Abstract
We evaluated the prognostic impact of clonal circulating plasma cells (cPCs) detected by six-color multi-parametric flow cytometry (MFC) in light chain (AL) amyloidosis at diagnosis. Of the 154 patients who underwent MFC, cPCs were detected in 42% (n = 65) patients. Median number of cPCs was 81 per 150 000 events (range: 6 – 17 844). High bone marrow plasma cell percentage was an independent predictor of presence of cPCs. Presence of cPCs at diagnosis was associated with inferior overall survival (OS) (90 vs. 98 months, p = 0.003) and inferior progression free survival (PFS) (31 vs. 52 months, p = 0.02). Estimated 1, 2 and 5 year OS in the two groups was: 74%, 64% and 57% and 89%, 87% and 80%, respectively. Estimated PFS at 1, 2 and 5 years was: 69%, 56% and 23% and 80%, 74% and 37%, respectively. Furthermore, the presence of cPCs at diagnosis was an independent adverse predictor of OS in multivariable analysis. Achieving a very good partial response or better was able to overcome the adverse impact of cPCs at diagnosis. Patients with cPCs at diagnosis may warrant closer monitoring post-treatment, especially if they do not achieve a deep hematologic response.
Keywords: circulating plasma cells, light chain amyloidosis, flow cytometry
INTRODUCTION
Light chain (AL) amyloidosis is a clonal plasma cell disorder characterized by organ dysfunction secondary to deposition of misfolded light chains and most commonly involves the heart, kidney, liver, nerves and gastrointestinal tract.1–3 The clonal plasma cell burden within the bone marrow in AL amyloidosis is lower than that observed in patients with multiple myeloma (MM).4 However, the presence of excess clonal bone marrow plasma cells has been shown to have an adverse impact in AL amyloidosis, both in the presence and absence of active MM as defined by the CRAB (hypercalcemia, renal insufficiency, anemia and bone lesions) criteria.5, 6
Multi-parametric flow cytometry (MFC) is a reliable tool to assess clonal plasma cells in both the bone marrow and peripheral blood compartments of plasma cells disorders, including AL amyloidosis.7, 8 The impact of circulating clonal plasma cells (cPCs) detected by MFC has been shown to impart a poor prognosis in patients with monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma, as well as active multiple myeloma (MM) in both the newly diagnosed and relapsed setting.9–14 Similarly, the presence of cPCs was also found to be an independent adverse prognostic factor for survival in patients with newly diagnosed systemic AL amyloidosis.15 However, this assessment of cPCs in AL amyloidosis has only been performed using a slide-based immunofluorescence assay and not via MFC. The former methodology tends to be labor intensive and hence unavailable for widespread clinical use. On the other hand, MFC is more reproducible and widely available in the clinical laboratory setting.
Since the impact of cPCs as detected by MFC has not been explored in AL amyloidosis, we report on the prognostic utility of quantifying cPCs in patients with AL amyloidosis as detected by MFC at the time of diagnosis.
METHODS
Following Institutional Review Board approval, patients with AL amyloidosis who were seen within 90 days of diagnosis at our institution and underwent testing for cPCs by MFC were identified from a prospectively maintained institutional database. All patients provided informed consent for review of medical records for research. Data pertaining to demographics, diagnosis, treatment and follow-up was collected from the database as well as abstracted from patients’ Electronic Medical Record. Patients were risk stratified by revised 2012 Mayo staging.16 AL amyloidosis with CRAB features refers to patients who had systemic AL amyloidosis with the presence CRAB (hypercalcemia, renal failure, anemia and bone disease) criteria.6
Six-color MFC for patients with plasma cell disorders has been used at Mayo Clinic since 2008 as previously described.7, 10, 11 In brief, six color MFC was performed on peripheral blood mononuclear cells isolated by Ficoll gradient using BD FACSCantos II instruments (Becton Dickinson, Franklin Lakes, NJ, USA) with a target of collecting 150,000 cellular events, and data were analyzed using the BD FACSDiva software (Beckton Dickinson). Antibodies to CD19, CD45, CD38, CD138 and cytoplasmic kappa and lambda light chains were employed to detect clonal plasma cells. cPCs detected were reported as number of clonal events per 150,000 events. For samples where less than 150 000 cells were gated or examined, the number of clonal events was adjusted for 150,000. The lower limit of detection by this method is 20 cells per 150 000 (~ 0.01%). Supplementary Figure 1S demonstrates an example of a MFC assessment of peripheral blood in a patient with AL amyloidosis that quantifies the cPCs.
The statistical analysis for this study was carried out using JMP® 12 (SAS Institute Inc., Cary, NC) statistical software.17 The primary endpoints of the study were overall survival (OS) from the date of diagnosis based on presence of cPCs in patients with newly diagnosed AL amyloidosis. Chi-Square and Fischer Exact tests were used to carry out univariate analysis for categorical variables and Wilcoxon Rank Sum/Kruskal Wallis for continuous variables. Survival analysis was carried out using the Kaplan-Meier method and the log-rank test was used to compare survival curves. Progression free survival (PFS) was defined as time from diagnosis to progression requiring treatment or death, whichever occurred earlier. For patients who did not have a hematology evaluation within six months of death or date of last contact, PFS was censored at date of last hematologic follow-up. Multivariate survival analysis was carried out by Cox proportional hazards model.
RESULTS
Baseline Characteristics
Of the 154 patients diagnosed with AL amyloidosis who underwent testing for cPCs at time of diagnosis, cPCs were detected in 42% (n=65) of the patients. Median number of cPCs in patients with detectable cPCs was 81 per 150 000 events (range: 6 – 17 844). In patients who had detectable cPCs without associated CRAB features (36%, 46/128), the median number of cPCs present was 62 per 150 000 events (range: 6 – 4 582). Baseline characteristics of patients with and without detectable cPCs are described in Table 1. Patients with cPCs had higher dFLC (median: 38 vs. 16 mg/dL, p = 0.005) and higher bone marrow plasma cell percentage (median: 15% vs. 10%, p < 0.001) compared to patients without any cPCs. A higher proportion of patients with detectable cPCs had associated CRAB features (29% vs. 8%, p = 0.0005). There were no differences noted in other baseline characteristics including age, sex, 2012 Mayo stage, involved light chain type, organ involvement, NT-Pro-BNP level, 24 hour urine protein, or associated FISH (fluorescence in-situ hybridization) abnormalities including t(11;14) and presence of trisomy/tetrasomy.
Table 1.
Baseline Characteristics based on presence or absence of circulating clonal plasma cells (cPCs) at diagnosis
| cPCs Present N=65 |
cPCs Absent N=89 |
P value | |
|---|---|---|---|
| Median age, years (range) | 63 (43-82) | 62 (44-85) | 0.96 |
| Sex, males, n(%) | 39 (60) | 57 (64) | 0.6 |
| Involved light chain (lambda), n(%) | 44 (69)# | 61 (70)## | 0.9 |
| Median dFLC, mg/dL (IQR) | 38 (15-116) | 16 (6-58) | 0.005 |
| Median bone marrow plasma cells, % (IQR) | 15 (10-35) | 10 (5-10) | <0.001 |
| Median NT-ProBNP, pg/mL (IQR) | 922 (241-6404) | 541 (181-2240) | 0.08 |
| Median 24 hour urine protein, mg (IQR) | 1142 (159-3344) | 1254 (203-4466) | 0.5 |
| FISH abnormalities | N=48 | N=76 | |
| t (11;14) | 20 (42) | 37 (49) | 0.4 |
| trisomy/tetrasomy | 15 (31) | 19 (25) | 0.5 |
| Mayo Stage | N=61 | N=88 | |
| 1/2/3/4 | 17/17/9/19 | 37/21/14/16 | 0.2 |
| n (%) | (27/27/15/31) | (42/24/16/18) | |
| Organ involvement, n (%) | |||
| Cardiac involvement | 39 (60) | 50 (56) | 0.6 |
| Renal involvement | 37 (59)## | 56 (62) | 0.7 |
| Liver involvement | 5 (8) | 10 (11)# | 0.4 |
| Gastrointestinal involvement | 10 (15) | 21 (24) | 0.2 |
| Autonomic nervous system involvement | 9 (14) | 14 (16) | 0.8 |
| Multi-organ involvement | 30 (47) | 47 (52) | 0.4 |
| CRAB features present, n (%) | 19 (29) | 7 (8) | 0.0005 |
| Treatment, n (%) | N=62 | N=89 | |
| ASCT based | 33 (53) | 64 (72) | 0.03 |
| Bortezomib based | 9 (15) | 19 (21) | |
| Alkylator | 14 (23) | 6 (7) | |
| IMiD | 4 (6) | – | |
| None | 2 (3) | – | |
P-value for comparison of ASCT between two groups
Abbreviations: dFLC: difference in involved and uninvolved light chains; IQR: interquartile range; FISH: fluorescence in-situ hybridization; IMiD: immunomodulatory drug ; ASCT: autologous stem cell transplantation. CRAB features: hypercalcemia, renal insufficiency, anemia and lytic lesions
Data missing for 1 patient
Data missing for 2 patients
Factors associated with cPCs
In univariate analysis the following factors were associated with presence of cPCs: high bone marrow plasma cell percentage (p < 0.001), including plasma cells >10% (p = 0.001), presence of CRAB features (p = 0.0005) and higher dFLC at diagnosis (p = 0.04). Mayo stage (p = 0.2), multi-organ involvement (p = 0.4) and presence of t(11;14) (p = 0.4) or trisomy/tetrasomy (p = 0.5) were not associated with presence of cPCs. In multivariable analysis, only high bone marrow plasma cells retained statistical significance (p=0.001), while dFLC (p=0.6) and presence of CRAB features (0.6) were not significantly associated with cPCs.
Therapy and Response
Treatment received by the patients in both groups is described in Table 1. Autologous stem cell transplant (ASCT) based therapy was the most common treatment in both groups, though notably fewer patients in the group with detectable cPCs underwent ASCT (53% vs. 70%, p = 0.03) For patients not undergoing ASCT, alkylator and bortezomib-based therapy were most commonly used. Hematologic responses rates were similar in both groups (Table 2), with very good partial response (VGPR) or better rate in the two groups being 64% vs. 65% (p=0.9), respectively. There was no difference in cardiac (47% vs. 57%) and renal response (80% vs. 73%) rates and time taken to achieve these responses. There were very few patients who could be evaluated for liver response (11 evaluable of 15 patients with liver involvement) and proportion of patients achieving a liver response was higher in the groups with detectable cPCs (100%, n=4/4) compared to that in patients without cPCs (14%; n=1/7) (p = 0.002).
Table 2.
Hematologic and Organ response with treatment in patients with newly diagnosed AL
| CPCs Present N=60 N (%) |
CPCs Absent N=89 N (%) |
P value | |
|---|---|---|---|
| Hematologic Response | |||
| Evaluable | N=36 | N=67 | |
| Complete Response | 12 (33) | 26 (39) | |
| VGPR | 11 (31) | 18 (27) | |
| Partial Response | 8 (22) | 15 (22) | |
| No response/progression | 5 (14) | 8 (12) | |
| VGPR or better rate | 23 (64) | 44 (65) | 0.9* |
| Not evaluable | 9 | 16 | |
| Not known/died before assessment | 15 | 6 | |
| Organ Response | |||
| Cardiac response, n/N (%) | 9/19 (47) | 20/35 (57) | 0.5 |
| Renal response, n/N (%) | 20/25 (80) | 35/48 (73) | 0.5 |
| Liver response, n/N (%) | 4/4 (100) | 1/7 (14) | 0.002 |
| Median time to cardiac response, months (IQR) | 9 (4-17) | 9 (5-13) | 1 |
| Median time to renal response, months (IQR) | 7 (3-14) | 6 (3-12) | 0.8 |
| Median time to liver response, months (IQR) | 4 (3-8) | 4 (4-4) | 1 |
p value for comparison of VGPR or better response
Abbreviations: VGPR: very good partial response; IQR: interquartile range
Survival Outcomes
Median follow-up of the entire cohort was 44 months and inferior survival outcomes were observed for patients with cPCs at diagnosis. Median OS in patients with detectable cPCs was 90 months [95% confidence interval (CI): 25 - not reached] vs. 98 months (95% CI: 79 - 98) in patients without any detectable cPCs, p = 0.003. Estimated survival at 1, 2 and 5 years in patients with cPCs was: 74%, 64% and 57% and in those without cPCs was 89%, 87% and 80%, respectively. Overall survival remained inferior for patients with cPCs even after excluding the 26 patients with CRAB features, with median OS of 90 vs. 98 months, p=0.02. Similarly, estimated survival at 1, 2 and 5 years in patients with cPCs was: 76%, 67% and 63% and in those without cPCs was 90%, 89% and 82%, respectively. (Figure 1) In a multivariate Cox proportional hazards model incorporating age at diagnosis, bone marrow plasma cells >10% and revised 2012 Mayo stage, presence of cPCs were independently associated with inferior OS (hazard ratio: 2.0, 95% CI 1.1 - 3.9), p = 0.03. (Table 3)
Figure 1.
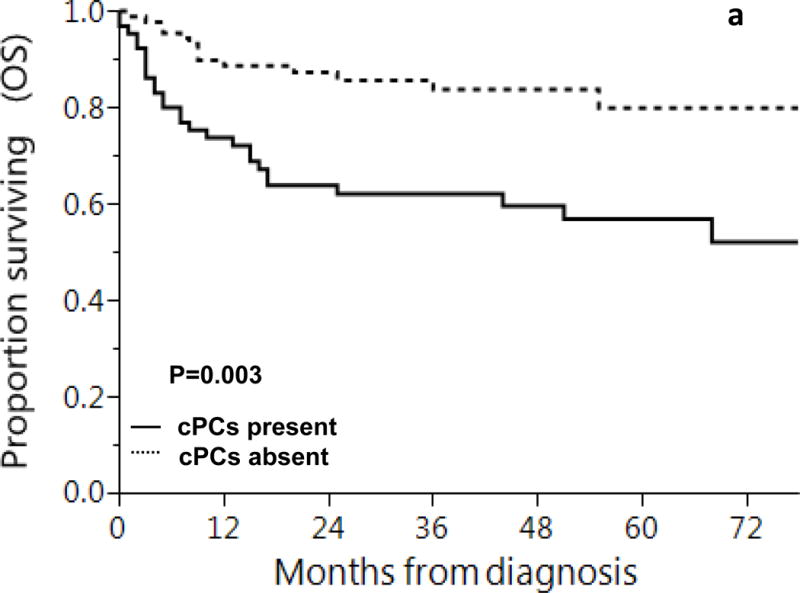
Overall Survival based on presence of clonal circulating plasma cells (a) all newly diagnosed AL amyloidosis patients and (b) newly diagnosed AL amyloidosis patients, excluding those with CRAB features
Shown is overall survival based on presence or absence of cPCs at diagnosis in patients with light chain amyloidosis
1, 2 and 5 year overall survival rates based on presence of cPCs were:
a: cPCs present: 74%, 64% and 57%
cPCs absent: 89%, 87% and 80%
b: cPCs present: 76%, 67% and 63%
cPCs absent: 90%, 89% and 82%
Table 3.
Multivariable analysis predicting for worse OS in patients with AL amyloidosis at diagnosis
| Hazard Ratio with 95% confidence interval | P value | |
|---|---|---|
| Age at diagnosis | 2.8 (0.7-11.3) | 0.1 |
| cPCs present vs. absent | 2.1 (1.1-4.0) | 0.03 |
| Mayo 2012 Stage (advanced vs. early) | 5.7 (2.9-11.9) | <0.001 |
| BMPCs >10% (yes vs. no) | 0.97 (0.5-2.0) | 0.9 |
Abbreviations: cPC: circulating plasma cells; BMPCs: bone marrow plasma cells
Advanced Mayo Stage= Stage 3 and 4 and Early Mayo Stage=stage 1 and 2
Patients with detectable cPCs had median PFS of 31 months (95% CI: 15 - 41) compared to 52 months (95% CI: 41 - 62) in the group without cPCs, p = 0.02. Estimated PFS at 1, 2 and 5 years in patients with cPCs was 69%, 56% and 23% and in those without cPCs was 80%, 74% and 37%, respectively. When patients with CRAB features were excluded, the group with detectable cPCs still had a lower PFS, though the difference was not statistically significant (median PFS: 32 months vs. 52 months, p = 0.16). Estimated 1,2 and 5 year PFS in patients with CPCs was 72%, 59% and 26% and in those without cPCs was: 80%, 74% and 35%. (Figure 2)
Figure 2.
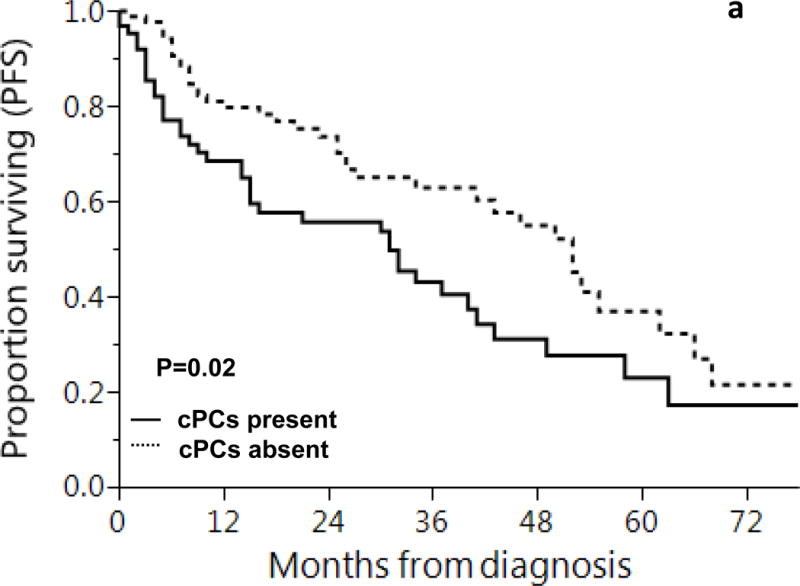
Progression Free Survival (PFS) based on presence of clonal circulating plasma cells (a) all newly diagnosed AL amyloidosis patients and (b) newly diagnosed AL amyloidosis patients, excluding those with CRAB features
Shown is progression free survival (PFS) based on presence or absence of cPCs at diagnosis in patients with light chain amyloidosis
1, 2 and 5 year progression free survival rates based on presence of cPCs were:
a: cPCs present: 69%, 56% and 23%
cPCs absent: 80%, 74% and 37%
b: cPCs present: 72%, 59% and 26%
cPCs absent: 80%, 74% and 35%
In the sub-group of 67 patients who achieved a VGPR or better response, there was no difference in overall survival amongst patients with and without cPCs at diagnosis (not reached vs. 98 months, p=0.9). Similarly, no difference in PFS was observed (49 vs. 53 months, p=0.9) amongst patients achieving a VGPR or better response.
Survival for patients in our study cohort was higher than that observed for all patients (n = 998) with AL amyloidosis seen at our institution during the study period. The median OS in the entire cohort (n = 998) was 43 months (95% CI: 33 - 54), with 27% (n = 267) patients undergoing upfront transplant. (Supplementary Figure 2S)
DISCUSSION
Previous studies from our institution have observed inferior outcomes in patients with AL amyloidosis who have cPCs using a slide-based immunofluorescence assay.15 However, given the lack of wide spread availability of the slide-based immunofluorescence assay, assessment of cPCs by MFC is a more feasible and clinically useful methodology. Our results show that presence of cPCs at diagnosis using six-color MFC at diagnosis is an independent adverse prognostic feature in AL amyloidosis, though the impact on PFS was lower when patients with co-existing CRAB criteria were excluded. Importantly, amongst patients who achieved a VGPR or better, presence of cPCs at diagnosis was not prognostic for survival, suggesting that achieving a deep hematologic response may overcome this adverse prognostic feature. We also observed that high bone marrow plasma cells independently predict for presence of circulating plasma cells in peripheral blood.
Our study cohort consisted of patients with a generally good prognosis, with higher median OS than that observed in all patients with AL amyloidosis seen at our institution over the study period (2008-2015). The median OS for patients in our cohort who had cPCs tested at time of diagnosis was 98 months compared to 43 months in all patients seen over that time period. This is likely due to higher number of patients in the study cohort being transplant eligible compared to all patients with AL amyloidosis. The difference in transplant eligibility of our study cohort is because testing for cPCs is not yet routine for all AL amyloidosis patients, but is often done for patients undergoing evaluation for an ASCT. Even in patients with an excellent prognosis, presence of cPCs at time of diagnosis was associated with an inferior PFS and OS. There was no difference in hematologic response or rates of organ response for cardiac and renal response between patients with and without cPCs. Although we observed a statistically significant difference in the rates of liver response, there were too few patients in both groups to make a meaningful comparison. Given the prognostic impact of presence of cPCs in AL amyloidosis, it remains reasonable to consider assessment for cPCs in patients with AL amyloidosis at the time of diagnosis. Patients who have cPCs at time of diagnosis may be considered for a more risk-adapted therapy.
We acknowledge that our study has limitations owing to its retrospective design and testing of cPCs in non-contiguous patients. Our study cohort includes a higher proportion of patients who are transplant-eligible given our current practice pattern. However, the baseline characteristics of patients with and without cPCs at diagnosis were comparable, except for factors predictive of presence of cPCs. Therefore, our results are valid despite having a selected cohort. Given our small sample size, we were unable to determine an optimal cutoff for the absolute number of cPCs that would predict for worse outcomes; further studies are needed to determine such an appropriate cut-off for cPCs. A recent study from our group also established the prognostic role of MFC on bone marrow in patients with AL amyloidosis at diagnosis and at end of therapy.18 Future studies are warranted to establish correlation between bone marrow clonal plasma cells detected by MFC and presence of cPCs by MFC. Detection of cPCs in peripheral blood offers a more convenient method of monitoring, especially at end of therapy. Further investigation is also warranted to determine if early clearance of cPCs can predict for hematologic response and if it is associated with improved outcomes.
In conclusion, presence of cPCs as detected by six-color MFC in patients with AL amyloidosis is an independent adverse prognostic factor at time of diagnosis. Our results suggest that patients with detectable cPCs at diagnosis, especially those who do not achieve a deep hematologic response may require closer monitoring.
Supplementary Material
Acknowledgments
The Mayo Clinic Hematological Malignancies Program and in part by grants K23CA218742, CA107476, CA168762 and CA186781 from the National Cancer Institute, Rockville, MD, USA. It is also supported in part by the Jabbs Foundation, Birmingham, United Kingdom, the Henry J. Predolin Foundation, USA and the Marion Schwartz Foundation for Multiple Myeloma.
Footnotes
Conflict of Interest: The authors have no conflicts of interest in relation to this manuscript
AUTHORSHIP CONTRITBUTIONS:
S.S, S.K.K. and W.I.G. designed the study, collected the data, analyzed the data and wrote the manuscript; N.T. collected the data and contributed to writing and reviewing the manuscript. A.D., M.A.G., D.D., D.J., W.J.M., P.K., T.V.K., M.Q.L., S.R.H., F.K.B., N.L., R.S.G., Y.L., S.J.R., J.A.L., S.R.Z., R.W., Y.L.H., M.A.H., A.L.F., R.A.K. and S.V.R., contributed to writing and reviewing the manuscript.
References
- 1.Gertz MA. Immunoglobulin light chain amyloidosis: 2016 update on diagnosis, prognosis, and treatment. Am J Hematol. 2016;91(9):947–56. doi: 10.1002/ajh.24433. [DOI] [PubMed] [Google Scholar]
- 2.Wechalekar A, Wechalekar AD, Mahmood SA, Youngstein T, Coyne M, Foard D, et al. Interim analysis of ALCHemy – a prospective study of 1000 patients with Systemic AL amyloidosis. Clinical Lymphoma, Myeloma and Leukemia. 15:e60. [Google Scholar]
- 3.Mahmood S, Palladini G, Sanchorawala V, Wechalekar A. Update on treatment of light chain amyloidosis. Haematologica. 2014;99(2):209–21. doi: 10.3324/haematol.2013.087619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006;108(8):2520–30. doi: 10.1182/blood-2006-03-001164. [DOI] [PubMed] [Google Scholar]
- 5.Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(34):4319–24. doi: 10.1200/JCO.2013.50.8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morice WG, Hanson CA, Kumar S, Frederick LA, Lesnick CE, Greipp PR. Novel multi-parameter flow cytometry sensitively detects phenotypically distinct plasma cell subsets in plasma cell proliferative disorders. Leukemia. 2007;21(9):2043–6. doi: 10.1038/sj.leu.2404712. [DOI] [PubMed] [Google Scholar]
- 8.Lisenko K, Schonland SO, Jauch A, Andrulis M, Rocken C, Ho AD, et al. Flow cytometry-based characterization of underlying clonal B and plasma cells in patients with light chain amyloidosis. Cancer medicine. 2016;5(7):1464–72. doi: 10.1002/cam4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonsalves WI, Rajkumar SV, Dispenzieri A, Dingli D, Timm MM, Morice WG, et al. Quantification of circulating clonal plasma cells via multiparametric flow cytometry identifies patients with smoldering multiple myeloma at high risk of progression. Leukemia. 2017;31(1):130–5. doi: 10.1038/leu.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonsalves WI, Rajkumar SV, Gupta V, Morice WG, Timm MM, Singh PP, et al. Quantification of clonal circulating plasma cells in newly diagnosed multiple myeloma: implications for redefining high-risk myeloma. Leukemia. 2014;28(10):2060–5. doi: 10.1038/leu.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonsalves WI, Morice WG, Rajkumar V, Gupta V, Timm MM, Dispenzieri A, et al. Quantification of clonal circulating plasma cells in relapsed multiple myeloma. British journal of haematology. 2014;167(4):500–5. doi: 10.1111/bjh.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paiva B, Perez-Andres M, Vidriales MB, Almeida J, de las Heras N, Mateos MV, et al. Competition between clonal plasma cells and normal cells for potentially overlapping bone marrow niches is associated with a progressively altered cellular distribution in MGUS vs myeloma. Leukemia. 2011;25(4):697–706. doi: 10.1038/leu.2010.320. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi G, Kyle RA, Larson DR, Witzig TE, Kumar S, Dispenzieri A, et al. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia. 2013;27(3):680–5. doi: 10.1038/leu.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty R, Muchtar E, Kumar SK, Jevremovic D, Buadi FK, Dingli D, et al. Risk stratification in myeloma by detection of circulating plasma cells prior to autologous stem cell transplantation in the novel agent era. Blood Cancer J. 2016;6(12):e512. doi: 10.1038/bcj.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardanani A, Witzig TE, Schroeder G, McElroy EA, Fonseca R, Dispenzieri A, et al. Circulating peripheral blood plasma cells as a prognostic indicator in patients with primary systemic amyloidosis. Blood. 2003;101(3):827–30. doi: 10.1182/blood-2002-06-1698. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–95. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Kimlinger T, Morice W. Immunophenotyping in multiple myeloma and related plasma cell disorders. Best practice & research Clinical haematology. 2010;23(3):433–51. doi: 10.1016/j.beha.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muchtar E, Jevremovic D, Dispenzieri A, Dingli D, Buadi FK, Lacy MQ, et al. The prognostic value of multiparametric flow cytometry in AL amyloidosis at diagnosis and at the end of first-line treatment. Blood. 2017;129(1):82–7. doi: 10.1182/blood-2016-06-721878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


