) | 2 (8%) | 1.37 | 0.29–6.47 | 0.69 |
| Antihistamine, n (%) | 23 (11%) | 7 (29%) | 3.33 | 1.25–8.88 | 0.02 |
| Analgesics, n (%) | 26 (12%) | 3 (13%) | 1.01 | 0.28–3.61 | 0.99 |
DEQ5, dry eye questionnaire 5; n, number in each group; SD, standard deviation; OR, odds ratio; CI, confidence interval
A odds ratio greater than 1 represents an increased likelihood of association; -- refers to an odds ratio with unstable estimates
DE symptoms correlated with pain complaints elsewhere in the body; r=0.35 for intensity of non-ocular pain averaged over the past week, r=0.31 for number of chronic pain conditions; r=0.23 for number of chronic pain locations (p<0.001). DE symptoms also correlated with depression (r=0.34, p<0.001) (Figure 3) and anxiety scores (r= 0.21, p= 0.001).
Figure 3.
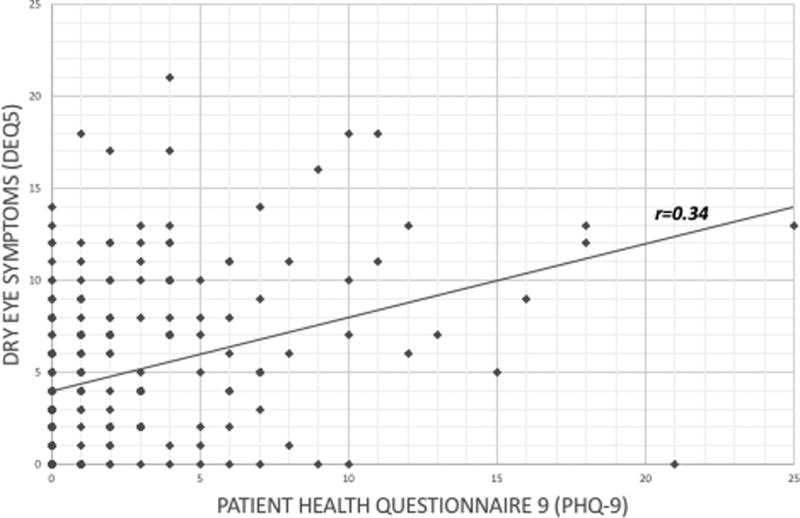
Correlation between dry eye symptoms (dry eye questionnaire 5, DEQ5) and depression (patient health questionnaire 9, PHQ-9). (r= Pearson’s correlation)
Similar to our findings in the VA population, mild and severe DE symptoms were co-morbid with chronic pain conditions, including back pain, muscle pain, headaches, and arthritic pain (Table 3; data for severe DE symptoms versus none, mild or moderate symptoms is not shown).
Table 3.
Non-ocular pain conditions by dry eye (DE) status (mild or greater DE symptoms, DEQ5≥6, versus no symptoms,DEQ5<6)
| No DE symptoms (n=139) |
Mild or greater DE symptoms (n=94) |
OR | CI | p Value | |
|---|---|---|---|---|---|
| Primary central sensitization* | |||||
| Back pain, n (%) | 24 (17%) | 32 (34%) | 2.47 | 1.34–4.56 | <0.001 |
| Muscle pain, n (%) | 10 (7%) | 25 (27%) | 4.63 | 2.10–10.22 | <0.001 |
| Headaches, n (%) | 25 (18%) | 30 (32%) | 2.14 | 1.16–3.95 | 0.02 |
| Tendonitis, n (%) | 7 (5%) | 5 (5%) | 1.06 | 0.33–3.44 | 0.92 |
| Trigeminal neuralgia, n (%) | 0 (0%) | 1 (1%) | -- | -- | 1.00 |
| TMJ pain, n (%) | 0 (0%) | 6 (6%) | -- | -- | 1.00 |
| Fibromyalgia, n (%) | 1 (1%) | 1 (1%) | 1.48 | 0.09–24.02 | 0.78 |
| Migraines, n (%) | 18 (13%) | 19 (20%) | 1.70 | 0.84–3.45 | 0.14 |
| Secondary central sensitization* | |||||
| Arthritis, n (%) | 5 (4%) | 18 (19%) | 6.34 | 2.27–17.78 | <0.001 |
| Chronic postsurgical pain, n (%) | 5 (4%) | 6 (6%) | 1.83 | 0.54–6.17 | 0.33 |
| Diabetic neuropathy, n (%) | 1 (1%) | 1 (1%) | 1.48 | 0.09–24.02 | 0.78 |
| Sciatica, n (%) | 8 (6%) | 5 (5%) | 0.92 | 0.29–2.90 | 0.89 |
| Burn pain, n (%) | 1 (1%) | 3 (3%) | 4.52 | 0.46–44.10 | 0.20 |
OR=odds ratio; CI=confidence interval; DEQ5, dry eye questionnaire 5; TMJ, temporomandibular joint pain
Classification from Yunus MB. Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. CurrRheumatol Rev 2015;11:70–85.
Multivariable regression analyses
To test the robustness of the relationship between DE symptoms and non-ocular pain, we performed forward stepwise linear regression analyses that controlled for variables significant in the univariable models: systemic co-morbidities (arthritis, ocular and non-ocular allergies, contact lens wear, diabetes, muscle pain, headache, back pain), medication (antihistamines), and depression and anxiety indices. When controlling for these covariates, depression score (OR 1.2, 95% confidence interval (CI) 1.1–1.3), eye allergies (OR 3.1, 95% CI 1.1–8.5), arthritis (OR 5.4, 95% CI 1.6–18.2), muscle pain (OR=2.9, 95% CI 1.1–7.1), and contact lens wear (OR 2.6, 95% CI 1.3–5.5) remained significantly correlated with mild or greater DE symptoms. Depression score (OR 1.2, 95% CI 1.1–1.3) and allergies not involving the eye (OR 3.1, 95% CI 1.2–7.8) remained significantly correlated with severe DE symptoms.
DISCUSSION
To summarize, consistent with our previous study consisting of older (mean age of 65 years), predominantly male (90%) veteran population, DE symptoms correlated with NOP complaints, non-ocular pain conditions, depression and anxiety in a mostly middle-aged, female population.11 In a similar manner, DE diagnosis and symptoms also significantly associated with chronic pain conditions and depression in a British cohort of female twins.22 The replication of these correlations in a novel population strengthens the connection between DE symptoms, neuropathic-like ocular pain, and non-ocular pain conditions.
While associations between depression, anxiety, post-traumatic stress disorder (PTSD) and DE have been studied,21, 30, 31 less information is available on the link between DE and non-ocular pain conditions.32, 33 The latter association, however, is not surprising given the emerging concept that pain does not exist in isolation and that patients tend to have more than one chronic pain conditions, a term known as chronic overlapping pain conditions (COPC).34 Interestingly, we did not find an association between DE symptoms and the use of antidepressants or anxiolytics. However, patients reporting symptoms of depression and anxiety may not be on treatment, and conversely, patients who are on adequate treatment may not have significant mood symptoms.
Our current data further supports the postulation that in some cases, DE symptoms may be one manifestation of a COPC, with central sensitization as a potential unifying factor tying in multiple pain conditions. Central sensitization, a mechanism of neuroplasticity, describes the physiological phenomenon in which prolonged, intense input of pain signals into the dorsal horns of the spinal cord,35 the spinal trigeminal subnucleus caudalis in the medulla,36 or higher centers,37, 38 alters neuronal phenotype resulting in amplified synaptic transmission of pain signaling. This contributes to generation of spontaneous pain signaling, and/or enhanced responsiveness to painful stimuli with sensory gain amplification that clinically manifests as hyperalgesia, allodynia.39
In this study, ocular allergies and contact lens wear were associated with DE symptoms. Ongoing ocular surface damage such as from allergens or microtrauma from contact lens use may lead to inflammation, tear film thinning (evaporation or dewetting), and resultant hyperosmolarity;40 which, in turn, can activate and sensitize nociceptive fibers that signal pain in the cornea. Corneal pain fibers project to the trigeminal subnucleus caudalis33 and then, through multiple synaptic connections, relay information to brain areas that process the sensation of pain, such as various subcortical centers (limbic system, anterior cingulate cortex, amygdala, parabrachial nuclei, etc.) and the somatosensory cortex. Central sensitization leads to increased excitability of neurons in the dorsal horns, trigeminal nuclei, and higher brain centers,41 amplifying the sensory perception of pain or resulting in a variety of unpleasant sensations, including spontaneous pain. Due to the convergence of various afferent nociceptive pathways onto the same pain processing centers (i.e. trigeminal, glossopharyngeal and cervical pathways in the medulla and higher cervical cord, and pain signaling from the trigeminal and somatic afferents at more proximal centers), the excitability and gain increase in these centers may be a shared factor explaining the presence of various coexistent chronic pain conditions in the setting of central sensitization. Furthermore, descending inhibitory pain pathways modulate somatosensory processing, and if compromised (as is the case in several chronic neuropathic states), further amplification and distortion of pain signals can occur.13 Finally, it should be noted that inflammatory cytokines and neuroinflammation play a role both in the pathogenesis of neuropathic pain and mental illnesses such as depression.42–44 Our findings suggest that similar to other COPC, patients with DE may have a predisposition to chronic pain that may be explained by central sensitization, neuroinflammation, and maladaptive neuroplasticity.
Both genetic susceptibility and environmental factors are thought to play pivotal roles in dictating phenotypic manifestation of ocular pain. For example, genetic polymorphisms in a number of targets including ion channels, hormone receptors,45 and cytokines33 have been identified, and haplotypes of catecholamine-O-methyltransferase (COMT) have been associated with chronic pain.46 In the setting of DE, Vehof et al. demonstrated a heritability of 29% for DE symptoms and 40% for the clinical diagnosis of DED in a British female twin cohort study.47 As found with non-ocular pain,48–50 three proinflammatory gene polymorphisms have been associated with DE symptoms in Korean non-Sjögren patients.51 This propensity for inflammation may partially explain the link between ocular surface inflammatory cytokines (interleukins, tumor necrosis factor-α, and matrix metalloproteinase 9) and DE.52–54 Propensity for similar proinflammatory mechanisms and aberrant plasticity in the brain, as stated above, may also partly explain affective disorders (depression, anxiety) in the same populations.42, 43, 55
As in all studies, our findings must be considered bearing in mind the study limitations. First, South Florida has a unique population, with a large proportion of Hispanics compared to other US cities. Second, we focused on DE symptoms and objective findings were not recorded since studies have shown a dissociation between signs and symptoms of DE.1 Furthermore, previous studies have demonstrated that dry eye symptoms correlate more closely with non-ocular comorbidities than tear film parameters, such as TBUT or corneal fluorescein staining.10, 11 Third, DE symptoms have many potential contributors and it was not possible to capture all confounders (e.g. diet, environmental factors) in one intake form. Fourth, each subject in this study was surveyed once on a single day. Subjective responses can vary day to day, and the retest reliability is unknown. Despite these limitations, this study expands our previous work to a novel population and demonstrates that the link between DE symptoms and pain elsewhere in the body is robust. This suggests that we need to redefine “dry eye” and separate those who have ocular surface dryness from those whose DE symptoms are driven by somatosensory dysfunction such as neuropathy.
This separation has implications for DE treatment. Currently, DE therapies focus on improving various components of tear function, such as meibum (e.g. with oral antibiotics), inflammation (e.g. with topical anti-inflammatories), and anatomy (e.g. with conjunctivoplasty). In patients in whom DE symptoms co-exist with non-ocular pain conditions, treatments that focus on attenuating the excitability of the somatosensory function may be needed, especially in those with persistent DE symptoms despite adequate topical therapy. Gabapentin and pregabalin have been used in some cases to treat ocular pain, most often in the setting of post refractive surgery pain.56 However, recent reports have noted that treatments that are used to treat chronic non-ocular pain may be applied to ocular pain.57 This includes, for example, neurostimulation and/or intrathecal infusion of fentanyl and bupivacaine in a patient with intractable corneal pain after refractive surgery. More research is needed, however, to identify patients with NOP and optimize treatment of both ocular and associated non-ocular conditions.
Acknowledgments
Funding: Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006-15S (Dr. Galor), R01EY026174 (Dr. Galor), NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, The Ronald and Alicia Lepke Grant, The Lee and Claire Hager Grant, The Jimmy and Gaye Bryan Grant, The H. Scott Huizenga Grant, The Robert Baer Family Grant, The Gordon Charitable Foundation and The Richard Azar Family Grant (institutional grants).
Footnotes
Conflict of Interest: No conflicting relationship exists for any author.
Bibliography
- 1.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 2.Schaumberg DA, Dana R, Buring JE, et al. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch Ophthalmol. 2009;127(6):763–8. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaumberg DA, Sullivan DA, Buring JE, et al. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–26. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 4.Schein OD, Munoz B, Tielsch JM, et al. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124(6):723–8. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 5.Jie Y, Xu L, Wu YY, et al. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye (Lond) 2009;23(3):688–93. doi: 10.1038/sj.eye.6703101. [DOI] [PubMed] [Google Scholar]
- 6.Lin PY, Tsai SY, Cheng CY, et al. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2003;110(6):1096–101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 7.Pouyeh B, Viteri E, Feuer W, et al. Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. Am J Ophthalmol. 2012;153(6):1061–66. e3. doi: 10.1016/j.ajo.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412–9. doi: 10.1016/S0161-6420(03)00462-7. [DOI] [PubMed] [Google Scholar]
- 9.Galor A, Feuer W, Lee DJ, et al. Ocular surface parameters in older male veterans. Invest Ophthalmol Vis Sci. 2013;54(2):1426–33. doi: 10.1167/iovs.12-10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satitpitakul V, Kheirkhah A, Crnej A, et al. Determinants of Ocular Pain Severity in Patients With Dry Eye Disease. Am J Ophthalmol. 2017;179:198–204. doi: 10.1016/j.ajo.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Galor A, Felix ER, Feuer W, et al. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol. 2015;99(8):1126–9. doi: 10.1136/bjophthalmol-2014-306481. [DOI] [PubMed] [Google Scholar]
- 12.Kalangara JP, Galor A, Levitt RC, et al. Burning Eye Syndrome: Do Neuropathic Pain Mechanisms Underlie Chronic Dry Eye? Pain Med. 2016;17(4):746–55. doi: 10.1093/pm/pnv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128–34. doi: 10.1136/bjophthalmol-2014-306280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crane AM, Levitt RC, Felix ER, et al. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2015-308214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galor A, Zlotcavitch L, Walter SD, et al. Dry eye symptom severity and persistence are associated with symptoms of neuropathic pain. Br J Ophthalmol. 2015;99(5):665–8. doi: 10.1136/bjophthalmol-2014-306057. [DOI] [PubMed] [Google Scholar]
- 17.Galor A, Batawi H, Felix ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol. 2016;100(6):745–9. doi: 10.1136/bjophthalmol-2015-307094. [DOI] [PubMed] [Google Scholar]
- 18.Crane AM, Levitt RC, Felix ER, et al. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol. 2017;101(2):227–31. doi: 10.1136/bjophthalmol-2015-308214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spierer O, Felix ER, McClellan AL, et al. Corneal Mechanical Thresholds Negatively Associate With Dry Eye and Ocular Pain Symptoms. Invest Ophthalmol Vis Sci. 2016;57(2):617–25. doi: 10.1167/iovs.15-18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galor A, Levitt RC, McManus KT, et al. Assessment of Somatosensory Function in Patients With Idiopathic Dry Eye Symptoms. JAMA Ophthalmol. 2016;134(11):1290–8. doi: 10.1001/jamaophthalmol.2016.3642. [DOI] [PubMed] [Google Scholar]
- 21.Galor A, Feuer W, Lee DJ, et al. Depression, post-traumatic stress disorder, and dry eye syndrome: a study utilizing the national United States Veterans Affairs administrative database. Am J Ophthalmol. 2012;154(2):340–6. e2. doi: 10.1016/j.ajo.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Vehof J, Kozareva D, Hysi PG, et al. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol. 2014;98(12):1712–7. doi: 10.1136/bjophthalmol-2014-305201. [DOI] [PubMed] [Google Scholar]
- 23.Farrand KF, Fridman M, Stillman IO, et al. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol. 2017 doi: 10.1016/j.ajo.2017.06.033. In press. [DOI] [PubMed] [Google Scholar]
- 24.Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33(2):55–60. doi: 10.1016/j.clae.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Crane AM, Feuer W, Felix ER, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol. 2017;101(9):1238–1243. doi: 10.1136/bjophthalmol-2016-309658. [DOI] [PubMed] [Google Scholar]
- 26.Yunus MB. Editorial review: an update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev. 2015;11(2):70–85. doi: 10.2174/157339711102150702112236. [DOI] [PubMed] [Google Scholar]
- 27.Sidebottom AC, Harrison PA, Godecker A, et al. Validation of the Patient Health Questionnaire (PHQ)-9 for prenatal depression screening. Arch Womens Ment Health. 2012;15(5):367–74. doi: 10.1007/s00737-012-0295-x. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prinz U, Nutzinger DO, Schulz H, et al. Comparative psychometric analyses of the SCL-90-R and its short versions in patients with affective disorders. BMC Psychiatry. 2013;13:104. doi: 10.1186/1471-244X-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim KW, Han SB, Han ER, et al. Association between depression and dry eye disease in an elderly population. Invest Ophthalmol Vis Sci. 2011;52(11):7954–8. doi: 10.1167/iovs.11-8050. [DOI] [PubMed] [Google Scholar]
- 31.Na KS, Han K, Park YG, et al. Depression, Stress, Quality of Life, and Dry Eye Disease in Korean Women: A Population-Based Study. Cornea. 2015;34(7):733–8. doi: 10.1097/ICO.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 32.Vehof J, Sillevis Smitt-Kamminga N, Kozareva D, et al. Clinical Characteristics of Dry Eye Patients With Chronic Pain Syndromes. Am J Ophthalmol. 2016;166:203–4. doi: 10.1016/j.ajo.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Galor A, Covington D, Levitt AE, et al. Neuropathic Ocular Pain due to Dry Eye is Associated with Multiple Comorbid Chronic Pain Syndromes. J Pain. 2016;17(3):310–8. doi: 10.1016/j.jpain.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maixner W, Fillingim RB, Williams DA, et al. Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. J Pain. 2016;17(9 Suppl):T93–T107. doi: 10.1016/j.jpain.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–8. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 36.Sessle BJ. Peripheral and central mechanisms of orofacial pain and their clinical correlates. Minerva Anestesiol. 2005;71(4):117–36. [PubMed] [Google Scholar]
- 37.Noseda R, Constandil L, Bourgeais L, et al. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci. 2010;30(43):14420–9. doi: 10.1523/JNEUROSCI.3025-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci. 2006;26(16):4426–36. doi: 10.1523/JNEUROSCI.5298-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol. 2012;32(1):68–81. doi: 10.1097/WNO.0b013e3182474548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nichols JJ, Sinnott LT. Tear film, contact lens, and patient-related factors associated with contact lens-related dry eye. Invest Ophthalmol Vis Sci. 2006;47(4):1319–28. doi: 10.1167/iovs.05-1392. [DOI] [PubMed] [Google Scholar]
- 41.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lees JG, Fivelman B, Duffy SS, et al. Cytokines in Neuropathic Pain and Associated Depression. Mod Trends Pharmacopsychiatri. 2015;30:51–66. doi: 10.1159/000435932. [DOI] [PubMed] [Google Scholar]
- 43.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rial D, Lemos C, Pinheiro H, et al. Depression as a Glial-Based Synaptic Dysfunction. Front Cell Neurosci. 2015;9:521. doi: 10.3389/fncel.2015.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mogil JS, Wilson SG, Chesler EJ, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003;100(8):4867–72. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14(1):135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 47.Vehof J, Wang B, Kozareva D, et al. The heritability of dry eye disease in a female twin cohort. Invest Ophthalmol Vis Sci. 2014;55(11):7278–83. doi: 10.1167/iovs.14-15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omair A, Mannion AF, Holden M, et al. Age and pro-inflammatory gene polymorphisms influence adjacent segment disc degeneration more than fusion does in patients treated for chronic low back pain. Eur Spine J. 2016;25(1):2–13. doi: 10.1007/s00586-015-4181-x. [DOI] [PubMed] [Google Scholar]
- 49.Milosevic N, Nikolic N, Djordjevic I, et al. Association of Functional Polymorphisms in Matrix Metalloproteinase-9 and Glutathione S-Transferase T1 Genes with Temporomandibular Disorders. J Oral Facial Pain Headache. 2015;29(3):279–85. doi: 10.11607/ofph.1343. [DOI] [PubMed] [Google Scholar]
- 50.Kovacs D, Eszlari N, Petschner P, et al. Interleukin-6 promoter polymorphism interacts with pain and life stress influencing depression phenotypes. J Neural Transm (Vienna) 2016;123(5):541–8. doi: 10.1007/s00702-016-1506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Na KS, Mok JW, Kim JY, et al. Proinflammatory gene polymorphisms are potentially associated with Korean non-Sjogren dry eye patients. Mol Vis. 2011;17:2818–23. [PMC free article] [PubMed] [Google Scholar]
- 52.Lee SY, Han SJ, Nam SM, et al. Analysis of tear cytokines and clinical correlations in Sjogren syndrome dry eye patients and non-Sjogren syndrome dry eye patients. Am J Ophthalmol. 2013;156(2):247–53. e1. doi: 10.1016/j.ajo.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Massingale ML, Li X, Vallabhajosyula M, et al. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28(9):1023–7. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 54.Sambursky R, Davitt WF, Latkany R, et al. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013;131(1):24–8. doi: 10.1001/jamaophthalmol.2013.561. [DOI] [PubMed] [Google Scholar]
- 55.Fiore NT, Austin PJ. Are the emergence of affective disturbances in neuropathic pain states contingent on supraspinal neuroinflammation? Brain Behav Immun. 2016;56:397–411. doi: 10.1016/j.bbi.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Lichtinger A, Purcell TL, Schanzlin DJ, et al. Gabapentin for postoperative pain after photorefractive keratectomy: a prospective, randomized, double-blind, placebo-controlled trial. J Refract Surg. 2011;27(8):613–7. doi: 10.3928/1081597X-20110210-01. [DOI] [PubMed] [Google Scholar]
- 57.Hayek SM, Sweet JA, Miller JP, et al. Successful Management of Corneal Neuropathic Pain with Intrathecal Targeted Drug Delivery. Pain Med. 2016;17(7):1302–7. doi: 10.1093/pm/pnv058. [DOI] [PubMed] [Google Scholar]


