Abstract
The study of the airway microbiome in children is an area of emerging research, especially in relation to the role microbial diversity may play in acute and chronic inflammation. Three such pediatric airway diseases include cystic fibrosis, asthma, and chronic lung disease of prematurity. In cystic fibrosis, the presence of Pseudomonas spp. is associated with decreased microbial diversity. Decreasing microbial diversity is also associated with poor lung function. In asthma, early viral infections appear to drive changes in bacterial diversity which may be associated with asthma risk. Premature infants with Ureaplasma spp. are at higher risk for chronic lung disease due to inflammation. Microbiome changes due to prematurity also appear to affect the inflammatory response to viral infections post-natally. Importantly, microbial diversity can be measured using metataxonomic (e.g., 16S rRNA sequencing) and metagenomic (e.g., shotgun sequencing) approaches. A metagenomics approach may be preferable as it can provide further granularity of the sample composition, identifying the bacterial species or strain, information on additional microbial components, including fungal and viral components, information about functional genomics of the microbiome, and information about antimicrobial resistance mutations. Future studies of pediatric airway diseases incorporating these techniques may provide evidence for new treatment approaches for these vulnerable patient populations.
Keywords: microbiome, asthma, cystic fibrosis, pediatrics, translational medicine
1. Introduction
The study of the airway microbiome in adults and children is an area of emerging research (Huang et al., 2013). There has been an increased recognition of the diversity of bacteria, fungi, and viruses within both healthy and diseased airways, and the role this diversity may play in acute and chronic inflammation (Huang et al., 2013). Furthermore, characterizing the role of the lung microbiome in chronic airway diseases is important to allow for the development of improved approaches in the treatment of these conditions.
Three such chronic airway diseases are cystic fibrosis, asthma, and chronic lung disease of prematurity. Cystic fibrosis is an autosomal recessive disease that affects more than 30,000 people in the United States (MacKenzie et al., 2014). Recurrent and chronic pulmonary infections are associated with morbidity and mortality (Ramsey, 1996). Adolescents and adults with the most severe genetic mutations often have at least one pulmonary exacerbation per year, and three-quarters require hospitalization or intravenous antibiotics for treatment (O’Sullivan et al., 2015). Furthermore, treatment load and other co-morbidities contribute to overall psychological burden (Jamieson et al., 2014; Quittner et al., 2016). Childhood asthma is a major public health problem in the US, currently affecting 9.6% of US children, and prevalence continues to rise (Centers for Disease Control and Prevention, 2011). Twenty-six percent of all persons with asthma visited an emergency department in 2008, and 7% were hospitalized (Centers for Disease Control and Prevention, 2011). Additionally, the total cost of asthma to society has been estimated to be as high as $56 billion in one year (Barnett & Nurmagambetov, 2011). Approximately one in ten infants per year are born premature (< 37 weeks gestation) (Purisch & Gyamfi-Bannerman, 2017). Prematurity is associated with many lifelong health problems, including bronchopulmonary dysplasia (Purisch & Gyamfi-Bannerman, 2017). The cost of hospital readmission for chronic respiratory illness in preterm infants in California was $45.4 million over 8 years (Underwood et al., 2007).
In cystic fibrosis, the introduction of sequencing to identify bacteria beyond standard culture based techniques led to the discovery of much more complex communities within the airway (Harris et al., 2007; Carmody et al., 2013; Fodor et al., 2012, Lim et al., Tunney et al., 2008; Zemanick et al., 2013, Zhao et al., 2012). This included the presence of many anaerobes, such as, Gemella spp., Prevotella spp., and Veillonella spp. (Carmody et al., 2013; Fodor et al., 2012; Zemanick et al., 2015). The presence of Pseudomonas aeruginosa has consistently been associated with a decrease in overall microbial diversity (Carmody et al., 2013; Smith et al., 2014; Zemanick et al., 2015). The impact of antibiotics on short-term diversity remains mixed (Fodor et al., 2012; Price et al., 2013; Smith et al., 2014; Zemanick et al., 2015), while long-term impact appears to be associated with decreasing microbial diversity (Zhao et al., 2012). Decreasing microbial diversity has also consistently been associated with increasing age and progressive lung disease (Cox et al., 2010; Coburn et al., 2012, Flight et al., 2015).
The airway microbiome has also been studied for associations with the development of asthma and asthma severity. The microbiome of children with asthma has been shown to have a different composition than healthy controls (Hilty et al., 2010). Early infections which perturb the microbiome have been associated with subsequent persistent wheeze later in childhood (Gern, 2009; Holt et al., 2010; Kusel et al., 2008, 2007). Additional external environmental factors may also alter the microbiome and lead to changes in risk for asthma development (Ege et al., 2011; Depner et al., 2015). It has also been suggested that there may be differences in the airway microbiome related to asthma severity (Huang et al., 2015; Zhang et al., 2016). The impact of treatments for severe asthma on the airway microbiome, such as corticosteroids, requires further study (Marri et al., 2013; Zhang et al.; Hilty et al., 2016). Similar to the findings in persons with cystic fibrosis, antibiotic use alters the airway microbiome which may further affect asthma development and severity (Khalkhali et al., 2014; Reiter et al., 2013; Stokholm et al., 2016).
Lastly, the airway microbiome may also provide insight into the post-natal problems faced by premature infants. These infants are at an increased risk to develop chronic lung disease of prematurity, also known as bronchopulmonary dysplasia (BPD). They are at risk of having a prolonged need for oxygen therapy, frequent and severe pulmonary infections that possibly require hospitalization, asthma, exercise intolerance, and pulmonary hypertension (Jobe 2011; Northway et al., 1990). There are limited studies examining the role of the airway microbiome in the chronic lung disease of prematurity.
Fully understanding the microbiome in these pediatric diseases, as well as others, could provide an opportunity to further personalize therapies and improve patient outcomes. With this review, we discuss the role of airway microbiome diversity in three pediatric diseases: cystic fibrosis, asthma, and chronic lung disease of prematurity and point to areas where further study is needed to take full advantage of microbiome information in developing approaches to improve patient outcomes associated with these diseases.
2. Cystic Fibrosis
2.1 The airway microbiome in children with cystic fibrosis
Harris et al. (2007) were the first to capitalize on sequencing approaches to characterize microbiome diversity in cystic fibrosis patients. Their group used Sanger sequencing to look at the difference between standard culture techniques versus a sequence based molecular approach to identify the pathogens within cystic fibrosis bronchoalveolar lavage samples and within control bronchoalveoloar lavage samples in children. They found that molecular based methods and culture based methods were similar in identifying conventional pathogens, such as Staphylococcus aureus and Pseudomonas aeruginosa, which were present in over 85% of their cohort. However, molecular based techniques also identified other families and orders of bacteria, such as Coxiellaceae and Rickettsiales, that otherwise would not have been detected in 46% of the samples. The establishment of sequencing as a viable method to identify the diversity of microbes within the cystic fibrosis airway provided an opportunity for hypothesis generation.
Subsequent studies of the cystic fibrosis lung microbiome have also identified traditionally considered pathogens, specifically Pseudomonas spp., Staphylococcus spp., Haemophilus spp., and Burkholderia spp. (Carmody et al., 2013; Fodor et al., 2012, Lim et al., Tunney et al., 2008; Zemanick et al., 2013, Zhao et al., 2012). These studies also further showed the prevalence of anaerobic and facultative anaerobic bacteria, including Prevotella spp., Veillonella spp., and Gemella spp. (Carmody et al., 2013; Fodor et al., 2012, Lim et al., Tunney et al., 2008; Zemanick et al., 2015, Zhao et al., 2012). While some studies have suggested that the presence of anaerobic bacteria, including Prevotella spp. and Veillonella spp., was associated with lower rates of inflammation (Zemanick et al., 2013), others have noted certain anaerobic organisms, such as Gemella spp., to be associated with onset of pulmonary exacerbations (Carmody et al., 2013). In our own cohort of children with cystic fibrosis, we also detected the presence of previously known pathogens (including Pseudomonas spp., Staphylococcus spp., and Haemophilus spp.) and more recently discovered anaerobes (including Prevotella spp. and Gemella spp.) using 16S rRNA NGS (see Figure 1) (Hahn et al., 2016).
Figure 1. Taxonomic profiles based on 16S targeted amplicon data of a cohort of eight children with cystic fibrosis.
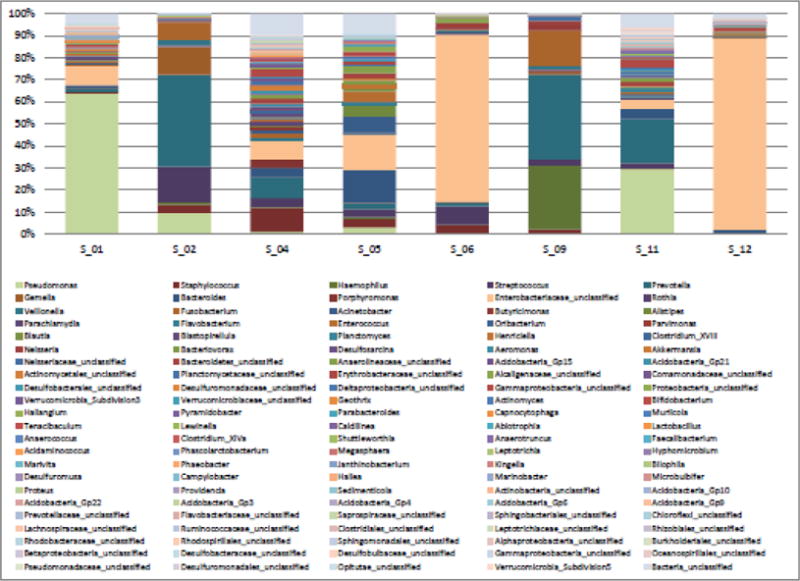
Only genera with a minimum total observation count of 0.1% per subject are shown. Pseudomonas, Staphylococcus, Haemophilus, Streptococcus, Prevotella, Gemella, Bacteroides, Fusobacterium, and Enterobacteraiaceae_unclassified were identified at relative abundances of at least 10% in one or more of the eight subjects.
2.2 The airway microbiome during pulmonary exacerbation
As alluded to above, several studies built upon the capabilities of sequencing, including next generation sequencing (NGS) to look at a particular area of interest within cystic fibrosis disease, pulmonary exacerbations. One research center compared 68 paired baseline and exacerbation sputum samples collected from 28 patients with cystic fibrosis between the ages of 10 and 53 years (Carmody et al., 2013). Overall, they found that significant differences in bacterial community diversity between baseline and exacerbation samples were not observed. However, a subset of research subjects showed considerable changes between baseline and exacerbation samples. The dominant taxa and initial level of diversity were significant predictors of the magnitude of community structure changes between baseline and exacerbation samples. Specifically, samples containing Pseudomonas aeruginosa at baseline had a greater dissimilarity between baseline and exacerbation samples. In addition, those samples with greater baseline diversity were more dissimilar from their paired exacerbation sample than those with lower diversity at baseline.
A study that also incorporated the detection of inflammatory markers in 37 samples from 21 subjects found that the presence of Pseudomonas spp. and Staphylococcus spp. at the onset of pulmonary exacerbation were associated with elevated neutrophil elastase and C- reactive protein (Zemanick et al., 2013). They also similarly found that Pseudomonas spp. was associated with lower microbial diversity. Further corroborating the impact of Pseudomonas spp. on the composition of the airway microbiome in cystic fibrosis, Smith and colleagues (2014) also found that the relative abundance of Pseudomonas spp. showed a strong negative correlation with microbial diversity at the onset of a pulmonary exacerbation.
Despite any trends found of changes in diversity from baseline to onset of pulmonary exacerbation, it is important to note that temporal variability in microbial composition still exists. Carmody et al. (2015) followed daily changes in the lung microbiome during clinical stability and through pulmonary exacerbations, collected daily sputum samples from four adult subjects with cystic fibrosis for 20–27 days. They found that even when subjects were clinically stable, there was day to day variation of the bacterial community structure.
2.3 Impact of antibiotic exposure on microbial diversity
Further research has been done comparing changes in the airway microbiome during treatment of acute exacerbations, and some conflicting results have been reported. Fodor et al. (2012) followed 23 adult patients with cystic fibrosis and obtained sputum samples at multiple time points, including at the onset of an acute exacerbation, after completing a course of antibiotic treatment, and when they were stable. They found that the cystic fibrosis microbiota remained stable over time and infection type, and were highly resilient to antibiotic treatment of exacerbations. Antibiotic use was associated with a small decrease in bacterial richness, but there was a minimal change in the overall community structure. The community composition remained similar during an acute exacerbation and when the patient was stable. They did, however, find a strong correlation between low species richness and poor lung function.
Another study followed 17 patients with cystic fibrosis through an acute pulmonary exacerbation, treatment, and recovery also found that the total and relative abundance of bacterial genera at the population level were stable regardless of clinical status (Price et al, 2013). While they identified more than 170 bacterial genera, 12 of them accounted for more than 90% of the bacterial load amongst all of the samples. A distinct microbial signature associated with the different stages of disease was not identified in this cohort.
However, another study was performed that collected 37 sputum samples in 21 children and adults at two time points: early treatment (day 1–3) and late treatment (day 7–14) (Zemanick et al., 2013). They studied both inflammation and airway microbiota during pulmonary exacerbations. With early treatment, they found that lower diversity was associated with a high relative abundance of Pseudomonas aeruginosa, decreased pulmonary function (forced expiratory volume in one second, FEV1), and increased inflammation (C reactive protein, CRP). The presence of obligate and facultative anaerobes were associated with less inflammation and a higher FEV1. During late treatment, they found that the amount of Pseudomonas aeruginosa present in the samples decreased while anaerobic genera showed marked variability. They also found that the change in the amount of Prevotella spp. present was associated with more variability in FEV1 response to treatment.
An additional study of the short-term effects of antibiotics was performed in 23 adults who experienced 24 exacerbations (Smith et al., 2014). They found a decrease in diversity of the airway microbiome three days after initiating IV antibiotic therapy for pulmonary exacerbation. However, diversity was again increased by day 8–10 of therapy. The authors hypothesized that this might be due to selective killing of Pseudomonas spp. species with IV antibiotic in their cohort, which has been previously shown to have a strong association with sample diversity.
The impact of repeated antibiotic use was explored in a study by Zhao et al. (2012) who followed six patients with cystic fibrosis for 8–9 years. They found that antibiotic use, rather than age or lung function, was most strongly associated with decreasing diversity. Bacterial communities showed both short- and long-term resilience after antibiotic administration. While the bacteria present within the airways showed decreasing diversity over time with disease progression, the total density of bacteria (measured by quantitative PCR) remained stable.
More recent studies have focused on the microbiome within the nasopharynx of infants with cystic fibrosis. In one cohort, antibiotic use over the first 6 months of life was associated with increased colonization of gram negative bacteria (Preveas et al., 2016). Specifically, they found increased colonization of Burkholderia spp. and members of the Enterobacteriaceae family and decreased presence of other commensal organisms which might be beneficial. Another study of infants with cystic fibrosis was performed by Mika and colleagues (2016) also evaluated the effects of antibiotic administration. They found that there was higher diversity and richness after the first antibiotic administration, possibly related to transient colonizers. This change was also associated with a decrease in the relative abundance of the Moralxellaceae family of bacteria and an increase in other bacterial families.
Finally, a proof of principle study for disease monitoring was performed using metagenomic sequencing of 10 sputum samples in three adult subjects with cystic fibrosis (Lim, et al., 2014). They found that the relative abundances of bacterial species changed as clinical treatment was altered. They also found that the bacteria present within all samples encoded a diversity of mechanisms to resist antibiotic treatment. The metabolic potentials of the bacteria differed by the health status and recovery route of each patient.
All of these studies suggest that there are certain core bacteria that remain stable during the onset and treatment of pulmonary exacerbations in cystic fibrosis. They also suggest that antibiotic exposure plays a role in the variability of the airway microbiome both between patients and within patients over time.
2.4 Association of microbial diversity with age and disease severity in cystic fibrosis
In a cohort of 45 children age 2 to 16 years, oropharyngeal swabs were obtained and analyzed to describe their microbial community profile using phylochip (Klepac-Ceraj, et al., 2010). They found an inverse correlation between age and microbial richness (r2=0.61, p<0.001). Another cohort of 20 pediatric patients was compared to a cohort of 23 adult patients, each having respiratory samples obtained at multiple time-points (Boutin, et al., 2015). They found that high inter-individual variation occurred in both pediatrics and adults, and clustering of ecotypes appeared to be dependent upon the presence of Pseudomonas spp., Streptococcus spp., and Burkholderia spp.
Further studies of longitudinal samples from children to adults have established that microbial diversity is also associated with disease progression (Cox et al., 2010). In a study using phylochip that ranged in children 9 months to adults 72 years old, older patients with decreased lung function have more uneven bacterial communities, and that this diversity was lost over time. Pseudomonas aeruginosa and Stenotrophomonas maltophilia abundance was greatest in older cystic fibrosis patients who also exhibited lower bacterial diversity. Haemophilus influenzae was more commonly abundant in younger patients when community diversity was at its peak.
Additional studies of a broad range of age and disease stage of persons with cystic fibrosis found similar results. Coburn and colleagues (2015) also studied a wide range of children as young as 4 years of age through late adulthood. There was significant inter- individual variability in bacterial community diversity and composition. They identified five core genera (Actinomyces spp., Prevotella spp., Rothia spp., Streptococcus spp., and Veillonella spp.) that seemed consistent amongst all patients. Pathogens typically associated with pulmonary exacerbation (Achromobacter spp., Burkholderia spp., Pseudomonas spp., and Stenotrophomonas spp.) were less common, but tended to be a large portion of the bacterial community when present. Bacterial diversity and pulmonary function were greatest in children younger than 10 years of age, appearing to plateau around 25 years of age. Additionally, lower bacterial diversity correlated with worsening pulmonary function. The relationship between microbial diversity and disease severity in cystic fibrosis was further solidified in a study of 93 adults (Flight et al., 2015). Distinct microbial diversity profiles clustered according to pathogen, with Achromobacter spp., Burkholderia spp., and Pseudomonas spp. dominated samples all showing significant decreases in diversity compared to samples not dominated by those pathogens (p<0.001). Furthermore, loss of lung function (specifically FEV1) was also associated with decreasing diversity (p<0.05).
These studies thus suggest that high inter-individual variability exists in the airway microbiome in both pediatric and adult patients, and pathogen dominance effectively drives decreasing diversity no matter what the age of the patient. Decreasing pulmonary function has also been associated with decreasing diversity in both children and adults with cystic fibrosis.
New knowledge gained over the last decade about microbial dysbiosis is altering the traditional understanding of the role of infection in progressive lung disease. Future studies that explore the relationship between community diversity and advancing lung disease may establish whether maintaining diverse communities might improve health and/or whether more targeted anti-microbial therapy might positively impact the patient clinical course (Huang & LiPuma, 2016).
3. Asthma
3.1 Role of the airway microbiome on the development of asthma
The study by Hilty et al. (2010) challenged the traditional thought that the lower airways were sterile and found that the lower airway microbiome differed between asthmatics and controls. The composition of the airway microbiome is one of the many factors involved in the development of asthma. A cohort study found that the early respiratory microbiota composition at ten weeks of life correlated strongly to the stability of the microbiota over the first two years of life, and these early microbiota profiles related to the overall respiratory health of the child (Biesbroek et al., 2014). Those infants with early presence and high abundance of Moraxella- and Corynebacterium/Dolosigranulum-dominated communities had lower rates of respiratory infections in the first two years of life.
It is widely accepted that the frequency and severity of lower respiratory infections in early life are associated with an increased risk of persistent wheeze later in childhood (Gern, 2009; Holt et al., 2010; Kusel et al., 2008, 2007). A longitudinal birth cohort study of high risk infants, with mothers with asthma, found an association between viral infections and acute exacerbations in asthma (Bisgaard et al., 2010). The study found episodes of wheezing to be associated with bacterial infection, from Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae, and independently associated with viral infection. Though this study did not find direct causation between the presence of bacteria or viruses and the exacerbation of asthma-like symptoms, it may have clinical implications if it can be determined that treatment with antibiotics, for example, has an impact on episodes of wheezing.
Another recent birth cohort study found that the bacterial pathogens present in the nasopharyngeal microbiome in infants during upper respiratory viral infections were significant determinants of the spread of the infection to lower airways and may increase the risk of development of persistent asthma (Teo et al., 2015). Previous to this study, viral infections, specifically rhinovirus infections, in early childhood have been associated with the development of asthma later in childhood (Jackson et al., 2008). Another study by Hyde et al. (2014) of infants and children < 2 years of age with viral bronchiolitis found an association with the presence of Proteobacteria (specifically Haemophilus influenzae and Moraxella catarrhalis) and respiratory syncytial virus (RSV) infection, RSV/rhinovirus(RV) co-infection, and acute wheezing. This corroborates the findings in other studies that show an association between Proteobacteria and asthma in older children (Hilty et al., 2010), and suggests a possible link between the two life events. A more recent study found that specific classes of lower respiratory infections, febrile lower respiratory infection and RVs-C positive lower respiratory infections associated with wheezing, were associated with an increased risk of later chronic wheeze by age 5, especially among atopic children (Teo et al., 2015).
Additionally, microbe-host interactions are predicted to play a major role in the development of complex diseases, such as asthma. Dual transcriptomic profiling has been used to examine the interaction between host gene expression and the microbiome in regards to the immune response in asthma (Pérez-Losada et al., 2015). Their analysis showed that functional and compositional changes in the microbiome modulate host mucosal inflammation and immune response during asthma (Pérez-Losada et al., 2015). Similarly, a more recent study examined the host-viral interaction and found that viruses greatly altered the host transcriptome through the down-regulation of ciliated gene cell expression, modulation of Th2 and other asthma associated gene patterns (Wesolowska-Andersen et al., 2017). In another study, the throat microbiota was not found to have a significant association to the development of asthma; while the nasal microbiota composition was associated with asthma and was more susceptible to environmental influences (Depner et al., 2015). Additional infant cohort studies are needed to assess further the role of the airway microbiota in asthma development.
There are many different additional variables that have been found to perturb the composition of the airway microbiome and the associated risk of asthma development, including antibiotic use in early childhood, home environment, older siblings, and pets. The study by Depner et al. (2015) found that the development of asthma was associated decreasing microbial diversity and an increase in Moraxella spp. colonization for non-farm children, but the same relationship was not found in farm children. Children who grow up on a farm are exposed to a greater variety of environmental microorganisms compared to children who do not grow up on farms, and are also found to have less occurrences of asthma (Ege et al., 2011). Infants with older siblings are typically another group less likely to have asthma, but Hasegawa et al. (2016), found infants with older siblings to have a Moraxella-dominated nasal microbiota. The findings of this study suggest that the presence of older siblings in the home and a Moraxella spp.- dominant airway microbiota overall lead to a lower risk of developing asthma, potentially due to fewer lower respiratory infections during the critical window of lung development in early life, though the molecular mechanism behind this remains unknown. Additionally, it has been found that exposure to other children in general, whether through siblings or day care, leads to a Moraxella spp.-dominated nasopharyngeal microbiome (Teo et al., 2015). In their study, Teo et al. (2015) did not find Moraxella spp. to be associated with the risk for asthma development. However, in a cohort of asthma patients at our own institution, we found their nasopharyngeal microbiomes to be dominated by Moraxella spp. compared to controls (Pérez-Losada et al, 2017). Thus, the role of Moraxella spp. specifically still remains unclear; it is possible the impact is species or strain specific.
The role of the airway microbiome in the development of asthma is complicated, and we still do not fully understand the role it plays. Asthma is multi-factorial disease; more research needs to be done to better understand the role the airway microbiome plays in the development of asthma in order to propose better treatments, and possibly prevent the development of this chronic disease. Furthermore, microbial composition and function have been consistently neglected in the inference of asthma phenotypes (i.e., asthma groups defined by a list of clinically observable characteristics) and asthma endotypes (i.e., subtypes of asthma driven by a distinct functional or pathophysiological mechanism). Similarly, host-microbe interactions during pediatric asthma have only recently begun to be studied and inter-relations proposed (Goleva et al., 2013; Perez-Losada et al., 2015). These are topics that should be further investigated.
3.2 Relationship between microbial diversity and asthma severity
It is known that the composition of the airway microbiome of asthmatics is inherently different from non-asthmatics both in pediatric and adult cases (Hilty et al., 2010). Some adult studies have also shown a differing microbial composition between cases of asthma of differing severities (Huang et al., 2015), but similar studies have not yet been published for children.
A recent study found significant differences in the bronchial microbiome between cases of healthy adults, adults with mild-moderate asthma, and adults with severe asthma (Huang et al., 2015). Severe asthmatics were enriched in certain taxa compared to healthy controls and compared to mild-moderate cases, with the largest fold-difference for the Klebsiella spp. Their findings suggest that airway dysbiosis may be specific to cases of severe asthma and that response to treatments, such as corticosteroids may be affected by airway microbial composition and diversity.
An additional study found clear differences in the distributions of phyla between healthy adults, non-severe asthmatic adults and severe asthmatic adults (Zhang et al., 2016). They examined the lower airway microbiota and found that Bacteroidetes and Fusobacteria phyla were reduced in non-severe and severe asthmatic groups compared to healthy controls. In severe asthmatics only, Firmicutes phyla were significantly increased compared to non-severe asthmatics and controls. Proteobacteria phyla levels were more common in non-severe asthmatics compared to healthy controls.
The two studies had discrepancies in the specific differences among the three groups, which may be due to the region of the lung from which the samples were taken. The first study sampled the bronchial microbiome using bronchial brushings while the second sampled the lower airway using induced sputum. It is thought that the respiratory microbiome has complex biogeography, and, therefore, further study needs to be done to investigate within lung microgeographic differences in microbial diversity (Dickson et al., 2015).
Strong similarities have been found between the adult and pediatric asthmatics and controls, despite different sampling methods (Hilty et al., 2010). Thus, examination of adult airway microbiome diversity in relation to asthma severity may hold some merit in pediatric cases. That being said, the relationships found between airway microbial diversity and asthma severity in adults may not hold true in pediatric cases; therefore, it should also be examined in pediatric cases of asthma.
3.3 Effects of asthma treatments on the airway microbiome
The effect of current asthma treatments, such as corticosteroids, on the airway microbiota remains unclear; more study needs to be done to better understand how traditional asthma treatments affect the composition of the airway microbiota. Meta-analysis of data from Hilty et al. (2016) and Marri et al. (2013) indicated that there were no major differences between patients on corticosteroids and controls (Zhang et al., 2016). Further study is required to better understand the airway microbiota’s possible role in corticosteroid treatment resistant cases of asthma.
In addition to understanding the effectiveness of current treatments such as steroids based on the airway microbiome, there is also the possibility of new treatments being developed from new insights. One such area is using antibiotics to alter the airway microbiome in the hopes of affecting the development and/or severity of asthma. Macrolides are a specific class of antibiotics thought to have a potential role in the treatment of asthma. Meta-analysis of different studies found that extended treatment with macrolides led to significant improvement of various clinical asthma outcomes (Reiter et al., 2013). A more recent clinical study found that treatment with azithromycin significantly reduced the duration of asthma-like symptoms in 1–3 year old children with a history of recurrent asthma-like symptoms and that azithromycin had no significant effect on the long-term risk of future episodes of asthma-like symptoms (Stokholm et al., 2016). The effects of macrolides specifically on the airway microbiome have not yet been examined. Current pediatric guidelines do not recommend the use of antibiotics for asthma-like symptoms, though these studies shed light on the possibility of their use for these types of symptoms in the future. However, the use of macrolides for the treatment of asthma and other lung diseases remains controversial (Stokholm et al., 2016). Another potential treatment for asthma often discussed is the use of probiotics to increase the patient’s microbial diversity with the expectation that this will reduce asthmatic episodes. Meta-analysis of studies that administered probiotics prenatally, postnatally or both pre- and postnatally revealed that the administration of probiotics did not significantly reduce asthma/wheeze in children (Elazab et al., 2013). While they did not find a significant reduction in asthma symptoms with the administration of probiotics, they recommended that future trials consider specific strains of probiotics used, use longer follow-up times, and consider associations with oligosaccharides when examining the effects of probiotics on the reduction of asthma risk. While antibiotics or probiotics may be effective in manipulating the microbiome, many caution its use in that role as it is not a complete solution and we have, at best, only a basic understanding behind the mechanisms at work (Robinson & Van Asperen, 2013).
Manipulation of the viral portion of the microbiome has also been theorized as a possible treatment or prevention of asthma in high-risk children and, in theory, sounds promising. Prevention of viral respiratory infections in high-risk children, through use of vaccinations or immunoprophylaxis, have been proposed to prevent the development, or limit exacerbations, of asthma (Gern, 2009; Wu & Hartert, 2011). However, based on a recent study and the absence of effective antiviral therapies, targeting specific pathogenic bacteria in the nasopharyngeal microbiome of high-risk infants could be a more effective way of preventing the development of asthma (Teo et al., 2015).
Pediatric cases of asthma offer a unique opportunity to possibly manipulate the microbiome and prevent the development and exacerbation of the disease (Robinson & Van Asperen, 2013). The potential effects of such manipulation of the airway microbiome by antibiotics or probiotics remain widely unknown (Edwards et al., 2017). Further analysis into the specific modulations of the microbiome resulting from treatment with antibiotics or probiotics will help to improve treatment of pediatric asthma. An improved understanding of the role of the airway microbiome in the development and phenotyping of asthma could lead to the discovery of better treatments for this patient population.
4. Prematurity
4.1 The role of the microbiome in chronic lung disease of prematurity
The early studies regarding the role of the airway microbiome and BPD were severely limited in size and scope. A study of eight intubated preterm infants used terminal restriction fragment length polymorphism (T-RFLP) to characterize the airway bacterial composition and found a diverse array of bacteria in the lower respiratory tract within the first week of life (Stressmann et al., 2010). The limited size of this study prevents further determination of whether these bacteria are transient or more permanently colonized. Another study examined the bacterial composition of the respiratory tract using pyrosequencing over the first 21 days of life for ten intubated preterm infants (Mourani et al., 2011). They found that in the first 72 hours after birth, the majority of tracheal aspirates had an undetectable bacterial load. Within the first week of life, bacterial colonization of the airway occurred with nearly all tracheal aspiration samples having a dominant organism, most frequently either Staphylococus spp. or Ureaplasma spp. The presence of Ureaplasma spp. is important, as Ureaplasma spp. has been found to be associated with a pro-inflammatory response by the immune system and an increased risk for BPD when detected by PCR or culture in humans and in experimental animal models as outlined in a review by Viscardi & Hasday) (2009).
A slightly larger cohort of 25 premature infants, with 10 eventually developing BPD, was the first to characterize the airway microbiome of premature infants and examine its relationship to bacterially mediated inflammation and the development of BPD using 16S rRNA sequencing (Lohmann et al., 2014). Infants with BPD had significant differences in microbiome diversity compared to infants without BPD. At the time of intubation, neonates who eventually developed BPD had lower bacterial diversity that those who did not develop BPD. Analysis at the phylum- level showed that the evolution of the microbiome differed between infants with BPD and infants without BPD; the airway microbiome of infants with BPD showed an increased in Firmicutes phyla and a decrease in Proteobacteria phyla, whereas, infants without BPD had a relatively diverse and more stable microbiome.
Another recent study compared the nasopharyngeal microbiome of premature versus term infants using 16S rRNA sequencing (Perez et al., 2017). In their study of 13 infants (7 born <32 weeks gestation), they found a contrasting profile from Lohmann and colleagues (2014), detecting increased Proteobacteria phyla and decreased Firmicutes phyla in the premature infants. These infants were followed longitudinally up to 2 years of age, and the microbiome changes associated with prematurity persisted during subsequent rhinovirus infection. This might suggest the microbiome changes due to prematurity may modulate the airway inflammatory response to viral infections past the neonatal period.
Recent research also examined the relationship between the airway microbiome and the severity of BPD in ventilated preterm infants using 16S rRNA sequencing (Wagner et al., 2017). At seven days, no relationship was found between microbiome composition and later severity of BPD. Examination of the airway microbiome composition over time revealed that infants who developed more severe BPD had greater bacterial community turnover, increasing from birth, and acquired less Staphylococcus spp. in the first days after birth, and had higher initial levels of Ureaplasma spp. These findings reveal the possibility of using the patterns and evolution of the airway microbiome as a predictor of BPD severity.
A better understanding of the role of the airway microbiome in BPD and lung development in premature infants is important to be able to develop better, more directed, therapies that can lead to better prognoses and late respiratory outcomes of preterm infants. Early respiratory infection and inflammation have been shown to impair alveolar development and contribute to the development of BPD (Kallapur & Jobe, 2006; Speer, 2009). Transgenic mouse modes have been used to show that abnormal alveolarization occurs when TNF-alpha, TGF-alpha, IL-11 and IL-6 are over-expressed, which are cytokines typically expressed as part of the host immune response to an infection (Jobe & Bancalari, 2001).
The development of BPD is a severe risk in premature infants, and a better understanding of the role the airway microbiome plays in the development of BPD may lead to a better prognosis for these premature infants. More research needs to be done to examine and understand the role the airway microbiome plays in BPD.
5. Conclusions
5.1 Insights
- Cystic Fibrosis
-
○Pseudomonas aeruginosa is associated with lower microbial diversity.
-
○While antibiotic use is associated with decreasing bacterial richness, it produces minimal changes in overall community structure over time.
-
○There is a strong correlation between decreasing bacterial diversity and richness and poor lung function.
-
○High inter-individual variability exists in the airway microbiome in both pediatric and adult patients, and pathogen dominance is associated with decreasing diversity at all patient ages.
-
○
- Asthma
-
○The frequency and severity of lower respiratory infections in early life are associated with an increased risk of persistent wheeze later in childhood.
-
○Bacterial pathogens present in the nasopharyngeal microbiome in infants during upper respiratory viral infections may increase the risk of development of persistent asthma.
-
○Higher microbial diversity appears to be associated with a lower risk of developing asthma, which is driven through exposure to older children (such as siblings or daycare) or to the environment (such as farms).
-
○The role of Moraxella spp. appears important but remains unclear, as some studies have noted increased asthma risk, while others have found it to be protective.
-
○
- Prematurity
-
○The presence of Ureaplasma spp. may increase the risk for BPD, likely due to its stimulation of a pro-inflammatory response.
-
○Airway microbiome changes due to prematurity may modulate the airway inflammatory response to viral infections past the neonatal period.
-
○
5.2 Commonalities
In cystic fibrosis, asthma, and BPD, studies of the airway microbiome have suggested that children with these chronic lung diseases might have different microbial communities than their healthy counterparts. A wider variety of bacteria are present than previously thought, and this knowledge has expanded the opportunities for hypothesis driven research, including potential alterations in treatment. Haemophilus influenzae is a bacterial pathogen common in children with either cystic fibrosis or asthma early in the disease process. In cystic fibrosis and BPD, the presence or absence of certain bacteria appear to have a role in either promoting or protecting from airway inflammation.
Another commonality in all respiratory microbiome studies is the impact of the site of sample collection on research findings. Studies in CF have shown differences and similarities between the microbiome characteristics of oropharyngeal swab and sputum samples (Zemanick et al., 2015). Another study of an explanted CF lung showed spatial variability of the microbiome (Brown et al., 2014). While there are no specific studies of asthma or BPD, studies of healthy lung have also shown that the respiratory microbiome has complex biogeography (Dickson et al., 2015). Regional variability is also not unique to the airway microbiome, as it has been shown in other applications and other lung disease states such as the transcriptome in emphysema (Steiling et al., 2013). Thus, this geographic variability needs to be considered when comparing findings across research studies of lung diseases.
5.3 Differences
In cystic fibrosis, it has been shown that Pseudomonas spp., a long known pathogen associated with more severe lung disease, is also associated with microbial diversity. Furthermore, this diversity is associated with the presence of anaerobic organisms. It remains to be shown if anaerobes have positive or negative influences on disease progression, and how antibiotic treatment might be altered to address this. Decreasing microbial diversity in cystic fibrosis has also been associated with age and disease progression, again providing opportunities for further research.
Moraxella spp. is a bacterial pathogen that appears to have opposite effects in children with cystic fibrosis and asthma. The Moraxellacae family of bacteria has been associated with higher levels of microbial diversity in children with cystic fibrosis. Conversely, in healthy children Moraxella spp. has been associated with lower diversity. It seems to have an important role in asthma development, but what that role is remains unclear. In children with asthma, it appears that viral infections play a role in altering the microbiome and thereby affect the development of asthma. The role of antibiotics in asthma development and severity remains unclear.
In premature infants, Ureaplasma spp. may increase the risk for developing BPD. Conversely from asthma, where viruses drive microbiome changes, in prematurity it appears the altered microbiome impacts the inflammatory response to viral infection.
5.4 Recommendations and future directions
Microbial diversity can be measured using metataxonomic and metagenomic approaches (Hilton, et al., 2016). Metataxonomic approaches use 16S rRNA targeted sequencing (e.g., Figure 1) and are able to describe the composition of the microbial community down to the genus or species level. A metagenomics approach, however, uses shotgun sequencing and thus can provide further granularity of the sample composition, identifying the bacterial species or strains, and is also able to provide information about antimicrobial resistance mutations (Hilton, et al., 2016) (see Table 2 and Figure 2). RNA metagenomic profiling could provide even more information about microbial activity and the metabolism of the microbiomes (Castro-Nallar et al., 2015; Pérez-Losada, et al., 2015) (see Figure 3). Future studies of pediatric airway diseases, such as cystic fibrosis, asthma, and BPD that incorporated these techniques would provide further evidence that could be garnered in improving treatment approaches for these vulnerable patient populations.
Table 2.
Comparison of Different Sequencing Approaches for Evaluation of Microbiome
| Technique | Accuracy | Fungus/Virus detection | Resistance mutation detection | Metabolic activity | Limitations of technique |
|---|---|---|---|---|---|
| Metataxonomic (e.g., 16S or ITS) | Genus | Fungus with ITS | No | No | Differences in variable regions (V1–V9) used for sequencing |
| Metagenomic (e.g., shotgun) | Species or Strain | Yes | Yes | No | Need deep sequencing for adequate coverage |
| RNA-seq | Species | Yes | Yes | Yes | RNA less stable than DNA and requires careful processing |
Figure 2. Metagenomic profiles comparing cohorts of asthma (AS) and control (CN) subjects.
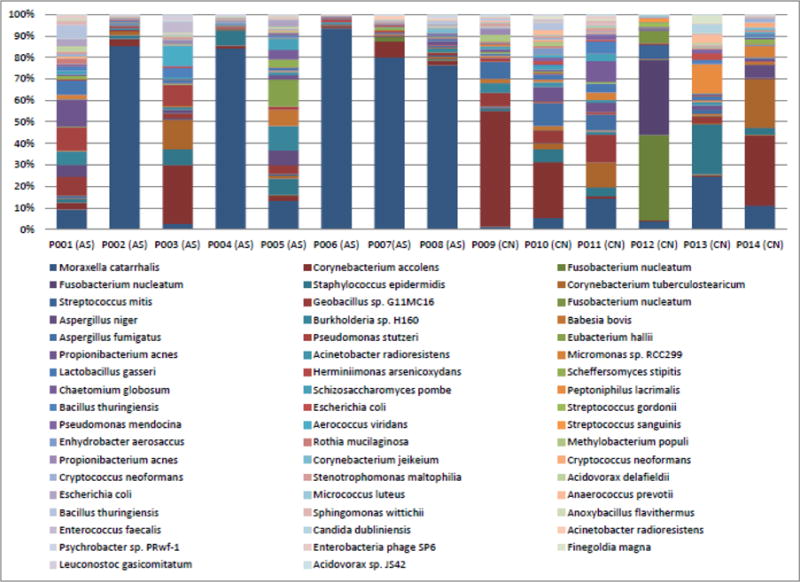
Only species with a minimum total observation count of 0.1% are shown. Asthma patients have a dominance of Moraxella catarrhalis within their microbiomes. In addition to being able to report bacteria at the species level, fungi such as Aspergillus niger and Aspergillus fumigatus, and yeast such as Candida dublinensis are also reported as part of the microbial composition using this approach.
Figure 3. Functional profiles and functional properties mapped to 171 SEED metabolic pathways comparing cohorts of asthma and control subjects.
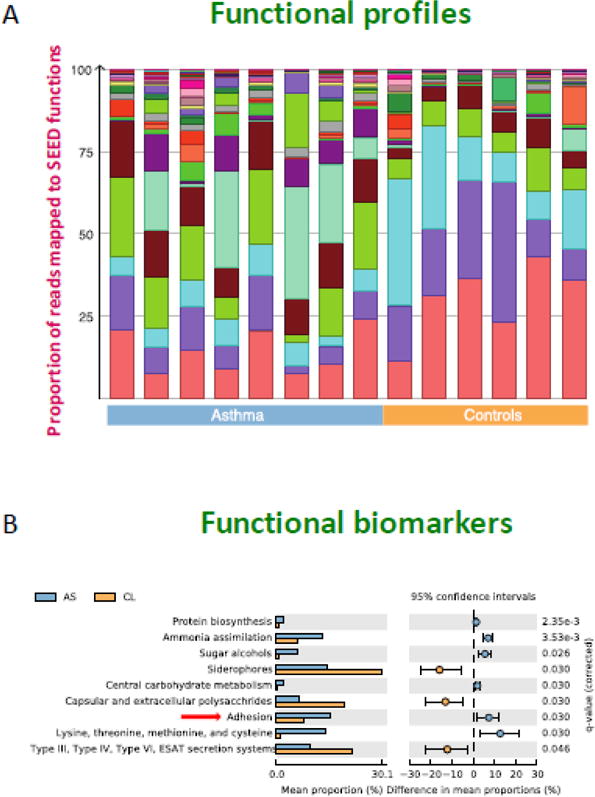
Panel A. Each color in the functional profile represents different SEED functions that were identified in the cohort, demonstrating the similarities and differences between asthmatics and healthy controls. Panel B. This extended error bar plot shows functional properties differing significantly between asthmatics (AS) and healthy controls (CL) with an effect size of one percent. Several pathways are significant, including one involved in virulence functions (adhesion).
Table 1.
Airway Microbiome Insights
| Frequent Bacterial Species | Diversity Trends | |
|---|---|---|
| Cystic Fibrosis | Pseudomonas spp., Staphylococcus spp., Haemophilus spp., Burkholderia spp., Prevotella spp., Veillonella spp., and Gemella spp. | Lower diversity is associated with Pseudomonas spp. and decreased pulmonary function |
| Asthma | Moraxella spp. and Haemophilus spp. | Higher diversity is associated with a lower risk of developing asthma |
| Prematurity | Staphylococcus spp. and Ureaplasma spp. | Increasing bacterial turnover over time is associated with the development of more severe bronchopulmonary dysplasia |
Highlights.
Microbial diversity may play a role in inflammatory pediatric airway diseases.
In cystic fibrosis, decreasing bacterial diversity correlates with poor lung function.
Higher microbial diversity is associated with a lower risk of developing asthma.
Microbiome changes due to prematurity modulate the response to viral infections.
Acknowledgments
This publication was supported by Award numbers UL1TR001876 and KL2TR001877 from the NIH National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. ALH and MP-L were funded in part by a K12 Career Development Program K12HL119994 and the Margaret Q. Landenberger Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127(1):145–52. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Biesbroek G, Tsivtsivadze E, Sanders EAM, Montijn R, Veenhoven RH, Keijser BJF, Bogaert D. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med. 2014;190:1283–1292. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Hermansen MN, Bønnelykke K, Stokholm J, Baty F, Skytt NL, Johnston SL. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin S, Graeber SY, Weitnauer M, Panitz J, Stahl M, Clausznitzer D, Dalpke AH. Comparison of microbiomes from different niches of upper and lower airways in children and adolescents with cystic fibrosis. PLOS One. 2015;10:e0116029. doi: 10.1371/journal.pone.0116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PS, Pope CE, Marsh RL, Qin X, McNamara S, Hoffman LR. Directly sampling the lung of a young child with cystic fibrosis reveals diverse microbiota. Ann Am Thorac Soc. 2014;11(7):1049–55. doi: 10.1513/AnnalsATS.201311-383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody LA, Zhao J, Schloss PD, Petrosino JF, Murray S, Young VB, LiPuma JJ. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Annals of the American Thoracic Society. 2013;10(3):179–187. doi: 10.1513/AnnalsATS.201211-107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody LA, Zhao J, Kalikin LM, LeBar W, Simon RH, Venkataraman A, LiPuma JJ. The daily dynamics of cystic fibrosis airway microbiota during clinical stability and at exacerbation. Microbiome. 2015;3(12):1–11. doi: 10.1186/s40168-015-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Nallar E, Shen Y, Freishtat RJ, Perez-Losada M, Manimaran S, Liu G, Johnson WE, Crandall KA. Integrating microbial and host transcriptomics to characterize asthma-associated microbial communities. BMC Medical Genomics. 2015;8:50. doi: 10.1186/s12920-015-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (CDC) Vital signs: Asthma prevalence, disease characteristics, and self-management education - United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2011;60(17):547–52. [PubMed] [Google Scholar]
- Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S, Guttman DS. Lung microbiota across age and disease stage in cystic fibrosis. Scientific Reports. 2015;5:10241. doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MJ, Allgaier M, Taylor B, Baek MS, Huang YJ, Daly RA, Lynch SV. Airway microbiota and pathogen abudance in age-stratified cystic fibrosis patients. PLoS One. 2010;5:e11044. doi: 10.1371/journal.pone.0011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depner M, Ege MJ, Cox MJ, Dwyer S, Walker AW, Birzele LT, Legatzki A. Bacterial microbiota of the upper respiratory tract and childhood asthma. J Allergy Clin Immunol. 2017;139(3):826–834 e13. doi: 10.1016/j.jaci.2016.05.050. [DOI] [PubMed] [Google Scholar]
- Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB, Curtis JL. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc. 2015;12:821–830. doi: 10.1513/AnnalsATS.201501-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Relman DA. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: A molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MR, Walton RP, Jackson DJ, Feleszko W, Jartti T, EAACI Anti- infectives in Asthma and Asthma Exacerbations Task Force The potential of anti-infectives and immunomodulators as therapies for asthma and asthma exacerbations. Allergy. 2017 doi: 10.1111/all.13257. Jul 19, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WOCM, Braun-Fahrländer C, von Mutius E. Exposure to Environmental Microorganisms and Childhood Asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E, Gorbach S. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics. 2013;132:e666–76. doi: 10.1542/peds.2013-0246. [DOI] [PubMed] [Google Scholar]
- Flight WG, Smith A, Paisey C, Marchesi JR, Bull MJ, Norville PJ, Mahenthiralingam E. Rapid detection of emerging pathogens and loss of microbial diversity associated with severe lung disease in cystic fibrosis. J Clin Microbiol. 2015;53(7):2022–9. doi: 10.1128/JCM.00432-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor AA, Klem ER, Gilpin DF, Elborn JS, Boucher RC, Tunney MM, Wolfgang MC. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One. 2012;7(9):e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gern JE. Rhinovirus and the initiation of asthma. Curr Opin Allergy Clin Immunol. 2009;9:73–78. doi: 10.1097/ACI.0b013e32831f8f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, Singh PK. Direct sampling of cystic fibrosis lungs indicates that DNA-based analysis of upper-airway specimens can misrepresent lung microbiota. Proceedings of the National Academy of Sciences. 2012;109(34):13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goleva E, Jackson LP, Harris JK, Robertson CE, Sutherland ER, Hall CF, Good JT, Jr, Gelfand EW, Martin RJ, Leung DY. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med. 2013;188(10):1193–201. doi: 10.1164/rccm.201304-0775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Sanyal A, Perez GF, Colberg-Poley AM, Campos J, Rose MC, Perez- Losada M. Different next generation sequencing platforms produce different microbial profiles and diversity in cystic fibrosis sputum. J Microbial Methods. 2016;130:95–99. doi: 10.1016/j.mimet.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton TH, Green DM, Cutting GR, Morrison HG, Sogin ML, Gifford AH, O’Toole GA. The microbiome in pediatric cystic fibrosis patients: the role of shared environment suggests a window of intervention. Microbiome. 2014;2(14):1–5. doi: 10.1186/2049-2618-2-14. Retrieved from http://microbiomejournal.com/content/2/1/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JK, De Groote MA, Sagel SD, Zemanick ET, Kapsner R, Penvari C, Pace NR. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proceedings of the National Academy of Sciences. 2007;104(51):20529–20533. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Linnemann RW, Mansbach JM, Ajami NJ, Espinola JA, Fiechtner LG, Petrosino JF, Camargo CA. Household siblings and nasal and fecal microbiota in infants. Pediatr Int. 2017;59:473–481. doi: 10.1111/ped.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton SK, Castro-Nallar E, Perez-Losada M, Toma I, McCaffrey TA, Hoffman EP, Crandall KA. Metataxonomic and metagenomic approaches vs. culture- based techniques for clinical pathology. Front Microbiol. 2016;7:484. doi: 10.3389/fmicb.2016.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Cookson WOC. Disordered microbial communities in asthmatic airways. PLOS ONE. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Rowe J, Kusel M, Parsons F, Hollams EM, Bosco A, Sly PD. Toward improved prediction of risk for atopy and asthma among preschoolers: A prospective cohort study. J Allergy Clin Immunol. 2010;125:653–659.e7. doi: 10.1016/j.jaci.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Charlson ES, Collman RG, Colombini-Hatch S, Martinez FD, Senior RM. The role of the lung microbiome in health and disease. A national heart, lung, and blood institute workshop report. Am J Respir Crit Care Med. 2013;187:1382–7. doi: 10.1164/rccm.201303-0488WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, Boushey H. The Airway Microbiome in Severe Asthma: Associations with Disease Features and Severity. J Allergy Clin Immunol. 2015;136(4):874–84. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, LiPuma JJ. The Microbiome in Cystic Fibrosis. Clin Chest Med. 2016;37:59–67. doi: 10.1016/j.ccm.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde ER, Petrosino JF, Piedra PA, Camargo CA, Espinola JA, Mansbach JM. Nasopharyngeal Proteobacteria are associated with viral etiology and acute wheezing in children with severe bronchiolitis. J Allergy Clin Immunol. 2014;133:1220–2. doi: 10.1016/j.jaci.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Lemanske RF. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson N, Fitzgerald D, Singh-Grewal D, Hanson CS, Craig JC, Tong A. Children’s experiences of cystic fibrosis: a systematic review of qualitative studies. Pediatrics. 2014;133:e1683–97. doi: 10.1542/peds.2014-0009. [DOI] [PubMed] [Google Scholar]
- Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–72. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed. 2006;91:F132–5. doi: 10.1136/adc.2004.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac-Ceraj V, Lemon KP, Martin TR, Allgaier M, Kembel SW, Knapp AA, Kolter R. Relationship between cystic fibrosis respiratory tract bacterial communities and age, genotype, antibiotics, and Pseudomonas aeruginosa. Environmental Microbiology. 2010;12:1293–1303. doi: 10.1111/j.1462-2920.2010.02173.x. [DOI] [PubMed] [Google Scholar]
- Kusel MMH, De Klerk N, Holt PG, Sly PD. Antibiotic use in the first year of life and risk of atopic disease in early childhood. Clin Exp Allergy. 2008;38:1921–1928. doi: 10.1111/j.1365-2222.2008.03138.x. [DOI] [PubMed] [Google Scholar]
- Kusel MMH, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YW, Evangelista JS, Schmieder R, Bailey B, Haynes M, Furlan M, Conrad D. Clinical insights from metagenomic analysis of sputum samples from patients with cystic fibrosis. J Clin Microbiol. 2014;52(2):425–437. doi: 10.1128/JCM.02204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann P, Luna RA, Hollister EB, Devaraj S, Mistretta TA, Welty SE, Versalovic J. The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatr Res. 2014;76:294–301. doi: 10.1038/pr.2014.85. [DOI] [PubMed] [Google Scholar]
- Lu W, Yu J, Ai Q, Liu D, Song C, Li L. Increased Constituent Ratios of Klebsiella sp., Acinetobacter sp., and Streptococcus sp. and a Decrease in Microflora Diversity May Be Indicators of Ventilator-Associated Pneumonia: A Prospective Study in the Respiratory Tracts of Neonates. PLoS One. 2014;9:e87504. doi: 10.1371/journal.pone.0087504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, Marshall BC. Longevity of Patients With Cystic Fibrosis in 2000 to 2010 and Beyond: Survival Analysis of the Cystic Fibrosis Foundation Patient Registry. Ann Intern Med. 2014;161:233–241. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131:346–352.e3. doi: 10.1016/j.jaci.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika M, Korten I, Qi W, Regamey N, Frey U, Casaulta C, Latzin P, Hilty M, SCILD study group The nasal microbiota in infants with cystic fibrosis in the first year of life: a prospective cohort study. Lancet Respir Med. 2016;4:627–35. doi: 10.1016/S2213-2600(16)30081-9. [DOI] [PubMed] [Google Scholar]
- Mourani PM, Harris JK, Sontag MK, Robertson CE, Abman SH. Molecular identification of bacteria in tracheal aspirate fluid from mechanically ventilated preterm infants. PLoS One. 2011;6(10):e25959. doi: 10.1371/journal.pone.0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northway WH, Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, Brown BW. Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;323:1793–1799. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- O’Sullivan AK, Signorovitch JE, Fang A, Wagener J, Hodgkins P. Clinical and economic burden of pulmonary exacerbations in patients with cystic fibrosis who are homozygous for the F508del mutation. Value Health. 2015;18:A658–9. doi: 10.1016/j.jval.2015.09.2386. [DOI] [Google Scholar]
- Perez GF, Perez-Losada M, Isaza N, Rose MC, Colberg-Poley AM, Nino G. Nasopharyngeal microbiome in premature infants and stability during rhinovirus infection. J Investig Med. 2017 doi: 10.1136/jim-2017-000414. Mar 31 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Losada M, Alamri L, Crandall KA, Freishtat RJ. Nasopharyngeal microbiome diversity changes over time in children with asthma. PLOS One. 2017;12:e0170543. doi: 10.1371/journal.pone.0170543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Losada M, Castro-Nallar E, Bendall ML, Freishtat RJ, Crandall KA. Dual transcriptomic profiling of host and microbiota during health and disease in pediatric asthma. PLoS One. 2015;10(6):e0131819. doi: 10.1371/journal.pone.0131819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preveas SMPJ, de Winter-de Groot KM, Janssens HM, deSteenjuijsen Piters WAA, Tramper-Stranders GA, Wyllie AL, Bogaert D. Development of the Nasopharyngeal Microbiota in Infants with Cystic Fibrosis. AmJ Resp Crit Care Med. 2016;193:504–515. doi: 10.1164/rccm.201509-1759OC. [DOI] [PubMed] [Google Scholar]
- Price KE, Hampton TH, Gifford AH, Doben EL, Hogan DA, Morrison HG, O’Toole GA. Unique microbial communities persist in individual cystic fibrosis patients throughout clinical exacerbation. Microbiome. 2013;1(27):1–11. doi: 10.1186/2049-2618-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol. 2017 doi: 10.1053/j.semperi.2017.07.009. Aug 30, pii: S0146-0005(17)30081-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Quittner AL, Saez-Flores E, Barton JD. The psychological burden of cystic fibrosis. Curr Opin Pulm Med. 2016;22:187–91. doi: 10.1097/MCP.0000000000000244. [DOI] [PubMed] [Google Scholar]
- Ramsey BW. Management of Pulmonary Disease in Patients with Cystic Fibrosis. N Engl J Med. 1996;335:179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- Reiter J, Demirel N, Mendy A, Gasana J, Vieira ER, Colin AA, Quizon A, Forno E. Macrolides for the long-term management of asthma - a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040–1049. doi: 10.1111/all.12199. [DOI] [PubMed] [Google Scholar]
- Robinson PD, Van Asperen P. Newer treatments in the management of pediatric asthma. Pediatr Drugs. 2013;15:291. doi: 10.1007/s40272-013-0020-x. [DOI] [PubMed] [Google Scholar]
- Sirvent JM, Torres A, Vidaur L, Armengol J, de Batlle J, Bonet A. Tracheal colonisation within 24 h of intubation in patients with head trauma: risk factor for developing early-onset ventilator-associated pneumonia. Intensive Care Med. 2000;26:1369–1372. doi: 10.1007/s001340000611. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Badrick AC, Zakrzewski M, Krause L, Bell SC, Anderson GJ, Reid DW. Pyrosequencing reveals transient cystic fibrosis lung microbiome changes with intranvenous antibiotics. Eur Respir J. 2014;44:922–30. doi: 10.1183/09031936.00203013. [DOI] [PubMed] [Google Scholar]
- Speer CP. Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology. 2009;95:353–361. doi: 10.1159/000209301. [DOI] [PubMed] [Google Scholar]
- Steiling K, Lenburg ME, Spira A. Personalized management of chronic obstructive disease via transcriptomic profiling of the airway and lung. Ann Am Thorac Soc. 2013;10:S190–6. doi: 10.1513/AnnalsATS.201306-190AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Pedersen TM, Vinding RK, Bisgaard H. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: A randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19–26. doi: 10.1016/S2213-2600(15)00500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stressmann FA, Connett GJ, Goss K, Kollamparambil TG, Patel N, Payne MS, Rogers GB. The use of culture-independent tools to characterize bacteria in endo-tracheal aspirates from pre-term infants at risk of bronchopulmonary dysplasia. J Perinat Med. 2010;38:333–337. doi: 10.1515/JPM.2010.026. [DOI] [PubMed] [Google Scholar]
- Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, Inouye M. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunney MM, Field TR, Moriarty TF, Patrick S, Doering G, Muhlebach MS, Elborn JS. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;177:995–1001. doi: 10.1164/rccm.201210-1937OC. [DOI] [PubMed] [Google Scholar]
- Underwood MA, Danielsen B, Gilbert WM. Costs, causes, and rates of rehospitalization of preterm infants. J Perinatol. 2007;27:614–619. doi: 10.1038/sj.jp.721180. [DOI] [PubMed] [Google Scholar]
- Viscardi RM, Hasday JD. Role of Ureaplasma species in neonatal chronic lung disease: Epidemiologic and experimental evidence. Pediatr Res. 2009;65:84R–90R. doi: 10.1203/PDR.0b013e31819dc2f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BD, Sontag MK, Harris JK, Miller JI, Morrow L, Robertson CE, Mourani PM. Airway Microbial Community Turnover Differs by BPD Severity in Ventilated Preterm Infants. PLoS One. 2017;12:e0170120. doi: 10.1371/journal.pone.0170120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowska-Andersen A, Everman JL, Davidson R, Rios C, Herrin R, Eng C, Seibold MA. Dual RNA-seq reveals viral infections in asthmatic children without respiratory illness which are associated with changes in the airway transcriptome. Genome Biol. 2017;18:12. doi: 10.1186/s13059-016-1140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9:731–745. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalaz M, Altun-Koroglu O, Ulusoy B, Yildiz B, Akisu M, Vardar F, Ozinel MA, Kultursay N. Evaluation of device-associated infections in a neonatal intensive care unit. Turk J Pediatr. 2012;54:128–135. [PubMed] [Google Scholar]
- Zemanick ET, Harris JK, Wagner BD, Robertson CE, Sagel SD, Stevens MJ, Laguna TA. Inflammation and airway microbiota during cystic fibrosis pulmonary exacerbations. PLoS One. 2013;8(4):e62917. doi: 10.1371/journal.pone.0062917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemanick ET, Wagner BD, Robertson CE, Stevens MJ, Szefler SJ, Accurso FJ, Harris JK. Assessment of airway microbiota and inflammation in cystic fibrosis using multiple sampling methods. Annals of the American Thoracic Society. 2015;12(2):221–229. doi: 10.1513/AnnalsATS.201407-310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cox M, Liang Z, Brinkmann F, Cardenas PA, Duff R, Chung KF. Airway microbiota in severe asthma and relationship to asthma severity and phenotypes. PLoS One. 2016;11(4):e0152724. doi: 10.1371/journal.pone.0152724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, LiPuma JJ. Decade-long bacterial community dynamics in cystic fibrosis airways. Proceedings of the National Academy of Sciences. 2012;109(15):5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]


