Abstract
Under current guidelines hepatitis C virus (HCV)-positive livers are not transplanted into HCV-negative recipients because of adverse post-transplant outcomes associated with allograft HCV infection. However, HCV can now be cured post liver transplant (LT) using direct-acting antivirals (DAAs) with >90% success; therefore, HCV-negative patients on the liver transplant (LT) waiting list may benefit from accepting HCV-positive organs with preemptive treatment. Our objective was to evaluate if and in which HCV-negative patients the potential benefit of accepting an HCV-positive (i.e., viremic) organ outweighed the risks associated with HCV allograft infection. We developed a Markov-based mathematical model that simulated a virtual trial of HCV-negative patients on the LT waiting list to compare long-term outcomes in patients: 1) willing to accept any (HCV-negative or HCV-positive) liver versus 2) those willing to accept only HCV-negative livers. Patients receiving HCV-positive livers were treated preemptively with 12 weeks of DAA therapy and had a higher risk of graft failure than those receiving HCV-negative livers. The model incorporated data from published studies and the United Network for Organ Sharing (UNOS). We found that accepting any liver regardless of HCV status versus accepting only HCV-negative livers resulted in an increase in life expectancy when MELD was ≥ 20, and the benefit was highest at MELD 28 (0.172 additional life years). The magnitude of clinical benefit was greater in UNOS regions with higher HCV-positive donor organ rates, i.e. Regions 1, 2, 3, 10, and 11. Sensitivity analysis demonstrated that model outcomes were robust.
Conclusions
Transplanting HCV-positive livers into HCV-negative patients with preemptive DAA therapy could improve patient survival on the LT waiting list. Our analysis can help inform clinical trials and minimize patient harm.
Keywords: Sustained virologic response (SVR), Markov model, Direct-acting antivirals (DAAs), Allograft infection, United Network for Organ Sharing (UNOS)
INTRODUCTION
In the United States the prevalence of patients with complications of cirrhosis in need of transplantation has continued to rise, while the number of annual liver transplants performed has remained unchanged over the last decade (1). Donor liver availability continues to be the limiting factor. As this definite shortage of transplant viable organs in the United States persists, it is of paramount importance that all transplantable organs are utilized to their maximum potential (2).
Hepatitis C virus (HCV)-positive organs have been used successfully for liver transplantation into HCV-positive recipients for years without an increase in all-cause graft loss or mortality (3, 4). An advantage of using HCV-positive livers is a potential decrease in wait time and waitlist mortality. A disadvantage of using HCV-positive livers is the possibility of accelerated decompensation in those patients who are unable to complete HCV therapy and achieve sustained virologic response (SVR) following transplantation (5–7). Historically, with the use of pegylated interferon (IFN)-based HCV treatment regimens, SVR rates were low (in the 20–30% range), in large measure the result of significant side effects leading to high discontinuation rates (8, 9). With the advent of well tolerated, oral direct-acting antivirals (DAAs), HCV can now be treated with high success rates (SVR greater than 90%) post liver transplant (LT), which is associated with fibrosis stabilization or regression and improved graft survival (10, 11). As a result, we are now beginning to see an increase in the utilization of HCV-positive donor organs in the era of DAA therapy (3).
The supply of HCV-positive organs has increased in the recent years because of the rise of opioid epidemic; persons who inject drugs are now the fastest-growing category of donor (2). Despite this, it is not uncommon for HCV-positive donor livers to be rejected for transplantation and subsequently discarded; this can happen for a variety of reasons, including the lack of an HCV-positive recipient on the waiting list. Current guidelines do not recommend the use of these HCV-positive livers for transplantation into HCV-negative recipients. One of the primary reasons continues to be the risk associated with liver allograft HCV infection, including the possible increased rates of rapidly progressive HCV related disease. However, while there are limited data on the use of DAAs in this context, it is postulated that they would continue to demonstrate excellent efficacy and have the potential to play an important role in curtailing the current discard rates for HCV-positive donor livers (12, 13). Several ongoing studies are evaluating DAA use in transplantation (14, 15), and future recommendations may involve more routine consideration of HCV-nucleic acid positive donor organs into HCV-negative recipients with preemptive antiviral treatment with DAAs (2).
As the burden on the LT waiting list shifts from HCV to nonalcoholic steatohepatitis and alcoholic liver disease (16–18), it has become even more important to consider such changes in transplant practice. A number of these HCV-negative patients on the liver transplant waiting list may benefit from accepting HCV-positive donor organs and receiving treatment with DAAs. Particularly, accepting HCV-positive organs could reduce patients’ time to transplant and waitlist associated mortality; however, it could also increase potential post-LT complications associated with HCV allograft infection. Approximately 5% may fail to achieve SVR with initial treatment, and be at risk of developing severe cholestatic hepatitis or chronic progressive HCV leading to the need for re-transplantation.
Ideally, a randomized controlled trial would inform such trade-offs. However, such a trial will be prohibitively large, time consuming, or even unethical in some cases (2). In such situations, outcomes from mathematical modeling can inform clinical decision-making as well as future trial design to reduce any potential harm to patients (19–23). Our objective was to evaluate this clinical question and determine if and in which HCV-negative patients (based on their model for end-stage liver disease [MELD] score) the potential benefit of accepting an HCV-positive (nucleic acid test positive) organ with preemptive DAA therapy would outweigh the risks associated with HCV allograft infection by using decision-analytic modeling. Preemptive treatment in our analysis was defined as antiviral treatment at the time of or shortly after liver transplantation (i.e. administered within the days to weeks following transplant).
METHODS
Model Overview
We used and modified a previously validated Markov-based mathematical model, SIM-LT (Simulation of Liver Transplant Candidates), to perform our analysis (24, 25). The SIM-LT model was revised for this study to simulate a virtual trial of HCV-negative patients on the LT waiting list to compare long-term outcomes in patients willing to accept only HCV-negative livers versus those willing to accept any, i.e. HCV-negative or HCV-positive, livers. Patients receiving HCV-positive livers were treated preemptively after LT with 12 weeks of DAA therapy. We utilized data from published studies and the United Network for Organ Sharing (UNOS). The model’s outcomes were validated with the reported outcomes of the Organ Procurement and Transplantation Network (OPTN) data. SIM-LT was developed in the Java programming language.
Baseline population
We created multiple cohorts of HCV-negative decompensated cirrhotic patients (without hepatocellular carcinoma) who were listed on the LT waiting list. At baseline, patients had MELD scores between 12 and 40 and a mean age of 50.
SIM-LT waiting list
We simulated the natural history of patients on the LT waiting list. Patients’ MELD scores could change as they waited for an acceptable liver for transplant. We used a previously published study based on UNOS data to estimate weekly increase or decrease in MELD score, i.e., the natural course of the disease (20, 26). Specifically, data from 1,997 HCV-infected patients on the liver transplant waiting list from UNOS were used to create cubic splines of bilirubin, creatinine, albumin level and prothrombin time for each patient. The splines were then sampled at regular intervals to obtain a complete longitudinal history of each patient over time. Data was aggregated in groups of paired consecutive MELD scores starting with 12–13, 14–15, etc. We also estimated mortality on the waiting list depending on the MELD score from the same data source (Supplementary Table S1). Based on patients’ MELD scores, they could undergo LT, remain on the waiting list, or die because of liver-related or background mortality.
Interventions
For each patient on the LT waiting list, we simulated and compared the long-term outcomes under two scenarios: 1) accept only HCV-negative livers, the current practice, and 2) accept any liver, HCV-negative or HCV-positive, with a plan to initiate preemptive DAA therapy in patients receiving an HCV-positive organ.
In the ‘accept only HCV-negative livers’ scenario, patients’ likelihood of receiving an organ was dependent on their MELD score (Figure 1). We used a published study to estimate weekly probability of receiving a liver transplant based on patient’s MELD score (27), (Supplementary Table S2). After a successful transplant, patients moved to Post-LT (non-viremic) health state. Patients could experience graft failure anytime after LT. If a patient had acute-graft failure. i.e. in the first 2 weeks of liver transplant, s/he was placed at the top of the waiting list and made eligible for retransplantation. We assigned the average probability of liver transplant and liver-related mortality to these patients, which were estimated from UNOS data (24). At any time these patients could die from liver-related mortality as well as background mortality.
Figure 1. Model schematic showing the pathway of patients accepting HCV-positive or HCV-negative livers.
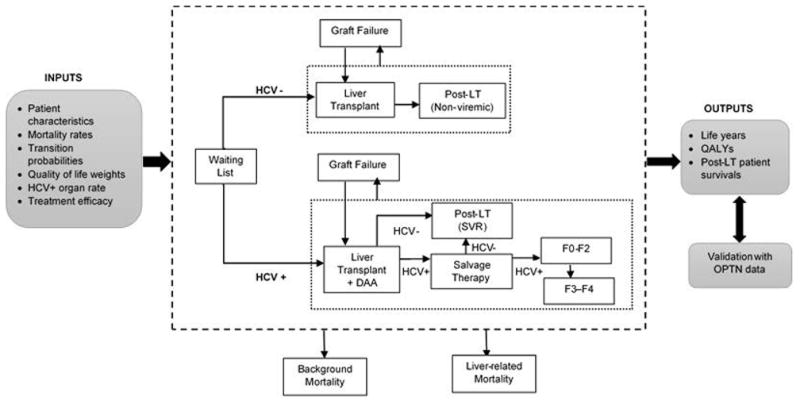
For each patient profile, the model simulated two scenarios: (1) accept any liver, and (2) accept only HCV-negative livers. Patient open to accepting any liver had a higher likelihood of receiving a LT. Patients receiving an HCV-positive liver were treated preemptively with DAAs. These patients had a higher risk of graft failure and could remain viremic (if treatment was not successful) after LT.
Abbreviations: LT, Liver transplant; DAA, Direct-acting antivirals; HCV, hepatitis C virus; QALYs, quality-adjusted life years; SVR, sustained virologic response; OPTN, Organ Procurement Transplant Network.
In the ‘accept any liver’ scenario, patients were willing to receiving the offered liver irrespective of the HCV-infection status—positive or negative. As a result, their likelihood of receiving a liver was higher than if they were not open to accepting an HCV-positive liver—we therefore increased the rate of receiving a liver by 5.9% (28), which corresponds to the proportion of HCV-infected livers among all liver donors in the U.S. In the sensitivity analysis, we varied this percentage using a wide range of 2.9–26.7%, corresponding to the lowest and highest HCV-positive organ rates observed in different UNOS regions (29). If a patient received an HCV-negative liver, s/he followed the path described in the above scenario (Figure 1). If a patient received an HCV-positive liver, preemptive antiviral treatment with a DAA was initiated. We incorporated data from recent trials and used the SVR rates of sofosbuvir and ledipasvir plus ribavirin for 12 weeks in post-LT patients (Table 1) (11, 30–32). Though we used the outcomes of sofosbuvir-based therapy, our analysis is not limited to any specific regimen and can be applied to other regimens that are currently used or will be approved in the near future for treatment of post-LT patients. We conducted a sensitivity analysis on a wide range of SVR rates. We assumed that patients receiving an HCV-positive liver could have a higher risk of graft failure within three months of LT as compared to those receiving an HCV-negative liver, and therefore, we increased the probability of graft failure by a hazard ratio of 1.44 (range: 1.08–1.80) (33). After graft failure, patients were eligible for retransplantation and placed back on the LT waitlist.
Table 1.
List of model variables used in SIM-LT, shown base case values and minimum and maximum values considered in the sensitivity analysis
| Parameter | Base Case | Min | Max | References |
|---|---|---|---|---|
| Baseline Age | 50 | 35 | 65 | Assumption |
| Sustained virologic response rate | ||||
| Preemptive Therapy SVR rate | 0.950 | 0.900 | 0.980 | (11, 30–32) |
| Salvage Therapy SVR rate | 0.950 | 0.900 | 0.980 | Assumption |
| Transition probabilities | ||||
| Liver transplant to liver-related death (1st year of 1st LT) | 0.0820 | 0.0615 | 0.1025 | (28) |
| Liver transplant to liver-related death (1st year of repeat LT) | 0.1900 | 0.1425 | 0.2375 | (28) |
| Liver transplant to graft failure (1st year of 1st LT) | 0.1050 | 0.0788 | 0.1313 | (28) |
| Liver transplant to graft failure (1st year of repeat LT) | 0.2140 | 0.1605 | 0.2675 | (28) |
| Post-Liver Transplant to liver-related death (1st year) * | 0.0742 | 0.0556 | 0.0927 | (40) |
| Post-Liver Transplant to liver-related death (subsequent year) * | 0.0303 | 0.0227 | 0.0378 | (40) |
| Post-Liver Transplant to graft failure * | 0.0494 | 0.0371 | 0.0618 | (41) |
| F0–F2 to liver-related death (1st year of 1st LT) | 0.0820 | 0.0615 | 0.1025 | (28) |
| F0–F2 to liver-related death (Subsequent year of 1st LT | 0.0461 | 0.0346 | 0.0577 | (28) |
| F0–F2 to liver-related death (1st year of repeat LT) | 0.1900 | 0.1425 | 0.2375 | (28) |
| F0–F2 to liver-related death (Subsequent year of repeat LT) | 0.0532 | 0.0399 | 0.0665 | (28) |
| F3–F4 to liver-related death (1st year of 1st LT) | 0.0820 | 0.0615 | 0.1025 | (28) |
| F3–F4 to liver-related death (Subsequent year of 1st LT | 0.0461 | 0.0346 | 0.0577 | (28) |
| F3–F4 to liver-related death (1st year of repeat LT) | 0.1900 | 0.1425 | 0.2375 | (28) |
| F3–F4 to liver-related death (Subsequent year of repeat LT) | 0.0532 | 0.0399 | 0.0665 | (28) |
| F0–F2 to graft failure (1st year of 1st LT) | 0.1050 | 0.0788 | 0.1313 | (28) |
| F0–F2 to graft failure (1st year of repeat LT) | 0.2140 | 0.1605 | 0.2675 | (28) |
| F3–F4 to graft failure (1st year of 1st LT) | 0.1937 | 0.1453 | 0.2422 | (5) |
| F3–F4 to graft failure (1st year of repeat LT) | 0.2140 | 0.1920 | 0.2370 | (28) |
| F0–F2 to graft failure (subsequent year of 1st LT) | 0.0503 | 0.0377 | 0.0629 | (28) |
| F0–F2 to graft failure (subsequent year of repeat LT) | 0.0587 | 0.0440 | 0.0734 | (28) |
| F3–F4 to graft failure (subsequent year of 1st LT) | 0.0503 | 0.0377 | 0.0629 | (28) |
| F3–F4 to graft failure (subsequent year of repeat LT) | 0.0587 | 0.0440 | 0.0734 | (28) |
| Graft failure to liver-related death | 0.652 | 0.489 | 0.815 | UNOS data |
| Graft failure to repeat transplant | 0.805 | 0.604 | 1.000 | UNOS data |
| F0–F2 to F3–F4 | 0.2000 | 0.1500 | 0.2500 | (42) |
| HCV-positive organ rate | 0.0590 | 0.0292 | 0.2670 | (28) |
| Hazard Ratio for increased graft failure | 1.44 | 1.08 | 1.80 | (33) |
| Health-related quality-of-life weights | ||||
| Transplant waiting list | 0.800 | 0.570 | 0.990 | (43, 44) |
| Liver transplant | 0.600 | 0.370 | 0.730 | (45) |
| F0–F2 | 0.828 | 0.716 | 0.865 | (44, 45) |
| F3–F4 | 0.801 | 0.693 | 0.837 | (44, 45) |
| Salvage therapy | 0.890 | 0.770 | 0.930 | (45) |
| Virus-free Post-LT | 0.890 | 0.770 | 0.930 | (45) |
| Sustained virologic response | 0.890 | 0.770 | 0.930 | (45) |
| Graft failure | 0.800 | 0.570 | 0.990 | (43, 46) |
Post-Liver Transplant probabilities correspond to those in Post-LT (Non-viremic) and Post-LT (SVR) stages in the model.
Abbreviations: SIM-LT, simulation of liver transplant candidates; HCV, Hepatitis C Virus; SVR, Sustained virologic response; LT, Liver transplant
If patients received an HCV-positive liver, the post-LT status was determined by their response to antiviral treatment—ongoing viremia or achievement of SVR. If patients cleared the virus, they moved to the Post-LT (SVR) state (Figure 1); otherwise, they were given salvage therapy—the SVR rate was assumed to be the same as with preemptive therapy. If patients failed to achieve SVR after salvage therapy, they were assumed to have chronic HCV and followed the natural progression of the disease.
Health-related quality of life
For each health state in our model, we also assigned health-related quality-of-life (QoL) weights, with 0 denoting death and 1 denoting perfect health, and adjusted them with age and sex. We derived EuroQol-5D instrument (Rotterdam, Netherlands) values from a previous study and adjusted them to the US population norm (Supplementary Table S3) (34–36).
Outcomes
We projected life years and quality adjusted life years (QALYs) for each MELD score (aggregated in groups of two) for both scenarios and estimated increase, or decrease, in life expectancy if patients were open to accepting any liver versus accepting only HCV-negative livers. We further projected these outcomes separately for each of the 11 UNOS regions. For that purpose, we adjusted the likelihood of LT and mortality on the waiting list for each UNOS region using region-specific transplantation and death rates (Supplementary Table S4). We also incorporated differential HCV-positive organ rates within each region (Supplementary Table S5) and further adjusted the increased likelihood of a getting transplant when patients were open to accepting any liver. Specific details are provided in Supplementary Section S1. We also performed one-way sensitivity analysis to determine which parameters had the most impact on the model outcomes.
We validated our model’s predicted post-LT survival with 1-year, 3-year and 5-year survival rates reported by OPTN data (Supplementary Figure S1). Survival rates predicted by our model matched well with reported data.
RESULTS
Benefits of accepting an HCV-positive liver
Figure 2 shows change in life years for patients on the LT waiting list when they are willing to accept an HCV-positive liver versus waiting for only HCV-negative livers. The clinical benefits were dependent on the MELD scores at which patients were willing to accept an HCV-positive liver. We found that patients would benefit from accepting an HCV-positive liver at MELD score 20 and beyond. In these patients, the increased risks associated with potential HCV allograft infection were offset by the benefit of receiving an LT sooner. The highest benefit was obtained when patients accept an HCV-positive liver at MELD 28—patients willing to accept any liver at MELD 28 gained 0.172 additional life years. In patients with MELD below 20, the risk of HCV allograft infection was not offset by the benefits of receiving LT sooner; instead of accepting an HCV-positive liver they would be better off waiting for an HCV-negative liver until their MELD score increased to at least 20. Supplementary Table S6 shows the expected life years for patients willing to accept an HCV-positive liver versus waiting for only HCV-negative livers. When we accounted for QoL of patients in the model, the outcomes and conclusions did not change (Supplementary Figure S2).
Figure 2. Change in life years if HCV-negative patients on the transplant waiting list are willing to accept any liver versus accept only HCV-negative livers.
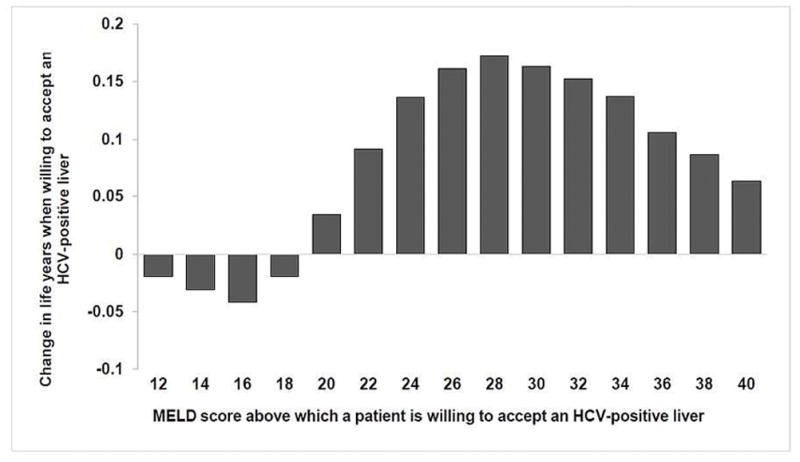
The clinical benefits are dependent on the MELD scores at which patients are willing to accept an HCV-positive liver. Starting at a MELD ≥ 20, patients benefit from accepting any liver. The highest clinical benefit of accepting any liver is seen at MELD 28.
Abbreviations: MELD, model for end-stage liver disease; HCV, hepatitis C virus.
Analysis by UNOS regions
We further analyzed the results for each UNOS region to account for regional differences in HCV-positive donor organ rates, and time spent on the LT waiting list. We observed that patients would benefit from accepting an HCV-positive liver at MELD 20 and beyond, irrespective of the UNOS region (Figure 3), although the magnitude of benefits varied by region. Furthermore, the magnitude of clinical benefit was proportional to the region-specific HCV-positive donor organ rates—highest in regions having a larger number of HCV-positive donor organs (Figure 4). For instance, a patient having MELD 28 in Region 1, which has one of the highest HCV-positive organ rate, would gain 0.36 life years if s/he is willing to accept HCV-positive liver, whereas the corresponding gain in life years would be 0.10 in Region 7, which has the lowest HCV-positive organ rate.
Figure 3. Change in life years by UNOS region if HCV-negative patients on the transplant waiting list are willing to accept any liver versus accept only HCV-negative livers.
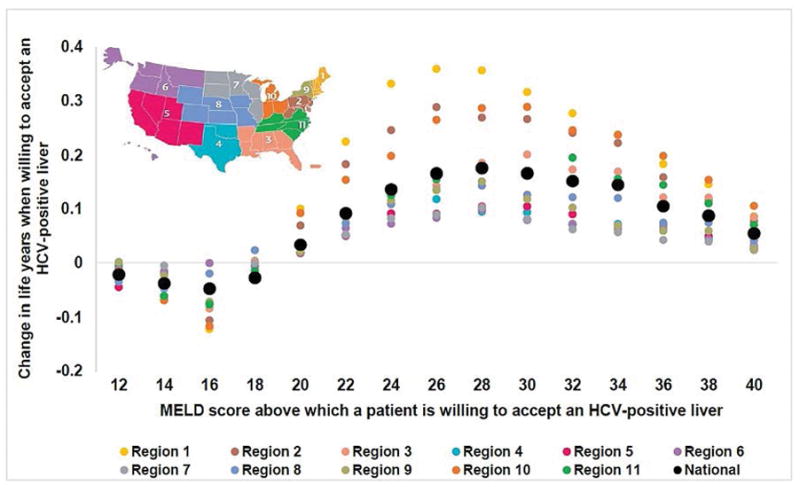
Patients benefit from becoming open to accepting an HCV-positive liver at MELD 20 and beyond, irrespective of the UNOS region, although the magnitude of benefit varied by region.
Abbreviations: UNOS, United Network for Organ Sharing, MELD, model for end-stage liver disease; HCV, hepatitis C virus
Figure 4. Regional results showing the correlation between HCV-positive organ rate and the health benefits within a UNOS region.
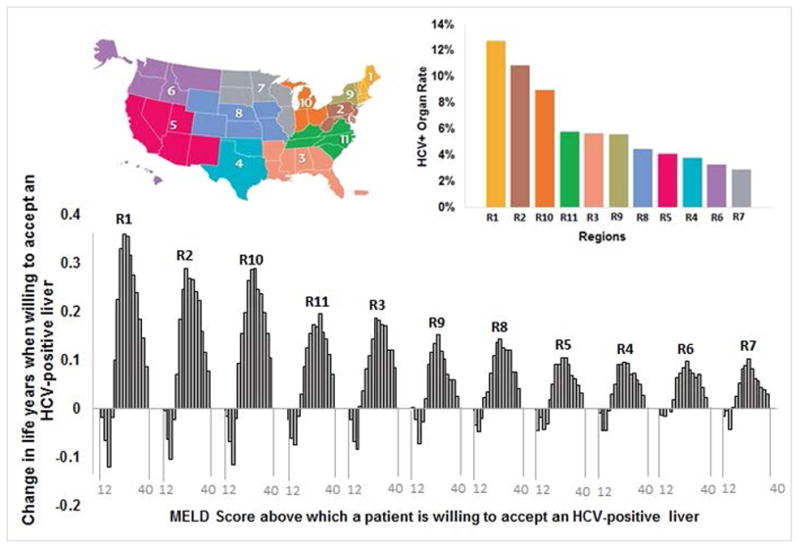
The magnitude of clinical benefit was proportional to the HCV-positive organ rates of the region—larger in regions having higher HCV-positive organ rates including Regions 1, 2, 3, 10 and 11.
Abbreviations: HCV, Hepatitis C Virus; MELD, Model for end-stage disease; UNOS, United Network for Organ Sharing; R1–11, UNOS Regions 1–11
Sensitivity and Scenario Analyses
We analyzed our model outcomes for each blood type to account for donor eligibility in different blood types. We found that though the clinical benefit in each MELD score was variable by blood type, the MELD threshold to accept any liver remained the same in all blood types, i.e. MELD 20 (Supplementary Figure S3). We further analyzed the results by incorporating the effect of Regional Share 35 policy. For that purpose, we increased the transplant rates of patients having MELD ≥ 35 by 11.8% and decreased the rates in patients having MELD < 35 (37). We found that the Regional Share 35 rule did not change our results—HCV-negative patients with MELD ≥ 20 would still benefit from willing to accept HCV-positive liver (Supplementary Figure S4). We also conducted an additional analysis to evaluate the impact of the recently reported HCV-positive organ rate of 26.7% observed in Ohio, which belongs to UNOS Region 10 (29). We found that though the clinical benefit increased for national as well as Region 10 results, the MELD threshold above which a patient is willing to accept HCV-positive livers did not change, i.e. MELD 20 (Supplementary Figures S5 and S6).
We conducted deterministic or one-way sensitivity analysis to determine the parameters that have the highest impact on our model’s primary outcome, i.e., change in life years under the two scenarios. Because MELD 20 was the cut-off score for accepting any liver and MELD 28 had the highest clinical benefit, we conducted 1-way sensitivity analyses on these scores. Supplementary Tables S7 and S8 show all model parameters in a decreasing order based on their impact on change in life years at MELD scores 20 and 28. The 10 parameters that the model results are most sensitive to are shown in the tornado diagrams (Figure 5). We found that the HCV-positive donor organ rate had the highest impact on change in life years; however, change in life years always remained positive—implying that patients would still benefit from accepting an HCV-positive liver regardless of the variability in parameter values.
Figure 5.
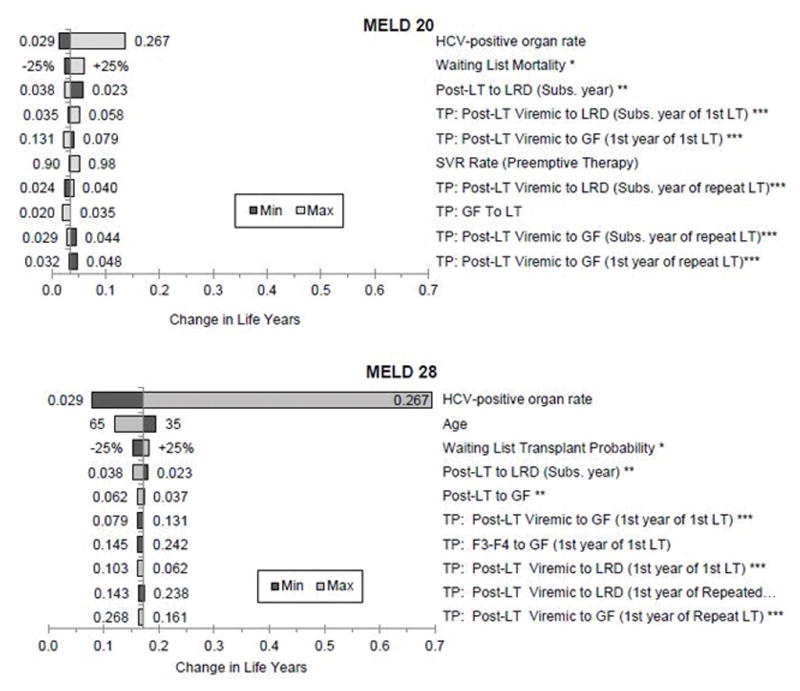
Tornado diagram showing the 10 most sensitive model parameters in MELD 20, i.e. the cutoff score to accept HCV-positive liver, and in MELD 28, i.e. the MELD score with the highest gain in life years. In both cases, HCV-positive organ rate had the highest impact on the primary model outcome, i.e. change in life years.
*Parameters having a value by each MELD score. Basically, we used +/− 25% change from baseline values
**Post-LT corresponds to Post-LT (Non-viremic) and Post-LT (SVR) stages in the model.
***Post-LT Viremic correspond to stages including salvage therapy, F0–F2, and F3–F4 in HCV-positive arm in the model
Abbreviations: HCV, hepatitis C virus; LT, liver transplant; SVR, sustained virologic response; GR, graft failure; TP, transition probability
DISCUSSION
As the shortage of transplant viable organs in the United States persists, it is of paramount importance that all transplantable organs are utilized to their maximum potential. With the advent of DAAs, it may now be time to consider the use of HCV-positive organs for transplantation into HCV-negative recipients. In this modeling-based study, we found that HCV-negative patients awaiting LT with MELD scores 20 and above may benefit from the clinical practice of accepting an HCV-positive liver. In this population, the risks associated with HCV allograft infection are offset by the benefits of receiving earlier transplantation. The benefits were highest for patients having a MELD score of 28, and for patients in UNOS regions having higher HCV-positive donor organ rates including Regions 1, 2, 3, 10 and 11. Given that a large number of patients awaiting LT in the U.S. have MELD scores greater than 20, a change in practice towards a willingness to accept HCV-positive livers would benefit the majority of the waitlist population.
Though DAAs have been shown to be highly effective in successfully treating HCV in the post-transplant setting when used in HCV-positive patients receiving HCV-positive livers, no such data exists on their use in HCV-negative patients receiving HCV-positive livers. While published clinical trials are still needed to confirm DAA efficacy in preemptively treating HCV-negative patients accepting viremic livers, recruitment and early case reports are actively ongoing. Encouragingly, two recent clinical trials in renal transplantation demonstrated that HCV-negative patients who received HCV-positive kidneys were successfully treated with 100% SVR rates (38, 39). After investigations confirm DAA efficacy in the liver transplant setting, we anticipate the potential addition of HCV-positive (i.e., viremic) livers to the routine “extended donor criteria” discussed with patients at the time of transplant listing. In our study, by simulating a virtual trial using computer modeling, we sought to identify which patients may benefit most from a willingness to accept HCV-positive livers. The results of our modeling-based analysis can thus inform efficient design of future trials and clinical practice in liver transplantation.
It is important to note that our analysis is not drug-regimen specific nor does it impose specific restrictions on treatment groups, including those with renal failure and non-genotype 1 infection. It is in essence SVR-specific. It was developed using SVR data from trials on ledipasvir/sofosbuvir for treatment of patients who develop recurrent HCV post liver transplantation (i.e. HCV-positive to HCV-positive liver transplant recipients), but model conclusions hold true for alternative DAA regimens as long as the SVR rates continue to fall within the range of our sensitivity analysis. We recognize that clinically there is clearly an important decision to be made around 1) what DAA to choose (e.g., pan-genotypic regimen may be preferred in some settings) and 2) the administration timing for a given therapy, but with our current model that treatment plan can be left to clinician discretion.
There are some caveats to the generalizability of the model results. There are several factors, such as refractory complications of portal hypertension, that determine a patient’s urgency for LT that are not reflected in MELD alone. These subsets of patients may experience significant morbidity and higher mortality at lower MELD scores than the more general LT waitlist population. As such, the benefit of decreased wait time may outweigh the risks of accepting an HCV-positive organ at MELD values lower than 20 in these patient populations. This helps highlight an important discussion point – while the results of this model can help guide shared patient-physician clinical decision making, it is not meant for use as a standalone algorithm for all LT waitlist patients. Instead, it should be used as a tool to help inform individual patient management. Patients who are risk averse may wait until their MELD is 28 before choosing to accept an HCV-positive organ, or determine that they only want to accept HCV-negative organs irrespective of MELD score. Alternatively, there may be patients who are willing to take on the increased risk of accepting an HCV-positive organ at lower MELD scores if it results in a decrease in wait time. These continue to remain personal choices that can be incorporated into a patient’s LT listing preferences through an informed decision making process.
Though our study provides empirical evidence on the benefits of transplanting HCV-positive livers to HCV-negative recipients on the LT waiting list, further data are needed on the cost-effectiveness of preemptive HCV treatment in HCV-negative recipients receiving HCV-positive livers. Second, clinical studies are needed to determine the optimal duration of DAA therapy—12 weeks or shorter, in transplant recipients (32). Third, changes in reimbursement structure may be needed to successfully implement transplantation of HCV-positive livers to negative recipients. Finally, further analyses are required to evaluate the trade-offs of accepting HCV-positive livers for transplant into HCV-negative recipients in other countries.
Our study also has a number of limitations. First, we assumed that the efficacy of preemptive treatment with DAAs would be similar to what is seen in HCV-positive patients who received an HCV-positive LT. We do not have real-world data on DAA use in this specific setting; however, there is no reason to believe that the efficacy of DAAs would be lower in HCV-negative treatment naïve recipients as compared with HCV-positive recipients. We addressed this limitation by conducting sensitivity analysis on SVR rates. Second, long-term data on transplant recipients who achieved SVR is extremely limited, especially when considering different MELD groups. Third, our analysis did not include decompensated cirrhotic patients with HCC. Such patients will have a different natural history of the disease and should be best evaluated in a separate analysis. Finally, we did not factor in post-LT mortality rates by MELD score.
Despite these limitations, our study provides some of first empirical data on the benefits and harms of transplanting HCV-positive livers in HCV-negative recipients. It is important that further research in this area continues, as we expect that the supply of HCV-positive organs may continue to increase in light of the growing opioid epidemic, while the number of HCV-positive patients on the LT waitlist decreases with the use of DAAs (2). Growing evidence on the successful use of HCV-positive organs into HCV-negative recipients can help ensure we utilize all organs to their maximum potential and curtail the HCV-positive donor organ discard rate.
Conclusion
Transplanting HCV-positive livers into HCV-negative patients receiving preemptive DAA therapy could be a viable option for improving patient survival on the LT waiting list, especially in UNOS regions with high HCV-positive donor organ rates. Patients with MELD ≥ 20 benefit from a willingness to accept an HCV-positive liver, with the greatest benefit seen at a MELD score of 28. Clinical data are needed to confirm the efficacy of DAA therapy in this setting, and our analysis can help inform future trials and minimize patient harm by recognizing the LT waitlist populations that may benefit most from accepting HCV-positive donor organs.
Supplementary Material
Acknowledgments
Funding Support
This study was supported in part by Research Scholar Grant, RSG-17-022-01-CPPB, from the American Cancer Society; Health Resources and Services Administration contract 234-2005-37011C; National Institutes of Health grant DK078772; National Science Foundation award 1722665; and the MGH Research Scholars Program. Dr. Kanwal’s effort was supported in part by the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413) and PHS grant P30DK05633. Dr. Ayer’s effort was supported in part by the National Science Foundation under award number 1452999. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the sponsors.
List of Abbreviations
- HCV
Hepatitis C virus
- SVR
Sustained virologic response
- LT
Liver transplant
- MELD
Model for end-stage liver disease
- SIM-LT
Simulation of liver transplant candidates
- UNOS
United Network for Organ Sharing
- OPTN
Organ Procurement and Transplantation Network
- QoL
Quality-of-life
- QALYs
Quality adjusted life years
Footnotes
Author contribution:
Study concept and design: Chhatwal, Samur, Bethea, Chung
Drafting of manuscript: Chhatwal, Samur, Bethea
Critical revision of the manuscript for important intellectual content: Chhatwal, Samur, Bethea, Ayer, Kanwal, Hur, Roberts, Terrault, Chung
Statistical analysis: Samur
Interpretation of data: Chhatwal, Samur, Bethea, Ayer, Kanwal, Hur, Roberts, Terrault, Chung
References
- 1.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant. 2017;17(Suppl 1):174–251. doi: 10.1111/ajt.14126. [DOI] [PubMed] [Google Scholar]
- 2.Levitsky J, Formica RN, Bloom RD, Charlton M, Curry M, Friedewald J, Friedman J, et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. American Journal of Transplantation. doi: 10.1111/ajt.14381. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 3.Bowring MG, Kucirka LM, Massie AB, Luo X, Cameron A, Sulkowski M, Rakestraw K, et al. Changes in Utilization and Discard of Hepatitis C-Infected Donor Livers in the Recent Era. Am J Transplant. 2017;17:519–527. doi: 10.1111/ajt.13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Northup PG, Argo CK, Nguyen DT, McBride MA, Kumer SC, Schmitt TM, Pruett TL. Liver allografts from hepatitis C positive donors can offer good outcomes in hepatitis C positive recipients: a US National Transplant Registry analysis. Transpl Int. 2010;23:1038–1044. doi: 10.1111/j.1432-2277.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 5.Berenguer M, Prieto M, Rayon JM, Mora J, Pastor M, Ortiz V, Carrasco D, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32:852–858. doi: 10.1053/jhep.2000.17924. [DOI] [PubMed] [Google Scholar]
- 6.Berenguer M. What determines the natural history of recurrent hepatitis C after liver transplantation? J Hepatol. 2005;42:448–456. doi: 10.1016/j.jhep.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer M. Treatment of hepatitis C after liver transplantation. Clin Liver Dis. 2005;9:579–600. vi. doi: 10.1016/j.cld.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Berenguer M, Palau A, Aguilera V, Rayon JM, Juan FS, Prieto M. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008;8:679–687. doi: 10.1111/j.1600-6143.2007.02126.x. [DOI] [PubMed] [Google Scholar]
- 9.Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49:274–287. doi: 10.1016/j.jhep.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Kremers WK, Rosen CB, et al. Impact of pegylated interferon and ribavirin treatment on graft survival in liver transplant patients with recurrent hepatitis C infection. Am J Transplant. 2008;8:2426–2433. doi: 10.1111/j.1600-6143.2008.02362.x. [DOI] [PubMed] [Google Scholar]
- 11.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, Jr, Fried MW, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Kling CE, Limaye AP, Landis CS, Sibulesky L. Expanding access to transplantation with hepatitis C-positive donors: A new perspective on an old issue. Clin Transplant. 2017:31. doi: 10.1111/ctr.12884. [DOI] [PubMed] [Google Scholar]
- 13.Martini S, Tandoi F, Terzi di Bergamo L, Strona S, Lavezzo B, Sacco M, Maione F, et al. Negativization of viremia prior to liver transplant reduces early allograft dysfunction in hepatitis C recipients. Liver Transpl. 2017 doi: 10.1002/lt.24772. [DOI] [PubMed] [Google Scholar]
- 14.Shah AP, Cameron A, Singh P, Frank AM, Fenkel JM. Successful treatment of donor-derived hepatitis C viral infection in three transplant recipients from a donor at increased risk for bloodborne pathogens. Transpl Infect Dis. 2017:19. doi: 10.1111/tid.12660. [DOI] [PubMed] [Google Scholar]
- 15.Khan B, Singer LG, Lilly LB, Chaparro C, Martinu T, Juvet S, Pipkin M, et al. Successful Lung Transplantation From Hepatitis C Positive Donor to Seronegative Recipient. Am J Transplant. 2017;17:1129–1131. doi: 10.1111/ajt.14137. [DOI] [PubMed] [Google Scholar]
- 16.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, Charlton M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 152:1090–1099.e1091. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guirguis J, Chhatwal J, Dasarathy J, Rivas J, McMichael D, Nagy LE, McCullough AJ, et al. Clinical impact of alcohol-related cirrhosis in the next decade: estimates based on current epidemiological trends in the United States. Alcohol Clin Exp Res. 2015;39:2085–2094. doi: 10.1111/acer.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, Kuntz KM. State-transition modeling: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force. Medical Decision Making. 2012;32:690–700. doi: 10.1177/0272989X12455463. [DOI] [PubMed] [Google Scholar]
- 20.Alagoz O, Maillart LM, Schaefer AJ, Roberts MS. The optimal timing of living-donor liver transplantation. Management Science. 2004;50:1420–1430. [Google Scholar]
- 21.Van Arendonk KJ, Chow EKH, James NT, Orandi BJ, Ellison TA, Smith JM, Colombani PM, et al. Choosing the Order of Deceased Donor and Living Donor Kidney Transplantation in Pediatric Recipients: A Markov Decision Process Model. Transplantation. 2015;99:360–366. doi: 10.1097/TP.0000000000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow EK, Massie AB, Muzaale AD, Singer AL, Kucirka LM, Montgomery RA, Lehmann HP, et al. Identifying appropriate recipients for CDC infectious risk donor kidneys. Am J Transplant. 2013;13:1227–1234. doi: 10.1111/ajt.12206. [DOI] [PubMed] [Google Scholar]
- 23.Schaubel DE, Dykstra DM, Murrayc S, Ashby VB, McCullough KP, Dickinson DM, Hulbert-Shearon TE, et al. Analytical approaches for transplant research, 2004. American journal of transplantation. 2005;5:950–957. doi: 10.1111/j.1600-6135.2005.00837.x. [DOI] [PubMed] [Google Scholar]
- 24.Chhatwal J, Samur S, Kues B, Ayer T, Roberts MS, Kanwal F, Hur C, et al. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology. 2017;65:777–788. doi: 10.1002/hep.28926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samur S, Kues B, Ayer T, Roberts MS, Kanwal F, Hur C, Donnell DMS, et al. Cost-Effectiveness of Pre versus Post Liver Transplant Hepatitis C Treatment with Direct-Acting Antivirals. Clin Gastroenterol Hepatol. 2017 doi: 10.1016/j.cgh.2017.06.024. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shechter SM, Bryce CL, Alagoz O, Kreke JE, Stahl JE, Schaefer AJ, Angus DC, et al. A clinically based discrete-event simulation of end-stage liver disease and the organ allocation process. Medical Decision Making. 2005;25:199–209. doi: 10.1177/0272989X04268956. [DOI] [PubMed] [Google Scholar]
- 27.Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, Schnitzler MA, et al. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant. 2011;11:2362–2371. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.A national research agenda for public health services and systems. Am J Prev Med. 2012;42:S72–78. doi: 10.1016/j.amepre.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez SA, Trotter JF. The Rise of the Opioid Epidemic and Hepatitis C Positive Organs: A New Era in Liver Transplantation. Hepatology. doi: 10.1002/hep.29572. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 30.Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. The Lancet Infectious Diseases. 2016;16:685–697. doi: 10.1016/S1473-3099(16)00052-9. [DOI] [PubMed] [Google Scholar]
- 31.Gane E, Manns M, McCaughan G, Curry MP, Peck-Radosavljevic M, Vlierberghe HV, Arterburn S, et al. Ledipasvir/sofosbuvir with ribavirin in patients with decompensated cirrhosis or liver transplantation and HCV infection: SOLAR-1 and −2 trials. AASLD Abstract. 1049;2015:2015. [Google Scholar]
- 32.Levitsky J, Verna EC, O’Leary JG, Bzowej NH, Moonka DK, Hyland RH, Arterburn S, et al. Perioperative Ledipasvir–Sofosbuvir for HCV in Liver-Transplant Recipients. New England Journal of Medicine. 2016;375:2106–2108. doi: 10.1056/NEJMc1611829. [DOI] [PubMed] [Google Scholar]
- 33.Cholankeril G. The Use of HCV Positive Donors in HCV Negative Liver Transplant Recipients. AASLD Liver Meeting. 2016 Abstract ID: 1630. [Google Scholar]
- 34.Chong CAKY, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, Krahn M. Health-state utilities and quality of life in hepatitis C patients. The American Journal of Gastroenterology. 2003;98:630–638. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 35.Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth B, Manns M, et al. Cost effectiveness of peginterferon-2b plus ribavirin versus interferon -2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52:425. doi: 10.1136/gut.52.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanmer J, Lawrence WF, Anderson JP, Kaplan RM, Fryback DG. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Medical Decision Making. 2006;26:391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 37.Edwards EB, Harper AM, Hirose R, Mulligan DC. The impact of broader regional sharing of livers: 2-year results of “Share 35”. Liver Transplantation. 2016;22:399–409. doi: 10.1002/lt.24418. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg DS, Abt PL, Blumberg EA, Van Deerlin VM, Levine M, Reddy KR, Bloom RD, et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. New England Journal of Medicine. 2017;376:2394–2395. doi: 10.1056/NEJMc1705221. [DOI] [PubMed] [Google Scholar]
- 39.Durand C, Brown D, Wesson R, Bhair N, Naqvi F, Ostrander D, Bowring M, et al. EXPANDER-1: Exploring Renal Transplants Using Hepatitis-C Infected Donors for HCV-Negative Recipients. [abstract] [Accessed August 12, 2017];Am J Transplant. 2017 17(suppl 3) http://atcmeetingabstracts.com/abstract/expander-1-exploring-renal-transplants-using-hepatitis-c-infected-donors-for-hcv-negative-recipients/ [Google Scholar]
- 40.Thuluvath P, Guidinger M, Fung J, Johnson L, Rayhill S, Pelletier S. Liver transplantation in the United States, 1999–2008. American Journal of Transplantation. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 41.Berenguer M, Prieto M, San Juan F, Rayon JM, Martinez F, Carrasco D, Moya A, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002;36:202–210. doi: 10.1053/jhep.2002.33993. [DOI] [PubMed] [Google Scholar]
- 42.Samuel D, Feray C. Recurrent hepatitis C after liver transplantation: clinical and therapeutical issues. J Viral Hepat. 2000;7:87–92. doi: 10.1046/j.1365-2893.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 43.Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, Krahn M. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–638. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 44.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162:397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, Ryder S, et al. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis. Br J Cancer. 2008;98:1166–1175. doi: 10.1038/sj.bjc.6604301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chhatwal J, Ferrante SA, Brass C, El Khoury AC, Burroughs M, Bacon B, Esteban-Mur R, et al. Cost-Effectiveness of boceprevir in patients previously treated for chronic hepatitis C genotype 1 Infection in the United States. Value in Health. 2013;16:973–986. doi: 10.1016/j.jval.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


