Abstract
Endometriosis is ectopic growth of endometrial tissue traditionally thought to arise through retrograde menstruation. We aimed to determine if cells derived from endometriosis could enter vascular circulation and lead to hematogenous dissemination. Experimental endometriosis was established by transplanting endometrial tissue from DsRed+ mice into the peritoneal cavity of DsRed− mice. Using flow cytometry, we identified DsRed+ cells in blood of animals with endometriosis. The circulating donor cells expressed CXCR4 and mesenchymal stem cell (MSC) biomarkers, but not hematopoietic stem cell markers. Nearly all the circulating endometrial stem cells originated from endometriosis rather than from the uterus. Cells expressing DsRed, CXCR4 and MSCs markers were identified in the peritoneal wall and surrounding vessels of recipient mice, contributing to both endometriosis and angiogenesis. Cells originating in endometriosis lesions migrated and implanted in lung tissue and displayed makers of differentiation, indicating retained multipotency. In vitro these cells demonstrated multipotency and were able to differentiate into adipogenic, osteogenic, and chondrogenic lineages. Endometriosis lesions also expressed high levels of CXCL12, the CXCR4 receptor ligand. Serum CXCL12 levels were greater than in sham control mice. In humans with endometriosis, serum CXCL12 levels were significantly higher than controls, suggesting that the CXCL12/CXCR4 axis is operational in women with spontaneous endometriosis as well. Stem cells, rather than differentiated cells from endometriosis, enter the circulation in response to CXCL12. We identify an endometriosis-derived stem cell population, a potential mechanism of dissemination of this disease and a potential target for treatment of endometriosis.
Graphical abstract
Introduction
Endometriosis is a chronic, estrogen-dependent condition in which ectopic endometrial glands and stroma are present outside the uterine cavity. Endometriosis has been estimated to affect approximately 10% of reproductive-age women, 25% to 50% of women with infertility, and up to 50% of women with pelvic pain [1,2]. While endometriosis lesions are primarily located in the pelvis they have also been found in areas remote from the peritoneal cavity including pericardium, pleura, lung parenchyma, liver, spine, and the brain [3,4].
The definitive pathogenesis of endometriosis remains uncertain; the most commonly accepted mechanism is Sampson's theory of retrograde menstruation, whereby endometrial cells are shed through the fallopian tubes into the peritoneal cavity [5]. While this theory explains intraperitoneal endometriosis lesions, to account for the presence of endometriosis at distant sites outside the pelvis it has been suggested that lymphatic and hematogenous migration of endometrial tissue contributes to the pathogenesis of endometriosis [5,6]. In previous studies we demonstrated in both humans and a murine endometriosis model that bone marrow-derived mesenchymal stem cells contribute to the makeup of the eutopic endometrium and to endometriosis lesions, likely traveling through the circulatory system [7,8].
Here we hypothesized that vascular metastasis of endometriosis-derived mesenchymal stem cells may be a source of new lesions and contribute to pre-existing distant endometriosis [9]. To investigate the potential contribution of circulating ectopic endometrial-derived cells to the pathogenesis of endometriosis, we used an established mouse model of surgically induced endometriosis to identify and characterize circulating endometriosis derived cells.
Experimental Procedures
Experimental murine endometriosis
All murine experiments were approved by the Yale Institutional Animal Care and Use Committee (IACUC, 2014-07113). The endometriosis mouse model was created using forty C57BL/6 female mice obtained from Charles River Laboratories (Wilmington, MA) as tissue recipients and ten DsRed mice [Tg(CAGDsRed* MST)1Nagy/J (Jackson Laboratories, Bar Harbor) [10–12]. In the endometriosis group (EMS group, n=10), each recipient animal received one DsRed donor uterus cut into three one-centimeter segments. Each segment was sutured into the peritoneum at separate sites so that the uterine serosa was in contact with the peritoneal wall. In the endometriosis plus ovariectomy group (EMS+OVX group, n=10), each host underwent simultaneous ovariectomy as well as endometriosis induction as described above. In control groups, sham surgeries were conducted either with (n=10) or without ovariectomy (n=10), placing sutures in the peritoneal wall. Animals were treated with 1 mg/kg/day SQ of Meloxicam as an analgesic for 72hrs post-operatively.
Flow Cytometry Analysis and Cell Sorting (FACS) of Circulating Ectopic Cells
FACS analysis was performed after surgeries at set intervals to determine the presence of donor DsRed-positive cells in the peripheral blood. We collected 100µl of peripheral blood from each mouse by cheek puncture into tubes pre-treated with unfractionated heparin. Blood was kept on ice until processing, and red blood cells were lysed using ACK Lyse Solution (Life Technique, New York, USA), per the manufacturer's directions.
Flow cytometry was performed using a Beckman Coulter MoFlo (Beckman Coulter, SanJose, CA) and acquired data were analyzed with FLOWJO flow cytometry analysis software v9.5 (Treestar, Ashland, OR) with analysis gates designed to remove residual platelets and cellular debris. RFP-positive cells were defined by setting gates to exclude any background DsRed signaling found in control animals and IgG isotype staining. Between 500,000 and 1,000,000 events were counted in each sample, and duplicate measurements were performed for each sample. DsRed positive cells were sorted into sterile PBS on ice for further qRT-PCR and fluorescent-immunocytochemistry analysis.
Fluorescent -Immunocytochemistry (FICC)
Sorted cells were smeared on slides and fixed using 4% paraformaldehyde overnight and blocked with 5% BSA for two hours. Slides were incubated with primary antibody (1:200 diluted in PBS with 1% donkey serum) in a humidified chamber overnight at 4°C, followed by and secondary antibodies for 1 h at 37 °C. Primary antibodies used included goat polyclonal DsRed (Santa Cruz Biotechnology, sc-33354, Dallas, TX), rabbit polyclonal anti-CD140B (abcam, ab32570, Cambridge, UK), rat polyclonal anti-CD146 (abcam, ab75769), followed by donkey-anti-goat secondary antibody AlexaFluor® 633 (Life Technologies, A-21082, Carlsbad, CA), donkey-anti-rabbit secondary antibody AlexaFluor® 568 (Life Technologies, A-10012) and donkey anti-rat secondary antibody AlexaFluor® 488 (Life Technologies, A-21208) conjugate for 1 h at 37 °C respectively. Vectashield/4, 6-diamidino-2-phenylindole (DAPI, Vector laboratories, Peterborough, UK) was added onto the cells before observation on a Zeiss LSM 710 Duo NLO/Multiphoton Confocal Microscope (Zeiss Company, Germany). Negative and positive controls for DsRed consisted of smears of sorted DsRed negative from C57BL/6 female mice and DsRed positive DsRed mice uterine cells, respectively. Negative and positive control cells were processed simultaneously in each staining run. For each group, at least 2000 cells were counted. Cells were counted by two observers blinded to the experimental group.
Paraffin-embedded Tissue Immunofluorescence
As described previously [13], one uterine horn, one lung and the spleen were harvested from each mouse. Tissues were fixed in 4% paraformaldehyde for 24 hours, transferred into 70% ethanol, and then paraffin embedded. Three-micrometer thick sections of the mounted formalin-fixed paraffin-embedded uterine, lung and spleen sections were deparaffinized and rehydrated, followed by 10 min boiling in sodium citrate (pH 6) for antigen retrieval and 30min blocking using 2% donkey serum (Invitrogen, Carlsbad, CA). Slides were incubated with primary antibodies (1:200, diluted in 1% donkey serum in PBS) in a humidified chamber overnight at 4°C, followed by secondary antibodies (1:500, diluted in 1% BSA in PBS) for 45 min at 37°C. Primary antibodies used were goat polyclonal DsRed (Santa Cruz Biotechnology, sc-33354), rabbit polyclonal CXCR4 (Santa Cruz Biotechnology, sc-9046) and rabbit anti-Prosurfactant Protein C (abcam, ab90716). Secondary antibodies used were donkey-anti-goat secondary antibody, AlexaFluor® 633 (Life Technologies, A-21082) and donkey-anti-rabbit secondary antibody, AlexaFluor® 568 (Life Technologies, A-10012) conjugates for 1 h at 37° C. Alternatively, slides were incubated with 1:200 rat polyclonal anti-CD140B (abcam, ab23914) or 1:200 rat polyclonal anti-CD146 (abcam, ab75769) at 4° C overnight, followed by donkey anti-rat secondary antibody, Alexa Fluor 488 (Life Technologies, A-21208). Vectashield containing 4, 6-diamidino-2-phenylindole (DAPI, Vector laboratories, Peterborough, UK) was added onto the cells and allowed to incubate for 3 hours before confocal microscopy and photography using a Zeiss LSM 710 Duo NLO/Multiphoton Microscope (Zeiss Company, Germany). Negative and positive controls for DsRed tissue consisted of sections uteri of from C57BL/6 female mice and DsRed positive mice, respectively. Spleen tissue was used as a negative control for anti-Prosurfactant Protein C. Negative and positive control cells were processed simultaneously in each staining run. Microscopy of lung tissue was performed by individuals blinded to the treatment group.
In-vitro Mesenchymal Cell Differentiation and Imaging
Diestrous uteri from 7–8 weeks old cycling female mice were isolated, horns longitudinally dissected and the lumen exposed. Four 3-mm2 pieces were sutured onto the peritoneum (4 in each side) of C57BL/6J females, with the luminal side facing the peritoneum. Females were kept cycling by changing the cages’ bedding 3 time per week with fresh male urine. On day 8 of endometriosis induction, corresponding to the day dsRed+/CD146+/CD140b+/CD45− cells demonstrated highest levels in circulation, lesions were extracted, finely minced and digested in a solution of Hanks’ balanced salt solution (HBSS) containing 25 mM HEPES (Life Technologies), 1 mg/mL collagenase B (Roche Diagnostics) and 0.1 mg/mL deoxyribonuclease I (Sigma-Aldrich) for 45 minutes at 37°C with periodic pipetting. Samples were filtered using 70-µm mesh, centrifuged at 2000 rpm at 4°C for 8 minutes, and washed in PBS. Cells were resuspended in DMEM/F12 10% FBS with 1% antibiotic and cultured on gelatin-coated 24-well plates. Differentiation into multiple mesenchymal lineages was performed using the Mouse Mesenchymal Stem Cell Functional Identification Kit (R&D, catalog# SC010). Mature phenotype of adipocytes, osteocytes, and chondrocytes was defined by staining for anti-mFABP4, anti-Osteopontin, and anti-Collagen II, respectively, according to manufacturer’s instructions. Images of osteocytes and adipocytes were viewed in the Zeiss Observer.Z1 fluorescence microscope and images were capture using the Volocity Software. Paraffin sections of cell pellets undergoing chondrogenic differentiation were imaged using laser scanning confocal microscope (LSM 710; Zeiss) and captured using ZEN software (Carl Zeiss).
Human Study Population
The human portion of our study was approved by the institutional review board of Gangnam Severance Hospital, where deidentified samples were collected, and by Yale School of Medicine (HIC Waiver 9808010450). Twenty-one women aged 22 to 45 years participated in this study after giving written informed consent. As described in our previous study [14], volunteers were recruited from patients who underwent laparoscopy for various indications including, pelvic masses, pelvic pain, endometriosis, infertility, and diagnostic evaluation of benign gynecologic disease between June 2010 and March 2013. Women were recruited based on the following inclusion criteria: aged 20 to 50 years, no hormone therapy for at least 3 months, nonsmoker, no history or signs of any inflammatory disease, and who were undergoing surgical treatment/exploration. Participants were rejected from the study based on the following exclusion criteria: postmenopausal status; previous hormone or gonadotropin-releasing hormone (GnRH) agonist use; adenomyosis; endometrial cancer, uterine hyperplasia, or endometrial polyps; infectious diseases; chronic or acute inflammatory diseases; malignancy; autoimmune disease; and cardiovascular disease. Eleven patients had histologically confirmed peritoneal and/or ovarian endometriosis, and ten patients without endometriosis participated as controls. Blood samples were collected into 10 mL sterile tubes with no additives and were immediately centrifuged at 1000 g for 10 minutes. Sediment-free serum samples were obtained and aliquots were frozen at −80°C for further analysis.
CXCL12 (SDF-1) Enzyme-Linked Immunosorbent Assay (ELISA) analysis
To study circulating CXCL12 (SDF-1) concentration after induction, blood samples were taken from murine EMS, EMS+OVX and control groups and serum stored at −20 °C until use. Mouse and human Serum CXCL12 concentrations were determined with ELISA per the manufacturer's instructions (R&D Systems, Wiesbaden Norderstedt, Germany).
Quantitative Real-time reverse transcription-polymerase chain reaction (real-time RT-PCR)
The mRNA of sorted cells by FACS was extracted using mirVana PARIS RNA and Native Protein Purification Kit (Life technologies, AM1556) per the manufacturer's protocol. Complementary cDNA was synthesized using reverse transcriptase iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) in a 20 µL reaction mixture. Real-time quantitative RT-PCR using the CFX Connect Real-Time PCR Detection System (Bio-Rad) was performed with 20 µL of the single stranded cDNA sample with iQ SYBR Green supermix (Bio-Rad) following the manufacturer's protocol. Melting curve analysis was performed after the real-time RT-PCR to monitor PCR specificity and primer efficacy. Samples were run in technical triplication, and samples from all 20 animals were analyzed. Samples were evaluated for the presence of β-actin, CD90, CD105, CD9, CD45, CD34, Oct-3/4, CXCR4 and DsRed transcripts. We define samples as negative that had not crossed the automated Cq threshold after 40 cycles and had valid β-actin signals. All samples that are positive for a marker crossed the Cq threshold by 35 cycles and had valid β-actin signals.
Western blotting
Protein extracts from endometriosis lesions were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The blots were transferred to PVDF membrane, blocked with 5% BSA in PBS-Tween-20 (PBS-T) and processed for immunodetection with rabbit polyclonal anti-SDF-1a (1:1000) (Abcam, ab25117) and rabbit polyclonal anti-GAPDH (1:1000) (Cell signaling, USA, D16H11) at 4 °C overnight. Bands were visualized with the corresponding secondary antibody conjugated with horseradish peroxidase diluted at 1:8,000 (Cell signaling, USA) by enhanced chemiluminescence (Amersham Life Sciences, Little Chalfont, UK). The density of specific bands was quantified using Quantity One software (Bio-Rad). The relative band density was calculated as a ratio of sample to GAPDH.
Results
Endometriosis-Derived Cells in the Circulation of mice
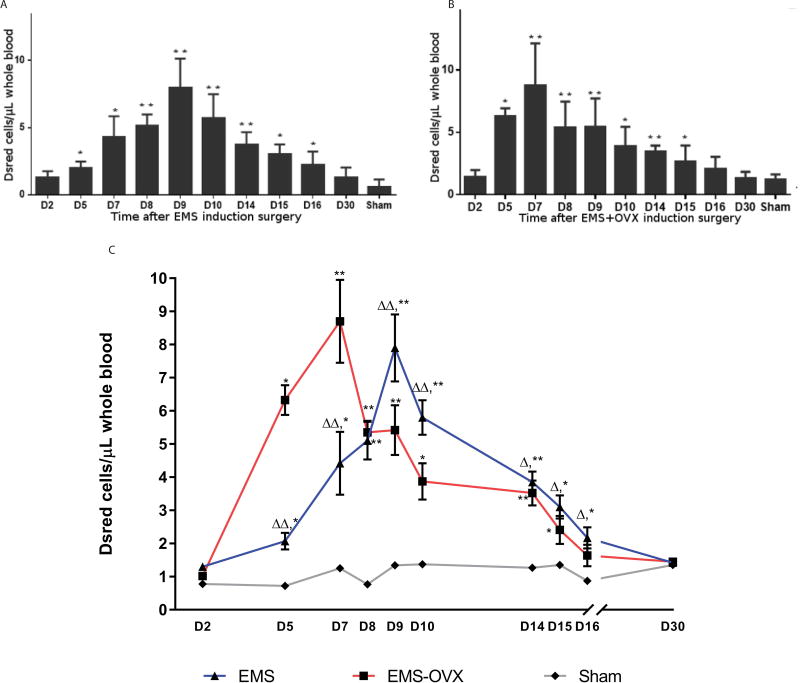
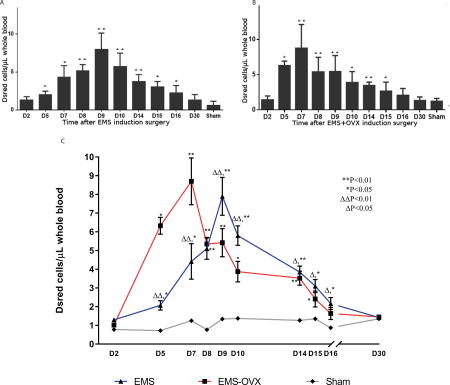
To determine if endometriosis-derived cells could be detected in the circulation, DsRed positive cells in blood were measured by flow cytometry in the EMS and EMS+OVX mouse models. We detected endometriosis-derived cells in the circulation of all animals with experimental endometriosis and none in the controls. Circulating endometriosis-derived cell counts rose from day 5 after disease induction and remained elevated for approximately two weeks (Figure 1). We recorded a significant overall mean elevation in endometriosis-derived cell counts within the EMS and EMS+OVX groups from day 5 through day 16, with peaks at day 7 in the EMS+OVX group (8.7 cells/µL) and day 9 for the EMS group (7.9 cells/µL). Circulating endometriosis-derived cells levels in the EMS+OVX group were significantly higher from D2 to D8 but decreased after D8 compared with the EMS group. No significant difference was detected between sham surgery animals, or between sham surgeries versus non-treated C57BL/6 mice.
Figure 1. Endometriosis-derived cells circulate in the vascular system after lesion implantation.
A: Circulating DsRed total cell counts in endometriosis induced mice compared to sham surgery control group, ** P < 0.01, * P < 0.05; B: Circulating DsRed cells in endometriosis induced and OVX mice. Compared to sham surgery group, ** P < 0.01, * P < 0.05; C: Circulating DsRed cells in EMS vs. EMS+OVX mice, compare to sham surgery group, ** P < 0.01, * P < 0.05; compare to EMS+OVX group, ΔΔ P < 0.01, Δ P < 0.05. N=10 per treatment group.
Circulating Endometriosis-Derived Cells Expressed CXCR4 and Mesenchymal Stem Cell Biomarkers
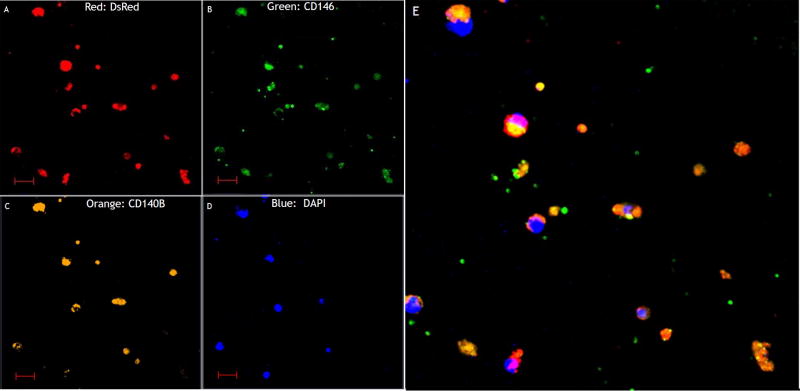
Fluorescent-ICC was performed on sorted cells to confirm DsRed expression as well as endometrial MSC biomarkers. Co-expression of CD146 and platelet-derived growth factor receptor beta (PDGF-Rb, also known as CD140B) was also evaluated here as a well characterized marker used to identify endometrial mesenchymal stem cells [15]. When circulating cells were sorted using CD146 and CD140B in the EMS and EMS+OVX groups, 90.1% and 89.3%, respectively, (P=0.35) of sorted cells were DsRed positive. When sorted for DsRed, 96% and 97% (P=0.41) of the positive cells expressed both CD146 (green) and CD140B (orange), respectively (Figure 2 A–E). We did not find any DsRed signal in cells from control C57BL/6 female mice. These data indicate that a majority of the circulating endometrial stem cells are derived from endometriosis rather than from the endometrium.
Figure 2. Endometriosis-derived stem cells co-express mesenchymal stem cell markers.
Fluorescent -ICC Analysis of sorted circulating DsRed Cells in experimental endometriosis without OVX. Sorted cells were confirmed to be DsRed positive cells, and proved to express endometrial mesenchymal stem cell markers CD146 & CD104B. Scale bars are 20µM.
Further characterization was done by real-time RT-PCR measuring CXCR4, mesenchymal stem/stromal cell biomarkers CD90, CD105, CD9, and Oct3/4, endometrial mesenchymal stem cell markers CD146 and CD140B as well as hematopoietic stem cell markers CD34 and CD45. Circulating DsRed cells in both EMS and EMS+OVX mice expressed CXCR4 and CD90, CD105, CD9 and Oct3/4 mesenchymal stem cell biomarkers as well as CD146 and CD140b but not hematopoietic stem cell markers CD34 and CD45[16–18]. The presence of known endometrial and mesenchymal stem cell markers and a lack of hematopoietic stem cell biomarkers indicates that circulating endometriosis-derived cells are likely endometrial mesenchymal stem cells.
Endometriosis Derived Cells Retain Potency after Hematogenous Dissemination
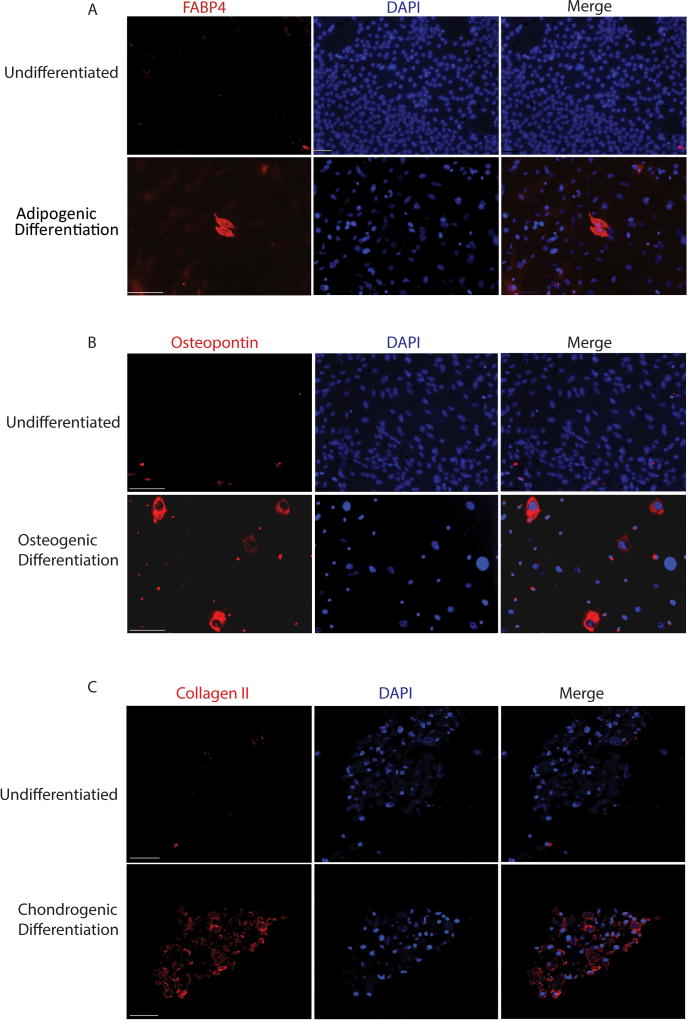
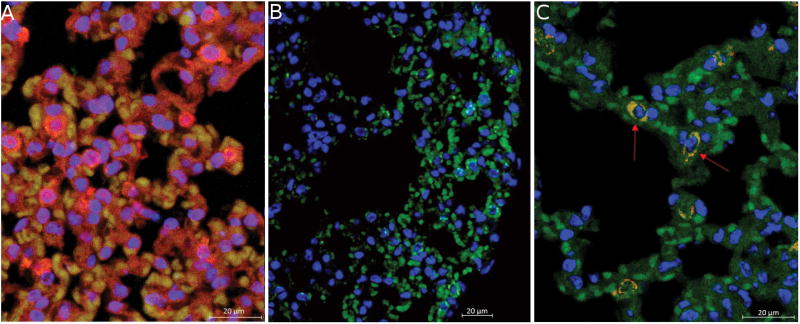
To confirm that populations of cells within endometriosis lesions retain multipotency, we performed a mesenchymal stem cell differentiation assay on lesions after 8 days of implantation, the same time frame where we see maximal dissemination of mesenchymal stromal cells via FACS. Mesenchymal stem cells from endometrial lesions were able to differentiate into adipogenic, osteogenic and chondrogenic lineages, demonstrating retained potency at the time of hematogenous dissemination (Figure 3). We verified that endometriosis-derived cells exhibit stem-cell properties in vivo by examining regions accessible only by vascular dissemination for the presence of Dsred cells, and for evidence of differentiation into non-endometrial cells. Cells that co-express Dsred and Prosurfactant Protein C were identified within the lung of EMS animals 8 weeks after endometriosis induction (Figure 4). Prosurfactant Protein C is a membrane bound protein expressed by type II alveolar cells but not expressed in uterine endometrial cells or leukocytes [19].These differentiated cells provide evidence that endometriosis-derived cells retain multipotency, and implant in regions accessible only by vascular dissemination. Clusters of cells were found in four different animals that co-expressed both DsRed (red) and Prosurfactant Protein C (Green), indicating that cells originating from endometriosis lesions have traveled to the lung, engrafted, and differentiated in vivo within the lung. These data indicate that a population of cells disseminating from the endometriosis lesion are likely endometriosis-derived stem cells.
Figure 3. Differentiation of Endometriosis Derived Cells in vitro.
Cells derived from endometriosis lesions eight days after implantation differentiate in vitro into adipogenic lineages expressing FABP4 (A), osteogenic lineages expressing osteopontin (B) and chondrogenic lineages expressing collagen II (C). Scale Bars for A, B= 110µM; C=50µM
Figure 4. Endometriosis Derived Stem Cells Differentiate in vivo into Alveolar Cells.
A: DsRed positive lung tissue shows global DsRed and Prosurfactant Protein C expression. Staining shows Prosurfactant Protein C (green), DsRed (red), and nuclei (DAPI; blue). B: Sham surgery animals that received suture only show no DsRed staining in the lung. C: Concurrent DsRed and Prosurfactant Protein C staining in the lung indicates that cells from a DsRed endometriosis lesion have traveled from the lesion in the peritoneum to the lung and differentiated into Type II alveolar epithelial cells in vivo. Scale bars = 20µM (N=4)
Elevated CXCL12 (SDF-1) Levels in Serum and Ectopic lesions
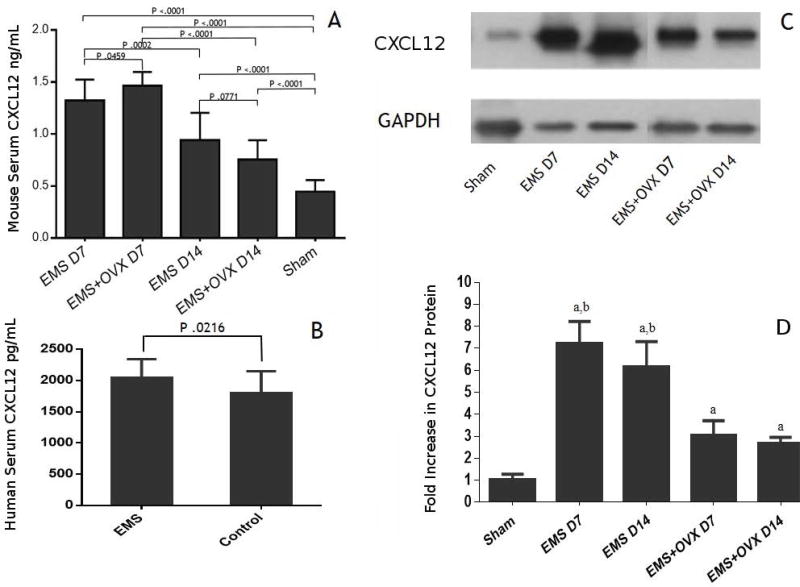
The level of serum CXCL12 (SDF-1) was determined in EMS, EMS+OVX and sham control groups. As shown in Figure 5, we found a significantly higher concentration of serum CXCL12 (SDF-1) at D7 after surgeries in EMS+OVX (1.46±0.134 ng/ml) and EMS (1.31±0.208 ng/ml) than sham control mice (0.44±0.187 ng/ml). Serum CXCL12 abundance diminished over time; at D14 CXCL12 was significantly higher in EMS (0.94±0.262 ng/ml) and EMS+OVX (0.75±0.182 ng/ml) than sham control mice (0.44±0.187 ng/ml) (Figure 5A). Levels of CXCL12 were also elevated in the serum of women with endometriosis compared to disease free controls (Figure 5B), however to a far lesser extent than in the murine model.
Figure 5. Serum CXCL12 (SDF-1) levels are increased in animals with endometriosis lesions.
A: Serum CXCL12 expression in endometriosis induced (EMS) and endometriosis induced and ovariectomy (EMS+OVX) mice at one and two weeks after induction. Compared to the sham surgery group, high serum CXCL12 levels were detected in all four experimental groups (P < 0.01). B: Similar to the murine model, human serum CXCL12 levels were increased in women with endometriosis. C: Western blotting analysis of ectopic lesions in EMS and EMS+OVX mice. Compared to the sham group higher levels of CXCL12 was detected in endometriosis; a indicates sham vs EMS P < 0.01; b indicates sham vs EMX+OVX P < 0.01.
To identify the potential source of the elevated serum CXCL12, ectopic lesions in EMS, EMS+OVX and sham groups were analyzed using western blotting. CXCL12 was expressed at high levels in the endometriosis lesions (Figure 4C). The expression decreased in parallel with the serum levels when the ectopic and serum SDF-1a levels were compared (Figure 5A and C).
CXCR4 positive Endometriotic-Derived Cells Contribute to the growth and Angiogenesis of Endometriotic Implants
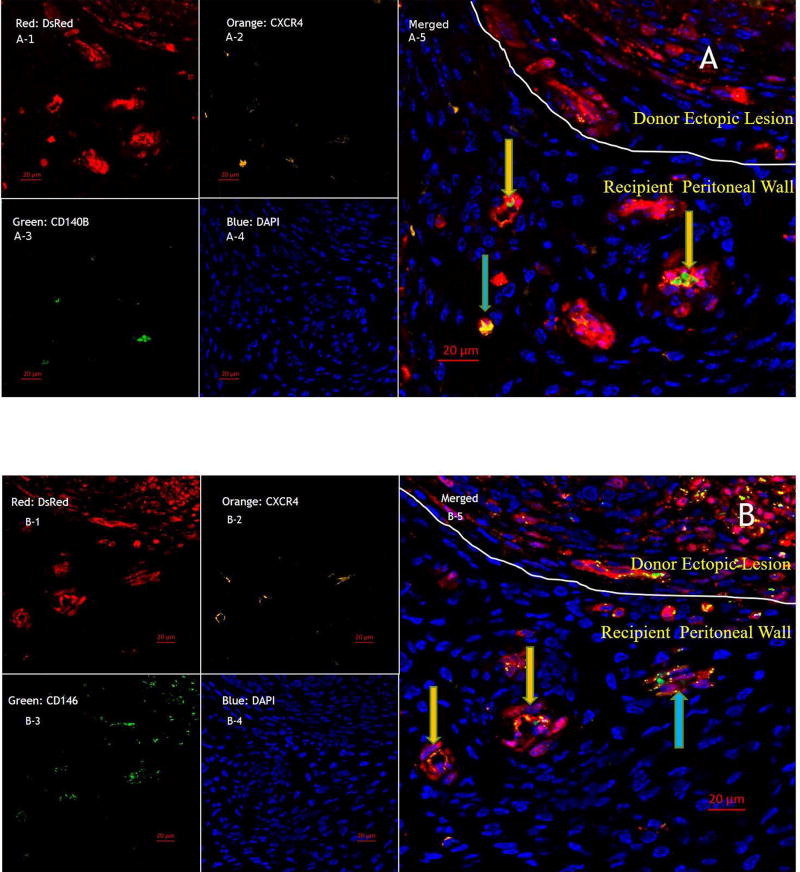
To investigate whether donor endometriosis derived CXCR4+ cells can contribute to the implantation of ectopic endometrium, uterine segments from DsRed mice were transplanted into the peritoneal walls of same strain CD57BL/6 mice. Histologic study of ectopic lesions with immunofluorescence staining demonstrated the presence of new vessels and single cells from donor tissue in the peritoneal wall of recipient mice. Those new vessels and single cells expressed DsRed, CXCR4 and CD146 and CD140B [15] (Figure 6). These data demonstrate that endometriotic-derived CXCR4 positive stem cells likely contributed to endometriosis growth and angiogenesis.
Figure 6. The CXCR4/CXCL12 pathway is involved in lesion expansion and proliferation.
Immunofluorescence Analysis of ectopic lesions in endometriosis induced mice. A: To investigate the contribution of CXCR4 positive ectopic endometrial-derived stem cells to the expansion of ectopic lesion, DsRed(A-1,B-1), CXCR4 (A-2,B-2), CD140B (A-3), and CD146(B-3) were used to mark mesenchymal stem cells that give rise to blood vessels within a lesion and lesion expansion. B: CXCR4 positive new blood vessels (yellow arrow) and single cells expressing mesenchymal stem cell markers (blue arrows) were found in recipient peritoneal wall. Scale bars = 20µM.
Discussion
Endometriosis-derived cells circulate, express MSCs markers and implant in remote locations
In this study, we demonstrated that cells from endometriosis lesions entered the circulation and had several characteristics of mesenchymal stem cells including proliferation, the immunophenotype of endometrial mesenchymal stem cells, as well as the ability to differentiate into type II alveolar cells in vivo. We additionally confirmed that cells within endometriosis lesions have potent stem cell populations 8 days after lesion implantation as defined by in vitro differentiation assay per ISTC guidelines. Endometriosis lesions have been identified in organs remote from the peritoneal cavity, and we speculate that hematogenous spread of stem cells derived from the endometriosis lesions contributes to the distant spread [3,4]. In our experiments, almost all the cells identified in circulation that expressed endometrial stem cell markers CD140b and CD146 were derived from the donor tissue that comprised the endometriosis. This suggests that populations of potent mesenchymal stem cells from endometriosis lesions, and not just the native uterus, can spread over large distances. Treatment of peritoneal lesions may prevent the propagation of further disease as well as the occurrence of remote lesions outside of the peritoneal cavity.
Endometriosis-derived circulating cells were consistently found in the blood of animals with endometriosis, and may be useful as a biomarker of disease in humans. Interestingly, the number of circulating donor endometriosis-derived cells increased during lesion establishment in the mouse endometriosis model, indicating active vascular trafficking of endometrial-derived cells in the early stages of lesion development. In the murine model, endometriosis is established by suturing portions of the endometrium to the peritoneal cavity, which is a different pathogenesis than in humans. This subjects the developing lesions to a potentially more profound hypoxic and inflammatory environment, possibly enhancing cell mobilization. A similar study using a mouse endometriosis model showed that donor bone marrow derived circulating endothelial progenitor cells were found to be elevated acutely after disease induction [20]. Both cell types may be involved in disease establishment and serve as biomarkers of active disease.
Here the majority of circulating endometriosis derived cells were likely mesenchymal stem cells given their expressed biomarkers and ability to differentiate in vivo. The circulating donor endometriosis-derived stem cells expressed mesenchymal stem cell markers but lacked hematopoietic stem cell markers. Several lines of experimental evidence suggest that endometrial stem cells function in the development of endometriosis, providing a another mechanism of disease origin [7,9,13,21–25]. Endometrial stem cells are capable of self-renewal, multipotency, immunomodulation, homing, and are well positioned to migrate and differentiate under the influence of different stimuli from the surrounding environment [26,27]. Similarly, we have demonstrated that endometriosis-derived stem cells retain their potency after leaving the lesion. We identified at least one site in each animal studied where endometriosis-derived mesenchymal cells implanted and differentiated into type II alveolar cells. Additionally we demonstrate through in vitro differentiation into chondrogenic, osteogenic and audiogenic lineages, that mesenchymal stem cells within endometriosis lesions retain their potency at the time of vascular dissemination. These findings support the hypothesis that that a population of the circulating endometriosis derived cells have retained the ability to differentiate remotely.
Endometriosis may be regarded as a stem cell disease; these endometrial stem cells differentiate into local tissue types, but cells may also differentiate into epithelium, glands, and stroma to form functional ectopic endometrial tissue [9,28]. Endometrial-derived stem cell vascular metastasis as described in this study might provide a valuable explanation for those cases of distant, deep infiltrating and recurrent endometriosis.
CXCR4/CXCL12 (SDF-1) axis and Endometriosis-Derived MSCs
Circulating endometriosis-derived stem cells expressed CXCR4 in our murine endometriosis model. CXCR4 is the receptor for CXCL12, a prototype of several chemotactic factors and membrane receptors involved in the migration of mesenchymal stem cells [29]. Our results are consistent with another study cataloging gene expression of endometrial mesenchymal stem cells in the uterus which showed that those cells had a high expression of CXCR4 [26]. Here we show that the cells that enter the circulation are likely stem cells and nearly all expressed CXCR4. It is possible that CXCR4 is a primary means by which these cells are directed to endometriosis which expressed high levels of CXCL12. We found that CXCL12 was increased in serum as well as in the endometriosis lesions. The role of the CXCR4/CXCL12 axis in both migration/circulation of normal stem cells and metastasis of malignant stem cells has been demonstrated in several laboratory model systems [23,30–38]. While the difference in serum CXCL12 levels between women with and without endometriosis is less than that between murine groups, human lesions are often small; the effect may be more important locally where the microenvironment surrounding endometriosis may contain high levels of CXCL12 [39–41]. Here, the elevated levels of CXCL12 produced by the ectopic lesions likely contribute to the pool of circulating CXCL12, however it is likely that locally elevated levels and large concentration gradients surrounding the lesions serve to attract cells that engraft and propagate the disease.
Stem cell metastasis is mediated by the CXCR4/CXCL12 axis through a multistep process in which CXCR4 positive cells first leave their stem cell niche, then are transported via the peripheral blood or lymph to the target tissues following the gradient of CXCL12 [31,42–45]. This axis may also explain the presence of CXCR4+ endometriosis-derived cells in the circulation as well as in ectopic sites in this study. Further, endometriosis-derived CXCR4 positive single cells and newly-formed vessels with endometrial and mesenchymal stem cells biomarkers were detected in the recipient peritoneal wall of ectopic sites, indicating that the CXCR4/CXCL12 axis may underlie the pathogenesis of ectopic endometrial stem cell mobilization as well as angiogenesis. These cells are capable of contributing to both the endometriosis itself as well as the new vasculature required to support the lesion. We speculate that aberrant CXCL12 expression may also attract these cells to new sites and lead to additional lesions of endometriosis.
The number of circulating donor endometriosis-derived cells peaked and decreased earlier in EMS+OVX mice. Consistent with the number of circulating endometriosis-derived cells, serum CXCL12 levels were also higher in EMS+OVX mice than EMS mice 7 days after induction. Although several in vitro studies have shown that estrogen positively regulates CXCL12 in human breast and ovarian cancer cells as well as endometrial cells in vitro [46–48], surprisingly, in our in vivo study serum CXCL12 levels were increased in OVX mice. Increased CXCL12 levels following OVX indicate that estrogen is not the only factor that controls CXCL12 production [46]. Endometriosis derived stem cells from normal tissue and endometriosis lesions were reported to lack estrogen receptors that were then acquired only during differentiation, suggesting that their survival and growth were estrogen independent [24]. CXCL12 mRNA levels paralleled numbers of circulating endometriosis-derived stem cells in animals with or without ovaries; therefore serum CXCL12 is elevated in active disease, independent of regulation by estrogen. Endometriosis results in CXCL12 mediated ectopic endometriosis-derived stem cell mobilization.
In conclusion, endometriosis-derived cells circulate during the establishment of endometriosis. These CXCR4 positive endometriosis-derived cells are likely endometriosis-derived stem cells and respond to serum CXCL12. They contribute to the endometriosis and angiogenesis in ectopic sites. We speculate that circulating endometriosis stem cells propagate the disease through vascular dissemination, and may also serve as biomarkers of active lesion establishment. Blocking endometriosis derived stem cell migration, potentially through the CXCL12/CXCR4 axis is a novel approach to control the spread of this common disease.
Acknowledgments
We thank Hanyia Naqvi for assistance in animal husbandry, Yuping Zhou for Western blotting, Ysabel Ilagan for qPCR analysis, Nickolina Doran and Jack Briano for immunofluorescence staining. We also thank the Gil Mor lab for the use of their microscopes and image analysis software. This work was supported by NIH R01HD076422 and a grant from OvaScience. We would like to acknowledge the Yale CCMI Confocal Facility, Yale FACS facility and the Yale Pathology Tissue Services (YPTS).
References
- 1.Verkauf BS. Incidence, symptoms, and signs of endometriosis in fertile and infertile women. J Fla Med Assoc. 1987;74:671–675. [PubMed] [Google Scholar]
- 2.Olive DL, Pritts EA, Pritts EA. Treatment of endometriosis. N Engl J Med. 2001;345:266–275. doi: 10.1056/NEJM200107263450407. [DOI] [PubMed] [Google Scholar]
- 3.Counseller V. Endometriosis. Am J Obs Gynecol. 1938;36:877–888. [Google Scholar]
- 4.Augoulea A, Lambrinoudaki I, Christodoulakos G. Thoracic Endometriosis Syndrome. Respiration. 2007;75:113–119. doi: 10.1159/000105102. [DOI] [PubMed] [Google Scholar]
- 5.Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. 1927;3:93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 6.Schuld J, Justinger C, Wagner M, et al. Bronchobiliary fistula: a rare complication of hepatic endometriosis. Fertil Steril. 2011;95:804.e15–8. doi: 10.1016/j.fertnstert.2010.07.1087. [DOI] [PubMed] [Google Scholar]
- 7.Du H, Taylor HS. Contribution of Bone Marrow-Derived Stem Cells to Endometrium and Endometriosis. Stem Cells. 2007;25:2082–2086. doi: 10.1634/stemcells.2006-0828. [DOI] [PubMed] [Google Scholar]
- 8.Taylor HS. Endometrial Cells Derived From Donor Stem Cells in Bone Marrow Transplant Recipients. JAMA. 2004;292:81. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Sasson IE, Taylor HS. Stem Cells and the Pathogenesis of Endometriosis. Ann N Y Acad Sci. 2008;1127:106–115. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinleitner A, Lambert H, Suarez M, et al. Immunomodulation in the treatment of endometriosis-associated subfertility: use of pentoxifylline to reverse the inhibition of fertilization by surgically induced endometriosis in a rodent model. Fertil Steril. 1991;56:975–979. [PubMed] [Google Scholar]
- 11.Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694. [PubMed] [Google Scholar]
- 12.Zamah NM, Dodson MG, Stephens LC, et al. Transplantation of normal and ectopic human endometrial tissue into athymic nude mice. Am J Obstet Gynecol. 1984;149:591–597. doi: 10.1016/0002-9378(84)90240-0. [DOI] [PubMed] [Google Scholar]
- 13.Santamaria X, Massasa EE, Taylor HS. Migration of Cells from Experimental Endometriosis to the Uterine Endometrium. Endocrinology. 2012;153:5566–5574. doi: 10.1210/en.2012-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho S, Mutlu L, Grechukhina O, et al. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril. 2015;103:1252–1260.e1. doi: 10.1016/j.fertnstert.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargett CE, Ye L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98:11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 17.Zeineddine D, Hammoud AA, Mortada M, et al. The Oct4 protein: more than a magic stemness marker. Am J Stem Cells. 2014;3:74–82. [PMC free article] [PubMed] [Google Scholar]
- 18.Halfon S, Abramov N, Grinblat B, et al. Markers Distinguishing Mesenchymal Stem Cells from Fibroblasts Are Downregulated with Passaging. Stem Cells Dev. 2011;20:53–66. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 19.Uhlén M, Fagerberg L, Hallström BM, et al. Tissue-based map of the human proteome. Science (80-) 2015;347 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 20.Becker CM, Beaudry P, Funakoshi T, et al. Circulating endothelial progenitor cells are up-regulated in a mouse model of endometriosis. Am J Pathol. 2011;178:1782–1791. doi: 10.1016/j.ajpath.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Te Linde RW, Scott RB. Experimental endometriosis. Am J Obstet Gynecol. 1950;60:1147–1173. doi: 10.1016/0002-9378(50)90517-5. [DOI] [PubMed] [Google Scholar]
- 22.D’Hooghe TM, Bambra CS, Raeymaekers BM, et al. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis) Am J Obstet Gynecol. 1995;173:125–134. doi: 10.1016/0002-9378(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 23.Kao A-P, Wang K-H, Chang C-C, et al. Comparative study of human eutopic and ectopic endometrial mesenchymal stem cells and the development of an in vivo endometriotic invasion model. Fertil Steril. 2011;95:1308–15.e1. doi: 10.1016/j.fertnstert.2010.09.064. [DOI] [PubMed] [Google Scholar]
- 24.Moggio A, Pittatore G, Cassoni P, et al. Sorafenib inhibits growth, migration, and angiogenic potential of ectopic endometrial mesenchymal stem cells derived from patients with endometriosis. Fertil Steril. 2012;98:1521–1530.e2. doi: 10.1016/j.fertnstert.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Ma S, Xie N, Li W, et al. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitzer TLB, Rojas A, Zelenko Z, et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod. 2012;86:58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mambelli LI, Winter GHZ, Kerkis A, et al. A novel strategy of mesenchymal stem cells delivery in the uterus of mares with endometrosis. Theriogenology. 2013;79:744–750. doi: 10.1016/j.theriogenology.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Laganà AS, Sturlese E, Retto G, et al. Interplay between Misplaced Müllerian-Derived Stem Cells and Peritoneal Immune Dysregulation in the Pathogenesis of Endometriosis. Obstet Gynecol Int. 2013;2013:527041. doi: 10.1155/2013/527041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Son B-R, Marquez-Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 30.Gueron G, De Siervi A, Vazquez E. Advanced prostate cancer: reinforcing the strings between inflammation and the metastatic behavior. Prostate Cancer Prostatic Dis. 2012;15:213–221. doi: 10.1038/pcan.2011.64. [DOI] [PubMed] [Google Scholar]
- 31.Kucia M, Reca R, Miekus K, et al. Trafficking of Normal Stem Cells and Metastasis of Cancer Stem Cells Involve Similar Mechanisms: Pivotal Role of the SDF-1-CXCR4 Axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 32.Zannettino ACW, Farrugia AN, Kortesidis A, et al. Elevated serum levels of stromal-derived factor-1alpha are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Res. 2005;65:1700–1709. doi: 10.1158/0008-5472.CAN-04-1687. [DOI] [PubMed] [Google Scholar]
- 33.Liu P, Long P, Huang Y, et al. CXCL12/CXCR4 axis induces proliferation and invasion in human endometrial cancer. Am J Transl Res. 2016;8:1719–1729. [PMC free article] [PubMed] [Google Scholar]
- 34.Kidd LJ, Stephens AS, Kuliwaba JS, et al. Temporal pattern of gene expression and histology of stress fracture healing. Bone. 2010;46:369–378. doi: 10.1016/j.bone.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Toksoy A, Müller V, Gillitzer R, et al. Biphasic expression of stromal cell-derived factor-1 during human wound healing. Br J Dermatol. 2007;157:1148–1154. doi: 10.1111/j.1365-2133.2007.08240.x. [DOI] [PubMed] [Google Scholar]
- 36.Tögel F, Isaac J, Hu Z, et al. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Deng Y, Zhou G-Q. SDF-1α/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–112. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 38.Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1 /CXC chemokine receptor 4 pathway. Proc Natl Acad Sci. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogłodek EA, Szota AM, Moś DM, et al. Serum concentrations of chemokines (CCL-5 and CXCL-12), chemokine receptors (CCR-5 and CXCR-4), and IL-6 in patients with posttraumatic stress disorder and avoidant personality disorder. Pharmacol Reports. 2015;67:1251–1258. doi: 10.1016/j.pharep.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Kitahara CM, Trabert B, Katki HA, et al. Body Mass Index, Physical Activity, and Serum Markers of Inflammation, Immunity, and Insulin Resistance. Cancer Epidemiol Biomarkers Prev. 2014;23:2840–2849. doi: 10.1158/1055-9965.EPI-14-0699-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagheri V, Hassanshahi G, Mirzaee V, et al. CXC chemokine CXCL12 tissue expression and circulating levels in peptic ulcer patients with Helicobacter pylori infection. Cytokine. 2016;85:1–4. doi: 10.1016/j.cyto.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 42.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 43.Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 44.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 45.Gelmini S, Mangoni M, Serio M, et al. The critical role of SDF-1/CXCR4 axis in cancer and cancer stem cells metastasis. J Endocrinol Invest. 2008;31:809–819. doi: 10.1007/BF03349262. [DOI] [PubMed] [Google Scholar]
- 46.Zhang F, Kang H, Xu Q. Estrogen increases secretion of stromal cell derived factor-1 in human breast cancer cells. Int J Clin Exp Med. 2014;7:5529–5534. [PMC free article] [PubMed] [Google Scholar]
- 47.Hall JM, Korach KS. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003;17:792–803. doi: 10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Mamillapalli R, Mutlu L, et al. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015;15:14–22. doi: 10.1016/j.scr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]