SUMMARY
The L-arginine/NO pathway is an important regulator of pulmonary hypertension, the leading cause of mortality in patients with the chronic lung disease of prematurity, bronchopulmonary dysplasia. L-arginine can be metabolized by NO synthase (NOS) to form L-citrulline and NO, a potent vasodilator. Alternatively, L-arginine can be metabolized by arginase to form urea and L-ornithine, a precursor to collagen and proline formation important in vascular remodeling. In the current study, we hypothesized that C3H/HeN mice exposed to prolonged hyperoxia would have increased arginase expression and pulmonary vascular wall cell proliferation. C3H/HeN mice were exposed to 14 days of 85% O2 or room air and lung homogenates analyzed by western blot for protein levels of arginase I, arginase II, endothelial NOS (eNOS), ornithine decarboxylase (ODC), ornithine aminotransferase (OAT), and α-smooth muscle actin (α-SMA). Hyperoxia did not change arginase I or eNOS protein levels. However, arginase II protein levels were 15-fold greater after hyperoxia exposure than in lungs exposed to room air. Greater protein levels of ODC and OAT were found in lungs following hyperoxic exposure than in room air animals. α-SMA protein levels were found to be 7-fold greater in the hyperoxia exposed lungs than in room air lungs. In the hyperoxia exposed lungs there was evidence of greater pulmonary vascular wall cell proliferation by α-SMA immunohistochemistry than in room air lungs. Taken together, these data are consistent with a more proliferative vascular phenotype, and may explain the propensity of patients with bronchopulmonary dysplasia to develop pulmonary hypertension.
Keywords: L-arginine, nitric oxide synthase, ornithine decarboxylase, ornithine aminotransferase, proliferation, pulmonary hypertension, chronic lung disease, neonate
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is the most common complication of preterm birth and a leading cause of pediatric chronic lung disease (1, 2). BPD is characterized by significant lung remodeling with both fewer and larger alveoli, as well as a propensity for a dysmorphic pulmonary vasculature (3). Development of pulmonary hypertension (PH) significantly increases morbidity and mortality in BPD patients (3, 4). A recent review highlighted the paucity of data regarding molecular mechanisms associated with the altered vascular development that occurs in BPD (5).
Previously, our group has used a C3H/HeN mouse exposed to 85% O2 for 14 days after birth that demonstrates lung changes similar to those seen in BPD, including decreased alveolarization, and changes in lung function (6). Furthermore, neonatal C3H/HeN mice exposed to hyperoxia develop cardiac dysfunction (7) and right ventricular hypertrophy as evidenced by an increase in right ventricle to total ventricular weight ratio (8).
Arginase hydrolyzes L-arginine to L-ornithine and urea, and L-ornithine can be further metabolized by either ornithine decarboxylase (ODC) to produce polyamines or by ornithine aminotransferase (OAT) to produce L-proline important for both endothelial and smooth muscle cell proliferation indicative of vascular remodeling in pulmonary hypertension (9, 10). Recently, our group demonstrated in infants with BPD, differential expression of a single nucleotide polymorphism (SNP) in the arginase-1 gene (11) between those patients with BPD and those with BPD and PH. Furthermore, lymphocytes harvested from patients with the arginase-1 SNP had greater levels of NO production (12). We have also described that patients with BPD and PH have differential expression of a SNP in the dimethylarginine dimethylaminohydrolase-1 (DDAH-1) gene compared to patients with BPD alone (13). Furthermore, we found higher plasma levels of asymmetric dimethylarginine (ADMA) in infants that went on to develop BPD with PH than in those with BPD alone (14). Taken together these results suggest alterations in the L-arginine/NO pathway in BPD patients with PH that favor arginase activity over NOS activity. Therefore, we hypothesized that in our mouse model of BPD that exposure to hyperoxia would result in greater arginase expression and greater evidence of pulmonary vascular wall cell proliferation.
RESULTS
Hyperoxia results in greater levels of arginase II protein
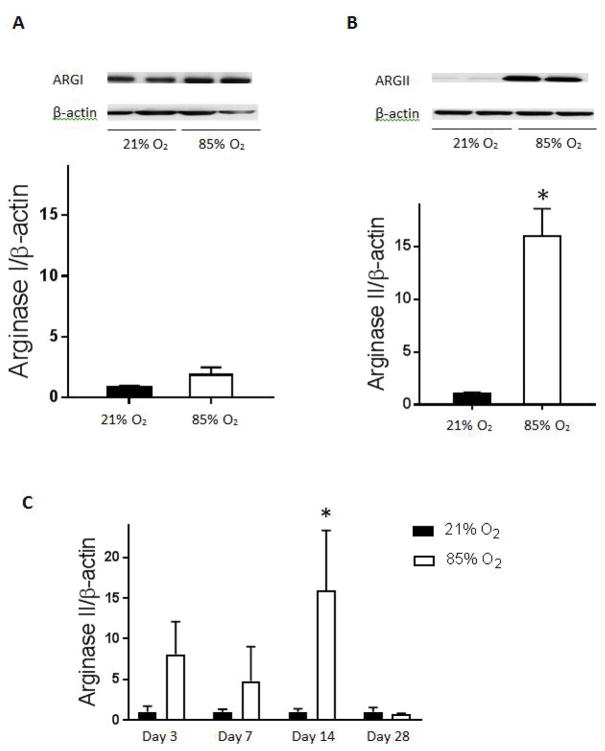
Mice were exposed to 85% O2 or room air for 14 days and their lungs harvested for western blotting. There was no difference in arginase I protein levels in the lungs between mice exposed to 85% O2 or room air (figure 1A). In mice exposed to 85% O2 there was approximately 15-fold greater levels of arginase II protein in the lungs (figure 1B) than in the lungs from normoxic controls. We then evaluated the time course of the hyperoxia-induced arginase II protein expression and found that arginase II protein levels were again significantly greater at day 14 in the lungs from hyperoxic exposed animals than in the lungs from animals exposed to room air (figure 1C).
Figure 1. Arginase protein levels in C3H/HeN mouse lung homogenates.
Protein was isolated from C3H/HeN mouse lung homogenates and arginase I and II protein levels measured by western blot analysis. Representative western blot and densitometry levels for arginase I in hyperoxia (n=6) compared to normoxic control (n=8) (A). Arginase I protein levels are not significantly different after 14 days of hyperoxic exposure as compared to normoxic control. Representative western blot and protein levels for arginase II in hyperoxia (n=6) compared to normoxic control (n=8) (B). Arginase II protein levels are significantly greater after 14 days of hyperoxic exposure than normoxic controls (*p<0.05). Time course of arginase II protein expression after hyperoxic exposure as compared to normoxic control at days 3, 7, 14, and 28. Arginase II protein levels are again greater at 14 days of hyperoxia than normoxic controls (*p<0.05) (C). All arginase protein levels are normalized to β-actin. Normoxia is represented by black bars and hyperoxia is represented by white bars.
eNOS protein levels are not different in C3H/HeN mouse lung after hyperoxic exposure
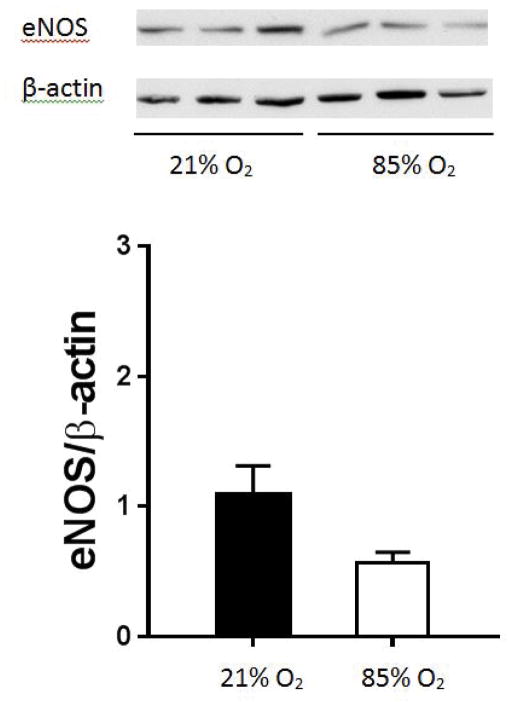
Mice were exposed to hyperoxia or room air and after 14 days the lungs were harvested for NOS western blotting. We found that the lungs from animals exposed to 85% O2 had eNOS protein levels similar to those found in lungs from animals exposed to room air (figure 2). We also found no difference in the lung levels of neuronal NOS (nNOS) between hyperoxia exposure and room air exposed animals (data not shown). We were unable to detect inducible NOS (iNOS) in any lung samples from these mice.
Figure 2. eNOS protein levels in C3H/HeN mouse lung homogenates.
Protein was isolated from C3H/HeN mouse lung homogenates and eNOS protein levels measured by western blot analysis. Representative western blot and densitometry levels for eNOS in hyperoxia (n=3) compared to normoxic control (n=3). eNOS protein levels are not significantly different after 14 days of hyperoxic exposure as compared to normoxic control. All eNOS protein levels are normalized to β-actin. Normoxia is represented by black bars and hyperoxia is represented by white bars.
Hyperoxia exposure results in greater protein levels of ODC and OAT
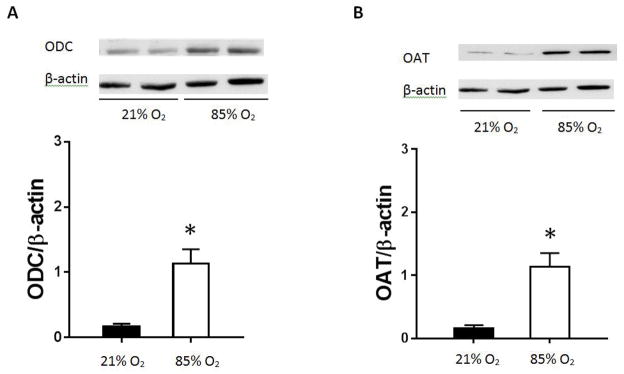
Mice were exposed to either 85% O2 or room air and protein harvested for western blotting for ornithine decarboxylase (ODC) or ornithine aminotransferase (OAT). We found that after 14 days of 85% O2 exposure, lung ODC protein levels were greater than in the lungs from normoxic controls (figure 3A). Similarly, lung OAT protein levels were greater in the lungs from mice exposed to 85% O2 than in the lungs from mice exposed to room air (figure 3B).
Figure 3. ODC and OAT protein levels in C3H/HeN mouse lung homogenates.
Protein was isolated from C3H/HeN mouse lung homogenates and ODC and OAT protein levels measured by western blot analysis. Representative western blot and densitometry levels for ODC in hyperoxia (n=3) compared to normoxic control (n=3) (A). ODC protein levels are significantly greater after 14 days of hyperoxic exposure than normoxic controls (*p<0.05). Representative western blot and protein levels for OAT in hyperoxia (n=3) compared to normoxic control (n=3) (B). OAT protein levels are significantly greater after 14 days of hyperoxic exposure than normoxic control (*p<0.05). All ODC and OAT protein levels are normalized to β-actin. Normoxia is represented by black bars and hyperoxia is represented by white bars.
α-SMA protein and immunohistochemistry are greater in the lungs from mice exposed to hyperoxia
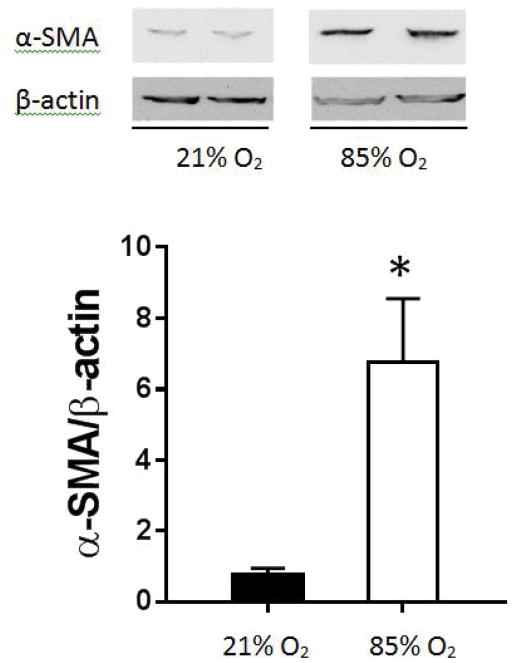
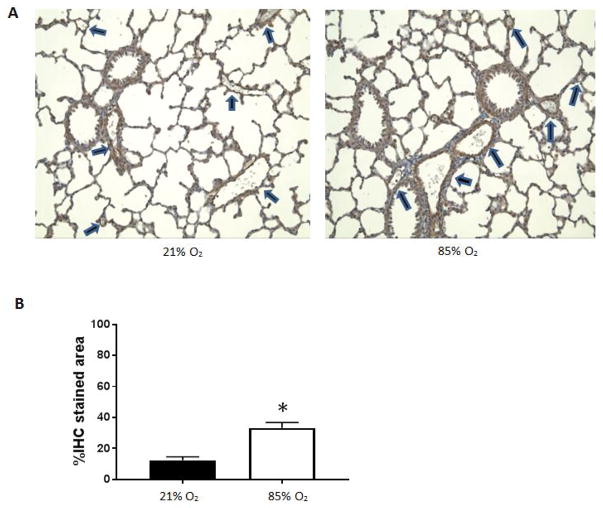
Mice were exposed to 85% oxygen or room air and the lungs were harvested for western blotting for α-SMA. We found approximately 7-fold greater α-SMA protein levels in the lungs from mice exposed to hyperoxia than in the lungs from mice exposed to normoxia (figure 4). In another set of experiments, mice were exposed to hyperoxia or room air and the lungs harvested for immunohistochemical determination of α-SMA. We found that lungs from mice exposed to hyperoxia had greater α-SMA staining than did lungs from normoxia (53% vs. 35%, p<0.01) exposed mice (data not shown). Similarly when we focused on the pulmonary blood vessels, we found that lungs from mice exposed to hyperoxia had greater pulmonary blood vessel α-SMA staining than did lungs from normoxia (33% vs. 12%, p<0.01) exposed mice (figure 5).
Figure 4. α-SMA protein levels in C3H/HeN mouse lung homogenates.
Protein was isolated from C3H/HeN mouse lung homogenates and α-SMA protein levels measured by western blot analysis. Representative western blot and densitometry levels for α-SMA in hyperoxia (n=5) compared to normoxic control (n=5). Experiment performed on a single western blot. α-SMA protein levels are significantly greater after 14 days of hyperoxic exposure than normoxic controls (*p<0.05). All α-SMA protein levels are normalized to β-actin. Normoxia is represented by black bars and hyperoxia is represented by white bars.
Figure 5. α-SMA immunohistochemistry of C3H/HeN mouse lung tissue sections.
Representative images from fixed lung sections (A). C3H/HeN mice were either exposed to 14 days of 21% O2 (control) or 85% O2 and lung tissue sections harvested and immune-stained for α-SMA. Pulmonary blood vessels are highlighted with arrows (six vessels per slide). Percent immunohistochemistry stain (%IHC) of α-SMA stained area was calculated from lung tissue sections as (IHC stained blood vessel area)/(total stained blood vessel area) × 100% (B). Lung tissue sections exposed to 85% O2 (5 slides) had greater %IHC blood vessel staining than control (4 slides, *p<0.01). Images were analyzed using Image J and mean ± standard error was calculated and graphed. Normoxia is represented by black bars and hyperoxia is represented by white bars.
DISCUSSION
The main findings of this study in neonatal mouse lungs were that following 85% O2 for 14 days, 1) arginase II protein levels were 15-fold greater, 2) ODC and OAT protein levels were greater, 3) α-SMA protein levels were greater, and 4) α-SMA immuno-staining in pulmonary vessels was greater than in normoxic controls. These findings are consistent with our hypothesis that in our mouse model of BPD, hyperoxia leads to greater arginase expression and greater expression of ornithine metabolizing enzymes, resulting in an overall net effect of greater vascular wall cell proliferation. Evidence for greater vascular wall cell proliferation comes from increased α-SMA protein levels in lungs and greater α-SMA staining of the vessel walls in the lungs. These findings are consistent with an alteration in the L-arginine/NO pathway favoring ornithine production by arginase II over NO production by NOS. This alteration in the L-arginine/NO pathway would be expected to lead to vascular remodeling, a hallmark of PH (9).
We found no differences in arginase I protein expression in lung homogenates from neonatal C3H/HeN mice exposed to 85% O2 for 14 days. Previous studies in our labs exposed adult C3H/HeN mice to >95% O2 for 72 or 96 hours and found greater hepatic arginase I and ornithine protein levels (15). These findings suggest that C3H/HeN mice have an organ specific arginase I response to hyperoxia.
To the best of our knowledge this is the first description of hyperoxic induction of arginase II and OAT proteins in the lungs of neonatal mice exposed to hyperoxia. Previous studies have reported greater hyperoxia-induced arginase II expression and greater arginase activity in adult Sprague-Dawley rats exposed to 100% oxygen for 60 hours, as well as decreased NO production with no changes in NOS protein expression (16). Similarly, in neonatal rats, Vadivel, et al. (17) demonstrated that 14 days of exposure to 95% oxygen resulted in greater arginase II expression and activity. Sopi, et al. (18) demonstrated greater arginase activity in the lungs from neonatal rats exposed to >95% oxygen for 12 days than in the lungs from neonatal rats exposed to room air for 14 days. Taken together, these results suggest that, at least in mice and rats, hyperoxia induces arginase expression and activity and increases the metabolism of L-arginine by arginase at the expense of L-arginine metabolism by NOS. We have previously shown in several different cell types that stimuli which up-regulate arginase result in decreased NO production due to competition for their common substrate L-arginine (19–21). Taken together these findings suggest that hyperoxia leads to increased arginase activity, which would favor the development of PH via at least two mechanisms. The first mechanism would be decreased NO production which would favor vasoconstriction; and the second mechanism would be greater arginase-mediated vascular wall cell proliferation which would favor vascular remodeling. The second mechanism, vascular wall cell proliferation, is supported by our findings that in the lungs of hyperoxia exposed animals, α-SMA protein and α-SMA vessel wall staining is greater than room air exposed animals.
L-ornithine production by arginase is an important first step towards the vascular remodeling that occurs in pulmonary hypertension. Ornithine decarboxylase (ODC) metabolizes L-ornithine to polyamine, an important positive regulator for both endothelial and smooth muscle cell growth (9). Ornithine aminotransferase (OAT) is necessary for the production of L-proline, an amino acid essential in collagen formation that occurs during the process of vascular remodeling leading to pulmonary hypertension (16). In the present study, we found that both ODC and OAT protein levels were greater after 85% O2 exposure for 14 days than in room air controls. Several other studies have demonstrated hyperoxic induction of ODC in both rat and mouse lung models of hyperoxic lung injury (16, 22, 23). However, hyperoxic induction of OAT has not been described previous to this study. Elevated levels of ODC and OAT support a vascular wall cell proliferative process.
The major limitation of this study is the use of lung homogenates. Although lung homogenates include pulmonary vascular smooth muscle cells, the samples are comprised of a mixture of several cell types. Therefore, our findings may reflect changes in protein expression of cells other than those of the pulmonary vasculature, including airway smooth muscle cells (24) and fibroblasts/myofibroblasts in the lung interstitium/parenchyma (25) that can also lead to increased production of L-proline and collagen formation. In order to attempt to address this specific limitation, we quantitated α-SMA immunohistochemistry focused on the pulmonary vasculature and excluding bronchioles. In the present study, we found that there was more α-SMA blood vessel staining after hyperoxia than normoxia, which is consistent with vascular remodeling indicative of pulmonary hypertension in BPD.
In conclusion, we describe for the first time in mouse lungs that hyperoxia leads to increased arginase II and OAT protein expression. We also found that hyperoxia increased protein expression of ODC and α-SMA. Taken together, these findings are consistent with a more proliferative vascular phenotype, and may explain the propensity of patients with BPD to develop PH.
METHODS
Animals
C3H/HeN mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Animals were maintained in the animal core facility of The Research Institute at Nationwide Children’s Hospital. The animals were permitted access to food and water ad libitum, and a 12:12-h day:night cycle was maintained throughout the study (26). The experimental protocols were approved by the Institutional Animal Care and Use Committee of The Research Institute at Nationwide Children’s Hospital. Mouse pups were exposed to 85% O2 delivered at 8L/min for 14 days or maintained in room air as previously described (15, 27). Animals in 85% O2 or room air were studied in parallel and nursing dams were switched daily. Oxygen concentrations were checked at least twice daily.
Protein sample preparation
After 14 days of hyperoxia exposure, mice were euthanasized, right lungs were tied with suture and were removed and frozen at −80 °C. Lung tissues were homogenized in 0.8 ml of ice cold 0.1 M Dulbecco’s phosphate-buffered saline (pH 7.4) using a Dounce homogenizer. Samples were centrifuged at 12,000 × g for 15 min, and the supernatants were collected and analyzed for total protein content, using the Bradford assay (Bio-Rad, Hercules, CA). The supernatants were stored at −80 °C.
Tissue preparation
At euthanasia, the trachea was cannulated using PE10 tubing and 10% neutral buffered formalin was instilled at 25 cm H2O pressure over 5 min. After 15 min, the trachea was tied and the left lung was removed and fixed overnight in 10% neutral buffered formalin. Lungs were serially dehydrated in increasing concentrations of ethanol and then paraffinized. Lung tissue sections were identically oriented and made into paraffin blocks and sections were cut transversely at the level of entry of the left main bronchus (26).
Western blot analysis
The lung homogenate supernatants were assayed for arginase I and II, endothelial NOS (eNOS), ornithine decarboxylase (ODC), ornithine aminotransferase (OAT), and alpha-smooth muscle actin (α-SMA) protein levels using standard western blot techniques (15, 28). The membranes were incubated with primary antibodies, arginase I, arginase II, eNOS, ODC, OAT, and α-SMA (1:1000 BD Transduction Laboratories, San Diego, CA) and the appropriate secondary antibodies (biotinylated IgG secondary antibodies (1:5000; Vector Laboratories, Burlingame, CA) and streptavidin-horseradish peroxidase conjugate (1:1500; Bio-Rad, Hercules, CA). The protein bands of interest were visualized using chemiluminescence (Amersham ECL) and quantified using densitometry (Sigma Gel, Jandel Scientific, San Rafael, CA). To control for protein loading, blots were reprobed for β-actin (1:10,000; Abcam, Cambridge, MA).
Immunohistochemistry
Fixed lung sections were stained with α-SMA antibody to visualize smooth muscle. Tissue sections were immunostained with α-SMA antibody (1:100 dilution; 2 μg/ml antibody protein concentration, Santa Cruz Biotechnology, Dallas, TX) to identify muscularized pulmonary vessels as previously described (29). Identically oriented tissue sections were visualized with an Olympus Optical (New York, NY) BX-41 microscope (10× magnification) and captured under identical lighting conditions and optical settings using an Insight digital camera. Images were analyzed using digital image analysis software (Image J 1.47v, U.S. National Institutes of Health, Bethesda, MD). Five slides containing lung tissue samples exposed to room air (21% O2) for 14 days were analyzed, as well as four slides containing tissue samples exposed to hyperoxia (85% O2) for the same duration. Six vessels were analyzed in all slides. Percent immunohistochemistry (%IHC) of α-SMA stained area was calculated by using the following formula: (IHC stained vessel area)/(total vessel area) × 100%.
Statistical analysis
The data are presented as mean ± standard errors of the mean of at least three independent experiments. Each experiment contained two groups (room air and 85% O2) and a Student’s t-test was used to compare groups. Significant differences were identified at p < 0.05.
Acknowledgments
This research was funded by the National Heart, Lung, and Blood Institute (Grant K08HL129080), and The Research Institute, Nationwide Children’s Hospital.
Footnotes
The authors declare no conflict of interest.
References
- 1.Mirza H, Ziegler J, Ford S, Padbury J, Tucker R, Laptook A. Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia. J Pediatr. 2014;165(5):909–14. e1. doi: 10.1016/j.jpeds.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 2.del Cerro MJ, Sabate Rotes A, Carton A, Deiros L, Bret M, Cordeiro M, et al. Pulmonary hypertension in bronchopulmonary dysplasia: clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol. 2014;49(1):49–59. doi: 10.1002/ppul.22797. [DOI] [PubMed] [Google Scholar]
- 3.Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol. 2013;37(2):124–31. doi: 10.1053/j.semperi.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. Journal of perinatology : official journal of the California Perinatal Association. 2011;31(10):635–40. doi: 10.1038/jp.2010.213. [DOI] [PubMed] [Google Scholar]
- 5.Silva DM, Nardiello C, Pozarska A, Morty RE. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. American journal of physiology Lung cellular and molecular physiology. 2015;309(11):L1239–72. doi: 10.1152/ajplung.00268.2015. [DOI] [PubMed] [Google Scholar]
- 6.Velten M, Britt RD, Jr, Heyob KM, Welty SE, Eiberger B, Tipple TE, et al. Prenatal inflammation exacerbates hyperoxia-induced functional and structural changes in adult mice. Am J Physiol Regul Integr Comp Physiol. 2012;303(3):R279–90. doi: 10.1152/ajpregu.00029.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velten M, Hutchinson KR, Gorr MW, Wold LE, Lucchesi PA, Rogers LK. Systemic maternal inflammation and neonatal hyperoxia induces remodeling and left ventricular dysfunction in mice. PLoS One. 2011;6(9):e24544. doi: 10.1371/journal.pone.0024544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velten MHK, Wold LE, Rogers LK. Perinatal Inflammation Induces Sex-related Differences in Cardiovascular Morbidities in Mice. American Journal of Physiology Heart and Circulatory Physiology. 2017 doi: 10.1152/ajpheart.00484.2017. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durante W. Role of arginase in vessel wall remodeling. Front Immunol. 2013;4:111. doi: 10.3389/fimmu.2013.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimoda LA, Laurie SS. Vascular remodeling in pulmonary hypertension. J Mol Med (Berl) 2013;91(3):297–309. doi: 10.1007/s00109-013-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trittmann JK, Nelin LD, Zmuda EJ, Gastier-Foster JM, Chen B, Backes CH, et al. Arginase I gene single-nucleotide polymorphism is associated with decreased risk of pulmonary hypertension in bronchopulmonary dysplasia. Acta Paediatr. 2014;103(10):e439–43. doi: 10.1111/apa.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trittmann JK, Jin Y, Chicoine LG, Liu Y, Chen B, Nelin LD. An arginase-1 SNP that protects against the development of pulmonary hypertension in bronchopulmonary dysplasia enhances NO-mediated apoptosis in lymphocytes. Physiol Rep. 2016;4(22) doi: 10.14814/phy2.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trittmann JK, Gastier-Foster JM, Zmuda EJ, Frick J, Rogers LK, Vieland VJ, et al. A single nucleotide polymorphism in the dimethylarginine dimethylaminohydrolase gene is associated with lower risk of pulmonary hypertension in bronchopulmonary dysplasia. Acta Paediatr. 2016;105(4):e170–5. doi: 10.1111/apa.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trittmann JK, Peterson E, Rogers LK, Chen B, Backes CH, Klebanoff MA, et al. Plasma asymmetric dimethylarginine levels are increased in neonates with bronchopulmonary dysplasia-associated pulmonary hypertension. J Pediatr. 2015;166(2):230–3. doi: 10.1016/j.jpeds.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malleske DT, Rogers LK, Velluci SM, Young TL, Park MS, Long DW, et al. Hyperoxia increases hepatic arginase expression and ornithine production in mice. Toxicol Appl Pharmacol. 2006;215(1):109–17. doi: 10.1016/j.taap.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Que LG, Kantrow SP, Jenkinson CP, Piantadosi CA, Huang YC. Induction of arginase isoforms in the lung during hyperoxia. Am J Physiol. 1998;275(1 Pt 1):L96–102. doi: 10.1152/ajplung.1998.275.1.L96. [DOI] [PubMed] [Google Scholar]
- 17.Vadivel A, Aschner JL, Rey-Parra GJ, Magarik J, Zeng H, Summar M, et al. L-citrulline attenuates arrested alveolar growth and pulmonary hypertension in oxygen-induced lung injury in newborn rats. Pediatr Res. 2010;68(6):519–25. doi: 10.1203/PDR.0b013e3181f90278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sopi RB, Haxhiu MA, Martin RJ, Dreshaj IA, Kamath S, Zaidi SI. Disruption of NO-cGMP signaling by neonatal hyperoxia impairs relaxation of lung parenchyma. Am J Physiol Lung Cell Mol Physiol. 2007;293(4):L1029–36. doi: 10.1152/ajplung.00182.2007. [DOI] [PubMed] [Google Scholar]
- 19.Stanley KP, Chicoine LG, Young TL, Reber KM, Lyons CR, Liu Y, et al. Gene transfer with inducible nitric oxide synthase decreases production of urea by arginase in pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(2):L298–306. doi: 10.1152/ajplung.00140.2005. [DOI] [PubMed] [Google Scholar]
- 20.Jin Y, Liu Y, Nelin LD. Extracellular signal-regulated kinase mediates expression of arginase II but not inducible nitric-oxide synthase in lipopolysaccharide-stimulated macrophages. The Journal of biological chemistry. 2015;290(4):2099–111. doi: 10.1074/jbc.M114.599985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talavera MM, Nuthakki S, Cui H, Jin Y, Liu Y, Nelin LD. Immunostimulated Arginase II Expression in Intestinal Epithelial Cells Reduces Nitric Oxide Production and Apoptosis. Front Cell Dev Biol. 2017;5:15. doi: 10.3389/fcell.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tierney DF, Hacker AD. Polyamines, DNA synthesis, and tolerance to hyperoxia of mice and rats. Am Rev Respir Dis. 1989;139(2):387–92. doi: 10.1164/ajrccm/139.2.387. [DOI] [PubMed] [Google Scholar]
- 23.Hacker AD, Tierney DF, O’Brien TK, Witschi HR. Hyperoxic lung injury and polyamine biosynthesis. Age-related differences. Am Rev Respir Dis. 1985;132(2):354–7. doi: 10.1164/arrd.1985.132.2.354. [DOI] [PubMed] [Google Scholar]
- 24.Moiseenko A, Kheirollahi V, Chao CM, Ahmadvand N, Quantius J, Wilhelm J, et al. Origin and characterization of alpha smooth muscle actin-positive cells during murine lung development. Stem Cells. 2017;35(6):1566–78. doi: 10.1002/stem.2615. [DOI] [PubMed] [Google Scholar]
- 25.Kitowska K, Zakrzewicz D, Konigshoff M, Chrobak I, Grimminger F, Seeger W, et al. Functional role and species-specific contribution of arginases in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L34–45. doi: 10.1152/ajplung.00007.2007. [DOI] [PubMed] [Google Scholar]
- 26.Stenger MR, Rose MJ, Joshi MS, Rogers LK, Chicoine LG, Bauer JA, et al. Inhaled nitric oxide prevents 3-nitrotyrosine formation in the lungs of neonatal mice exposed to >95% oxygen. Lung. 2010;188(3):217–27. doi: 10.1007/s00408-010-9235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffay TM, Locy ML, Hill CL, Jindal NS, Rogers LK, Welty SE, et al. Neonatal hyperoxic exposure persistently alters lung secretoglobins and annexin A1. Biomed Res Int. 2013;2013:408485. doi: 10.1155/2013/408485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelin LD, Chicoine LG, Reber KM, English BK, Young TL, Liu Y. Cytokine-induced endothelial arginase expression is dependent on epidermal growth factor receptor. Am J Respir Cell Mol Biol. 2005;33(4):394–401. doi: 10.1165/rcmb.2005-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryant AJ, Carrick RP, McConaha ME, Jones BR, Shay SD, Moore CS, et al. Endothelial HIF signaling regulates pulmonary fibrosis-associated pulmonary hypertension. American journal of physiology Lung cellular and molecular physiology. 2016;310(3):L249–62. doi: 10.1152/ajplung.00258.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]