Abstract
Two phenotypes have been proposed: insomnia with objective near-normal sleep duration (NNSD), related to increased psychological symptoms, and insomnia with objective short sleep duration (OSSD), associated with cardiometabolic morbidity. Reduced heart rate variability (HRV) has also been implicated in the pathophysiology of cardiometabolic disease; however, there is little data on whether cardiovascular function differs between patients with OSSD and NNSD. Participants (Mage=49.9±11.3; 62.8% female) were 180 adults with chronic insomnia (Mduration=15.7±13.6). Objective sleep duration was based on total sleep time (TST) averaged across two consecutive nights of polysomnography (PSG), and subjective sleep duration was based on two-week sleep diaries. The sample was divided into two groups with sleep duration shorter (PSG-TST: n=46; Sleep Diary: n=95) or equal/longer (PSG-TST: n=134; Sleep Diary: n=85) than 6hrs. Electrocardiogram data derived from PSG were used to obtain heart rate (HR) and HRV during stage 2 (N2) and rapid-eye movement (REM) sleep. HRV measures included absolute and normalized high frequency (HF) component, an index of parasympathetic activation, and the ratio of low to high frequency (LF/HF ratio), an index of sympathovagal balance. After controlling for covariates (e.g., co-morbidity), patients with OSSD had reduced HF (ps<.05) and elevated LF/HF ratio (p=.036) and HR (p=.051) compared to patients with NNSD. No differences were observed between phenotypes when subjective sleep duration was used. Insomnia patients with OSSD showed significantly dampened parasympathetic activation and increased sympathovagal imbalance relative to their counterparts with NNSD. These findings highlight the importance of treating insomnia, as treatment may reduce risk of cardiovascular disease.
Keywords: insomnia, phenotype, bjective short sleep duration, heart rate variability, heart rate
Introduction
It is proposed that objective sleep duration, defined by polysomnography (PSG) total sleep time (TST), can be used to identify different insomnia phenotypes. Based on this conceptualization, Vgontzas and colleagues (Vgontzas, Fernandez-Mendoza, Liao, & Bixler, 2013) have distinguished between two insomnia phenotypes: those with near-normal sleep duration (NNSD; PSG-defined TST ≥6hrs) and those with objective short sleep duration (OSSD; PSG-defined TST <6hrs). These authors have reported that objective sleep duration provides information on the natural course of insomnia, biological severity, as well as health and treatment outcomes (Vgontzas et al., 2013). Insomnia with NNSD is related with an anxious-ruminative profile (Vgontzas et al., 2013), whereas insomnia with OSSD is linked with physiological hyperarousal (Vgontzas et al., 2001), increased risk of type 2 diabetes, hypertension, as well as concurrent and future cerebrovascular and cardiovascular events (Fernandez-Mendoza et al., 2012; Vgontzas et al., 2009). Despite data on the link between insomnia and OSSD with cardiometabolic disease (Fernandez-Mendoza et al., 2012; Kalmbach, Pillai, Arnedt, & Drake, 2016; Vgontzas et al., 2009), information on potential underlying pathophysiological mechanisms, such as systematic inflammation (Fernandez-Mendoza et al., 2017), hormonal and metabolic dysregulation (D’Aurea et al., 2015; Fernandez-Mendoza et al., 2014), or alterations in the autonomic nervous system (Miller et al., 2016; Spiegelhalder et al., 2011) have been scant.
Non-invasive indicators of the autonomic nervous system include heart rate (HR), controlled by both the sympathetic and parasympathetic nervous systems, as well as heart rate variability (HRV), which is largely influenced by the parasympathetic nervous system (Task Force, 1996). HRV is a quantitative marker reflecting the variations between continuous heartbeats and is considered a quantifiable measure of cardiovascular autonomic function (Task Force, 1996). While elevated HRV is representative of a healthy cardiovascular autonomic function, dampened HRV (i.e., parasympathetic withdrawal) is significantly associated with a 32% to 45% increased risk of cardiovascular morbidity (Hillebrand et al., 2013). More specifically, nocturnal HRV is independently associated with increased risk of the initiation, progression (e.g., subclinical inflammation, glucose concentration, plasma fibrinogen) and development of cardiovascular disease in otherwise healthy populations (Jarczok, Li, Mauss, Fischer, & Thayer, 2013). While evidence suggests low HRV is associated with more severe sleep disturbances (Von Känel, Thayer, & Fischer, 2009), within the context of insomnia, findings vary across studies (Dodds, Miller, Kyle, Marshall, & Gordon, 2017). To the best of our knowledge, only two studies have investigated differences in HRV and HR between insomnia phenotypes (Miller et al., 2016; Spiegelhalder et al., 2011).
In a recent investigation, cluster analyses were conducted on several objective PSG variables, and while it was not the focus of the study, two insomnia clusters were identified. Unlike previous studies that used cut-off scores, these phenotypes were based on average PSG-TST of 4.82hrs and 6.5hrs (Miller et al., 2016). Authors documented significantly reduced HRV during sleep onset among insomnia volunteers with OSSD relative to those with NNSD; however, no differences were found for HR. In another study (Spiegelhalder et al., 2011), instead of PSG-TST, PSG-derived sleep efficiency (SE) was used to classify insomnia phenotypes. Nevertheless, significantly reduced HRV was observed in patients with PSG-SE <85% relative to patients with PSG-SE ≥85%; no differences in HR were found. Interestingly, these authors also assessed self-reported sleep duration; however, rather than compare HRV and HR between patients with subjective short and near-normal sleep duration, patients with subjective short sleep duration were only compared with healthy controls.
Given that objective (and not subjective) sleep duration is posited to be the most biologically severe phenotype of insomnia on future health morbidity and mortality (Vgontzas et al., 2013) and because there is limited data in the field thus far, the primary objective of the present study was to explore whether HRV and HR differ between clinical insomnia patients with OSSD (<6hrs) and NNSD (≥6hrs). To provide further evidence, a secondary objective of the present study was to examine whether HRV and HR differ between insomnia phenotypes with short or NNSD based on TST derived from sleep diaries.
Method
The present study is based on secondary analyses of data extracted from two clinical trials. The first trial was conducted in Université Laval (Québec) and examined the impact of different treatment sequences using cognitive behavioral therapy for insomnia (CBT-I) with and without zolpidem. The second trial was conducted in two sites: Université Laval (Québec) and University of California, Berkeley (Berkeley). This clinical trial examined the unique impact of behavior therapy and cognitive therapy compared to complete CBT-I. The detailed descriptions of study protocols, participants, and results pertaining to the primary research questions of these studies have been reported by Morin et al. (Morin et al., 2009) and Harvey and colleagues (Bélanger et al., 2016; Harvey et al., 2014), respectively.
Study context and participants
Recruitment strategies and inclusion criteria were similar in both parent studies. In general, participants were recruited through advertisements and referrals from health care practitioners. Following a telephone interview, eligible individuals completed a comprehensive clinical interview sessions, including the International Classification of Sleep Disorders evaluation (ICSD (American Academy of Sleep Medicine, 2005) and the Structured Clinical Interview for DSM-IV Axis-I Disorders (SCID-I) (First, Spitzer, Gibbon, & Williams, 1995), and the Duke Structured Interview for Sleep Disorders (Edinger et al., 2009).
All participants were ≥25 years old and diagnosed with chronic insomnia based on a combination of criteria from the DSM-IV-TR (American Psychiatric Association, 2013), the ICSD (American Academy of Sleep Medicine, 2005), and with quantitative cut-offs on the Insomnia Severity Index (ISI) (Morin, Belleville, Bélanger, & Ivers, 2011). Insomnia criteria included: (1) difficulties initiating and/or maintaining sleep, (2) insomnia duration ≥6 months, and (3) significant daytime distress/impairment.
Exclusion criteria included: (1) sleep-altering medication (e.g., steroids); patients using prescribed or over-the-counter sleep medications no more than twice weekly were enrolled after they withdrew from the medications, (2) evidence of sleep apnea (apnea/hypopnea index >15), restless legs syndrome, or periodic limb movements during sleep (movement index with arousal >15/hour), (3) night-shift work or irregular bed/wake time, (4) diagnosis of psychotic, bipolar disorder, or substance abuse; patients using alcohol as a sleep aid were also required to reduce or discontinue this practice for the study, (5) presence of unstable/progressive medical condition(s) or neurological degenerative disease; patients with stable medical (e.g., hypertension) or psychiatric disorders (e.g., anxiety) were included in the study provided that these conditions were not the primary cause of insomnia.
Additional exclusion criteria for the first trial (Morin et al., 2009) included history of suicide attempt. Additional exclusion criteria for the second clinical trail (Bélanger et al., 2016; Harvey et al., 2014) included body mass index (BMI) ≥35 or BMI of ≥32 and reporting at least three symptoms of breathing-related sleep disorder on the Duke Structured Interview for Sleep Disorders (Edinger et al., 2009) and current/past psychological treatment of insomnia within the past 5 years. Ethics committee approved the protocols for each clinical trial. All patients provided written informed consent and received financial compensation.
The sample for the first clinical trial (Morin et al., 2009) was composed of 80 participants, in which six dropped out, four had technical problems, and five had poor ECG data (>20% artifact), yielding a sample size of 65. The sample for the second clinical trial (Bélanger et al., 2016; Harvey et al., 2014) was comprised of 188 participants. From this sample, 22 participants had missing PSG data, 23 participants had technical problems, and 28 had poor ECG data. In total, 180 participants were included in the present study.
Measures
The present article focused exclusively on participant’s baseline measures prior to receiving treatment.
Sleep diary
Participants completed daily sleep diaries for two weeks during the baseline period. The sleep diary is a standard assessment instrument in insomnia research, which allows for prospectively monitoring sleep patterns in the patient’s home. The variables derived from sleep diaries included: sleep onset latency (SOL), wake time after sleep onset (WASO), total sleep time (TST), time spent in bed (TIB), SE, and self-rated sleep quality. TST from the daily sleep diaries were averaged over 2 weeks and used to create two groups with short sleep duration (<6hrs: n=95) or with NNSD (≥6hrs: n=85).
Insomnia Severity Index (ISI)
The ISI (Morin et al., 2011) is a 7-item, patient-reported outcome questionnaire used to assess the perceived severity of night-time and daytime symptoms of insomnia over the past month. Specifically, items addressed sleep problems, sleep (dis)satisfaction, interference of sleep difficulties with daytime functioning, noticeability of sleep problems, and distress/worry caused by sleep difficulties. Each item was rated on a 5-point Likert scale, with higher scores representative of severe insomnia. The ISI is a reliable and valid instrument to detect cases of insomnia in the population and is positively correlated with subjective sleep estimates (Morin et al., 2011).
Beck Depression Index (BDI)
The BDI (Beck, Steer, & Carbin, 1988) is a 21-item self-reported questionnaire that assesses the presence and the intensity of depressive symptoms experienced during the past two weeks. Each item was rated on a 4-point Likert scale, with higher scores indicative of greater depressive symptomatology.
Polysomnography
Standard PSG montage was used over three nights, where respiration and anterior tibialis electromyogram were monitored during the first night to screen for sleep apnea and periodic limb movements during sleep. On the nights of PSG, participants were not allowed to consume caffeine nor alcohol after 16h00. PSG-derived variables included SOL, WASO, TST, and SE. Additionally, sleep stages [i.e., N2, N3, rapid eye-movement (REM) sleep] were visually scored according to AASM standardized criteria (Iber, Ancoli-Isreal, Chesson, & Quan, 2007) by experienced sleep technologists. Given that one night of PSG is insufficient to derive stable estimates of sleep duration in insomnia patients (due to other factors such as sleeping environment, exercise, caffeine consumption) (Isreal et al., 2012), PSG-TST from the second and third nights of recording were averaged and used to create two groups: OSSD (n=46) and NNSD (n=134). Consistent with previous studies (Fernandez-Mendoza et al., 2012), OSSD was defined as PSG-TST shorter than 6hrs and NNSD was defined as PSG-TST equal to or more than 6hrs.
Heart rate variability (HRV) and heart rate (HR)
During PSG, continuous raw ECG data were acquired using the Stellate Harmonie 5.2 at a sampling rate of 1kHz. ECG data was derived from a modified Lead-II configuration. Research demonstrates that using one night of ECG data from PSG is sufficient to obtain reliable (i.e., high short-term stability) HRV estimates in insomnia patients (Isreal et al., 2012), and thus, ECG data of the last night of each evaluation were analyzed and the beat-by-beat RR intervals edited by visual inspection (Kubios® HRV 2.2).
As recommended by the Task Force (Task Force, 1996), 2-min HRV epochs were analyzed to obtain low frequency (LF)-HRV (0.04–0.15 Hz). Based on stringent criteria (Trinder et al., 2001), epochs for N2 and REM sleep stages were identified over the sleep period (wake periods were excluded) and all possible epochs were selected; a majority of the participants did not have sufficient N3 data, and thus, only data for N2 and REM sleep were analyzed in the present study. Identified 2-min artifact-free HRV epochs were then analyzed across the sleep period and averaged across sleep stages.
Power spectral estimates included LF-HRV and high-frequency power band (HF; 0.15–0.40 Hz). Normalized HF was used an index of parasympathetic activation and the ratio of low to high frequency (LF/HF ratio) was used as an index of sympathovagal balance (Task Force, 1996). While the physiological component for HF is well established, the LF/HF ratio is posited to represent important functional significance and adverse health consequences (Thayer & Fischer, 2013). HF absolute (ms2) and normalized units (nu) [i.e., HF (ms2)/(total power (ms2) – very low frequency (ms2)], LF/HF ratio [LF (ms2)/HF (ms2)], and HR (bpm) were generated for each patient for N2 and REM sleep.
Statistical analysis
Data were checked and analyzed using SAS and SPSS Statistics software (IBM Version 22). Frequency-domain variables were natural log transformed due to skewness (e.g., lnHF). One-way ANOVAs and Cronbachs were computed to identify potential covariates. Multilevel modeling (MLM) (also referred to as hierarchical linear modeling) (Raudenbush & Bryk, 2002) analyses were computed to examine whether HRV and HR during sleep differ as a function of short or NNSD using TST from PSG or sleep diaries. The two independent factors were sleep duration (between-subjects: OSSD and NNSD) and sleep stage (within-subjects: S2 and REM). Analyses included study site, sex, age, education, BMI, insomnia severity, depressive symptoms, and hypertensive status/medication as covariates. Statistical significance was set at 0.05 (2-tailed).
Results
Overall, patients were predominantly White (89.6%), female (62.8%), married (65.0%), and middle-aged (Mage=49.9, SD=11.3), completed an average of 15.5 years of education (SD=3.5) and had normal BMI (M=24.9, SD=3.6 kg/m2). Of the total sample, 40.0% had reported using hypnotic medication for sleep in the last month prior to the study. In terms of comorbidity, 42 (23.3%) of patients presented a comorbid psychiatric disorder (most commonly anxiety) and 110 patients (61.1%) presented at least one comorbid medical disorder (most commonly musculoskeletal disorders and hypertension). Within the subsample of patients with hypertension (n=14), 13 reported taking medication including beta-blockers (n=2), angiotension II receptor blockers (n=4), calcium channel blockers (n=2), diuretics (n=2), and angiotension converting enzyme inhibitors (n=3). Descriptive data of sample characteristics are summarized in Table 1.
Table 1.
Characteristics of insomnia patients with objective short and near-normal sleep duration
| Total (N=180) | PSG-TST <6h (n=46) | PSG-TST ≥6h (n=134) | |
|---|---|---|---|
| Age (years) | 49.9 (11.3) | 51.6 (10.8) | 49.3 (11.5) |
| Female (%) | 113 (62.8%) | 24 (52.2%) | 89 (66.4%) |
| Education (years) | 15.5 (3.5) | 14.3 (3.9) | 15.8 (3.3)* |
| BMI (kg/m2) | 24.9 (3.6) | 26.5 (4.5) | 24.3 (3.0)** |
| Insomnia duration (years) | 15.7 (13.6) | 18.2 (16.3) | 14.9 (12.6) |
| Insomnia Severity Index | 17.7 (3.5) | 17.5 (3.5) | 17.8 (3.5) |
| Depressive symptoms (Beck Depression Inventory) | 9.4 (6.7) | 9.0 (5.1) | 9.5 (7.1) |
| Psychological co-morbidity (% yes) | 42 (23.3%) | 11 (23.9%) | 31 (23.1%) |
| Medical co-morbidity (% yes) | 110 (61.1%) | 27 (58.7%) | 83 (61.9%) |
| Hypertension (% yes) | 14 (7.8%) | 6 (13.0%) | 8 (6.0%) |
| Sleep Measures | |||
| PSG-SOL (min) | 12.0 (11.0) | 16.9 (16.9) | 10.4 (7.5)** |
| PSG-WASO (min) | 55.7 (34.8) | 87.2 (40.9) | 45.0 (24.6)** |
| PSG-TST (min) | 380.3 (43.9) | 321.8 (34.6) | 400.4 (24.6)** |
| PSG-SE (%) | 83.1 (8.4) | 73.7 (8.9) | 86.3 (5.3)** |
| Diary-SOL (min) | 30.8 (28.4) | 31.1 (27.8) | 30.6 (28.7) |
| Diary-WASO (min) | 59.8 (34.3) | 67.4 (42.4) | 57.1 (30.7) |
| Diary-TST (min) | 345.2 (64.1) | 339.3 (68.2) | 347.3 (62.7) |
| Diary-SE (%) | 70.8 (12.9) | 69.1 (14.9) | 71.4 (12.1) |
| Diary sleep quality | 2.8 (0.6) | 2.8 (0.7 | 3.8 (0.6) |
Note. PSG = polysomnography (averaged over two nights). Diary = sleep diary (averaged over 14 nights). TST = total sleep time. BMI = body mass index. SOL = sleep onset latency. WASO= wake after sleep onset. SE = sleep efficiency.
p<.05,
p<.001
No significant group differences were observed for age (p=.226), respiration (p=.294), sex (p=.085), insomnia duration (p=.158), ISI (p=.647), BDI (p=.616), use of hypnotic medication for sleep (p=.577), medical co-morbidity (p=.697), psychological co-morbidity (p=.914), hypertensive status (p=.122), or in sleep diary variables: SOL (p=.920), WASO (p=.080), TST (p=.468), SE (p=.298), or sleep quality (p=.998). However, insomnia patients with OSSD had significantly less years of education (p=.014), and greater BMI (p<.001) than those with NNSD. As expected, they also had longer PSG-SOL (p<.001), longer PSG-WASO (p <.001), shorter PSG-TST (p<.001), and less PST-SE (p<.001) than those with NNSD.
Sleep duration based on PSG
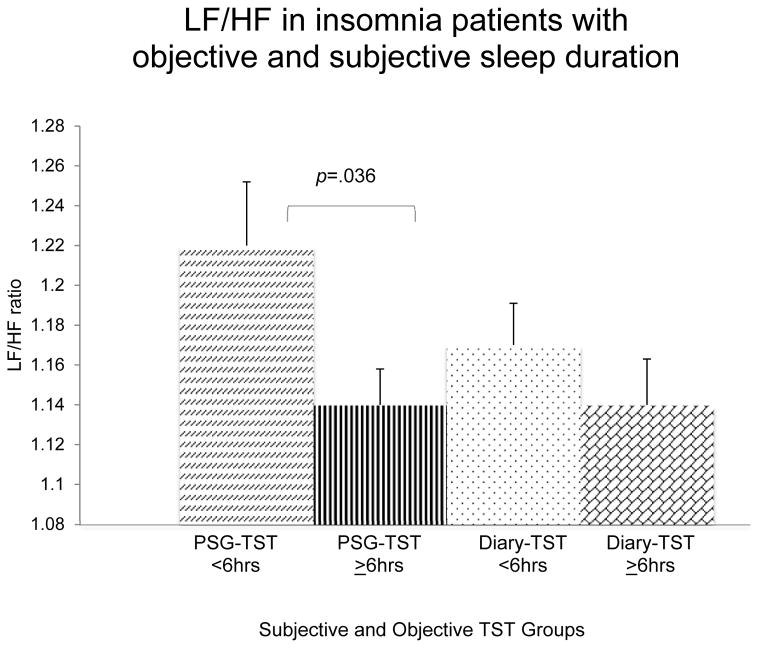
When TST was based on PSG, multilevel modeling analyses revealed significant differences between phenotypes. After controlling for covariates, patients with OSSD had significantly reduced lnHF (5.13 vs. 5.48) F(1, 163)=4.329, p=.039 (Figure 1), HFn.u. (35.1 vs. 40.0) F(1, 167)=4.453, p=.036 (Figure 2), and elevated LF/HF ratio (1.22 vs. 1.14) F(1, 164)=4.457, p=.036 (Figure 3) relative to patients with NNSD. Patients with OSSD also showed a tendency of greater HR than those with NNSD (64.5 vs. 62.1 bpm) F(1, 162)=3.866, p=.051 (Figure 4).
Figure 1.
Adjusted LnHF means and standard errors for insomnia short and near-normal sleepers based on sleep diaries and polysomnography (PSG) total sleep time (TST) <6 or ≥6hrs. Adjusted for study site, age, sex, education, body mass index, insomnia severity, hypertensive status/medication, and depressive symptoms.
Figure 2.
Adjusted HF n.u. means and standard errors for insomnia short and near-normal sleepers based on sleep diaries and polysomnography (PSG) total sleep time (TST) <6 or ≥6hrs. Adjusted for study site, age, sex, education, body mass index, insomnia severity, hypertensive status/medication, and depressive symptoms.
Figure 3.
Adjusted LF/HF ratio means and standard errors for insomnia short and near-normal sleepers based on sleep diaries and polysomnography (PSG) total sleep time (TST) <6 or ≥6hrs. Adjusted for study site, age, sex, education, body mass index, insomnia severity, hypertensive status/medication, and depressive symptoms.
Figure 4.
Adjusted heart rate (HR) means and standard errors for insomnia short and near-normal sleepers based on sleep diaries and polysomnography (PSG) total sleep time (TST) <6 or ≥6hrs. Adjusted for study site, age, sex, education, body mass index, insomnia severity, hypertensive status/medication, and depressive symptoms.
Main effects for sleep stage were also observed, indicating that lnHF (5.60 vs. 5.00) F(1, 174)=108.836 and Hfnu (43.3 vs. 31.8) F(1, 189)= 131.083 were significantly elevated and LF/HF ratio (1.09 vs. 1.27) F(1, 174)=53.781 and HR (62.2 vs. 64.6 bpm) F(1, 162)=90.981 were significantly reduced during N2 compared to REM sleep (all ps<.001). No significant interactions (sleep duration x sleep stage) were observed.
Sleep duration based on sleep diaries
When TST was based on sleep diaries, no significant differences between patients with short sleep duration and NNSD were documented for lnHF (5.6 vs. 5.1) F(1, 161)=0.012, p=.911 (Figure 1), HFn.u. (38.5 vs. 38.8) F(1, 162)=0.020, p=.887 (Figure 2), LF/HF ratio (1.17 vs. 1.14) F(1, 161)=1.508, p=.221 (Figure 3), or for HR (63.2 vs. 62.2 bpm) F(1, 161)=0.676, p=.412 (Figure 4). However, main effects for sleep stage were observed, suggesting that lnHF (5.67 vs. 5.11) F(1, 165)=123.197 and Hfnu (44.6 vs. 32.7) F(1, 173)= 191.730 were significantly elevated, and LF/HF ratio (1.07 vs. 1.25) F(1, 161)=1.508) and HR (61.5 vs. 63.9) F(1, 166)=3.644 were significantly reduced during N2 compared to REM sleep (all ps<.001). No significant interactions (sleep duration x sleep stage) were detected.
Discussion
The present study aimed to investigate whether nocturnal HRV and HR differed between insomnia patients with OSSD or NNSD. When TST was averaged across two nights of PSG, nocturnal HRV and HR differed significantly between insomnia patients with less than 6hrs compared to those with 6hrs or more of sleep. Indeed, insomnia short sleepers exhibited significantly dampened parasympathetic activation and sympathovagal imbalance relative to counterparts, indicative of cardiovascular autonomic dysfunction. These results remained significant even after controlling for age, sex, education, BMI, hypertension status/medication, depressive symptoms, insomnia severity, and study site. Conversely, when TST was averaged across 14 days of sleep diaries, nocturnal HRV and HR did not significantly differ between insomnia phenotypes.
Similar to the present study, past research found evidence of parasympathetic withdrawal among short sleepers with insomnia. More specifically, reduced frequency- and time-domain HRV (i.e., rMSSD, pNN50) were significantly reduced among insomnia patients with OSSD during sleep initiation (Spiegelhalder et al., 2011) or with low PSG-SE over the course of sleep (Miller et al., 2016) relative to counterparts. However, unlike the present study, previous findings only found non-significant trends for LF/HF ratio and HR between insomnia phenotypes (Miller et al., 2016; Spiegelhalder et al., 2011), which could be explained by differences in methodology and/or measurement across studies (e.g., 2- vs. 5-min epoch lengths, HRV during sleep vs. sleep onset) (Dodds et al., 2017).
The present non-significant findings when subjective TST was used are consistent with the notion that insomnia with OSSD is the most biologically severe phenotype (Vgontzas et al., 2013). To the best of our knowledge, this is the first study to examine modifications in the autonomic nervous system in insomnia patients with subjectively-derived sleep duration. The non-significant findings may be explained by the incongruity of comparing subjectively- and objectively-measured variables. Indeed, similar non-significant results have been documented when sleep duration is based on self-reports and outcomes are based on objective indices (Shivashankar et al., 2017). Conversely, when sleep duration and health outcomes were both subjective, significant associations were observed (Kalmbach et al., 2016).
Given that the consequences associated with this phenotype (insomnia with OSSD) are related with hyperarousal and increased risk of medical morbidity/mortality (Vgontzas et al., 2013) (although, results are not ubiquitous; Johann et al., 2017), it is posited that there are a number of pathophysiological mechanisms contributing to this association. To date, there are some, albeit limited findings in participants with insomnia and OSSD, identifying systematic inflammation (i.e., C-reactive protein) (Fernandez-Mendoza et al., 2017), HPA-axis endocrine and metabolic imbalances (i.e., cortisol, glucose) (D’Aurea et al., 2015; Fernandez-Mendoza et al., 2014), and hemodynamic dysregulations (i.e., systolic and diastolic blood pressure variability) (Johansson, Kronholm, & Jula, 2011) as possible culprits.
Another possible contributory mechanism may involve alterations in the autonomic cardiovascular system, indexed by parasympathetic withdrawal and/or sympathovagal imbalance. Indeed, parasympathetic withdrawal and/or sympathetic hyperactivity contributes to elevated heart rate, blood pressure, promotes the formation of artery-clogging deposits, inhibits pancreatic beta-cell function and insulin secretion, and is associated with immune dysfunction and inflammation (e.g., proinflammatory cytokines); all of which have been associated with cardiometabolic morbidity and death (Thayer & Lane, 2007; Von Känel et al., 2009). Mounting evidence indicates that the withdrawal of parasympathetic activation is significantly associated with the increased risk in the onset and development of cardiometabolic diseases (Von Känel et al., 2009). Reduced HRV has also been implicated in a number of internalizing and externalizing psychopathology (e.g., depression), as observed in insomnia samples with OSSD (Task Force, 1996; Fernandez-Mendoza et al., 2015; Thayer & Lane, 2007). Additionally, reduced HRV is linked with poor performance on executive functioning tasks (Hansen, Johnsen, & Thayer, 2003), corroborating past findings on the neurocognitive impairments observed particularly in adults with insomnia with OSSD (Fernandez-Mendoza et al., 2010). Taken together, it is postulated that the pathophysiological responses related to both insomnia and OSSD might trigger a nocturnal stress response within the nervous and endocrine system, expediting the progression of cardiovascular morbidity.
Limitations, Strengths, and Future Recommendations
The first limitation was the cross-sectional nature of the study, which precluded the investigation of a causal relation to be assessed between cardiovascular autonomic function, insomnia, and short sleep duration. The second limitation was the lack of N3 samples, precluding the examination of this sleep stage, which under normal conditions, are known to have parasympathetic predominance (Trinder et al., 2001). Thirdly, only frequency-domain HRV at night was examined and there are other methods used to analyze and interpret HRV (e.g., 24-hr HRV, non-linear dynamics of HRV); however, the frequency bandwidth range and physiological component for HF are well-defined and established (Task Force, 1996). Also, HRV is one of the most widely used methods for measuring cardiac autonomic function, as it is a sensitive, reproducible, and non-invasive measure that is easily derived from continuous ECG recordings. Lastly, while some information was obtained on patients’ health status and medication (e.g., antihypertensive medication), we did not obtain more comprehensive, verified details (e.g., symptoms, duration, self- or physician-diagnosed) on all patients’ medical conditions and medication use that may have impacted cardiovascular autonomic function.
One strength of the present study was the relatively large sample comprised of patients suffering from insomnia on average for 15 years, which provided an opportunity to examine HRV and HR within a clinical sample. Second, this is (to the best of our knowledge) the first study to investigate autonomic cardiovascular function in insomnia patients with short and NNSD derived from sleep diaries (over 14 days) and two consecutive nights of PSG. Third, ECG data were not influenced by wake periods across the night, as stringent criteria were used to identify stable sleep stages with artifact-free epochs (i.e., no intrusion from wake/artifact into clean epochs). Finally, the present findings were robust, even when controlling for relevant covariates including socio-demographics, anthropometrics, medical and psychological co-morbidity, and insomnia severity.
Given that insomnia, OSSD, and autonomic cardiovascular function are involved in a complex regulatory system, it is important to consider the other factors that may contribute to these associations as casual variables, mediators, and/or moderators (e.g., health status, menopause, medication use). Thus, it is suggested that prospective study designs with both clinical and population-based samples should be conducted to obtain more precise identification of such variables. Future longitudinal research with ecological validity (e.g., ambulatory PSG) can be used to better elucidate and test the temporal nature of this relation. Future research should also examine these associations with other objective sleep parameters in conjunction with subjective ratings, as it would be beneficial to examine agreement across sleep variables between groups. Notably, while statistically significant differences were observed in HRV between phenotypes, to the best of our knowledge, there are yet no clinically-defined nocturnal HRV values that identify those at risk (similar to systolic and diastolic blood pressure values that identify hypertension). As such, studies assessing the clinical applicability of nocturnal HRV and HR in risk stratification of insomnia patients at high risk of morbidity/mortality are needed. Indeed, few studies have examined the impact of cognitive behavioural therapy on insomnia patients with OSSD and NNSD (Bathgate, Edinger, & Krystal, 2017; Lovato, Lack, & Kennaway, 2016; Rochefort, Jarrin, Ivers, & Morin, 2016). Thus, it is worthwhile to consider interventions that directly influence and promote autonomic functioning in insomnia patients with OSSD. For example, acupuncture, which has direct effects on the autonomic nervous system, has been associated with changes in blood pressure, pupil size, skin conductance, microneurography recorded muscle sympathetic nerve activity, HR, and HRV (Huang, Kutner, & Bliwise, 2011).
In sum, the present findings provide new evidence on the potential role parasympathetic withdrawal and/or sympathovagal imbalance may play in the increased risk of insomnia with OSSD.
Acknowledgments
Support: Supported by National Institute of Mental Health Grant (RO1MH079188 & R01MH60413) and by the Fonds de recherche du Québec - Santé (32207).
Footnotes
Conflict of interests: Dr. Morin is part of the consulting/advisory board of Merck, Valeant, and Novartis. He is also on Valeant’s Speaker’s Bureau. No other conflict of interests is reported.
Author Contributions: Jarrin, Ivers, Lamy, and Chen had access to the data pertinent to the present study’s objectives and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Jarrin and Morin. Acquisition of data: Morin and Harvey. Analysis and interpretation of data: Jarrin, Ivers, Lamy, and Morin. Drafting of the manuscript: Jarrin and Morin. Critical revision of the manuscript for important intellectual content: Morin and Harvey. Statistical analysis: Jarrin and Ivers.
References
- American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Bathgate CJ, Edinger JD, Krystal AD. Insomnia Patients With Objective Short Sleep Duration Have a Blunted Response to Cognitive Behavioral Therapy for Insomnia. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical psychology review. 1988;8(1):77–100. [Google Scholar]
- Bélanger L, Harvey AG, Fortier-Brochu É, Beaulieu-Bonneau S, Eidelman P, Talbot L, … Morin CM. Impact of comorbid anxiety and depressive disorders on treatment response to cognitive behavior therapy for insomnia. Journal of consulting and clinical psychology. 2016;84(8):659–667. doi: 10.1037/ccp0000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiology T. F. o. t. E. S. o. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- D’Aurea C, Poyares D, Piovezan RD, Passos G, Tufik S, Mello MT. Objective short sleep duration is associated with the activity of the hypothalamic-pituitary-adrenal axis in insomnia. Arq Neuropsiquiatr. 2015;73(6):516–519. doi: 10.1590/0004-282X20150053. [DOI] [PubMed] [Google Scholar]
- Dodds KL, Miller CB, Kyle SD, Marshall NS, Gordon CJ. Heart rate variability in insomnia patients: A critical review of the literature. Sleep Medicine Reviews. 2017;33:88–100. doi: 10.1016/j.smrv.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Edinger J, Wyatt J, Olsen M, Stechuchak K, Carney C, Chiang A, … Radtke R. Reliability and validity of the Duke Structured Interview for Sleep Disorders for insomnia screening. Paper presented at the Sleep.2009. [Google Scholar]
- Fernandez-Mendoza J, Baker JH, Vgontzas AN, Gaines J, Liao D, Bixler EO. Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain Behav Immun. 2017;61:110–116. doi: 10.1016/j.bbi.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao D, … Vgontzas AN. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33(4):459–465. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Shea S, Vgontzas AN, Calhoun SL, Liao D, Bixler EO. Insomnia and incident depression: role of objective sleep duration and natural history. J Sleep Res. 2015;24(4):390–398. doi: 10.1111/jsr.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Calhoun SL, Vgontzas A, Tsaoussoglou M, Gaines J, … Bixler EO. Insomnia symptoms, objective sleep duration and hypothalamic-pituitary-adrenal activity in children. Eur J Clin Invest. 2014;44(5):493–500. doi: 10.1111/eci.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, Bixler EO. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60(4):929–935. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part I: Description. Journal of Personality disorders. 1995;9(2):83–91. [Google Scholar]
- Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Bélanger L, Talbot L, Eidelman P, Beaulieu-Bonneau S, Fortier-Brochu É, … Soehner AM. Comparative efficacy of behavior therapy, cognitive therapy, and cognitive behavior therapy for chronic insomnia: a randomized controlled trial. Journal of consulting and clinical psychology. 2014;82(4):670. doi: 10.1037/a0036606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand S, Gast KB, de Mutsert R, Swenne CA, Jukema JW, Middeldorp S, … Dekkers OM. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. 2013;15(5):742–749. doi: 10.1093/europace/eus341. [DOI] [PubMed] [Google Scholar]
- Huang W, Kutner N, Bliwise DL. Autonomic activation in insomnia: the case for acupuncture. J Clin Sleep Med. 2011;7(1):95–102. [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Isreal b, Buysse DJ, Kraffy RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: For some measures, one night is enough. Sleep. 2012;35(9):1285–91. doi: 10.5665/sleep.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarczok MN, Li J, Mauss D, Fischer JE, Thayer JF. Heart rate variability is associated with glycemic status after controlling for components of the metabolic syndrome. Int J Cardiol. 2013;167(3):855–861. doi: 10.1016/j.ijcard.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Johann AF, Hertenstein E, Kyle SD, Baglioni C, Feige B, Nissen C, … Spiegelhalder K. Insomnia with objective short sleep duration is associated with longer duration of insomnia in the Freiburg Insomnia Cohort compared to insomnia with normal sleep duration, but not with hypertension. PloS One. 2017;12(7):e0180339. doi: 10.1371/journal.pone.0180339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JK, Kronholm E, Jula AM. Variability in home-measured blood pressure and heart rate: associations with self-reported insomnia and sleep duration. J Hypertens. 2011;29(10):1897–1905. doi: 10.1097/HJH.0b013e32834abccd. [DOI] [PubMed] [Google Scholar]
- Kalmbach DA, Pillai V, Arnedt JT, Drake CL. DSM-5 Insomnia and Short Sleep: Comorbidity Landscape and Racial Disparities. Sleep. 2016;39(12):2101–2111. doi: 10.5665/sleep.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato N, Lack L, Kennaway DJ. Comparing and contrasting therapeutic effects of cognitive-behavior therapy for older adults suffering from insomnia with short and long objective sleep duration. Sleep Med. 2016;22:4–12. doi: 10.1016/j.sleep.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Miller CB, Bartlett DJ, Mullins AE, Dodds KL, Gordon CJ, Kyle SD, … Grunstein RR. Clusters of Insomnia Disorder: An Exploratory Cluster Analysis of Objective Sleep Parameters Reveals Differences in Neurocognitive Functioning, Quantitative EEG, and Heart Rate Variability. Sleep. 2016 doi: 10.5665/sleep.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index Psychometric Indicators to Detect Insomnia. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Vallières A, Guay B, Ivers H, Savard J, Mérette C, … Baillargeon L. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Vol. 1. Sage; 2002. [Google Scholar]
- Rochefort A, Jarrin DC, Ivers H, Morin CM. Treatment response to insomnia as a function of objective sleep duration. Sleep. 2016;39:A199. doi: 10.1016/j.sleep.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Shivashankar R, Kondal D, Ali MK, Gupta R, Pradeepa R, Mohan V, … Prabhakaran D. Associations of Sleep Duration and Disturbances With Hypertension in Metropolitan Cities of Delhi, Chennai, and Karachi in South Asia: Cross-Sectional Analysis of the CARRS Study. Sleep. 2017 doi: 10.1093/sleep/zsx119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalder K, Fuchs L, Ladwig J, Kyle SD, Nissen C, Voderholzer U, … Riemann D. Heart rate and heart rate variability in subjectively reported insomnia. J Sleep Res. 2011;20(1 Pt 2):137–145. doi: 10.1111/j.1365-2869.2010.00863.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine, and plasma cholesterol in apparently healthy human adults. Int J Cardiol. 2013;162(3):240–244. doi: 10.1016/j.ijcard.2011.05.058. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74(2):224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Trinder J, Kleiman J, Carrington M, Smith S, Breen S, Tan N, Kim Y. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10(4):253–264. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, … Chrousos GP. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86(8):3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: A population-based study. Diabetes Care. 2009;32(11):1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Känel R, Thayer JF, Fischer JE. Nighttime vagal cardiac control and plasma fibrinogen levels in a population of working men and women. Annals of noninvasive electrocardiology. 2009;14(2):176–184. doi: 10.1111/j.1542-474X.2009.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]