Abstract
Coagulation is a critical component in the progression of liver disease. Identification of key molecules involved in the intrahepatic activation of coagulation (IAOC) will be instrumental in the development of effective therapies against liver disease. Using a mouse model of concanavalin A (Con A)-induced hepatitis, in which IAOC plays an essential role in causing liver injury, we uncovered a previously unknown procoagulant function of chitinase 3 like 1 (Chi3l1). Chi3l1 expression is dramatically elevated after Con A challenge, which is dependent on Con A-induced T cell activation, and the resulting IFN-γ and TNF-α productions. Compared to wild type mice, Chi3l1−/− mice show less IAOC, reduced tissue factor (TF) expression and attenuated liver injury. Reconstituting Chi3l1−/− mice with recombinant TF triggers IAOC and augments liver injury. Conclusion: Our data demonstrate that Chi3l1, through induction of TF via MAPK activation, promotes IAOC and tissue injury.
Keywords: Chi3l1, intrahepatic activation of coagulation, liver disease, tissue factor, concanavalin A
Introduction
The activation of blood coagulation cascade is evident in a variety of acute liver injury1–3. Growing evidence indicates that coagulation is not merely a process reactive to toxicity, but rather a critical determinant of acute liver disease progression4–10. A reduction in liver damage by administration of anticoagulant has been observed in a variety of animal models, including APAP-induced hepatotoxicity, hepatic ischemia reperfusion injury, as well as liver injury caused by lipopolysaccharide/D-galactosamine, anti-Fas antibody and concanavalin A (Con A)4–10. The alleviation of liver injury in hepatocyte TF deficient mice further demonstrates the significance of intrahepatic activation of coagulation (IAOC) in acute liver injury11,12. However, the mechanisms by which coagulation cascade exacerbates liver disease are not completely understood.
To gain mechanistic insights and identify key players involved in intrahepatic coagulation, we used a mouse model of Con A-induced liver injury (CILI), in which excessive IAOC drives tissue damage9,10,13,14. Con A is a lectin with agglutination activities resulting in prominent intra-sinusoidal stasis. This phenomenon is manifested by erythrocyte agglutination, adherence of lymphocytes and neutrophils to the endothelium, as well as platelet aggregation/degranulation. Together, these events result in a marked decrease of intrahepatic blood flow and elevated portal pressure, culminating in severe liver injury9,15. It has been demonstrated that anticoagulant treatment significantly attenuates CILI, supporting that the sinusoidal hypercoagulation with increased thrombin generation plays a paramount role in the injury process9,14. To identify novel mediators of IAOC, we screened a panel of soluble factors produced by innate and adaptive immune cells that are known to be activated by Con A. We found that both mRNA and protein levels of chitinase 3 like 1 (Chi3l1) were significantly elevated after Con A challenge.
Chi3l1 is a chitinase like soluble protein without chitinase activities16. It is produced by multiple cell types, including neutrophils, macrophages, chondrocytes, synovial cells, smooth muscle cells, endothelial cells, and tumor cells16,17. Increased levels of Chi3l1 has been correlated with poor prognosis in liver diseases, such as hepatic fibrosis, non-alcoholic fatty liver, alcoholic liver disease, and hepatocellular carcinoma18,19. However, the biological functions of Chi3l1 in liver remain mysterious. Our study indicates that Chi3l1 plays a critical role in promoting IAOC and liver injury through inducing tissue factor (TF) expression via MAPK activation. This discloses a previously unappreciated pro-coagulant function of Chi3l1. The findings suggest that Chi3l1 may serve as a therapeutic target in the treatment of liver disease associated with intrahepatic coagulation.
Results
Chi3l1 contributes to CILI
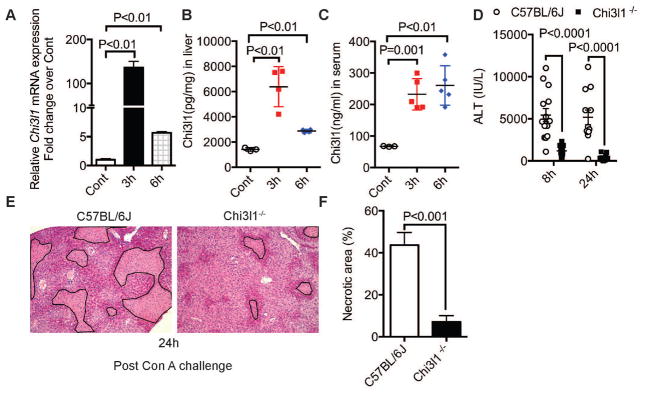
Con A challenge causes soluble mediators released by a variety of immune cells in the liver, including natural killer (NK) cell, NKT cell, neutrophils, macrophages, and T cells20–23. We measured the hepatic mRNA expression of a panel of these mediators in wild-type (WT) C57Bl/6J mice at 3h after Con A challenge (Fig. S1). Among these mediators, both mRNA and protein levels of Chi3l1 were dramatically increased post-Con A (Fig. 1, A–C). Interestingly, we found that mice deficient in Chi3l1 (Chi3l1−/−) were less susceptible to CILI than WT mice. The ALT levels in Chi3l1−/− mice were 1,200 and 600 IU/l at 8 and 24 h, respectively; whereas the levels in WT mice were as high as 5600 IU/l (Fig. 1D). In accordance with the reduced ALT activities, the histopathology of the liver showed less hepatocyte injury in Chi3l1−/− (Fig. 1, E&F). These results suggest that Chi3l1 is produced and released after Con A challenge and it contributes to CILI.
Fig. 1. Chi3l1 contributes to Con A-induced liver injury in mice.
Hepatic mRNA expression of Chi3l1 (A), and protein levels of Chi3l1 in liver (B) and serum (C) were measured in male WT (C57BL/6J) mice treated with 15 mg/kg Con A by intravenous injection (n=3–5 mice per group). (D) Serum ALT levels were determined at 8 and 24 h post-Con A treatment (n=15–22 mice per group). (E) Shown are representative H&E-stained liver sections from at least 4 mice per group at 24h after Con A treatment (magnification, 100×). Necrotic areas are outlined. (F) Necrotic areas are quantified by Image J. P values are as indicated. Two-tailed, unpaired t test in D and F; one-way ANOVA in A, B, C.
Con A-induced immune responses are not affected by Chi3l1
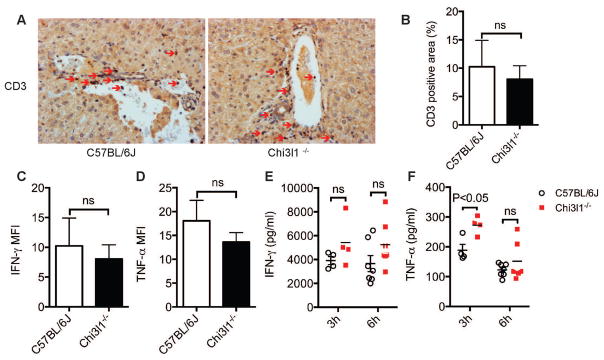
The activation of immune cells (T, NKT, neutrophils, macrophages) and their cytokine products, especially IFN-γ and TNF-α, have been reported to contribute to CILI20,21,24,25. We first investigated whether Chi3l1 could potentially affect these immune cells and their cytokine production. Our data showed no significant differences in the number of CD3+ T cells in the livers of WT and Chi3l1−/− mice (Fig. 2, A&B). The amounts of IFN-γ and TNF-α production by hepatic T cells were also similar between WT and Chi3l1−/− mice (Fig. 2, C–F). The serum levels of TNF-α were even higher in Chi3l1−/− than WT mice at 3h post-Con A (Fig. 2F). NKT cells and their IL-4 production are important in CILI20. However, our data (Fig. S3, A&B) showed that the numbers of hepatic NKT cells and their intracellular expression of IL-4 were comparable between WT and Chi3l1−/− mice. It has been reported that neutrophils and macrophages are important in promoting T cell recruitment and subsequent development of hepatitis21,24,26. However, we did not find any differences in the numbers of neutrophils or macrophages in the liver between WT and Chi3l1−/− mice (Fig. S4, A–D). Taken together, these data suggest that Chi3l1 does not affect Con A-induced activation of the innate and adaptive immune systems.
Fig. 2. Con A-induced T cell responses are not affected by Chi3l1.
Male WT and Chi3l1−/− mice were treated with Con A as described in Fig. 1. (A) At 6h after Con A administration, hepatic T cells were identified by immunohistochemical staining using anti-CD3 antibody (magnification: 200×). Red arrows indicate CD3+ T cells. Shown are representative images from at least 4 mice per group. (B) The number of hepatic CD3+ T cells are quantified by Image J. (C–D) At 3h after Con A administration, CD3+ T cell-derived IFN-γ (C) and TNF-α (D) were measured by intracellular staining and flow cytometry (n=5 mice per group). (E–F) At 3h and 6h after Con A administration, Serum levels of IFN-γ (E) and TNF-α (F) were measured by ELISA (n=4–7 mice per group). P values are as indicated. Two-tailed, unpaired t test were performed in B–F.
Chi3l1-deficiency attenuates CILI
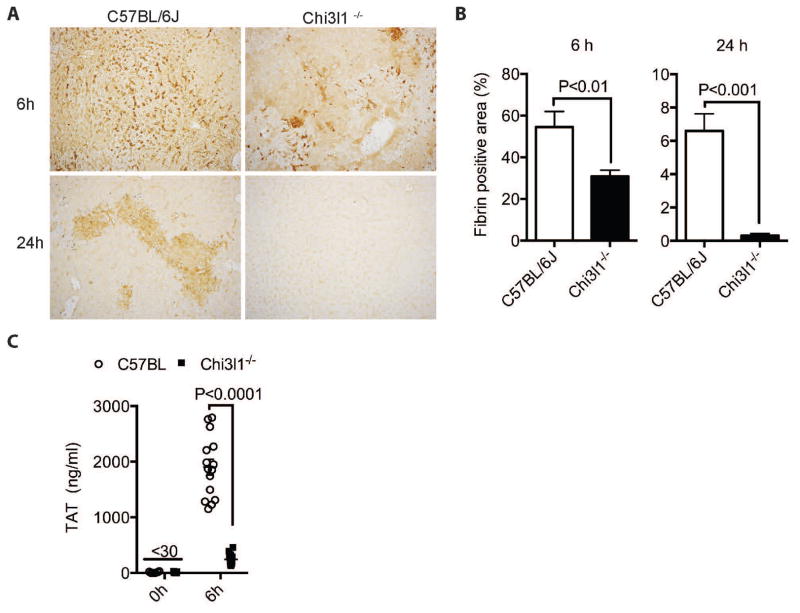
Aside from the pro-inflammatory immune responses, IAOC represents another critical underlying mechanism for CILI9,10. To explore whether Chi3l1 contributes to CILI through promoting coagulation, we compared fibrin(ogen) deposition, thrombin anti-thrombin complex (TAT) levels and platelets influx between WT and Chi3l1−/− mice. WT mice had higher levels of fibrin(ogen) deposition (Fig. 3, A&B) in liver tissues than Chi3l1−/− mice at both 6h and 24h post-Con A. TAT complexes are used as an indicator for thrombin formation in the circulation27. Our data showed that the plasma TAT levels were significantly elevated after Con A challenge in WT mice, but not in Chi3l1−/− mice (Fig. 3E). Moreover, platelets influx significantly increased at 3h post-Con A, but there was no difference between WT and Chi3l1−/− mice (Fig. S5, A&B). Taken together, these results demonstrate that Chi3l1 is critically involved in IAOC.
Fig. 3. Chi3l1−/− mice exhibit attenuated intrahepatic coagulation upon Con A challenge.
(A–B) Male WT and Chi3l1−/− were treated with Con A as described in Fig. 1 for 6h and 24h. Immunohistochemical staining for fibrin(ogen) (magnification: 200x) were performed and quantified. Images shown are representative of at least 4 mice per group. (C) The levels of TAT complex were measured in plasma of WT and Chi3l1−/− mice at 6h post-Con A treatment (n=14–15 per group). P values are as indicated. Two- tailed, unpaired t test were performed in B, D–F.
The effect of Chi3l1 on CILI depends on T cell-derived IFN-γ and TNF-α
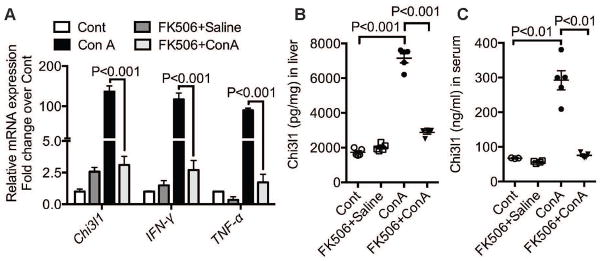
It has been reported that T cell activation and their production of IFN-γ and TNF-α contribute to CILI24. To determine if the Chi3l1 is a downstream mediator of this pathway, we treated WT mice with a T cell suppressor, FK506, 1 h before Con A challenge. As expected, FK506 treatment inhibited Con A-induced up-regulation of IFN-γ and TNF-α mRNA levels in the liver (Fig. 4A). Interestingly, the mRNA expression levels of Chi3l1 were also markedly reduced by FK506 (Fig. 4A). Consistently, the Chi3l1 protein expression in the liver and serum were also dramatically reduced by the FK506 treatment (Fig. 4, B&C). These results indicate that T cell activation is necessary to induce Chi3l1 production. Systematic time course studies also showed that the elevation of IFN-γ and TNF-α preceded that of Chi3l1 (Fig. 5A). Thus, to determine if IFN-γ and TNF-α induce Chi3l1 expression, we treated mice with anti-IFN-γ and TNF-α antibodies prior to Con A challenge. Blocking IFN-γ and TNF-α prevented Con A-induced up-regulation of both mRNA and protein expressions of Chi3l1 (Fig. 5, B–D).
Fig. 4. Chi3l1 production is dependent on T cell activity.
Male WT mice were orally treated with a T cell inhibitor FK506 at 1h prior to Con A administration. (A) Hepatic mRNA expression levels of Chi3l1, IFN-γ and TNF-α were measured at 3h post-Con A by qRT-PCR. (B–C) Protein levels of Chi3l1 in the liver (B) and serum (C) were measured by ELISA (n=3–5 mice per group). P values are as indicated. One-way ANOVA was performed in A–C.
Fig. 5. Chi3l1 production is dependent on IFN-γ and TNF-α.
(A) Con A-induced up-regulation of IFN-γ, TNF-α, and Chi3l1 mRNA levels in the liver were measured in WT mice at various time points (n=4 mice per group). (B–F) WT mice were treated with anti-IFN-γ and TNF-α antibodies by i.p. injection at the same time of Con A administration and sacrificed at 3h post-Con A treatment. The hepatic mRNA levels of Chi3l1 (B), and protein levels of Chi3l1 in the liver (C) and serum (D) were measured (n=3–5 mice per group). (E–F) LSEC and MΦ were isolated from naïve WT mice and treated with IFN-γ (50ng/ml) or TNF-α (50ng/ml) for 3h in vitro. Chi3l1 mRNA expression levels were measured by qRT-PCR. P values are as indicated. One-way ANOVA was performed in A–F.
In agreement with the notion that IFN-γ and TNF-α are key players causing CILI, our data showed that blocking IFN-γ and TNF-α significantly reduced fibrin(ogen) deposition, the degree of hepatic necrosis, and ALT activities (Fig. S6, A–C). To further demonstrate that Chi3l1 can be induced by IFN-γ and TNF-α, we isolated liver sinusoid endothelial cells (LSEC) and hepatic macrophages (MΦ) from naïve WT mice and treated the cells with either IFN-γ or TNF-α. We found that both IFN-γ and TNF-α could significantly up-regulate Chi3l1 mRNA expression in LSEC and hepatic MΦ (Fig 5, E&F). These data suggest that IFN-γ and TNF-α are potent regulators in inducing of Chi3l1 both in vivo and in vitro.
Chi3l1 plays a crucial role in the induction of TF
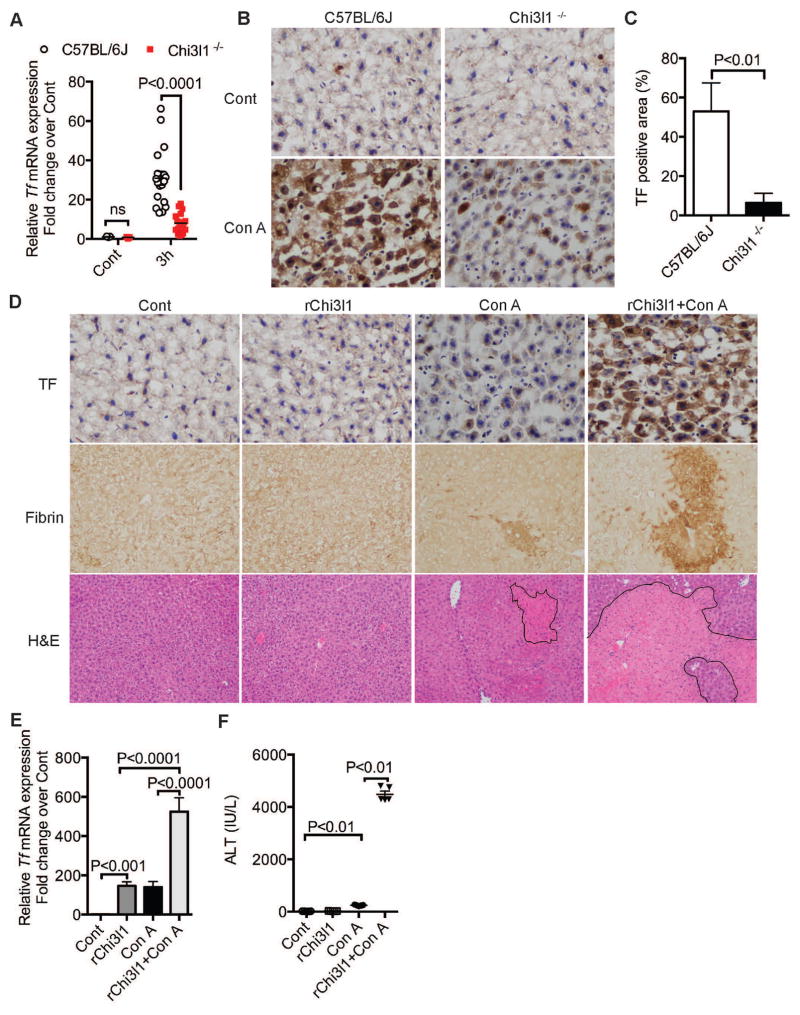
To understand the mechanism by which Chi3l1 contributes to CILI, we screened a number of coagulation factors after Con A treatment (Fig. S7). The baseline levels of the coagulation factors in WT and Chi3l1−/− mice were similar (Fig. S7A). At 3h after Con A treatment, the hepatic mRNA levels of TF, PAI-1, anti-thrombin, protein C and fibrinogen were significantly lower in Chi3l1−/− compared to WT mice among the factors we screened (Fig. S7B). It has been reported that TF-mediated IAOC contributes to CILI10. Through systematic time course studies, we also noticed that TF induction proceeds coagulation and liver injury caused by Con A (Fig. S8 A&B), indicating that Chi3l1 may be important in inducing TF production, thereby promoting coagulation. We found that compared with WT mice, Chi3l1−/− mice expressed significantly lower mRNA and protein levels of TF (Fig. 6, A–C; Fig. S9). To further demonstrate that Chi3l1 can induce TF, we reconstituted Chi3l1−/− mice with recombinant Chi3l1 (rChi3l1). As shown in Fig 6E, administration of rChi3l1 significantly increased the mRNA expression of TF in the liver. The combination of rChi3l1 and Con A dramatically up-regulated the mRNA and protein levels of TF (Fig. 6, D&E; Fig. S10A). Consistent with the induction of TF, reconstitution of Chi3l1−/− mice with rChi3l1 significantly increased the levels of fibrin(ogen) deposition in the liver and augmented CILI, evident by the increased ALT activities and worsened histology (Fig. 6, D&F; Fig. S10B). These data indicate that Chi3l1 plays a critical role in IAOC through the induction of TF. Interestingly, in the absence of Con A treatment, rChi3l1 alone did not cause coagulation in Chi3l1−/− mice, suggesting that Chi3l1 regulates the expression of TF, but the coagulant activity of TF is induced by Con A in a Chi3l1-independent manner.
Fig. 6. Chi3l1 plays a critical role in the induction of TF.
(A–C) Male WT and Chi3l1−/− mice were treated with Con A for 3h as described in Fig. 1. (A) Hepatic TF mRNA expression was measured by qRT-PCR (n=5–20 mice per group). (B) TF protein expression was measured by immunohistochemical staining (magnification: 200×). Shown are representative images from at with mouse recombinant Chi3l1 (rChi3l1) by i.p. injection at the same time of Con A administration. (D) The levels of TF and fibrin(ogen) were measured by immunohistochemical staining (magnification: 200×). Photomicrophages of H&E stained liver sections are shown with outlined necrotic areas (magnification: 100×). Representative images were from at least 4 mice per group. (E) Hepatic TF mRNA expression levels were measured at 3h post-Con A. (F) Serum ALT activities were determined at 24 h post Con A treatment (n=4–6 mice per group). P values are as indicated. Two-tailed, unpaired t test was performed in A, C and one-way ANOVA in E–F.
TF-induced by Chi3l1 drives activation of coagulation
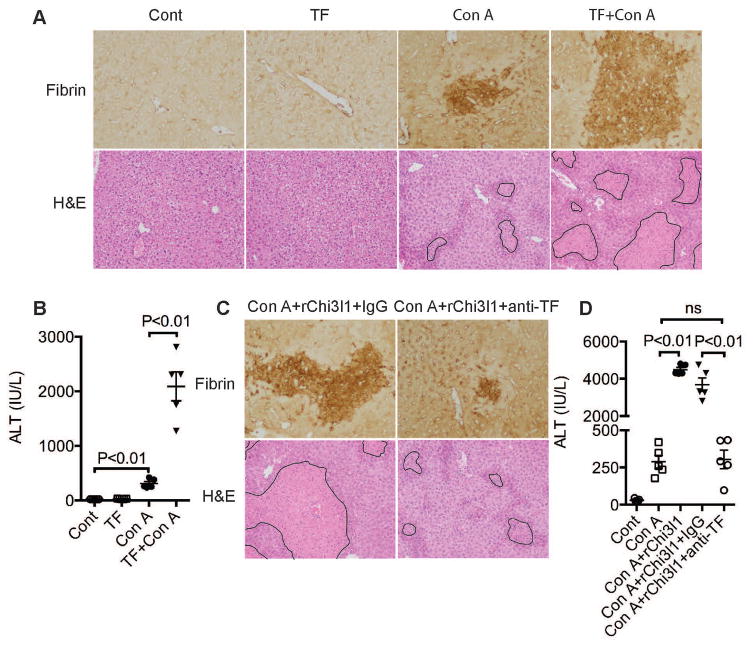
To confirm that TF drives IAOC, we blocked endogenous TF protein using an anti-TF (1H1) antibody and found that Con A-induced IAOC and liver injury were significantly decreased (Fig. S11, A&B). To further examine our hypothesis that in the Chi3l1−/− mice, it is the attenuated TF production that results in reduced liver injury, we administered exogenous rTF to Chi3l1−/− mice. Because the rTF we injected was not functionally activated by relipidation and lacked procoagulant activity, it alone did not cause systemic coagulation (Fig. S11C). However, in the presence of Con A treatment, likely caused activation of rTF, we observed significant increases of Con A-induced IAOC and liver injury in Chi3l1−/− mice (Fig. 7A and B). These data implicate that Chi3l1 induces TF expression but does not affect its activation. To further substantiate the role of Chi3l1 in TF induction, we reconstituted Chi3l1−/− mice with rChi3l1 and meanwhile neutralized TF. The data showed that rChi3l1-induced worsening of CILI and IAOC was abrogated by TF neutralization (Fig. 7).
Fig. 7. TF induced by Chi3l1 drives activation of coagulation.
(A–D) Male Chi3l1−/− mice were treated with Con A as described in Fig. 1 or recombinant TF(rTF), anti-TF (1H1) antibody or rChi3l1 as indicated. (A, C) Immunohistochemical staining for fibrin(ogen) (magnification: 200x), and H&E-stained liver sections (magnification, 100×) were performed at 24h after Con A treatment. Necrotic areas are outlined. (B, D) Serum ALT levels were determined at 24 h post-Con A treatment (n=5 mice per group). Images shown are representative of at least 4 mice per group. P values are as indicated. One-way ANOVA in A, C.
Chi3l1 induces TF expression through MAPK signaling pathway
It is reported that TF is expressed by hepatic MΦ, LSEC, and hepatocytes during CILI24. We examined TF mRNA expression in hepatic MΦ, LSEC, and hepatocytes from both naïve WT and Chi3l1−/− mice and mice treated with Con A for 3h. The baseline levels of TF mRNA in hepatocytes are about 8 fold higher than that in hepatic MΦ and LSEC in both WT and Chi3l1−/− mice (Fig. 8A). After Con A, TF mRNA increased 18-fold in hepatic MΦ of both WT and Chi3l1−/− mice. However, TF mRNA increased nearly 40-fold in LSEC and 45-fold in hepatocytes of WT mice, but only increased around 9-fold in LSEC and 19-fold in hepatocytes of Chi3l1−/− mice (Fig. 8A). To directly address whether Chi3l1 induces TF in these cells, we stimulated hepatic MΦ, LSEC, and hepatocytes isolated from naïve WT mice with rChi3l1. TF mRNA expression were induced in LSEC and hepatocytes but not in hepatic MΦ in vitro (Fig. 8B). Interestingly, hepatocytes are more responsive to rChi3l1. The elevation of TF mRNA levels is much more dramatic in hepatocytes (80 fold) than LSEC (3 fold) (Fig. 8B). The measurement of endotoxin levels and experiment using polymyxin B ruled out the possibility of TF induction due to endotoxin contamination of rChi3l1 (Fig. S12 A&B). To compare the potencies of IFN-γ, TNF-α and Chi3l1 in the induction of TF, we treated LSEC with the concentrations of these mediators detected in serum at 3h after Con A treatment. Among the three potential mediators, only rChi3l1 induced TF up-regulation (Fig. 8C). These data further suggest that Chi3l1 is an important downstream mediator of IFN-γ/TNF-α in inducing TF expression. To shed light on the molecular mechanisms by which Chi3l1 regulates TF expression, we screened several potential signaling pathways. Our data showed that Chi3l1 activated AKT and the mitogen-activated protein kinase (MAPK) family, including ERK1/2, c-Jun amino terminal kinases (JNKs), and p38 in transformed sinusoid endothelial cell line (TSEC) (Fig. 8D). However, rChi3l1 did not activate NF-κB or STAT1 (Fig. 8D). We then treated LSEC with rChi3l1 in combination with various MAPK inhibitors (JNK: SP600125, p38: SB203580, ERK: U0126), all of which significantly reduced TF expression levels (Fig. 8E). These results suggest Chi3l1 induces TF expression through activating MAPK signaling pathway.
Fig. 8. Chi3l1 induces TF expression through the MAPK signaling pathway.
(A) LSEC, MΦ and hepatocytes were isolated from naïve (Cont) and Con A-treated WT mice (n=6 per group). The TF mRNA expression levels in these cells were measured. (B) LSEC, MΦΦ and hepatocytes were isolated from naïve WT mice. The cells were treated with rChi3l1 for 5h, and TF mRNA expression levels were measured. (C) LSECs isolated from naïve WT mice were treated with indicated doses of IFN-γ, TNF-α, and rChi3l1 that match the levels of these factors detected in vivo (Fig. 2, E&F; Fig. 1C). After 5h, TF mRNA levels were measured. (D) TSEC cells (a mouse LSEC cell line) were treated with 100ng/ml of rChi3l1 for various time periods as indicated. Activation of various signaling pathways was measured by western blotting. (E) LSECs isolated from naïve WT mice were pretreated with MAPK inhibitors for 1h, followed by rChi3l1 treatment for 5h. TF mRNA expression was measured by qRT-PCR. (F) Schematic for the involvement of Chi3l1 in CILI and Con A-induced IAOC. Results shown are from three independent experiments. P values are as indicated. Two-tailed, unpaired t test was performed in B. One-way ANOVA was performed in A, C and E.
Discussion
In this study, we demonstrate that after Con A administration, activated T cells produce IFN-γ and TNF-α, which induce Chi3l1 production. The released Chi3l1 triggers MAPK activation and promotes TF production by LSEC and hepatocytes, thereby initiating IAOC and leading to liver injury (Fig. 8F).
Our observations that i) IFN-γ and TNF-α production precedes Chi3l1 release (Fig. 5A), ii) T cell suppression abrogates the elevation of Chi3l1 (Fig. 4, A–C), and iii) neutralization of IFN-γ and TNF-α dramatically reduces the levels of Chi3l1 (Fig. 5, B–D), suggest that Con A-triggered T cell activation and the production of IFN-γ and TNF-α play crucial roles in the up-regulation of Chi3l1. It has been reported that IFN-γ and TNF-α are important in inducing TF production and promoting hepatic thrombosis within the liver24. However, after Con A treatment, WT and Chi3l1−/− mice produce similar amount of IFN-γ and TNF-α (Fig. 2, C–F), but TF expression is much lower in Chi3l1−/− mice (Fig. 6, A–C, Fig. S9). Moreover, at the levels that can be detected in vivo, IFN-γ and TNF-α could not directly induce TF expression in vitro (Fig. 8C). These findings suggest that Chi3l1 is one of the downstream mediators of IFN-γ and TNF-α that plays an important role in inducing TF expression. Nonetheless, blocking IFN-γ/TNF-α did not completely abrogate Chi3l1 expression (Fig. 5). Thus, we cannot exclude the possibility that Con A may directly induce Chi3l1 expression, as Con A can activate LSEC and MΦs 9,26,28. Aside from IFN-γ and TNF-α, other cytokines produced by Con A-activated T cells may also induce Chi3l1 expression. For example, it is reported that the expression of Chi3l1 can be regulated by IL-6, IL-13, IL-18, IL-1β29–32. Our data show that IFN-γ and TNF-α induce Chi3l1 expression in both LSEC and hepatic MΦ (Fig. 5, E&F). However, it is possible that other cells in the liver may also produce Chi3l1. Therefore, questions such as the cellular source of Chi3l1 and the receptor(s) through which it signals, warrant further research.
T cell activation plays a critical role in the mechanism of CILI25. Moreover, IFN-γ and TNF-α, produced by Con A-activated T cells, are critical in mediating tissue damage. In addition to triggering T cell activation, Con A has agglutination properties that cause stasis of blood components in liver sinusoids, preceding liver damage9. Ample evidence suggests that Con A-induced microcirculatory disturbance is of paramount importance contributing to CILI9,10,13,14. Anti-coagulant (heparin, cyproheptadine and antithrombin III) pretreatment decrease hepatic injury through reducing hemostasis, but it does not affect inflammatory responses9,14. In agreement with these findings, our data demonstrate that Chi3l1-deficiency confers a profound resistance to CILI without impairing T cell activation or inhibiting IFN-γ and TNF-α production (Fig. 2). In contrast, Con A-induced IAOC is markedly attenuated in Chi3l1−/− mice (Fig. 3). Taken together, our study and published reports demonstrate that Con A-induced IAOC and T cell activation are both critical events contributing to liver injury.
TF initiates coagulation and plays an integral role in blood coagulation and thrombin generation11. Our data suggest that in Chi3l1−/− mice, it is the reduced TF expression that results in attenuated CILI (Fig. 6&7). Both mRNA and protein levels of TF are markedly lower in the livers of Chi3l1−/− compared to WT mice (Fig. 6, A–C; Fig. S9). Reconstituting Chi3l1−/− mice with rChi3l1 dramatically increased the TF mRNA and protein expressions, and also augmented intrahepatic coagulation and CILI (Fig. 6, D–F; Fig. S7). Our data showed that administering exogenous TF alone did not cause coagulation, as the TAT level was not increased (Fig. S11C). Although TF is important in initiating coagulation, endothelial activation/injury resulting in platelet aggregation and activation is also required33. In Chi3l1−/− mice treated with Con A, the platelets were recruited similarly as in WT mice (Fig. S5), but coagulation was suppressed (Fig. 3) due to the lack of TF (Fig. 7A). Thus, administering exogenous TF could promote coagulation in Con A-treated Chi3l1−/− mice, recapitulating the IAOC and liver injury observed in WT mice. Moreover, neutralizing TF abrogated the effect of rChi3l1 treatment in Chi3l1−/− mice (Fig. 7C–D), strongly suggesting that TF contributes to CILI and that the absence of Chi3l1 results in reduced TF expression and attenuated CILI in Chi3l1−/− mice. It is reported that hepatocyte-derived TF contributes to IAOC caused by APAP treatment 11,12. Our data show that Chi3l1 induces TF expression in both hepatocytes and LSEC (Fig. 8B; Fig. S9), suggesting that both cell types may be important source of TF production in CILI.
Liver diseases are consistently associated with coagulation activation mediated by TF24,34–36. TF-dependent activation of the coagulation cascade occurs and contributes to the progression of acute cholestatic hepatitis caused by α-naphthylisothiocyanate35. Myeloid cell-derived TF plays an important role in modulating hepatic necrosis during cholestasis36. In mice deficient of TF, overdose of acetaminophen-induced liver injury and activation of coagulation are significantly reduced12. Hematopoietic cell-derived TF is required for liver inflammation and steatosis in nonalcoholic fatty liver disease37. TF pathway contributes to liver fibrosis induced by chronic cholestasis by increasing expression of the αVβ6 integrin38. Administration of TF-antisense oligodeoxynucleotides successfully prevents monocrotaline/lipopolysaccharide-induced liver injury34. Therefore, the identification of Chi3L1 as a regulator of TF indicates an important role of Chi3l1 in liver diseases.
In summary, the present study demonstrates a novel pro-coagulant function of Chi3l1 and provides evidence that Chi3l1 induces TF production via MAPK activation. These findings identify Chi3l1 as a potential therapeutic target in treating coagulopathy in liver diseases and potentially other coagulation disorders.
Methods
Animal experiments and procedures
All animal experiments were performed according to guidelines from UC Denver Institutional Animal Care and Use Committee. 8–12 weeks old mice were injected with 15mg/kg Con A (Sigma, St. Louis, MO) intravenously (i.v.). Male and female mice respond to Con A treatment similarly, although their dose responses are different (Fig. 1D and Fig. S2)39. Because most of the published studies used male mice25, we used male mice in our studies. In some experiments, mice received Con A followed immediately injection with anti-murine IFN-γ antibody (500ng, Peprotech 500 p119), anti-murine TNF-α antibody (500ng, Peprotech 500-P64), normal rabbit IgG (Peprotech, 500-p00), recombinant Chi3l1 (rChi3l1 500ng, Sino Biological 50929-M08H) by intraperitoneal (i.p.) or TF protein (500ng, R&D 3178-PA-010), anti-TF antibody (1H1, 500μg, Genentech Inc.) by intravenous injection (i.v.). FK506 (32mg/kg body weight, Cayman 104987-11-3) was performed as previously described9. The levels of alanine aminotransferase (ALT) in serum and IFN-γ, TNF-α and Chi3l1 in serum and liver tissues were determined using commercially available kits (ALT, Teco Dignostics; ELISA, R&D).
Preparation of Liver Cells
Hepatic nonparenchymal cells and hepatocytes were isolated as previously described40,41.
Histology
Hematoxylin and eosin (H&E) were performed by the Histology core at CUAMC on paraffin sections. Fibrin(ogen) was performed as previously described24. TF were stained with anti-mouse TF antibody (abcam151748). CD41 were stained by anti-mouse CD41 antibody (BD Bioscience, Clone MWReg 30) followed by Alexa 488-conjugated donkey anti-rat secondary antibody(Invitrogen Life Technologies).
Thrombin Antithrombin III Complex (TAT) measurement
Plasma were collected by retro-orbital puncture into a 3.2% solution of sodium citrate. TAT was measured by a commercially available kit (AssayMax, cat#EMT1020-1).
Cell culture and treatment with cytokines or inhibitors
Isolated primary LSEC and MΦ were cultured in DMEM with 10% fetal bovine serum and treated with indicated amount of murine recombinant rChi3l1, IFN-γ (Peprotech 315-05), or TNF-α (Peprotech 500-P64) for various times. In some experiments, the cells were pre-treated with inhibitors for one hour before rChi3l1 treatment. The concentrations of the inhibitors used were as follows: c-JNK inhibitor (20μM SP600125, sigma S5567); P38 inhibitor (20μM SB203580, sigma S8307); ERK inhibitor (20μM U0126, sigma U120). The transformed sinusoids endothelial cell (TSEC) is a generous gift from Dr. Vijay Shah (Mayo Clinic, Minnesota). LSEC or TSEC cells were cultured in DMEM supplemented with 10% ECGS(SclenCell, cat#1052).
Flow cytometric analysis
Intracellular staining for IFN-γ, TNF-α, IL-4 was performed as previously described42.
Statistics
Data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism (GraphPad Software). Comparisons between two groups were analyzed using unpaired Student t test. Comparisons among multiple groups (n>=3) were analyzed using one-way ANOVA. P values are as labeled and less than 0.05 was considered significant. Positive areas of protein expression were analyzed by Image J software.
Supplementary Material
Acknowledgments
Financial support: This study was supported by U01AA021723, R21AA022387, R21AA024636 and R01DK109574 (C.J.)
Abbreviations
- Con A
concanavalin A
- Chi3l1
Chitinase 3 like 1
- IFN-γ
interferon gamma
- TNF-α
tumor necrosis factor alpha
- TF
tissue factor
- MAPK
mitogen-activated protein kinase
- CILI
concanavalin A (Con A)-induced liver injury
- NK cell
natural killer cell
- WT
wild-type
- TAT
thrombin anti-thrombin complex
- LSEC
liver sinusoid endothelial cells
- MΦ
macrophages
- rChi3l1
recombinant Chi3l1
- IAOC
Intrahepatic activation of coagulation
Footnotes
Author contributions
ZS designed and conducted experiments, analyzed and interpreted data, and wrote the manuscript; XDL conducted experiments and analyzed data; YC, MW, PC conducted experiments; WAD reviewed paper; CGL and JAE provided Chi3l1−/− mice; LGX and JP provided suggestions in experimental design; YG edited the manuscript; CJ conceived and supervised the project and wrote the manuscript.
References
- 1.James LP, Wells E, Beard RH, Farrar HC. Predictors of outcome after acetaminophen poisoning in children and adolescents. J Pediatr. 2002;140(5):522–526. doi: 10.1067/mpd.2002.122936. [DOI] [PubMed] [Google Scholar]
- 2.Lisman T, Bernal W. Hemostatic issues in pregnancy-induced liver disease. Thrombosis research. 2017;151:S78–S81. doi: 10.1016/S0049-3848(17)30073-7. [DOI] [PubMed] [Google Scholar]
- 3.Hugenholtz GCG, Adelmeijer J, Meijers JCM, Porte RJ, Stravitz RT, Lisman T. An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology. 2013;58(2):752–761. doi: 10.1002/hep.26372. [DOI] [PubMed] [Google Scholar]
- 4.Weerasinghe SVW, Moons DS, Altshuler PJ, Shah YM, Omary MB. Fibrinogen-γ proteolysis and solubility dynamics during apoptotic mouse liver injury: Heparin prevents and treats liver damage. Hepatology. 2011;53(4):1323–1332. doi: 10.1002/hep.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez M, Kopec AK, Joshi N, et al. Fas-Induced Apoptosis Increases Hepatocyte Tissue Factor Procoagulant Activity In Vitro and In Vivo. Toxicol Sci. 2014;141(2):453–464. doi: 10.1093/toxsci/kfu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyakawa K, Joshi N, Sullivan BP, et al. Platelets and protease-activated receptor-4 contribute to acetaminophen-induced liver injury in mice. Blood. 2015;126(15):1835–1843. doi: 10.1182/blood-2014-09-598656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson JM, Schultze AE, Schwartz KA, Scott MA, Davis JM, Roth RA. The thrombin inhibitor, hirudin, attenuates lipopolysaccharide-induced liver injury in the rat. J Pharmacol Exp Ther. 1996;278(1):378–383. [PubMed] [Google Scholar]
- 8.Harada N, Okajima K, Uchiba M. Dalteparin, a low molecular weight heparin, attenuates inflammatory responses and reduces ischemia-reperfusion-induced liver injury in rats. Critical Care Medicine. 2006;34(7):1883–1891. doi: 10.1097/01.CCM.0000220764.10155.03. [DOI] [PubMed] [Google Scholar]
- 9.Miyazawa Y, Tsutsui H, Mizuhara H, Fujiwara H, Kaneda K. Involvement of intrasinusoidal hemostasis in the development of concanavalin a–induced hepatic injury in mice. Hepatology. 1998;27(2):497–506. doi: 10.1002/hep.510270225. [DOI] [PubMed] [Google Scholar]
- 10.Kato J, Okamoto T, Motoyama H, et al. Interferon-gamma-mediated tissue factor expression contributes to T-cell-mediated hepatitis through induction of hypercoagulation in mice. Hepatology. 2013;57(1):362–372. doi: 10.1002/hep.26027. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan BP, Kopec AK, Joshi N, et al. Hepatocyte tissue factor activates the coagulation cascade in mice. Blood. 2013;121(10):1868–1874. doi: 10.1182/blood-2012-09-455436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganey PE, Luyendyk JP, Newport SW, et al. Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology. 2007;46(4):1177–1186. doi: 10.1002/hep.21779. [DOI] [PubMed] [Google Scholar]
- 13.Diao H, Kon S, Iwabuchi K, et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21(4):539–550. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Ito T, Yoneda M, et al. Antithrombin III prevents concanavalin A-induced liver injury through inhibition of macrophage inflammatory protein-2 release and production of prostacyclin in mice. Journal of Hepatology. 2002;36(6):766–773. doi: 10.1016/s0168-8278(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 15.Podolsky DK, Weiser MM, La Mont JT, Isselbacher KJ. Galactosyltransferase and concanavalin A agglutination of cells. PNAS. 1974;71(3):904–908. doi: 10.1073/pnas.71.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268(34):25803–25810. [PubMed] [Google Scholar]
- 17.Kawada M, Hachiya Y, Arihiro A, Mizoguchi E. Role of mammalian chitinases in inflammatory conditions. Keio J Med. 2007;56(1):21–27. doi: 10.2302/kjm.56.21. [DOI] [PubMed] [Google Scholar]
- 18.Pizano-Martínez O. YKL-40 expression in CD14 +liver cells in acute and chronic injury. WJG. 2011;17(33):3830–3836. doi: 10.3748/wjg.v17.i33.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansen JS, Christoffersen P, Møller S, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. Journal of Hepatology. 2000;32(6):911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko Y, Harada M, Kawano T, et al. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191(1):105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995;21(1):190–198. doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- 22.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45(2):475–485. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- 23.Kon S, Ikesue M, Kimura C, et al. Syndecan-4 protects against osteopontin-mediated acute hepatic injury by masking functional domains of osteopontin. J Exp Med. 2008;205(1):25–33. doi: 10.1084/jem.20071324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato J, Okamoto T, Motoyama H, et al. Interferon-gamma-mediated tissue factor expression contributes to T-cell-mediated hepatitis through induction of hypercoagulation in mice. Hepatology. 2013;57(1):362–372. doi: 10.1002/hep.26027. [DOI] [PubMed] [Google Scholar]
- 25.Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90(1):196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonder CS, Ajuebor MN, Zbytnuik LD, Kubes P, Swain MG. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J Immunol. 2004;172(1):45–53. doi: 10.4049/jimmunol.172.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Tomura S, Nakamura Y, Deguchi F, Ando R, Chida Y, Marumo F. Coagulation and fibrinolysis in patients with chronic renal failure undergoing conservative treatment. Thrombosis research. 1991;64(1):81–90. doi: 10.1016/0049-3848(91)90207-d. [DOI] [PubMed] [Google Scholar]
- 28.Kesherwani V, Sodhi A. Differential activation of macrophages in vitro by lectin Concanavalin A, Phytohemagglutinin and Wheat germ agglutinin: Production and regulation of nitric oxide. Nitric Oxide. 2007;16(2):294–305. doi: 10.1016/j.niox.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodeling, fibrosis and cancer. Dan Med Bull. 2006;53(2):172–209. [PubMed] [Google Scholar]
- 30.Recklies AD, Ling H, White C, Bernier SM. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem. 2005;280(50):41213–41221. doi: 10.1074/jbc.M510146200. [DOI] [PubMed] [Google Scholar]
- 31.Lee CG, Hartl D, Lee GR, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13–induced tissue responses and apoptosis. J Exp Med. 2009;206(5):1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang M-J, Yoon CM, Nam M, et al. Role of Chitinase 3–Like-1 in Interleukin-18–Induced Pulmonary Type 1, Type 2, and Type 17 Inflammation; Alveolar Destruction; and Airway Fibrosis in the Murine Lung. American Journal of Respiratory Cell and Molecular Biology. 2015;53(6):863–871. doi: 10.1165/rcmb.2014-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furie B. Thrombus formation in vivo. J Clin Invest. 2005;115(12):3355–3362. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammad MA, Abdel-Bakky MS, Walker LA, Ashfaq MK. Tissue factor antisense deoxyoligonucleotide prevents monocrotaline/LPS hepatotoxicity in mice. J Appl Toxicol. 2012;33(8):774–783. doi: 10.1002/jat.2728. [DOI] [PubMed] [Google Scholar]
- 35.Luyendyk JP, Cantor GH, Kirchhofer D, Mackman N, Copple BL, Wang R. Tissue factor-dependent coagulation contributes to -naphthylisothiocyanate-induced cholestatic liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G840–G849. doi: 10.1152/ajpgi.90639.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luyendyk JP, Flanagan KC, Williams CD, et al. Tissue factor contributes to neutrophil CD11b expression in alpha-naphthylisothiocyanate-treated mice. Toxicology and Applied Pharmacology. 2011;250(3):256–262. doi: 10.1016/j.taap.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassel KM, Owens AP, III, Rockwell CE, et al. Protease-activated receptor 1 and hematopoietic cell tissue factor are required for hepatic steatosis in mice fed a Western diet. The American Journal of Pathology. 2011;179(5):2278–2289. doi: 10.1016/j.ajpath.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan BP, Weinreb PH, Violette SM, Luyendyk JP. The Coagulation System Contributes to αVβ6 Integrin Expression and Liver Fibrosis Induced by Cholestasis. The American Journal of Pathology. 2010;177(6):2837–2849. doi: 10.2353/ajpath.2010.100425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takamoto S. Gender-related differences in concanavalin A-induced liver injury and cytokine production in mice. Hepatology Research. 2003;27(3):221–229. doi: 10.1016/s1386-6346(03)00263-8. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, You Q, Lor K, Chen F, Gao Bin, Ju C. Chronic alcohol ingestion modulates hepatic macrophage populations and functions in mice. J Leukoc Biol. 2014;96(4):657–665. doi: 10.1189/jlb.6A0114-004RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu M-J, Feng D, Wu H, et al. Liver is the major source of elevated serum lipocalin-2 levels after bacterial infection or partial hepatectomy: A critical role for IL-6/STAT3. Hepatology. 2015;61(2):692–702. doi: 10.1002/hep.27447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin H, Cheng L, Langenbach R, Ju C. Prostaglandin I2 and E2 mediate the protective effects of cyclooxygenase-2 in a mouse model of immune-mediated liver injury. Hepatology. 2007;45(1):159–169. doi: 10.1002/hep.21493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.