Abstract
Aging severely limits myocardial repair and regeneration. Delineating the impact of age-associated factors such as short telomeres is critical to enhance the regenerative potential of cardiac progenitor cells (CPCs). We hypothesized that short telomeres activate p53 and induce autophagy to elicit the age-associated change in CPC fate. We isolated CPCs and compared mouse strains with different telomere lengths for phenotypic characteristics of aging. Wild mouse strain Mus musculus castaneus (CAST) possessing short telomeres exhibits early cardiac aging with cardiac dysfunction, hypertrophy, fibrosis and senescence, as compared to common lab strains FVB and C57 bearing longer telomeres. CAST CPCs with short telomeres demonstrate altered cell fate as characterized by cell cycle arrest, senescence, basal commitment, and loss of quiescence. Elongation of telomeres using a modified mRNA for telomerase restores youthful properties to CAST CPCs. Short telomeres induce autophagy in CPCs, a catabolic protein degradation process, as evidenced by reduced p62 and increased accumulation of autophagic puncta. Pharmacological inhibition of autophagosome formation reverses the cell fate to a more youthful phenotype. Mechanistically, cell fate changes induced by short telomeres are partially p53 dependent, as p53 inhibition rescues senescence and commitment observed in CAST CPCs, coincident with attenuation of autophagy. In conclusion, short telomeres activate p53 and autophagy to tip the equilibrium away from quiescence and proliferation towards differentiation and senescence, leading to exhaustion of CPCs. The study provides the mechanistic basis underlying age-associated cell fate changes that will enable identification of molecular strategies to prevent senescence of CPCs.
Keywords: Cardiac Progenitor Cells, Aging, Telomeres, p53, Autophagy
Introduction
Advancing age limits myocardial regeneration and repair and is an influencing risk factor for heart disease[1, 2]. The age-associated loss of tissue homeostasis and function is caused, at least partly, by the accumulation of exhausted tissue-resident stem and progenitor cells[1, 3]. Niches of c-kit+ cardiac progenitor cells (CPCs) have been identified in the adult heart that possess the potential to proliferate, self-renew, commit to cardio-myogenic lineages and regenerate in response to injury[4, 5]. Autologous delivery of exogenously expanded CPCs has been widely tested in experimental animal models[5–7], making headway into Phase I clinical trials[8, 9]. However, cell-intrinsic and extrinsic changes induced by age drastically affect the cellular properties leading to senescence, loss of stemness and impaired regenerative potential of CPCs limiting their therapeutic application[1, 10, 11]. Delineating the molecular mechanisms that elicit the age-associated change in cell fate of CPCs is therefore critical to identify strategies to rejuvenate and enhance CPC-efficacy in autologous cell-based therapies.
An important hallmark of aging is the shortening of telomeres[3], which are complex ribonucleoprotein structures with tandem repeats of TTAGGG nucleotide sequences capping and protecting the ends of chromosomes[12]. Collective stem cell literature reveals a role for telomere length in regulating cell fate determinants such as proliferation, survival, commitment, and senescence of stem cells. Telomere shortening is the result of cell division as well as increased oxidative stress and is the cause, rather than consequence, of cellular senescence[13, 14]. Additionally, short/dysfunctional telomeres limit tissue renewal capacity and survival[15], and cause aberrant differentiation in pluripotent stem cells[16]. Conversely, telomere elongation mediated by ectopic expression of telomerase reverse transcriptase (TERT) enhances stem cell proliferation and survival, rescues impaired progenitor cell differentiation, and antagonizes senescence[16–18]. Specifically in the context of CPCs, telomere shortening shows a strong correlation with growth arrest, impaired proliferation and cellular senescence in CPCs isolated from aged mice as well as adult patients with end stage heart failure [10–12, 19]. However, a role for short dysfunctional telomeres in collectively causing the age-associated change in cell fate of CPCs, and the underlying molecular mechanism of action are not well established.
Transcription factor p53 functions as a cell-cycle inhibitor and is activated in response to telomere and DNA damage, aging and pathologic stress in the heart[2, 20–22]. p53 modulates stem cell fate by exerting pleiotropic roles that impact cell survival, differentiation, senescence and quiescence[23, 24]. Additionally, p53 regulates autophagy, a catabolic process of bulk protein degradation, in a cell-type specific manner[25]. The previously unknown influence of telomere length, p53 and autophagy in collectively modulating CPC fate will be delineated in this study. Specifically, this study tests the hypothesis that short telomeres activate p53 which induces autophagy and switches cell fate from reversible to ‘irreversible’ cell cycle arrest in CPCs. Reversible cell cycle arrest is quiescence, a state of metabolic dormancy that stem cells typically reside in, while senescence and differentiation are considered ‘irreversible’ forms of cell cycle arrest[26].
A naturally occurring, wild mouse strain Mus musculus castaneus (CAST) bearing short telomeres from birth[27] has been used to study the effects of short telomeres in CPCs. Telomere length in CAST mouse ranges from 18–20Kb, which is similar to humans (5–15Kb), and differs from common laboratory mouse strains such as FVB or C57/BL6 (C57) bearing long, hypervariable telomeres ranging from 30–120Kb (averages of 75 and 50Kb for FVB and C57 mice respectively)[27]. By comparing hearts and CPCs isolated from the 3 mouse strains, this study – a) demonstrates that CAST mice display phenotypic characteristics of early cardiac aging, b) establishes that CAST CPCs exhibit altered cell fate consistent with aged mouse and human CPCs, c) delineates the molecular mechanism by which short telomeres modulate cell fate in CPCs, and d) defines clinically-relevant strategies to rejuvenate aged CPCs.
Methods
A detailed, expanded Methods section has been included in the Online Supplement.
Experimental animals include male FVB, C57 and CAST mice that were purchased from Jackson Labs. c-kit+ CPCs were isolated from these mice strains and cultured as described previously[11]. All animal protocols and studies were approved by the review board of the Institutional Animal Care and Use Committee at San Diego State University and University of California, Davis.
Results
CAST Mice Display Functional, Cellular and Molecular Characteristics of Cardiac Aging
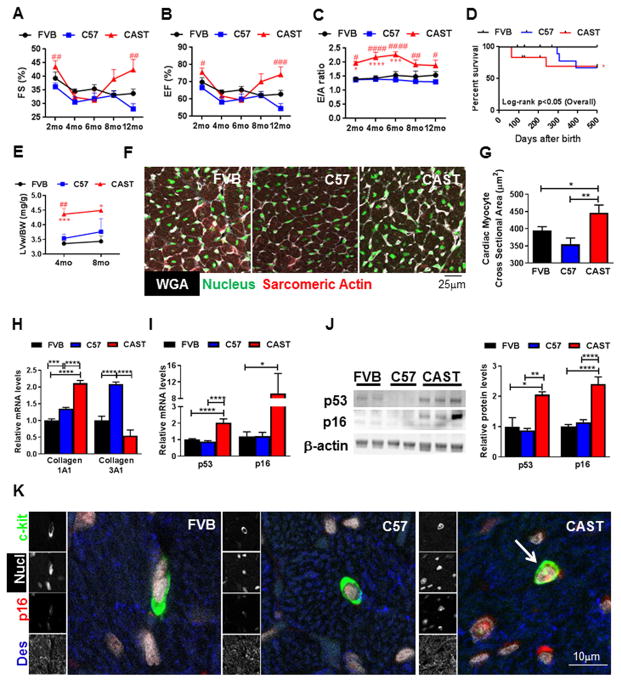
The first objective in the study was to establish the CAST mice as a model of cardiac aging and to this end the myocardium of 2–12 month old, male FVB, C57 and CAST mice were compared. Cardiac functional analyses revealed that CAST mice exhibit significantly decreased left ventricular (LV) inner diameter (LVID) both at diastole (d) and systole (s; Fig. S1AB), as well as reduced diastolic and systolic LV volume over 1 year (Fig. S1CD). CAST mice had preserved systolic function as measured by fractional shortening (FS) and ejection fraction (EF; Fig. 1AB). Interestingly however, diastolic dysfunction was evident in CAST mice as measured by clinically relevant parameters[28] such as the abnormally increased ratio of peak velocity of early to late filling of mitral inflow (E/A; Fig. 1C), a decrease in A wave velocity, reduction in the absolute value of early diastolic mitral annular tissue velocity (E′) and an increase in the absolute ratio of E/E′ (Supplemental Table 1). Survival rate of CAST mice was significantly decreased in comparison to FVB, but not C57 mice (Fig. 1D). LV hypertrophy is well documented during cardiac aging[29] and consistently, CAST mice displayed a significant increase in the LV weight to body weight (BW) ratio (LVw/BW) at 4 and 8 months after birth (26.6 and 25.7%; Fig. 1E), although no increases in diastolic or systolic LV posterior wall (LVPW) thickness were observed (Fig. S1EF). This was accompanied by a small (20%), but significant increase in the cardiac myocyte cross sectional area in the CAST mouse heart (Fig. 1FG), indicating cardiac myocyte hypertrophy. Markers of fibrosis were elevated in the CAST myocardium within 2 months after birth, as exemplified by increased collagen1A1 messenger ribonucleic acid (mRNA) levels (2.1-fold, Fig. 1H), higher collagen1/collagen3 ratio (4.9-fold, Fig. S1G) and more interstitial blue stain with Mason’s Trichrome staining (Fig. S1H). Plasma catecholamine levels were dramatically elevated in the CAST mice at 2 months of age (Fig. S1I), consistent with previous studies reporting their increased levels and reduced clearance during cardiac aging[30]. Expression of cell cycle inhibitors typically associated with cellular senescence, such as p53 and p16, were also significantly increased in the mRNA (2 and 9-fold; Fig. 1I) and protein levels (2 and 2.4-fold; Fig. 1J, Fig. S1J) in the CAST heart at 2–4 months. However, protein levels of cell cycle inhibitor Retinoblastoma1 (Rb) was not changed in the CAST myocardium (Fig. S1J). Additionally, activation of p53 and Rb as determined by phosphorylation at Serine 15 (P-p53) and Serine 780 (P-Rb) respectively were similar in FVB, C57 and CAST mouse hearts (Fig. S1K). Consistent with acquisition of senescence, telomere length was significantly shorter in the CAST myocardium, as determined by quantitative real time polymerase chain reaction (qPCR; Fig. S1L) and quantitative fluorescence in-situ hybridization (qFISH) assays (−89%; Fig. S1MN), corroborating with established evidence that wild mice bear short telomeres[27]. Furthermore, accumulation of senescent p16+ c-kit+ CPCs was observed in the CAST myocardium (Fig. 1K), suggesting that the CAST mouse exhibits molecular markers of senescence in the heart as well as endogenous CPCs therein. Collectively, the data indicates that CAST mice exhibit functional, cellular and molecular characteristics typical of cardiac aging.
Figure 1. A–B. Characterization of CAST mouse as a model of early cardiac aging.
M-mode echocardiographic measurements of fractional shortening (FS%) (A) and ejection fraction (EF%) (B) in mice at different ages (2 months (mo): N=13FVB, 13C57, 15CAST; 4mo: N=9/strain; 6mo: N=9FVB, 9C57, 6CAST; 8mo: N=9FVB, 8C57, 6CAST; 12mo: N=8FVB, 7C57, 4CAST) C. Pulse wave Doppler based measurement of peak velocity of early (E) and late (A) filling of mitral inflow (E/A) (2mo: N=6FVB, 4 C57 and CAST; 4mo: N=9/strain; 6mo: N=9FVB, 9C57, 6CAST; 8mo: N=8FVB, 9C57, 6CAST; 12mo: N=7FVB, 5C57, 4CAST)
D. Kaplan-Meier survival analyses (N=15FVB, 15C57, 18CAST). E. Left ventricular (LV) weight normalized to body weight (LVw/BW) (4mo: N=7FVB, 8C57, 10CAST; 8mo: N=3/strain). F–G. Micrographs of LV sections stained with wheat germ agglutinin (WGA, white), nuclear marker (green) and sarcomeric actin (red) (F) and quantification of cardiac myocyte size (G). H–I. mRNA levels of Collagen1A1 and Collagen3A1 (H) and p53 and p16 (I) as determined by qPCR in mouse hearts. J. Immunoblots and densitometric analyses of p53 and p16 expression in mouse hearts. Immunoblot for p16 with additional samples is included in the Supplement to address sample heterogeneity. K. Confocal micrographs of mouse hearts stained with c-kit (green), p16 (red), desmin (des, blue) and nucleus (nucl, white). Arrow indicates senescent (c-kit+, p16+) CPC. Male FVB, C57 and CAST mice at 2 months of age (N=3/strain) were used for experiments from F–K, except p16 in J, which includes samples from 2–4 months of age (N=8 FVB, 8 C57, 6 CAST). Units are listed in parantheses (A–B,E,G). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. * and # indicate comparison to FVB and C57 respectively and number of # represents p value similar to * (A–C, E). Two-way Anova (A–C, E), One-way Anova (D,G–J).
Altered Cell Fate in Aged CPCs with Short Telomeres
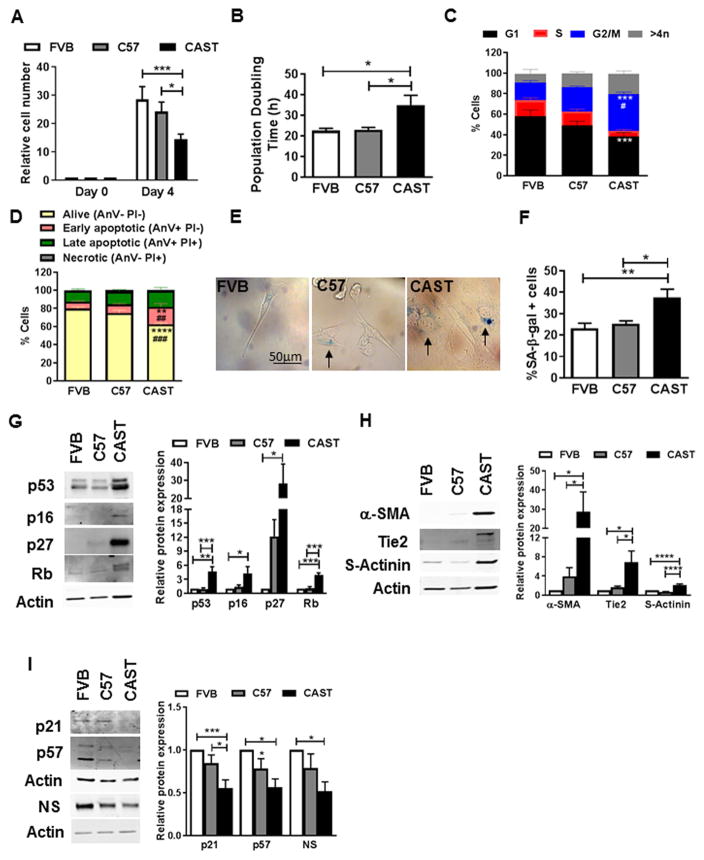
To specifically understand how short telomeres and cardiac aging affect myocardial stem cell biology, CPCs were isolated from FVB, C57 and CAST mice and cultured ex vivo. Flattened cellular morphology (Fig. S2A) coincident with significantly decreased proliferation rate (−49%, Fig. 2A) and elevated population doubling time (55.3%, Fig. 2B) were evidenced in CAST CPCs, indicating slower growth kinetics and cell cycle arrest. Cell cycle analyses confirmed arrest in the G2/M phase, marked by decreases in G1 (−33.5%) and S (−66.6%) concomitant with increase in G2/M phase (110%; Fig. 2C). Cell death was also increased in CAST CPCs, as demonstrated by a decline in the percentage of live cells (−21.8%) and increase in cells undergoing early apoptosis (148%, Fig. 2D) in comparison to FVB CPCs. Cell cycle arrest was accompanied by higher frequency of senescent CAST CPCs (62%), as measured by senescence associated β-galactosidase assay (SA-β-gal; Fig. 2EF). Additionally, molecular markers of senescence such as short telomeres (−43%, Fig. S2BC) as well as increased expression of cell cycle inhibitors p53 (4.6-fold), p16 (4.3-fold), p27 (28.3-fold) and Rb (3.9-fold, Fig. 2G) were evidenced in CAST CPCs. Expression of P-p53 was similar between FVB, C57 and CAST CPCs, while P-Rb was significantly decreased in C57 and CAST CPCs, indicating activation of Rb (Fig. S2D). Interestingly, cardio-myogenic lineage commitment markers were also elevated under basal conditions in CAST CPCs, as evidenced by increased expression of α-smooth muscle actin (α-SMA, 28.6-fold), Tie2 (6.9-fold), and sarcomeric actinin (s-actinin, 2-fold), indicative of smooth muscle, endothelial and cardiac myocyte lineages respectively (Fig. 2H). However, no further increase in expression of commitment markers was evidenced in CAST CPCs upon addition of dexamethasone, a non-specific inducer of differentiation of CPCs[11] (Fig. S3AB). Furthermore, expression of cell cycle inhibitors associated with stem cell quiescence such as p21[31] (−45%) and p57[32] (−44%), as well as ‘stemness’ marker nucleostemin[11] (NS, −49%) were dramatically reduced at baseline in CAST CPCs, suggesting loss of quiescence and stemness (Fig. 2I). Overall, the data indicates that CAST CPCs exhibit a switch from reversible (quiescence) to ‘irreversible’ (senescence and commitment) forms of cell cycle arrest, suggestive of a change in cell fate.
Figure 2. CAST CPCs exhibit altered cell fate.
A. Cell number normalized to day 0 and assessed by microscopic counting of live cells (N=9). B. Population doubling time calculated from live cell counts (N=12). C. Cell cycle stage assessed by measuring DNA content using flow cytometry (N=4). D. Flow cytometry based cell death analyses as assessed by propidium iodide (PI) and AnnexinV (Anv) staining of live cells (N=4). E–F. Phase contrast micrographs (E) and counts (F) of CPCs stained with senescence associated β-galactosidase (SA-β-gal). Arrows indicate senescent, blue cells (N=7). G–I. Immunoblots and densitometric analyses of senescence markers p53, p16, p27 and Rb (G), cardiomyogenic lineage commitment markers α-smooth muscle actin (α-SMA), Tie2 and sarcomeric actinin (s-actinin) (H), and quiescence/stemness markers p21, p57 and nucleostemin (NS) (I) (N=4–15). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, * and # indicate comparison to FVB and C57 respectively and number of # represents p value similar to * (C,D). Two-way Anova (A,C,D), One-way Anova (B,F–I).
CPCs isolated from young (YCPC) and old (OCPC) FVB mice were compared to further correlate the change in cell fate with short telomeres. Prior evidence from our group has established that OCPCs exhibit shorter telomeres[12], slower growth kinetics[11] and increased SA-β-gal positivity coincident with loss of NS[11], as compared to YCPCs. Corroborating with findings from CAST CPCs, expression of senescence markers p53 (2-fold), p27 (3.4-fold) and Rb (3.7-fold), as well as lineage commitment markers α-SMA (6.3-fold) and s-actinin (1.7-fold) were elevated in OCPCs, concurrent with loss of quiescence markers p21 (−34%) and p57 (−76%; Fig. S4ABC). Additionally, hypophosphorylation of Rb and p53 were evidenced in OCPCs, indicating activation of Rb, but not p53 (Fig. S4D). Altered cell fate with increased basal lineage commitment marker expression was also found in adult human CPCs with critically short telomeres (Fig. S4E), consistent with our prior findings establishing correlations between telomere length, proliferation rate and senescence marker expression in multiple adult human CPC lines[19].
Telomere Elongation Partially Reverses Cell Fate
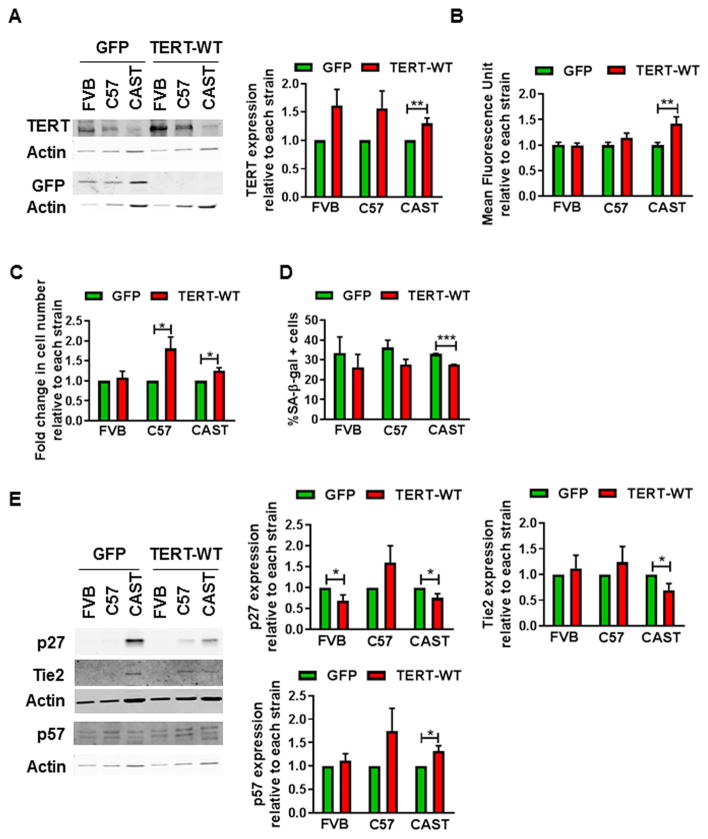
A causal role for short telomeres in eliciting the age-associated change in CPC fate was determined by transiently elongating telomeres using a modified mRNA (mmRNA) encoding wild type telomerase reverse transcriptase (TERT-WT), as described previously[33]. Coincident with a significant increase in endogenous TERT expression (30%, Fig. 3A) telomere fluorescence intensity was increased in CAST CPCs transfected with TERT-WT mmRNA (42%, Fig. 3B), suggesting telomere elongation. Increased proliferation rate (32%, Fig. 3C), reduced senescent cell frequency (−17%, Fig. 3D), concomitant with decline in expression of senescence and commitment markers p27 (−25%) and Tie2 (−24%) as well as increased expression of quiescence marker p57 (32%, Fig. 3E) were observed, collectively indicating attenuation of senescence and restoration of youthful cell fate in CAST CPCs transfected with TERT-WT mmRNA.
Figure 3. Elongation of telomeres partially restores youthful cell fate in CPCs.
A. Immunoblot and densitometric analyses of endogenous telomerase reverse transcriptase (TERT) and green fluorescent protein (GFP) in CPCs transfected with 0.6–2μg/ml modified mRNA expressing GFP or wild type TERT (TERT-WT) (N=8). GFP expression indicates success of transfection. B. Mean fluorescence intensity of telomere staining as determined by qFISH (N=39–56 cells from 3 experiments). C. Cell counts as determined by microscopic counting of live cells at 4 days after plating (N=4). D. Senescent cell counts as determined after staining with SA-β-gal (N=3). E. Immunoblots and densitometric analyses of p27, Tie2 and p57 expression (N=4–5). For all experiments, except D, data is expressed relative to GFP transfected CPCs of each strain. *p<0.05, **p<0.01, ***p<0.001. Multiple t-tests (A–E).
Autophagy Is Activated and Modulates Cell Fate of CPCs with Short Telomeres
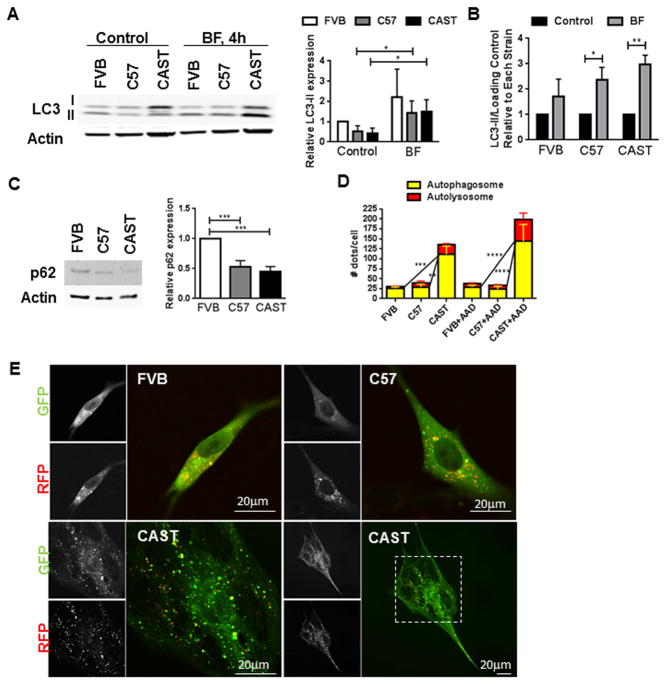
The role of autophagy in mediating the change in cell fate of CPCs was investigated following evidence establishing its involvement in cellular remodeling and stem cell aging[34, 35]. Expression of autophagy marker LC3-II declined under basal conditions in CAST CPCs (−59% Fig. 4A), but increased significantly in the presence of Bafilomycin A1 (BF; 3 fold, Fig. 4AB), an inhibitor of autophagome-lysosome fusion[36]. LC3-II/LC3-I ratio also increased in CAST CPCs treated with BF (Fig. S5A). Additionally, expression of p62 that gets sequestered in autophagosomes for lysosomal degradation decreased significantly (−55%, Fig. 4C), indicating increased autophagy flux. Presence of autophagic puncta was revealed by transducing CPCs with a baculovirus harboring tandem fluorescent proteins - red fluorescent protein (RFP)-green fluorescent protein (GFP)-tagged LC3. CAST CPCs exhibited increased number of autophagosomes and autolysosomes/cell (Fig. 4DE), identified respectively as yellow and red dots in overlaid micrographs, as described previously[36]. Autophagic puncta further increased in CAST CPCs following amino-acid deprivation (AAD; Fig. 4D), a positive inducer of autophagy[37]. Overall, the data demonstrates increased and rapid autophagy flux in CAST CPCs. Consistent findings of reduced p62 expression (−64%, Fig. S5B) is evidenced in OCPCs, collectively indicating enhanced activation of autophagy in CPCs bearing short telomeres.
Figure 4. Activation of autophagy in CAST CPCs.
A–B. Immunoblots and densitometric analyses of LC3-II expression in CPCs cultured under control conditions or treated with autophagy inhibitor bafilomycin (BF, 100nM, 4hours). LC3-II is normalized to Actin expression and expressed relative to FVB control CPCs (A) or control of each strain (B) (N=3–4). C. Expression of p62 as determined by immunoblots and densitometric analyses (N=8). D–E. CPCs were transduced with Premo Autophagy sensor, a baculovirus expressing RFP-GFP-LC3. Counts of autophagosomes and autolysosomes/cell under basal conditions and following amino acid deprivation (AAD), achieved by 1 hour treatment with Hanks balanced salt solution (D). Yellow dots in overlaid images indicate autophagosomes and red dots that do not overlay with green puncta indicate autolysosomes. Representative micrographs of autophagy puncta are shown (E) (N=4–5). *p<0.05, **p<0.01, ***p<0.001. Multiple t-tests (A–B), One-way Anova (C), Two-way Anova (D).
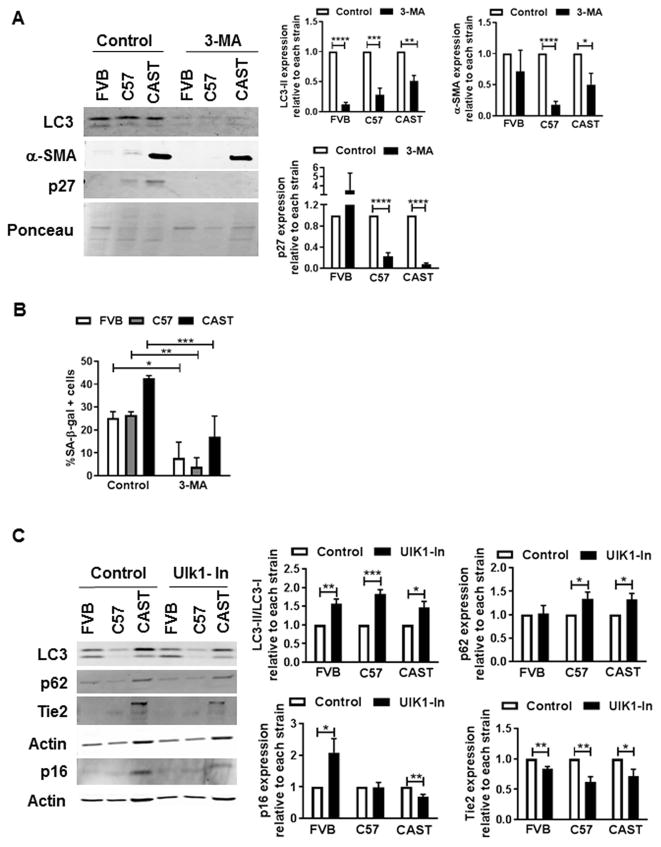
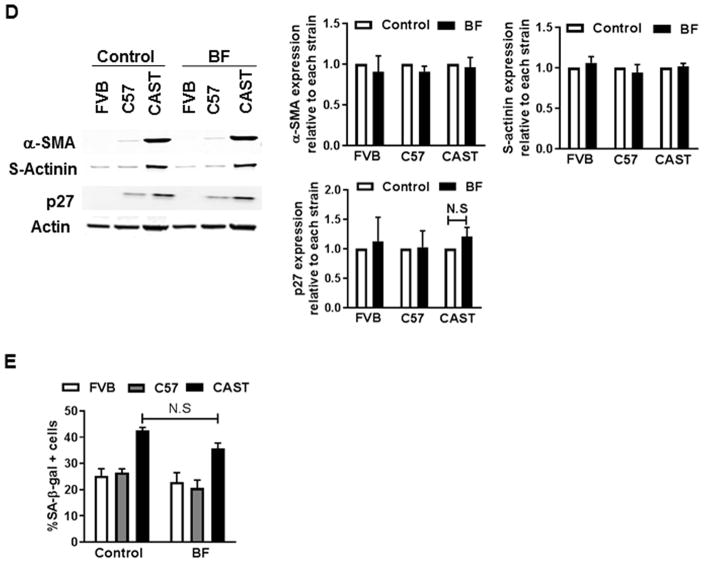
Mechanistic involvement of autophagy in regulating CPC fate was verified using pharmacological inhibitors that blunt different stages of the autophagy process. Coincident with reduced LC3-II levels (−54%), expression of commitment and senescence markers α-SMA (−47%) and p27 (−94%, Fig. 5A), as well as senescent cell frequency (−60%, Fig. 5B) were diminished in CAST CPCs treated with 3-methyladenine (3-MA), an inhibitor of autophagosome formation[38]. Similarly, reduced levels of p16 (−37%) and Tie2 (−40%) with increased LC3-II/LC3-I (46%) and p62 (32%, Fig. 5C) were evidenced in CAST CPCs treated with SBI-0206965, a small molecule inhibitor of Ulk1 kinase (Ulk1-In)[39] that regulates autophagosome initiation[40] and maturation[41]. Interestingly however, no reduction in expression of senescence and lineage commitment markers (Fig. 5D) or SA-β-gal activity (Fig. 5E) were observed upon blocking autophagosome-lysosome fusion via treatment with BF, suggesting that blunting the early stages of autophagy attenuates senescence and basal commitment and rejuvenates CAST CPCs.
Figure 5. Effect of autophagy inhibitors on cell fate of CPCs.
A. Immunoblots and densitometric analyses of CPCs cultured under control conditions or treated with 3-Methyladenine (3-MA, 10mM, 24h). Ponceau was used to determine loading (N=4–5). B. Senescent cell counts (N=3–5). C. Immunoblots and densitometric analyses of CPCs treated with Ulk1-inhibitor SBI-0206965 (Ulk1-In, 10μM, 2h, N=4–5) D. Immunoblots and densitometric analyses of CPCs treated with BF (100nM, 4hours, N=3–6). E. Counts of cells positive for SA-β-gal (N=4–5). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, N.S not significant. Data is expressed relative to control of each strain, except B. and E, where data is not normalized to control. Multiple t-tests (A, C, D), Two-way Anova (B, E).
p53 Regulates Cell Fate and Modulates Autophagy in CAST CPCs
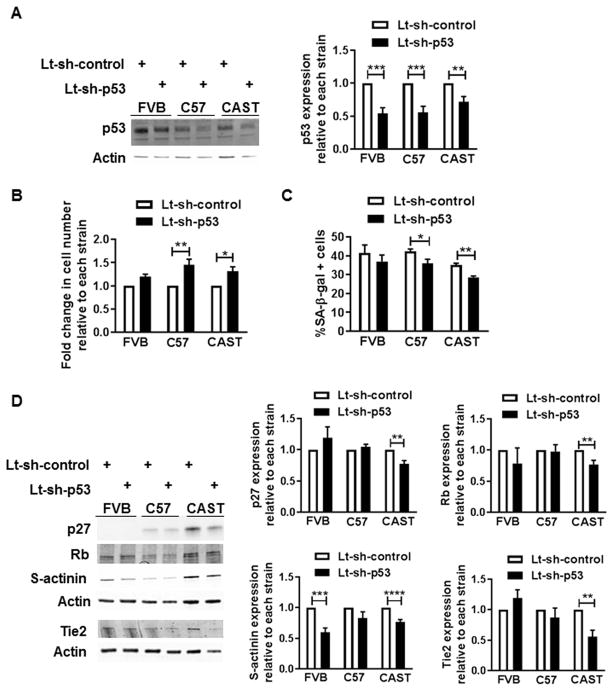
Establishing the signaling mechanism underlying autophagy regulation in CAST CPCs prompted examination of the role of p53, based on prior evidence demonstrating that p53 regulates senescence[11] and cell fate of CPCs[23] and induces autophagy in cardiac cells[42]. Stable knockdown of p53 (−28%, Fig. 6A) significantly increased the proliferation rate (31%, Fig. 6B) and decreased the frequency of senescent CAST CPCs (−19%, Fig. 6C). Additionally, p53 silencing reduced the expression of molecular markers of senescence and commitment in CAST CPCs, including p27, Rb, s-actinin (−23% each) and Tie2 (−44.5%, Fig. 6D), without affecting levels of P-Rb or P-p53 normalized to actin (Fig. S6A). The reversal of cell fate of CAST CPCs mediated by p53 inhibition correlates with blunting of autophagy activity, as evidenced by decreased LC3-II (−26%, Fig. S6A) and increased p62 levels (2 fold, Fig. S6B) upon gene knockdown or treatment with Pifithrin-α (PFT), a pharmacological inhibitor of p53. Overall, the data suggests that loss of p53 mediates youthful cell fate at least partially via autophagy suppression in CAST CPCs.
Figure 6. p53 inhibition attenuates senescence and basal commitment of CAST CPCs.
A. Immunoblots and densitometric analyses of CPCs treated with lentivirus harboring short-hairpin RNA against p53 (Lt-sh-p53) or scramble control (Lt-sh-control) (N=7). B. Cell counts as determined by microscopic counting of live cells at 1–2 days after plating (N=3). C. Senescent cell counts (N=3–4). D. Immunoblots and densitometric analyses of senescence (p27, Rb) and commitment markers (s-actinin, Tie2) in CPCs (N=5–6). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Data is expressed relative to Lt-sh-control transduced CPCs of each strain, except C. Multiple t-tests (A–D).
Discussion
An inherent limitation of CPC-based therapy is that the cells are often isolated from an aged, stressed source, which significantly impacts their regenerative potential and function[11]. CPCs isolated from aged mice and human patients exhibit impaired cellular and molecular characteristics including cell cycle arrest, reduced proliferation, increased susceptibility to cell death, senescence, loss of ‘stemness’, compromised ability to commit in response to stimulus, as well as defects in mitochondrial biogenesis and paracrine factor secretion[11, 19, 43]. The cell-autonomous biological age of the exogenous stem cells influences the efficiency and outcome of cellular therapy, as indicated by a strong positive correlation between CPC function and cardiac anatomy in patients with ischemic cardiomyopathy[44]. Biologically youthful CPCs with superior growth characteristics facilitate better repair and preserve cardiac structure, whereas biologically old, senescent cells are associated with negative remodeling of the heart[44]. Thus, understanding the basic biology of how aging affects stem cell maintenance is of paramount importance to enhance the regenerative potential of CPC-based therapy that is largely targeted to an elderly patient population.
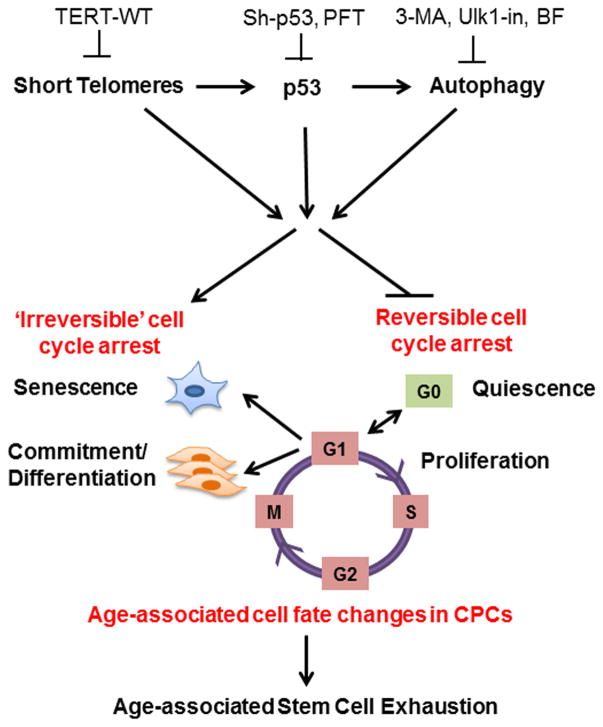
Results of our study delineate a novel mechanism involving short telomeres and protein homeostatic mechanisms in the modulation of cell fate of CPCs. Cell fate changes characterized by loss of quiescence, impaired proliferation, increased senescence and basal commitment are observed in CAST CPCs as well as old mouse and human CPCs bearing short telomeres (Fig. 2 and Fig. S4), corroborating with findings in geriatric skeletal muscle stem cells[45]. The switch from reversible to ‘irreversible’ cell cycle arrest likely accounts for exhaustion of the endogenous stem cell pool that occurs with aging (Fig. 7). Our results indicate that short telomeres cause the age-associated change in CPC fate, as elongating telomeres via overexpression of TERT-WT attenuates senescence and basal commitment while increasing proliferation and markers of quiescence in CAST CPCs (Fig. 3). CPCs have high endogenous telomerase activity that declines coincident with telomere shortening[10], suggesting that CAST CPCs have lower telomerase activity relative to FVB or C57 CPCs concomitant with reduced TERT expression (Fig. 3A). This study utilizes a modified mRNA to overexpress human TERT-WT, which represents a transient, clinically relevant, and safe strategy to elongate telomeres, delay senescence and enhance proliferation without immortalizing cells[33]. Single transfection with TERT-WT mmRNA partially restores youthful cellular characteristics to CAST CPCs (Fig. 3), which may be extended by repeated rounds of transfection, as demonstrated in fibroblasts and myoblasts[33]. Interestingly, TERT-WT increases cellular proliferation without elongating telomeres in C57 CPCs (Fig. 3BC). TERT-induced increase in cellular growth and proliferation may be mediated via mechanisms that are dependent or independent of telomere elongation[46]. In addition to its primary function as a reverse transcriptase that is crucial for telomere elongation, TERT interacts with other proteins, regulates chromatin structure and modulates gene expression leading to induction of pro-proliferative and inhibition of anti-proliferative genes, collectively resulting in increased cell number[46]. Strain-specific differences likely account for the disparate regulation of proliferation upon TERT-WT overexpression in CPCs, such that C57 CPCs exhibit increased cellular proliferation without telomere elongation, while CAST CPCs demonstrate increased cell number that correlates with elongated telomeres. Interestingly, TERT-WT overexpression also increases expression of quiescence marker p57 in C57 and CAST CPCs (Fig. 3E). High proliferation at the cost of quiescence is not always desirable, as it causes stem cell exhaustion leading to decline in regenerative potential. Our data indicates that TERT-WT enhances both proliferation and quiescence of C57 and CAST CPCs thus preventing their exhaustion. Additionally, TERT-WT transfection reduces senescent cell counts to varying degrees in all three strains of CPCs (albeit not significantly in FVB or C57 CPCs, Fig. 3D) that correlates with either reduced expression of cell cycle inhibitory proteins such as p27, as evidenced in FVB and CAST CPCs (Fig. 3E), or with increase in proliferation, as seen in C57 and CAST CPCs (Fig. 3C). Corroborating effects of enhanced proliferation, survival and attenuation of senescence are also evidenced in CPCs modified with upstream modulators of TERT, such as Pim1 kinase or Nucleostemin[11, 12, 19]. Thus, molecular mechanisms that elongate telomeres via activation of TERT hold robust potential to rejuvenate CPCs. Overall, our data establishes that telomere length is a determinant of CPC fate and function, consistent with findings in other adult stem cells [47]. It is however important to note that telomeres can be damaged or uncapped independent of length [48] and thus telomeric integrity and not just the length may also predict cellular outcome.
Figure 7. Schematic of hypothetical signaling cascade.
The cell fate of CPCs changes with age and is characterized by a switch away from proliferation and quiescence (reversible form of cell cycle arrest) towards senescence and increased basal commitment (‘irreversible’ forms of cell cycle arrest) accounting for age-associated stem cell exhaustion. Mechanistically, short telomeres activate p53 that induces autophagy and at least partially contributes to the age-associated change in cell fate. Blunting telomere shortening via overexpression of TERT-WT, silencing p53, or treating with pharmacological inhibitors of p53 (PFT) and autophagy (3-MA, Ulk1-In, BF) selectively attenuate senescence and basal commitment and reverse cell fate of aged CPCs.
Cell fate changes occurring during aging are accompanied by induction of autophagy in CPCs, as evidenced by increased accumulation of LC3-II following BF treatment, increased autophagic puncta upon starvation and reduced p62 levels in CAST CPCs (Fig. 4) and OCPCs (Fig. S5). Our data suggests that autophagy is induced differentially in all three strains of CPCs (Fig. 4B) and likely plays different roles in youthful and senescent CPCs. In relatively youthful FVB CPCs, autophagy likely contributes to maintenance of stemness and proliferation as seen in skeletal muscle and hematopoietic stem cells[49, 50]. Consequentially, inhibition with 3-MA and Ulk1-In increases expression of cell-cycle inhibitors p16 and p27 in FVB CPCs, while decreasing senescent cell number (Fig. 5ABC), suggesting impaired cellular proliferation. A plethora of studies report impairment of autophagy during aging as well as the alleviation of senescence, elongation of lifespan and restoration of cardiac structure and function in the aged heart upon stimulation of autophagy[49, 51]. Paradoxically, our data indicates that autophagy is activated and plays a role in inducing senescence and basal commitment of aged CPCs. Inhibiting autophagosome formation using 3-MA attenuates senescence and commitment, as evidenced by a reduction in senescent cell counts and expression of p27 and α-SMA in CAST CPCs (Fig. 5AB). Similarly, blunting autophagosome maturation via treatment with Ulk1-In also decreases expression of senescence and commitment markers in CAST CPCs (Fig. 5C). These findings are consistent with several recent studies demonstrating induction of autophagy during telomere dysfunction, oncogene-induced- and DNA damage-triggered senescence in fibroblasts as well as aged hematopoietic stem cells [52–57]. Cellular remodeling and reorganization of the endomembrane system occurs in senescent cells and activates autophagosome formation[54, 55]. The high flux of aminoacids and metabolites generated by induction of autophagy drives increased protein synthesis and the senescence-associated secretory phenotype typically seen in metabolically active, senescent cells[54–56]. Mechanisms of autophagy induction during senescence involve activation of Ulk1 and Ulk3 kinases[52, 53] as well as spatio-temporal coupling with mammalian target of rapamycin[54, 55]. Furthermore, blunting autophagy delays the acquisition of senescence while overexpression of Ulk3 is sufficient to induce both autophagy and senescence, suggesting a causal role for autophagy in modulating senescence in some mammalian cells [52, 54]. Similarly, autophagy is activated and necessary for stem cell commitment, as reported during differentiation of myocardial progenitors[58] and myoblasts[59]. Collectively, our data supports a role for autophagy as aged CPCs switch from reversible to ‘irreversible’ forms of cell cycle arrest (senescence and commitment), and demonstrate that blunting the early stages of autophagy may at least partially reverse the cell fate of CAST CPCs (Fig. 5 and Fig. 7). Thus, clearly the regulation and role of autophagy appears to be highly cell-type and stimulus specific.
A nodal signaling molecule acting downstream of short telomeres is p53, which likely serves as an intermediate and modulates autophagy in CAST CPCs (Fig. S6). p53 is highly expressed during senescence, as seen in CAST CPCs (Fig. 2), OCPCs (Fig. S4A), adult human CPCs[19], and in young mouse CPCs that are forced to undergo senescence via cell-culture passaging[12] or silencing of key signaling molecules such as NS[11]. Stable knockdown of p53 attenuates senescence by decreasing molecular marker expression and senescent cell counts and increasing proliferation in C57 and CAST CPCs (Fig. 6), consistent with our prior results in mouse and human CPCs with NS knockdown[11]. Additionally, p53 silencing also reduces expression of commitment markers in CAST CPCs (Fig. 6D), collectively suggesting that loss of p53 restores youthful cell fate in aged CPCs. Interestingly, a recent study by Kannappan et al demonstrates that CPCs isolated from transgenic mice with a single extra copy of p53 (super-p53 mice) display a younger phenotype with higher proliferation, lower senescence marker expression and increased DNA damage repair[23]. Unlike transgenic mice with constitutively active p53, the super-p53 mice exhibit moderately higher p53 activity and regulation by post-translational modifications that is similar to the endogenous gene[23]. Reconciling our findings with Kanappan et al, it appears that the dose of p53 and method of regulation is critical for modulation of CPC youth. Lt-sh-p53-mediated knockdown induces partial, but not complete ablation of p53 levels (Fig. 6) consistent with our prior study[11], suggesting that a moderate amount of p53 may indeed be beneficial for CPCs. This study also measures activation of p53 by detecting phosphorylation at Ser15, that is important in stimulating transactivation at p53-responsive promoters, thus regulating target gene expression[60]. Surprisingly, our data demonstrates no significant differences in P-p53 levels in the CAST mouse myocardium or CPCs, as compared to FVB or C57 (Fig. S1K, S2D). It is likely that under basal conditions in the absence of an external DNA damage stimulus, p53 recruitment to target gene promoters occurs independent of Ser15 phosphorylation, as shown in a recent study[60]. Future work detecting other post-translational modifications of p53 will provide more information about the activation status and the mechanistic basis of p53-mediated regulation of CPC fate. Our data reveals attenuation of autophagy upon p53 inhibition in CAST CPCs (Fig. S6), but the precise mechanism by which p53 modulates autophagy remains a topic of future investigation. To our knowledge, this is the first study that suggests a link between telomere shortening and activation of autophagy in CPCs. However, the direct effect of p53 silencing or autophagy inhibition on telomere length is unclear and remains to be investigated. Findings from other cell types demonstrate that p53 inhibition activate telomerase as well as recombination mechanisms known as alternative lengthening of telomeres that lead to telomere elongation[61, 62]. Clearly elucidating the mechanistic role of p53 in autophagy regulation and telomere elongation will pave way for future studies that focus on selectively modulating the pleiotropic functions of p53.
Hypophosphorylation of Rb at Ser 780 that indicates activation is also measured in this study. Inactivation of Rb occurs via phosphorylation by cyclin-dependent protein kinases and permits cell cycle progression[63]. Reduced P-Rb levels in CAST and OCPCs (Fig. S2D, S4D) is consistent with acquisition of cell cycle arrest and senescence.
It is important to note that the three strains of CPCs exhibit differential responses to genetic manipulation. In our hands, FVB CPCs are most sensitive to lentiviral transduction as evidenced by increased cell death (data not shown), higher senescent cell counts as well as increased p53 expression in FVB CPCs transduced with scramble control lentivirus (Lt-sh-control; Fig. 6) as compared to uninfected cells of the same strain under basal conditions (Fig. 2). The differential response of FVB, C57 and CAST CPCs to mmRNA transfection, treatment with autophagy inhibitors as well as lentivirus transduction in combination with puromycin treatment has required normalization of the data relative to each strain (Figs. 3, 5, 6).
The discovery that CAST mice have short telomeres has resulted in their use to study the effects of short telomeres on age-associated disorders[13, 15]. Homozygous knockout mice for telomerase acquire short telomeres and consequently display functional defects only after 5 generations of breeding and at 6–8 months of age[64, 65]. However, the naturally-occurring CAST mice which are not genetically engineered exhibit short telomeres from birth and are more susceptible to limited tissue renewal, degenerative diseases and increased cellular senescence that occurs with aging[13, 15]. Surprisingly, no known studies till date have delved into understanding the cardiac biology and pathogenesis of age-associated heart diseases using the CAST mice. Our findings demonstrate that relative to common lab mouse strains such as FVB or C57, CAST mice display phenotypic characteristics consistent with clinical manifestations of cardiac aging[29] as early as 2 months after birth, such as diastolic dysfunction, cardiac hypertrophy, fibrosis, elevated plasma catecholamines, and increased cellular senescence (Fig. 1, Fig. S1). The increase in systolic function in aged CAST mice is likely associated with elevated hypertension/vascular pressure concomitant with high adrenaline/noradrenaline levels, corroborating recent findings of significantly increased EF in patients with high blood pressure[66]. Interestingly, the survival rate of the CAST mice is significantly lower than FVB, but comparable to C57 (Fig. 1D), suggesting that telomere length has a partial role in regulating lifespan. Findings from a recent study demonstrate that telomere length in early life is a better predictor of organismal lifespan such that individuals with longer telomeres from birth continuing throughout life have relatively long lifespans while those with short telomeres from birth have a shorter lifespan [67]. Alternatively, cellular and organismal longevity are also impacted by the accumulation of dysfunctional telomeres, which can be independent of absolute telomere lengths [48]. Future research is needed to establish the clear link between telomere shortening/dysfunction and organismal lifespan. The effects of short telomeres are also tissue-specific with a rather limited role in post-mitotic tissues such as the heart and cardiac myocytes there in, as compared to proliferating cells such as CPCs. Coincident with aging of the myocardium, CAST CPCs isolated and expanded ex vivo demonstrate age-associated molecular changes in cell fate, which are similar to aged human CPCs (Fig. 2 and Fig. S4)[19]. Collectively, our findings indicate that CAST mice and CPCs therein closely recapitulate human biology and thus represent an innovative and superior model to study myocardial aging and regeneration.
Overall, this study uses CAST as a mouse model of short telomeres and delineates a signaling cascade involving telomere shortening, p53 and autophagy in modulating the age-associated-change in cell fate of CPCs. The switch from reversible to ‘irreversible’ forms of cell cycle arrest in CAST CPCs likely leads to exhaustion of the stem cell pool that correlates with aging cardiomyopathy evident in the CAST mouse heart (Fig. 7). Defining these cell-intrinsic processes regulating cell fate has revealed novel, therapeutically-relevant strategies including TERT-WT mmRNA-mediated transient telomere elongation, p53 silencing and autophagosome inhibition to rejuvenate and augment the reparative potential of aged CPCs. Future investigations will need to test if the strategies proposed herein reverse myocardial stem cell fate in vivo and if autologous infusion of the modified, rejuvenated CPCs holds potential to antagonize the cardiac aging process.
Supplementary Material
Acknowledgments
The authors thank Drs. Donald Bers and Julie Bossuyt and other members of the department of Pharmacology at University of California at Davis for sharing lab space and resources with the Hariharan Lab. This study has utilized services and resources of the UC Davis Flow Cytometry Shared Resource Laboratory.
Funding Sources
N.H is supported by the American Heart Association Scientist Development Grant (16SDG30970046) and Academic Federation Innovative Development Award from University of California, Davis. M.A.S is supported by NIH grants: R01HL067245, R37HL091102, R01HL105759, R01HL113647, R01HL117163, P01HL085577, and R01HL122525, as well as an award from the Fondation Leducq. The UC Davis Flow Cytometry Shared Resource Laboratory is funded by the UC Davis Comprehensive Cancer Center Support Grant awarded by the National Cancer Institute (NCI P30CA093373).
Footnotes
Disclosures
M.A.S is founder and co-owner of CardioCreate Inc. The other authors report no disclosures.
Author Contributions:
1. Conception and Design: NN, MAS, NH, 2. Financial Support: MAS, NH, 3. Administrative Support: CM, NH, 4. Provision of study material or patients: MAS, NH, 5: Collection and/or assembly of data: CM, YJ, JE, PQ, NN, ADLT, MM, JN, ABL, MS, NH, 6. Data Analysis and Interpretation: CM, YJ, JE, PQ, NN, NH, 7. Manuscript Writing: CM, YJ, NH, 8. Final Approval of Manuscript: NH
References
- 1.Hariharan N, Sussman MA. Cardiac aging - Getting to the stem of the problem. J Mol Cell Cardiol. 2015;83:32–36. doi: 10.1016/j.yjmcc.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 5.Ellison GM, Vicinanza C, Smith AJ, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 6.Mohsin S, Khan M, Toko H, et al. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–1287. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolli R, Tang XL, Sanganalmath SK, et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesselli D, Beltrami AP, D’Aurizio F, et al. Effects of age and heart failure on human cardiac stem cell function. Am J Pathol. 2011;179:349–366. doi: 10.1016/j.ajpath.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hariharan N, Quijada P, Mohsin S, et al. Nucleostemin rejuvenates cardiac progenitor cells and antagonizes myocardial aging. J Am Coll Cardiol. 2015;65:133–147. doi: 10.1016/j.jacc.2014.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cottage CT, Neidig L, Sundararaman B, et al. Increased mitotic rate coincident with transient telomere lengthening resulting from pim-1 overexpression in cardiac progenitor cells. Stem Cells. 2012;30:2512–2522. doi: 10.1002/stem.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldser DM, Greider CW. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer cell. 2007;11:461–469. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016;8:3–11. doi: 10.18632/aging.100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao LY, Armanios M, Strong MA, et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Pucci F, Gardano L, Harrington L. Short telomeres in ESCs lead to unstable differentiation. Cell stem cell. 2013;12:479–486. doi: 10.1016/j.stem.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonsen JL, Rosada C, Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 18.Bar C, Povedano JM, Serrano R, et al. Telomerase gene therapy rescues telomere length, bone marrow aplasia, and survival in mice with aplastic anemia. Blood. 2016;127:1770–1779. doi: 10.1182/blood-2015-08-667485. [DOI] [PubMed] [Google Scholar]
- 19.Mohsin S, Khan M, Nguyen J, et al. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ Res. 2013;113:1169–1179. doi: 10.1161/CIRCRESAHA.113.302302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Z, Ito S, Nishio N, et al. Characteristics of cardiac aging in C57BL/6 mice. Experimental gerontology. 2013;48:341–348. doi: 10.1016/j.exger.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Leri A, Franco S, Zacheo A, et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–139. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crow MT. Revisiting p53 and its effectors in ischemic heart injury. Cardiovasc Res. 2006;70:401–403. doi: 10.1016/j.cardiores.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Kannappan R, Matsuda A, Ferreira-Martins J, et al. p53 Modulates the Fate of Cardiac Progenitor Cells Ex Vivo and in the Diabetic Heart In Vivo. E Bio Medicine. 2017;16:224–237. doi: 10.1016/j.ebiom.2017.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solozobova V, Blattner C. p53 in stem cells. World J Biol Chem. 2011;2:202–214. doi: 10.4331/wjbc.v2.i9.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White E. Autophagy and p53. Cold Spring Harb Perspect Med. 2016;6:a026120. doi: 10.1101/cshperspect.a026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemann MT, Greider CW. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 2000;28:4474–4478. doi: 10.1093/nar/28.22.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitter SS, Shah SJ, Thomas JD. A Test in Context: E/A and E/e’ to Assess Diastolic Dysfunction and LV Filling Pressure. J Am Coll Cardiol. 2017;69:1451–1464. doi: 10.1016/j.jacc.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 29.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto A, Takeishi S, Kanie T, et al. p57 is required for quiescence and maintenance of adult hematopoietic stem cells. Cell stem cell. 2011;9:262–271. doi: 10.1016/j.stem.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Ramunas J, Yakubov E, Brady JJ, et al. Transient delivery of modified mRNA encoding TERT rapidly extends telomeres in human cells. FASEB J. 2015;29:1930–1939. doi: 10.1096/fj.14-259531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadandale P, Kiger AA. Role of selective autophagy in cellular remodeling: “self-eating” into shape. Autophagy. 2010;6:1194–1195. doi: 10.4161/auto.6.8.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan JL, Simon AK, Prescott M, et al. Autophagy in stem cells. Autophagy. 2013;9:830–849. doi: 10.4161/auto.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hariharan N, Maejima Y, Nakae J, et al. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 38.Hariharan N, Ikeda Y, Hong C, et al. Autophagy plays an essential role in mediating regression of hypertrophy during unloading of the heart. PLoS One. 2013;8:e51632. doi: 10.1371/journal.pone.0051632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egan DF, Chun MG, Vamos M, et al. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol Cell. 2015;59:285–297. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell RC, Tian Y, Yuan H, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petherick KJ, Conway OJ, Mpamhanga C, et al. Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J Biol Chem. 2015;290:11376–11383. doi: 10.1074/jbc.C114.627778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang EY, Gang H, Aviv Y, et al. p53 mediates autophagy and cell death by a mechanism contingent on Bnip3. Hypertension. 2013;62:70–77. doi: 10.1161/HYPERTENSIONAHA.113.01028. [DOI] [PubMed] [Google Scholar]
- 43.Castaldi A, Dodia RM, Orogo AM, et al. Decline in cellular function of aged mouse c-kit+ cardiac progenitor cells. J Physiol. 2017 doi: 10.1113/JP274775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Amario D, Leone AM, Iaconelli A, et al. Growth properties of cardiac stem cells are a novel biomarker of patients’ outcome after coronary bypass surgery. Circulation. 2014;129:157–172. doi: 10.1161/CIRCULATIONAHA.113.006591. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Sousa-Victor P, Gutarra S, Garcia-Prat L, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 46.Chiodi I, Mondello C. Telomere-independent functions of telomerase in nuclei, cytoplasm, and mitochondria. Front Oncol. 2012;2:133. doi: 10.3389/fonc.2012.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flores I, Canela A, Vera E, et al. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 2008;22:654–667. doi: 10.1101/gad.451008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hewitt G, Jurk D, Marques FD, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nature communications. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Prat L, Martinez-Vicente M, Perdiguero E, et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- 50.Phadwal K, Watson AS, Simon AK. Tightrope act: autophagy in stem cell renewal, differentiation, proliferation, and aging. Cellular and molecular life sciences: CMLS. 2013;70:89–103. doi: 10.1007/s00018-012-1032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shirakabe A, Ikeda Y, Sciarretta S, et al. Aging and Autophagy in the Heart. Circ Res. 2016;118:1563–1576. doi: 10.1161/CIRCRESAHA.116.307474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young AR, Narita M, Ferreira M, et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Narita M, Young AR, Narita M. Autophagy facilitates oncogene-induced senescence. Autophagy. 2009;5:1046–1047. doi: 10.4161/auto.5.7.9444. [DOI] [PubMed] [Google Scholar]
- 54.Narita M, Young AR, Arakawa S, et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zoncu R, Sabatini DM. Cell biology. The TASCC of secretion. Science. 2011;332:923–925. doi: 10.1126/science.1207552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang C, Elledge SJ. How autophagy both activates and inhibits cellular senescence. Autophagy. 2016;12:898–899. doi: 10.1080/15548627.2015.1121361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mar FA, Debnath J, Stohr BA. Autophagy-independent senescence and genome instability driven by targeted telomere dysfunction. Autophagy. 2015;11:527–537. doi: 10.1080/15548627.2015.1017189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Liu J, Huang Y, et al. FRS2alpha-mediated FGF signals suppress premature differentiation of cardiac stem cells through regulating autophagy activity. Circ Res. 2012;110:e29–39. doi: 10.1161/CIRCRESAHA.111.255950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sin J, Andres AM, Taylor DJ, et al. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy. 2016;12:369–380. doi: 10.1080/15548627.2015.1115172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loughery J, Cox M, Smith LM, et al. Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic Acids Res. 2014;42:7666–7680. doi: 10.1093/nar/gku501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stampfer MR, Garbe J, Nijjar T, et al. Loss of p53 function accelerates acquisition of telomerase activity in indefinite lifespan human mammary epithelial cell lines. Oncogene. 2003;22:5238–5251. doi: 10.1038/sj.onc.1206667. [DOI] [PubMed] [Google Scholar]
- 62.Razak ZR, Varkonyi RJ, Kulp-McEliece M, et al. p53 differentially inhibits cell growth depending on the mechanism of telomere maintenance. Mol Cell Biol. 2004;24:5967–5977. doi: 10.1128/MCB.24.13.5967-5977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knudsen ES, Wang JY. Dual mechanisms for the inhibition of E2F binding to RB by cyclin-dependent kinase-mediated RB phosphorylation. Mol Cell Biol. 1997;17:5771–5783. doi: 10.1128/mcb.17.10.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blasco MA, Lee HW, Hande MP, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 65.Yuan X, Ishibashi S, Hatakeyama S, et al. Presence of telomeric G-strand tails in the telomerase catalytic subunit TERT knockout mice. Genes to cells: devoted to molecular & cellular mechanisms. 1999;4:563–572. doi: 10.1046/j.1365-2443.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- 66.Khanji M, Balawon A, Boubertakh R, et al. Lb01.07: Elevated Blood Pressure without Hypertrophy Raises Left Ventricular Ejection Fraction. J Hypertens. 2015;33(Suppl 1):e46. [Google Scholar]
- 67.Heidinger BJ, Blount JD, Boner W, et al. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A. 2012;109:1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.