Abstract
Background
PDE10A is a member of the phosphodiesterase family whose brain expression is restricted to the striatum. PDE10A regulates cAMP and cGMP, which mediate responses to dopamine receptor activation, and the levels of these cyclic nucleotides are decreased in experimental models of L-Dopa-induced dyskinesia (LID). The elevation of cAMP/cGMP levels by PDE10A inhibition may thus be targeted to reduce LID.
Methods
Five 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine–treated macaques with advanced parkinsonism and reproducible LID were used. MR1916, a selective PDE10A inhibitor, at doses 0.0015–0.05 mg/kg, s.c. or its vehicle (control test) was co-administered with L-Dopa methyl ester acutely (predetermined optimal and suboptimal s.c. doses) and oral L-Dopa chronically as daily treatment for 5 weeks. Standardized scales were used to assess motor disability and LID by blinded examiners. Pharmacokinetics was also examined.
Results
MR1916 consistently reduced LID in acute tests of L-Dopa optimal and suboptimal doses. Significant effects were present with every MR1916 dose tested, but the most effective was 0.015 mg/kg. None of the MR1916 doses tested affected the antiparkinsonian action of L-Dopa at the optimal dose. The anti-LID effect of MR1916 (0.015 mg/kg, s.c.) was sustained with chronic administration, indicating that tolerance did not develop over the 5-week treatment. No adverse effects were observed after MR1916 administration acutely or chronically.
Conclusions
Results show that regulation of striatal cyclic nucleotides by PDE10A inhibition could be a useful therapeutic approach for LID, and therefore, data support further studies of selective PDE10A inhibitors for PD therapy.
Keywords: PDE10A inhibitor, L-Dopa-induced dyskinesia, striatum, cyclic nucleotides, non-human primate models
L-Dopa-induced dyskinesias (LID) are a common complication of long-term dopamine (DA) replacement in Parkinson’s disease (PD) in the large majority of patients treated for more than 5 years.1 LID can interfere with most activities of daily living and highly impact the general functioning of patients, but yet remain poorly treated. Currently, the treatment of LID relies on amantadine, a nonselective and weak N-methyl-D-aspartate (NMDA) antagonist with variable anti-dyskinetic effects and significant adverse effects,2,3 or the invasive alternative of surgery for deep brain stimulation (DBS).4,5 The pathophysiology of LID involves complex, adaptive changes of the striatal function1,6 including morphological and synaptic plasticity changes of striatal projection neurons (SPNs)7–10 as a result of chronic DA loss and replacement therapy. Studies in experimental models of LID have provided evidence for altered responses of SPNs to dopaminergic stimulation ultimately leading to imbalance of striatal outputs and the occurrence of involuntary movements.11,12 Clearly, the development of effective therapeutics depends on targeting these key LID mechanisms13,14 with selective pharmacological strategies.
DA signaling mechanisms in SPNs are mediated by the second messengers cAMP and cGMP, which are regulated by phosphodiesterases. A member of this enzyme family that hydrolyzes both cyclic nucleotides and is expressed only in the brain15 is the phosphodiesterase 10A (PDE10A). Critically, PDE10A is mainly restricted to the striatum and localized in SPNs15–17, a property that confers unique selectivity to regulate signaling cascades in SPNs and manipulate the striatal outputs. PDE10A inhibitors were shown to produce behavioral effects similar to D2 antagonists by increasing the activity of SPNs expressing D2 receptors (D2R).18–20 However, PDE10A inhibitors can have a distinct pharmacological profile compared with D2 antagonists because PDE10A is expressed in both subpopulations of SPNs expressing D1R or D2R and projecting into the direct and indirect striatal output pathways, respectively.21–23 Therefore, PDE10A inhibitors acting on both direct and indirect SPNs may have a unique impact in the dyskinetic responses to DA replacement in PD.12,24 Importantly, studies in rodent models showed that cAMP and cGMP significantly decrease at the peak of LID,25,26 and the administration of PDE inhibitor reduces LID,26,27 suggesting that upregulation of cAMP/cGMP could be an effective approach to treat LID.
A new potent and selective PDE10A inhibitor, MR1916 (Mochida Pharmaceuticals Co. LTD., Gotemba, Japan), has been developed in the course of extensive chemical optimization. MR1916 has potent inhibitory activities for human, rat, and monkey PDE10A with IC50 values of less than 0.1 nM and > 1,000-fold selectivity against other PDEs. Additionally, MR1916 has excellent brain penetration, and is active orally (see also Supplementary Information). Here, we investigated whether MR1916 effectively reduces LID in the non-human primate model of advanced PD for predicting anti-LID efficacy in patients. After profiling the pharmacokinetics of MR1916 and the individual optimal and suboptimal (s.c.) doses of L-Dopa, MR1916 effects were assessed in trials of acute and chronic L-Dopa co-administration, and compared with amantadine effects. The selective PDE10A inhibitor MR1916 produced a dose-dependent reduction of LID that was maintained following chronic administration. In comparison to amantadine, MR1916 had an improved pharmacological profile for the treatment of LID in parkinsonian primates.
Materials and methods
Non-Human Primate Model
Five adult monkeys (Macaca fascicularis, 3 males and 2 females, the characteristics of each animal are described in Supplementary Table 1) were used in this study following the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996). All monkeys were rendered parkinsonian by repeated systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) for 6 months or longer as previously described.28 After reaching stabilization with moderate to severe parkinsonism over a 2-month period, monkeys received oral L-Dopa (carbidopa/levodopa [Sinemet® 25/100]; Merck & Co., Inc., Whitehouse Station, NJ) as maintenance treatment. On average, after 2 months of daily L-Dopa treatment, all five animals developed consistent and reproducible LID. Subsequently, the subcutaneous (s.c.) optimal and suboptimal doses of L-Dopa methyl ester plus benserazide (one quarter of L-Dopa dose; both from Sigma-Aldrich, St Louis, MO) were determined for each animal based on their response to repeated tests of various doses. These optimal and suboptimal doses were defined as the doses that induce a reduction of motor disability scores (MDS) between 50% and 75% (optimal dose) or < 50% (suboptimal dose).
Acute MR1916 and L-Dopa co-administration
All five animals were used in the acute trials. In trials of acute co-administration with L-Dopa, MR1916 hydrobromide (Mochida Pharmaceuticals, Gotemba, Japan) was dissolved in DMSO and cremophor, and injected s.c. at four escalating doses (0.0015 mg/kg, 0.005 mg/kg, 0.015 mg/kg, and 0.05 mg/kg). In these trials, amantadine hydrochloride (Sigma-Aldrich, St Louis, MO) was dissolved in saline and injected s.c. at two doses (5 and 10 mg/kg).29 In each acute trial (optimal and suboptimal L-Dopa dose), a vehicle injection (MR1916 at 0 mg/kg) was included as control. On test days, MR1916 or amantadine was administered s.c. immediately before the L-Dopa methyl ester injection at the preselected s.c. dose. All tests were performed in the morning, and animals were fasting overnight on days of experiments. All tests were done with a minimum of 48 hr interval to ensure adequate washout of MR1916. Oral L-Dopa maintenance treatment was withheld on test days. In all acute tests, MR1916 doses (0 mg/kg, 0.0015 mg/kg, 0.005 mg/kg, 0.015 mg/kg, and 0.05 mg/kg) or amantadine doses (5 mg/kg and 10 mg/kg) were tested in random order. Each treatment was repeated 3 times, and averaged data were used as results of a given treatment (Supplementary Figure S1).
Chronic MR1916 treatment
All five animals received a s.c. injection of MR1916 (0.015 mg/kg) in the morning together with the regular oral L-Dopa dose every day for 5 weeks to determine the development of tolerance or the appearance of adverse effects with chronic treatment. The dose of MR1916 for the chronic trial was selected based on results in acute trials. Once every week during this five-week period, MR1916 was given with the suboptimal s.c. dose of L-Dopa methyl ester instead of the oral L-Dopa dose for assessment of reliable responses to s.c. L-Dopa and accurate testing of sustained MR1916 effects or development of tolerance.30 Responses to the treatment combination MR1916 plus s.c. L-Dopa methyl ester were compared across the following time points: baseline (day 0: s.c. L-Dopa methyl ester plus MR1916 0 mg/kg), every week (weeks 1 through 5; s.c. L-Dopa methyl ester plus MR1916 0.015 mg/kg), and 1 week after discontinuation of daily MR1916 as washout for a test of baseline recovery (day 42: s.c. L-Dopa methyl ester plus MR1916 0 mg/kg; see also Supplemental Figure S1). Responses to the treatment combination MR1916 plus oral L-Dopa were also scored once weekly during the trial. All assessments were done in the morning and after overnight fast.
Behavioral assessment
Motor evaluations were performed using a standardized primate motor scale (PMS) for MPTP- treated monkeys (see the scale in Supplementary Information).30–32 The PMS has 2 parts: motor disability is rated in Part I (which is similar to Part III of the Unified Parkinson Disease Rating Scale used for PD patients), and LIDs are rated in Part II.28 In addition, animals were assessed with “Drug Effects on the Nervous System” (DENS) scale33 to evaluate other potential neurological side effects of MR1916 or amantadine. This scale rates rapid changes in cortical (attentiveness and reactivity), motor (eye movement and involuntary movements), and autonomic functions (salivation, upper and lower gastrointestinal functions, and urinary function) after acute drug administration. The “Klüver board test” (KBT) was also used to assess hand movement speed and fine motor skills.34 In all trials, motor behavior in the “off” state (parkinsonian motor disability) was scored just before administration of MR1916 (or amantadine) followed by administration of L-Dopa (s.c. or oral). After L-Dopa administration, scores were taken starting at 30 min post-injection, and continuing every 20 min interval during the duration of the motor response induced by L-Dopa (“on” state). Animals were assessed directly by a blinded examiner and videotaped for deferred assessment by other blinded investigators.
Pharmacokinetics
The pharmacokinetic profile of MR1916 was examined in 3 Macaca fascicularis (all males, age 3–5 years old, weight 2–6 kg) under alert conditions (no sedative drugs were used). MR1916 was administered at doses of 0.005, 0.015, 0.05, and 0.15 mg/kg, s.c alone or at 0.15 mg/kg, s.c. in combination with L-Dopa methyl ester plus benserazide (25/6.25 mg/kg, s.c.). Blood samples were collected at 15, 30, 60, 120, 180, 240, and 480 min after drug administration and were processed in duplicates. Using aliquots of plasma samples, MR1916 and L-Dopa with internal standards were extracted with a protein precipitation method and analyzed by liquid chromatography with tandem mass spectrometry. Pharmacokinetic parameters were analyzed using Phoenix WinNonlin version 6.1 (Pharsight Corp, Palo Alto, CA). The detection limit of quantification was defined as 0.1 ng/mL for MR1916 and 10 ng/mL for L-Dopa.
Statistical analysis
Measurements of behavioral effects composed parametric data that were analyzed using 1- or 2- way analysis of variance (ANOVA) for repeated measures followed by Fisher’s Least Significant Difference (LSD) test when the F indicated significance.30 Non-processed and normalized data (to control values) were used for analysis. In both acute and chronic co-administration tests, time course of LID, peak-dose LID (at 50 min post injection), total LID (sum of LID scores obtained at all post injection time points), MDS (motor disability score) and its AUC (area under the curve), movement time in KBT (time to complete the task before [0 min] and 90 min after treatment, animals were given up to 60 s to perform the task), and DENS scores were analyzed. All data are presented as mean ± S.E.M. Statistical significance was determined at p < 0.05.
Results
MR1916 has anti-dyskinetic effect
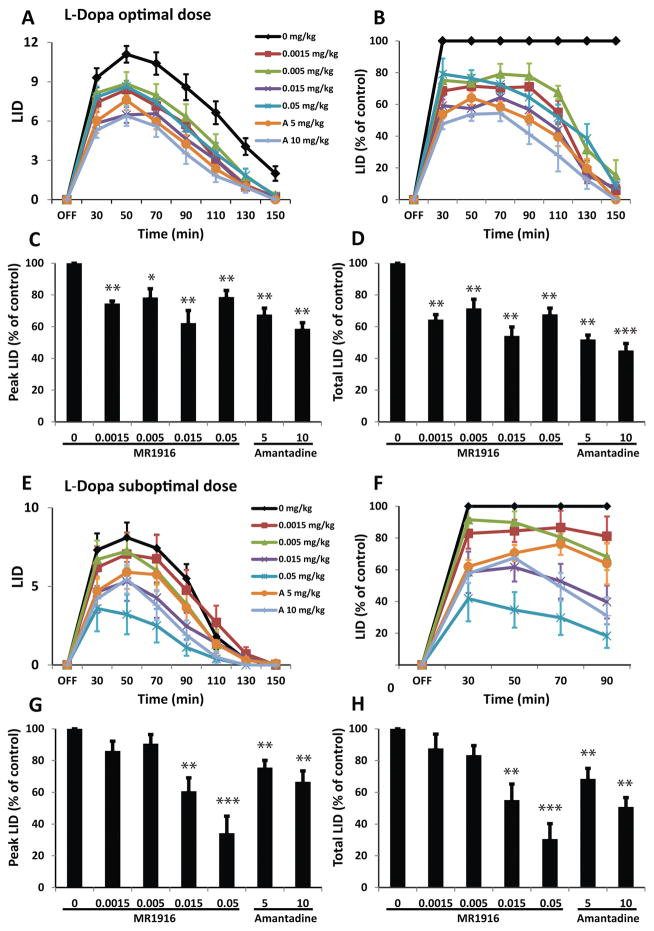
In combination with the optimal dose of L-Dopa, MR1916 at all the doses tested (ranging between 0.0015 and 0.05 mg/kg) significantly reduced LID. The most profound effect was found with 0.015 mg/kg (Fig. 1A, B). However, there was no significant difference in the effect size among the tested doses of MR1916. Both peak and total LID scores were significantly reduced by 38 and 46%, respectively by the most effective dose of MR1916 (0.015 mg/kg) (Fig. 1C, D). MR1916 effects on the dyskinesia induced by L-Dopa optimal dose were similar to those of amantadine at either dose 5 or 10 mg/kg (Fig. 1A–D). The effects of MR1916 on LID were consistent across these animals that exhibited moderate to severe dyskinesias with mean peak-dose LID scores between 9.5 and 12.5 following optimal doses of L-Dopa (see Supplementary Table 1), typically developed in monkeys with severe motor impairment and prolonged exposure to L-Dopa replacement treatment. MR1916 was effective in reducing choreodystonic diskinesias of the limbs as well as orofacial dyskinesias.
Figure 1. MR1916 co-administration with L-Dopa reduces LID.
(A) Time course of absolute LID scores following different doses of MR1916 or amantadine injected along with L-Dopa optimal dose (s.c.). (B–D) Normalized LID scores. All doses of MR1916 significantly reduced LID in all analyses: time course of LID (B), peak LID scored at 50 min after L-Dopa injection (C) and total LID scores calculated as the sum of scores from all postinjection time points (D). (E–H) LID scores following different doses of MR1916 or amantadine injected along with L-Dopa suboptimal dose (s.c.). Same graph details as in (A–D). MR1916 at 0.015 and 0.05 mg/kg significantly reduced LID in all analyses. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 versus control (MR1916 0 mg/kg or vehicle injection). A: amantadine.
In combination with the suboptimal dose of L-Dopa, MR1916 at 0.015 and 0.05 mg/kg significantly reduced LID (Fig. 1E, F). At 0.015 mg/kg, MR1916 reduced peak and total LID scores by 40 and 45%, respectively. The highest dose tested (0.05 mg/kg) reduced peak and total LID scores by 66 and 70%, respectively (Fig. 1G, H), which was more prominent than the effect of amantadine at 10 mg/kg (Fig. 1E–H). At lower doses (0.0015 and 0.005 mg/kg) MR1916 had non-significant effect on dyskinesia induced by L-Dopa suboptimal dose likely due to more subtle effects of MR1916 low doses and more variability in responses to suboptimal doses of L-Dopa (Fig. 1G, H).
MR1916 does not affect the therapeutic antiparkinsonian action of L-Dopa
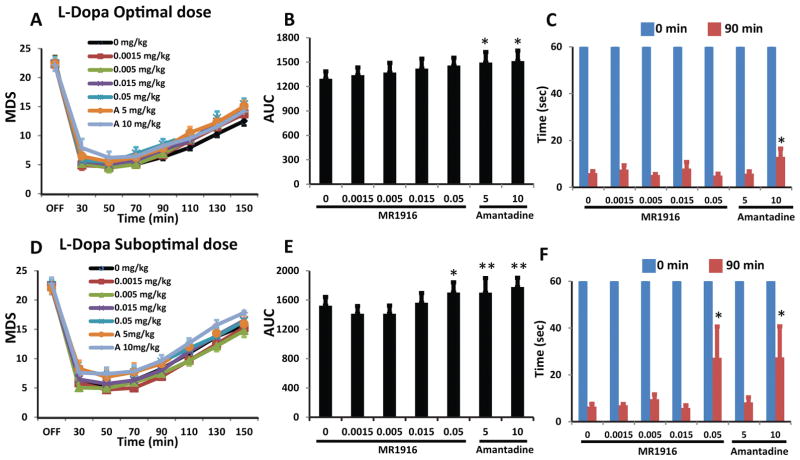
In combination with L-Dopa optimal dose, MR1916 co-administration at any of the doses tested did not change MDS in the “on” state (Fig. 2A, B). In contrast, amantadine (5 and 10 mg/kg) significantly increased MDS (Fig. 2B). Similarly, movement speed in KBT in the “on” state was not affected by MR1916 at any of the doses tested, but it was increased by amantadine at 10 mg/kg (Fig. 2C). In combination with L-Dopa suboptimal doses, MR1916 co-administration up to 0.015 mg/kg did not change MDS in the “on” state, but the highest dose of MR1916 (0.05 mg/kg) slightly increased MDS (Fig. 2D, E). In this trial, amantadine (5 and 10 mg/kg) also reduced the beneficial effect of L-Dopa on MDS (Fig. 2E). Movement speed in KBT was also increased by the highest doses of MR1916 and amantadine (Fig. 2F). These results indicate that the antiparkinsonian action of L-Dopa optimal dose is not attenuated by MR1916. However, at lower (suboptimal) L-Dopa doses its antiparkinsonian action could be affected by high doses of MR1916, but comparatively, amantadine effects present at a range of doses are more pronounced.
Figure 2. MR1916 cotherapy does not affect the antiparkinson action of L-Dopa.
(A) Time course and (B) AUC (area under the curve) of MDS with different doses of MR1916 or amantadine injected along with L-Dopa optimal dose (s.c.). (C) Movement time (sec) in the Klüver board test (KBT) at 0 min (baseline) and 90 min following MR1916 or amantadine in combination with L-Dopa optimal dose (s.c.). None of MR1916 doses affected MDS or the time in KBT. (D–F) MDS and time in KBT with different doses of MR1916 or amantadine injected along with L-Dopa suboptimal dose (s.c.). Same graph details as in (A–C). Only 0.05 mg/kg of MR1916 had a significant effect on MDS. Data are mean ± SEM. *p < 0.05, **p < 0.01, versus control (MR1916 0 mg/kg). A: amantadine.
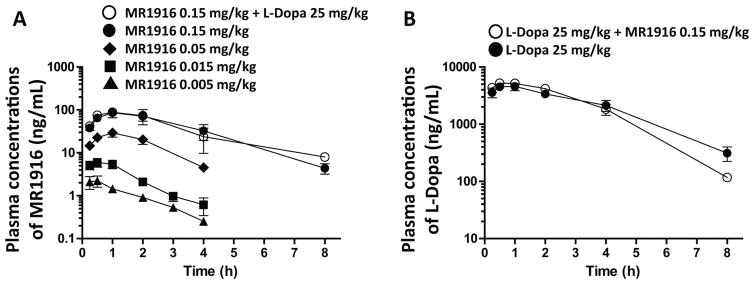
The analysis of plasma levels showed that MR1916 was rapidly absorbed with Tmax of less than 1 h, and had a dose-dependent increase of plasma concentrations (Cmax) at doses between 0.005 and 0.15 mg/kg. The half-life of MR1916 was between 1 and 2 h at the tested dose range. The pharmacokinetic profile of MR1916 was not changed by the addition of L-Dopa/benserazide (Fig. 3A and Supplementary Table 2). Critically for interpretation of MR1916 effects on dyskinesias, the plasma levels of L-Dopa did not change by the co-administration of MR1916 (Fig. 3B and Supplementary Table 2).
Figure 3. Pharmacokinetics.
(A) Plasma concentrations of MR1916. Plasma levels of MR1916 in primates vary dose-dependently, and was not changed by L-Dopa co-administration. (B) Plasma concentrations of L-Dopa. Plasma levels of L-Dopa in primates did not change following co-administration of MR1916 (0.15 mg/kg). Data are mean ± SEM.
Anti-LID effect of MR1916 is stable during chronic administration
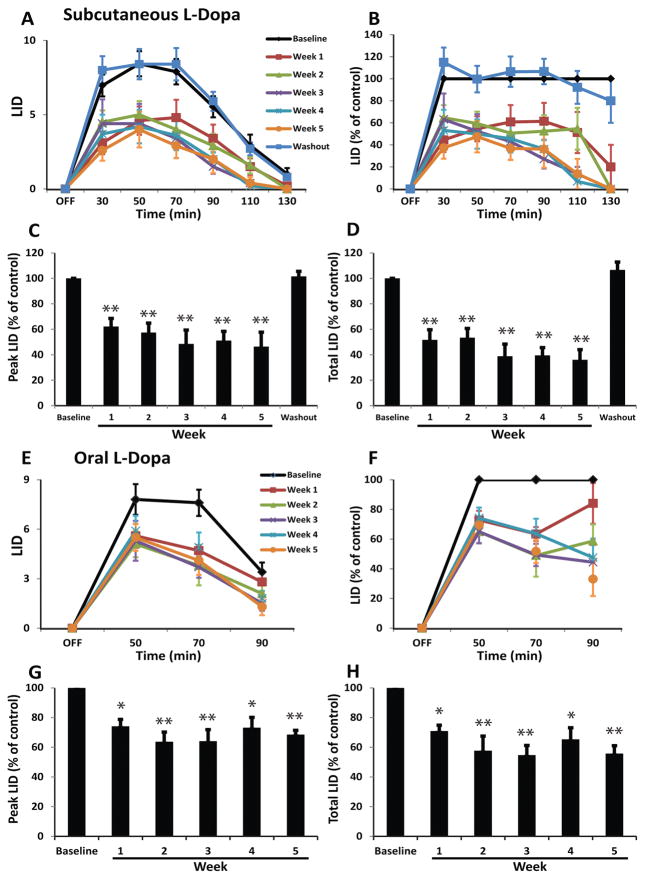
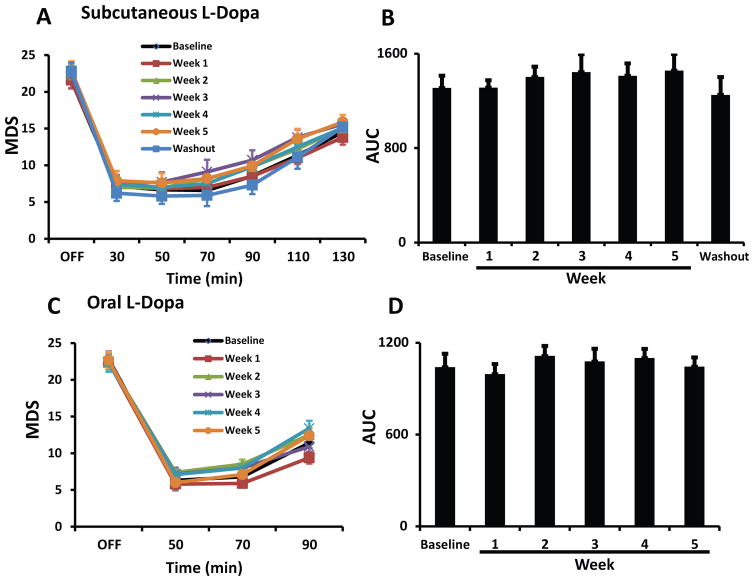
Chronic administration of MR1916 (0.015 mg/kg) significantly reduced dyskinesias from the first week of daily treatment, and the effect was sustained during the whole period of chronic treatment (Fig. 4A, B). Peak and total LID scores induced by the suboptimal dose of L-Dopa (s.c.) were significantly reduced across the weekly assessments of daily MR1916 treatment (Fig. 4C, D). Furthermore, the effect of MR1916 on LID had a tendency to augment with prolonged exposure towards the end of this chronic trial. As expected, after 1 week of MR1916 washout, LID scores returned to baseline levels (Fig. 4A–D). Although responses to oral L-Dopa are more variable in severely parkinsonian animals, the effect of daily MR1916 treatment on LID was also significant in the weekly comparisons of dyskinesias induced by the regular oral dose of L-Dopa (Sinemet25/100®; Fig. 4E–H).
Figure 4. Chronic administration of MR1916 has sustained anti-LID effect.
(A) Time course of absolute LID scores induced by (s.c.) suboptimal dose of L-Dopa alone at baseline or co-administered with 0.015 mg/kg of MR1916 (s.c.) after every week of daily MR1916 treatment for 5 weeks. (B–D) MR1916 significantly reduced LID at each time point during the 5-week treatment period in all analyses: time course of LID scored at every 20-minute interval (B), peak LID scored at 50 minutes following L-Dopa administration, and total LID scores calculated as the sum of scores from all post-administration time points. This effect was no longer detectable after a 1-week washout period (A–D). (E–H) LID scores induced by the oral maintenance dose of L-Dopa alone at baseline or co-administered with 0.015 mg/kg of MR1916 (s.c.) after every week of daily MR1916 treatment for 5 weeks. Same graph details as in (A–D). MR1916 significantly reduced LID in all analyses. Data are mean ± SEM. *p < 0.05, **p < 0.01, versus control (baseline, L-Dopa plus MR1916 0 mg/kg).
Chronic administration of MR1916 (0.015 mg/kg) that reduced LID had no significant effect on MDS across the weekly assessments with s.c. suboptimal dose or regular oral dose of L-Dopa (Fig. 5A–D). In addition, the improvement of movement speed (KBT) induced by L-Dopa (s.c.) was unchanged across the weekly assessments of chronic MR1916 treatment (data not shown). Therefore, results show that anti-LID effects of MR1916 at 0.015 mg/kg s.c. are sustained following chronic treatment without interfering with the antiparkinsonian action of L-Dopa.
Figure 5. Chronic administration of MR1916 has no impact on parkinsonian motor disability.
(A, C) Time course and (B, D) AUC (area under the curve) of MDS induced by (s.c.) suboptimal dose (A, B) or oral maintenance dose (C, D) of L-Dopa alone at baseline or co-administered with 0.015 mg/kg of MR1916 (s.c.) after every week of daily MR1916 treatment for 5 weeks. MDS induced by either s.c. or oral L-Dopa were not affected by chronic MR1916 therapy at any time point during the 5-week period.
MR1916 is well tolerated
MR1916 did not induce overt adverse effects at any of the doses tested following acute or chronic administration to parkinsonian monkeys. To quantitate potential side effects, we used the DENS scale that evaluates functions of the nervous system commonly affected by psychoneurotropic agents (cognitive, sensorimotor and autonomic functions). In acute treatment trials, items of the scale associated with cortical and autonomic function were unaffected (score 0) by MR1916 at any of the doses tested. There were no sedative effects as indicated by score 0 in the items of cortical function (attentiveness and reactivity). Only Involuntary Movements were scored in the DENS scale reflecting LID, which were reduced by MR1916 or amantadine. Thus, DENS scores were consistent with the PMS, Part II scores for LID. In chronic trials, again the only scores in the DENS scale were associated with Involuntary Movements, and showed also the sustained LID reduction with daily MR1916 treatment (0.015 mg/kg). Furthermore, prolonged observation of the animals on daily bases in the chronic co-administration trial confirmed the absence of other abnormalities in the animal’s behavior (such as increased aggressiveness or somnolence during day time) or clinical conditions (the animal’s parkinsonism and general health remained the same), indicating that chronic MR1916 treatment was well tolerated in parkinsonian monkeys.
Discussion
A novel PDE10A inhibitor, MR1916, significantly reduced LID in the advanced primate model of PD, and the effect was maintained over chronic treatment for 5 weeks. The anti-LID effect of this drug was larger than that induced by amantadine at a dose range. This anti-LID effect is present without compromising the antiparkinsonian action of L-Dopa or reducing its plasma levels. MR1916 was well tolerated by all animals even after prolonged exposure during more than 1 month of daily administration. No adverse reaction was observed, and the use of specific measurements (DENS scale) showed absence of sedation or other common side effects of psychoneurotropic agents.
Further analysis of results in the acute tests indicates that all MR1916 doses could effectively reduce LID leaving MDS unchanged when the inhibitor was combined with optimal doses of L-Dopa, which induce strong “on” responses and LID of consistent intensity. However, the therapeutic window of MR1916 changed with the suboptimal dose of L-Dopa in this group of severely parkinsonian primates. These observations suggest that D2R antagonistic effects might predominate over the D1R facilitating effects of PDE10A inhibition increasing cAMP/cGMP. The effects of MR1916 are consistent with those of other PDE10A inhibitors (TP-10 and papaverine),35,36 including antidyskinetic effects as shown in preliminary tests of TP-10 in rodents.37 It is important to consider the changes that occur as parkinsonism progresses and motor responses to L-Dopa evolve, particularly differential changes in sensitivity between D1R and D2R.1,6,38 The effect of MR1916 high dose on MDS was not observed with higher L-Dopa doses in this group of primates with similarly advanced parkinsonism, indicating that the anti-LID efficacy of PDE10A inhibition without impacting MDS is likely dependent on the relation between D1R and D2R stimulation by L-Dopa. Clearly, tests in models of milder parkinsonism as well as treatment combination with selective D1 or D2 agonists are necessary to fully address these points.
Of critical importance are the results of the chronic MR1916 tests in primates. The pronounced anti-LID effect of the PDE10A inhibitor was sustained during daily treatment. Indeed, repeated exposure to MR1916 at the most effective dose did not induce tolerance; on the contrary, the reduction of LID deepened toward the end of the chronic trial. Tests of responses to oral L-Dopa during the chronic co-administration also showed the MR1916 effect even though in severely parkinsonian animals there is more variability in responses to oral L-Dopa due to typical motor fluctuations.39 Altogether, data indicate that PDE10A inhibition can have efficacy against LID even in severely parkinsonian and dyskinetic stages.
PDE10A is restricted to the striatum and highly expressed in SPNs15–17 where its catabolic action leads to reduction of cAMP and cGMP levels. This regulation is present in both SPN subtypes, direct and indirect pathway SPNs that express dopamine D1R and D2R, respectively. Because DA activation of D1R or D2R has opposite effects on cAMP/cGMP signaling pathways, PDE10A and DA interact differently in the direct and indirect pathways. It thus follows that the increased cAMP/cGMP levels resulting from PDE10A inhibition is synergistic with D1R signaling and antagonistic with D2R signaling. However, PDE10A metabolic action may be up or down regulated depending on the levels of cyclic nucleotides, which likely change in SPNs of each pathway in conditions of chronic DA loss in PD. Studies in rodent models of PD have shown changes in cAMP/cGMP levels associated with the development of LID.25,26 Sancesario et al. showed that the anti-LID effect of amantadine in rodents is also related to changes in striatal levels of cAMP/cGMP.25 Nevertheless, the available data regarding the regulation of cyclic nucleotides in SPNs of each pathway are not clear.25–27 On the other hand, the roles of cAMP and cGMP for LID mechanisms may not be the same. Inhibitor of PDEs 5, 6 and 9 (zaprinast or UK343664), which increases cGMP, but not cAMP, improves AIMs (abnormal involuntary movements) in rodents.26,27 In the same line, upregulation of cGMP levels can rescue long-term depression at corticostriatal synapses, a form of synaptic plasticity that is lost in LID models.27 Of note, PDE10A levels were found to be lower in patients with PD in a recent PET study.40 However, these imaging data show enzyme reductions in striatum and globus pallidus but PDE10A is not expressed in the latter. Also important, the interpretation of PET scans obtained after L-Dopa treatment in relation to disease stage and motor states is unclear. PDE10A acts on both cGMP and cAMP, however, substrate affinity and other enzyme properties in the parkinsonian-dyskinetic setting are not known and may shed light on the mechanisms for its anti-LID effect.
The critical point of PDE10A inhibition having synergism with D1R but antagonism with D2R activation cannot be construed as a global, net effect in each pathway resulting in the inhibitor anti-LID effect. Stimulation of D1R, which increases cAMP via the olfactory type G-protein α subunit,14 is associated with multiple molecular markers of LID (Dopamine- and cAMP-regulated phosphoprotein, 32 kDa [DARPP-32], protein phosphatase 1 [PP-1], mitogen activated protein kinase [MAPK], extracellular signal related kinase [ERK1/2], and the transcription factor ΔFosB).41–45 In addition, a member of the D2-like subfamily, the D3R, that also inhibits adenylyl cyclase and suppresses cAMP, may participate in the mechanisms of LID. Interestingly, the D3R contribution has been associated with a D1R mediated mechanism according to a recent study in transgenic mice.46 But activation of D2R also has an important role in the development of LID. For example, the interactions between D2R and Ca2+/calmodulin-dependent protein kinase II (CaMKII) or RGS9-2 (the regulators of G-protein signaling) have been linked to LID.47–49 Important in relation to the present data, primate studies have shown both induction and control of LID by selective manipulation of either D1R or D2R mechanisms.50–53 It is thus likely that both striatal output pathways participate in the mechanisms of LID, which may result from multiple imbalances between individual SPN outputs between and within each pathway causing involuntary movements.12,24 It is thus likely that the dual action of PDE10A inhibition may blunt some of the signaling imbalances in either direction across single SPNs and thereby reduce LID.
The present tests of MR1916 in primates have clinical significance because results consistently showed anti-LID effects with a better pharmacological profile than the alternative treatment with amantadine. This study thus supports further development of the PDE10A inhibitor for the treatment of LID. A rapid path to advance MR1916 to clinical trials for PD is repurposing its clinically available form. Also importantly, this study provides key dose-response and pharmacokinetic data from primates to guide the experimental design of studies to assess efficacy in patients with PD.
Supplementary Material
Acknowledgments
We thank Drs. Keita Arakawa and Motohiko Kometani for their technical assistance as well as their discussion and comments on the manuscript.
Funding Sources;
NIH grants NS45962, NS073994, NCRR RR000165 and ORIP/OD OD011132 (S.M.P.)
Footnotes
Authors’ Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
G.B.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
S.M.: 1B, 1C, 3A, 3B
P.C.: 1C, 2B, 3B
S.M.P.: 1A, 1B, 2C, 3B
Financial Disclosure and Conflict of Interest;
Dr. Papa has received research support from NIH-NINDS, Michael J. Fox Foundation, Pfizer, Inc., EnVivo Pharmaceuticals, Inc., Forum Pharmaceuticals, Inc., GeneGraft, LTD., Key Neurosciences, and Mochida Pharmaceuticals Co. LTD. She has been a consultant for Teva Neuroscience. There is no conflict of interest with the present manuscript.
References
- 1.Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9:665–677. doi: 10.1038/nrn2471. [DOI] [PubMed] [Google Scholar]
- 2.Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology. 1998;50:1323–1326. doi: 10.1212/wnl.50.5.1323. [DOI] [PubMed] [Google Scholar]
- 3.Snow BJ, Macdonald L, McAuley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol. 2000;23:82–5. doi: 10.1097/00002826-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Herzog J, Pinsker M, Wasner M, et al. Stimulation of subthalamic fibre tracts reduces dyskinesias in STN-DBS. Mov Disord. 2007;22:679–684. doi: 10.1002/mds.21387. [DOI] [PubMed] [Google Scholar]
- 5.Hamani C, Neimat J, Lozano AM. Deep brain stimulation for the treatment of Parkinson’s disease. J Neural Transm Suppl. 2006:393–399. doi: 10.1007/978-3-211-45295-0_59. [DOI] [PubMed] [Google Scholar]
- 6.Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol. 2010;9:1106–1117. doi: 10.1016/S1474-4422(10)70218-0. [DOI] [PubMed] [Google Scholar]
- 7.Suarez LM, Solis O, Carames JM, et al. L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry. 2014;75:711–722. doi: 10.1016/j.biopsych.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon D, Petryszyn S, Sanchez MG, et al. Striatal neurons expressing D1 and D2 receptors are morphologically distinct and differently affected by dopamine denervation in mice. Sci Rep. 2017;7:41432. doi: 10.1038/srep41432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fieblinger T, Graves SM, Sebel LE, et al. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat Commun. 2014;5:5316. doi: 10.1038/ncomms6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villalba RM, Smith Y. Loss and remodeling of striatal dendritic spines in Parkinson’s disease: from homeostasis to maladaptive plasticity? J Neural Transm (Vienna) 2017 doi: 10.1007/s00702-017-1735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci. 2008;28:7537–7547. doi: 10.1523/JNEUROSCI.1176-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck G, Singh A, Papa SM. Dysregulation of striatal projection neurons in Parkinson’s disease. J Neural Transm (Vienna) 2017 doi: 10.1007/s00702-017-1744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solis O, Espadas I, Del-Bel EA, Moratalla R. Nitric oxide synthase inhibition decreases l-DOPA-induced dyskinesia and the expression of striatal molecular markers in Pitx3(−/−) aphakia mice. Neurobiol Dis. 2015;73:49–59. doi: 10.1016/j.nbd.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz-DeDiego I, Naranjo JR, Herve D, Moratalla R. Dopaminergic regulation of olfactory type G-protein alpha subunit expression in the striatum. Mov Disord. 2015;30:1039–1049. doi: 10.1002/mds.26197. [DOI] [PubMed] [Google Scholar]
- 15.Coskran TM, Morton D, Menniti FS, et al. Immunohistochemical localization of phosphodiesterase 10A in multiple mammalian species. J Histochem Cytochem. 2006;54:1205–1213. doi: 10.1369/jhc.6A6930.2006. [DOI] [PubMed] [Google Scholar]
- 16.Seeger TF, Bartlett B, Coskran TM, et al. Immunohistochemical localization of PDE10A in the rat brain. Brain Res. 2003;985:113–126. doi: 10.1016/s0006-8993(03)02754-9. [DOI] [PubMed] [Google Scholar]
- 17.Xie Z, Adamowicz WO, Eldred WD, et al. Cellular and subcellular localization of PDE10A, a striatum-enriched phosphodiesterase. Neuroscience. 2006;139:597–607. doi: 10.1016/j.neuroscience.2005.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt CJ, Chapin DS, Cianfrogna J, et al. Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther. 2008;325:681–690. doi: 10.1124/jpet.107.132910. [DOI] [PubMed] [Google Scholar]
- 19.Smith SM, Uslaner JM, Cox CD, et al. The novel phosphodiesterase 10A inhibitor THPP-1 has antipsychotic-like effects in rat and improves cognition in rat and rhesus monkey. Neuropharmacology. 2013;64:215–223. doi: 10.1016/j.neuropharm.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Uthayathas S, Masilamoni GJ, Shaffer CL, Schmidt CJ, Menniti FS, Papa SM. Phosphodiesterase 10A inhibitor MP-10 effects in primates: comparison with risperidone and mechanistic implications. Neuropharmacology. 2014;77:257–267. doi: 10.1016/j.neuropharm.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishi A, Kuroiwa M, Miller DB, et al. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–10471. doi: 10.1523/JNEUROSCI.2518-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sano H, Nagai Y, Miyakawa T, Shigemoto R, Yokoi M. Increased social interaction in mice deficient of the striatal medium spiny neuron-specific phosphodiesterase 10A2. J Neurochem. 2008;105:546–556. doi: 10.1111/j.1471-4159.2007.05152.x. [DOI] [PubMed] [Google Scholar]
- 23.Nishi A, Snyder GL. Advanced research on dopamine signaling to develop drugs for the treatment of mental disorders: biochemical and behavioral profiles of phosphodiesterase inhibition in dopaminergic neurotransmission. J Pharmacol Sci. 2010;114:6–16. doi: 10.1254/jphs.10r01fm. [DOI] [PubMed] [Google Scholar]
- 24.Singh A, Liang L, Kaneoke Y, Cao X, Papa SM. Dopamine regulates distinctively the activity patterns of striatal output neurons in advanced parkinsonian primates. J Neurophysiol. 2015;113:1533–1544. doi: 10.1152/jn.00910.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sancesario G, Morrone LA, D’Angelo V, et al. Levodopa-induced dyskinesias are associated with transient down-regulation of cAMP and cGMP in the caudate-putamen of hemiparkinsonian rats: reduced synthesis or increased catabolism? Neurochem Int. 2014;79:44–56. doi: 10.1016/j.neuint.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Giorgi M, D’Angelo V, Esposito Z, et al. Lowered cAMP and cGMP signalling in the brain during levodopa-induced dyskinesias in hemiparkinsonian rats: new aspects in the pathogenetic mechanisms. Eur J Neurosci. 2008;28:941–950. doi: 10.1111/j.1460-9568.2008.06387.x. [DOI] [PubMed] [Google Scholar]
- 27.Picconi B, Bagetta V, Ghiglieri V, et al. Inhibition of phosphodiesterases rescues striatal long-term depression and reduces levodopa-induced dyskinesia. Brain. 2011;134:375–387. doi: 10.1093/brain/awq342. [DOI] [PubMed] [Google Scholar]
- 28.Cao X, Liang L, Hadcock JR, et al. Blockade of cannabinoid type 1 receptors augments the antiparkinsonian action of levodopa without affecting dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys. J Pharmacol Exp Ther. 2007;323:318–326. doi: 10.1124/jpet.107.125666. [DOI] [PubMed] [Google Scholar]
- 29.Aron Badin R, Spinnewyn B, Gaillard MC, et al. IRC-082451, a novel multitargeting molecule, reduces L-DOPA-induced dyskinesias in MPTP Parkinsonian primates. PloS one. 2013;8:e52680. doi: 10.1371/journal.pone.0052680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potts LF, Park ES, Woo JM, et al. Dual kappa-agonist/mu-antagonist opioid receptor modulation reduces levodopa-induced dyskinesia and corrects dysregulated striatal changes in the nonhuman primate model of Parkinson disease. Ann Neurol. 2015;77:930–941. doi: 10.1002/ana.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papa SM, Chase TN. Levodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann Neurol. 1996;39:574–8. doi: 10.1002/ana.410390505. [DOI] [PubMed] [Google Scholar]
- 32.Potts LF, Uthayathas S, Greven AC, Dyavarshetty B, Mouradian MM, Papa SM. A new quantitative rating scale for dyskinesia in nonhuman primates. Behav Pharmacol. 2015;26:109–116. doi: 10.1097/FBP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uthayathas S, Shaffer CL, Menniti FS, Schmidt CJ, Papa SM. Assessment of adverse effects of neurotropic drugs in monkeys with the “drug effects on the nervous system” (DENS) scale. J Neurosci Methods. 2013;215:97–102. doi: 10.1016/j.jneumeth.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masilamoni GJ, Uthayathas S, Koenig G, Leventhal L, Papa SM. Effects of a novel phosphodiesterase 10A inhibitor in non-human primates: A therapeutic approach for schizophrenia with improved side effect profile. Neuropharmacology. 2016;110:449–457. doi: 10.1016/j.neuropharm.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giampa C, Laurenti D, Anzilotti S, Bernardi G, Menniti FS, Fusco FR. Inhibition of the striatal specific phosphodiesterase PDE10A ameliorates striatal and cortical pathology in R6/2 mouse model of Huntington’s disease. PloS one. 2010;5:e13417. doi: 10.1371/journal.pone.0013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardos G, Granacher RP, Cole JO, Sniffin C. The effects of papaverine in tardive dyskinesia. Prog Neuropsychopharmacol. 1979;3:543–550. doi: 10.1016/0364-7722(79)90008-0. [DOI] [PubMed] [Google Scholar]
- 37.Padovan-Neto FE, West AR. Regulation of Striatal Neuron Activity by Cyclic Nucleotide Signaling and Phosphodiesterase Inhibition: Implications for the Treatment of Parkinson’s Disease. Adv Neurobiol. 2017;17:257–283. doi: 10.1007/978-3-319-58811-7_10. [DOI] [PubMed] [Google Scholar]
- 38.Calabresi P, Pisani A, Rothwell J, Ghiglieri V, Obeso JA, Picconi B. Hyperkinetic disorders and loss of synaptic downscaling. Nat Neurosci. 2016;19:868–875. doi: 10.1038/nn.4306. [DOI] [PubMed] [Google Scholar]
- 39.Papa SM, Engber TM, Kask AM, Chase TN. Motor fluctuations in levodopa treated parkinsonian rats: relation to lesion extent and treatment duration. Brain Res. 1994;662:69–74. doi: 10.1016/0006-8993(94)90796-x. [DOI] [PubMed] [Google Scholar]
- 40.Niccolini F, Foltynie T, Reis Marques T, et al. Loss of phosphodiesterase 10A expression is associated with progression and severity in Parkinson’s disease. Brain. 2015;138:3003–3015. doi: 10.1093/brain/awv219. [DOI] [PubMed] [Google Scholar]
- 41.Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis. 1999;6:461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- 42.Santini E, Valjent E, Usiello A, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han C, Nie S, Chen G, et al. Intrastriatal injection of ionomycin profoundly changes motor response to l-DOPA and its underlying molecular mechanisms. Neuroscience. 2017;340:23–33. doi: 10.1016/j.neuroscience.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 44.Chen G, Nie S, Han C, et al. Antidyskinetic Effects of MEK Inhibitor Are Associated with Multiple Neurochemical Alterations in the Striatum of Hemiparkinsonian Rats. Front Neurosci. 2017;11:112. doi: 10.3389/fnins.2017.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 46.Solis O, Garcia-Montes JR, Gonzalez-Granillo A, Xu M, Moratalla R. Dopamine D3 Receptor Modulates l-DOPA-Induced Dyskinesia by Targeting D1 Receptor-Mediated Striatal Signaling. Cereb Cortex. 2017;27:435–446. doi: 10.1093/cercor/bhv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang S, Xie C, Wang Q, Liu Z. Interactions of CaMKII with dopamine D2 receptors: roles in levodopa-induced dyskinesia in 6-hydroxydopamine lesioned Parkinson’s rats. Sci Rep. 2014;4:6811. doi: 10.1038/srep06811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kovoor A, Seyffarth P, Ebert J, et al. D2 dopamine receptors colocalize regulator of G-protein signaling 9-2 (RGS9-2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways. J Neurosci. 2005;25:2157–2165. doi: 10.1523/JNEUROSCI.2840-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gold SJ, Hoang CV, Potts BW, et al. RGS9-2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson’s disease. J Neurosci. 2007;27:14338–14348. doi: 10.1523/JNEUROSCI.4223-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanchet P, Bedard PJ, Britton DR, Kebabian JW. Differential effect of selective D-1 and D-2 dopamine receptor agonists on levodopa-induced dyskinesia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- exposed monkeys. J Pharmacol Exp Ther. 1993;267:275–279. [PubMed] [Google Scholar]
- 51.Grondin R, Doan VD, Gregoire L, Bedard PJ. D1 receptor blockade improves L-dopa-induced dyskinesia but worsens parkinsonism in MPTP monkeys. Neurology. 1999;52:771–776. doi: 10.1212/wnl.52.4.771. [DOI] [PubMed] [Google Scholar]
- 52.Luquin MR, Laguna J, Obeso JA. Selective D2 receptor stimulation induces dyskinesia in parkinsonian monkeys. Ann Neurol. 1992;31:551–554. doi: 10.1002/ana.410310514. [DOI] [PubMed] [Google Scholar]
- 53.Koprich JB, Huot P, Fox SH, et al. The effects of fast-off-D2 receptor antagonism on L-DOPA-induced dyskinesia and psychosis in parkinsonian macaques. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:151–156. doi: 10.1016/j.pnpbp.2012.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.