Abstract
Background
Use of the immune checkpoint inhibitor ipilimumab is sometimes complicated by ipilimumab-associated colitis (Ipi-AC), an immune-mediated colitis that mimics inflammatory bowel disease.
Objective
We sought to characterize the histopathologic and immunophenotypic features of Ipi-AC, and to directly compare these features to ulcerative colitis (UC).
Methods
This is a retrospective cohort study of 22 patients with Ipi-AC, 12 patients with treatment-naïve UC, and 5 controls with diarrhea but normal endoscopic findings. Immunohistopathologic features were described, and quantitative immunohistochemistry (IHC) was performed for CD4, CD8, CD20, CD138, and FOXP3.
Results
Endoscopic findings in both the Ipi-AC and UC groups included ulcerated, edematous and erythematous mucosa. Involvement of the GI tract was more diffuse in Ipi-AC. As compared to UC, a smaller proportion of Ipi-AC biopsies had basal plasmacytosis (14% for Ipi-AC vs 92% for UC, p<0.0001) and crypt distortion (23% for Ipi-AC vs 75% for UC, p=0.003), whereas Ipi-AC biopsies had more apoptotic bodies in the left colon (17.6 ± 15.3 for Ipi-AC vs 8.2 ± 4.2 for UC, p=0.011). Cryptitis, ulcerations and crypt abscesses were common in both groups. Biopsy specimens from Ipi-AC had a lower density of CD20-positive lymphocytes than UC (275.8 ± 253.3 cells/mm2 for Ipi-AC vs 1173.3 ± 1158.2 cells/mm2 for UC, p=0.022), but had a similar density of CD4, CD8, CD138 and FOXP3-positive cells.
Conclusions
Ipi-AC is a distinct pathologic entity with notable clinical and histopathological differences compared to UC. These findings provide insights into the pathophysiology of immune-related adverse events (iAEs) from ipilimumab therapy.
Keywords: ipilimumab, colitis, inflammatory bowel disease, immunotherapy
Introduction
Immune checkpoints are molecules that modulate cellular immunity and can be co-opted by cancers to induce immune tolerance [1]. Over the past decade, the advent of immune checkpoint inhibitors has transformed the management of some cancers. Ipilimumab is an approved immune checkpoint inhibitor that targets cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4 or CD152), an inhibitory receptor that is constitutively expressed on CD25(+)CD4(+) T regulatory cells [2]. CTLA-4 is also upregulated on activated T-cells and transmits an inhibitory signal to down-regulate the immune response, therefore serving as an immune checkpoint [3]. By inhibiting signaling through CTLA-4, ipilimumab may deplete T regulatory cells or impair their function in the blood or tumor microenvironment, thereby maintaining T effector cell activation and increasing anti-tumor immunity [4–7]. Ipilimumab was the first systemic therapy to conclusively prolong life for patients with metastatic melanoma,[8] and anti-CTLA-4 therapies are currently being tested in clinical trials for a variety of other cancers alone or in combination with other immune checkpoints.
Treatment with ipilimumab is sometimes complicated by immune-related adverse events (iAE) resulting from immune activation in non-target tissues [9]. Such iAEs can be debilitating and in some cases life-threatening and include colitis, hepatitis, myocarditis, dermatitis, neuropathy, and endocrinopathies [10–15]. Colitis is the most common serious adverse event observed with ipilimumab therapy, and has been reported in as many as 21–40% of patients [16]. For reasons that are unclear, colitis is more common with anti-CTLA4 therapies than other immune checkpoint inhibitors such as program cell death protein 1 (PD-1) blocking therapies [17]. In most cases ipilimumab-associated colitis (Ipi-AC) develops weeks to a few months after starting therapy, and in severe cases it can lead to colonic perforation or death [16,18]. Patients with mild Ipi-AC can be managed with supportive therapies alone, but more severe cases require immune modulation with steroids and, in refractory cases, tumor necrosis factor (TNF) inhibitors such as infliximab [19,20].
The pathogenesis of colitis and other iAEs from ipilimumab remains unclear. Prior studies have described similar histopathological features in Ipi-AC as inflammatory bowel disease (IBD) [16,21,22], although no study to our knowledge has directly compared these two disease entities. Although Ipi-AC has been hypothesized to result from depletion of T regulatory (Treg) cells and activation of T effector cells in the gastrointestinal (GI) tract [23–25], studies describing the immunophenotypic features of the GI mucosa in Ipi-AC are limited and conflicting. Several studies have demonstrated an increase in cells expressing the Treg marker FOXP-3 in the GI mucosa of Ipi-AC [26,27], whereas another study of 9 patients with Ipi-AC did not find a difference in total FOXP3 expression [28]. In the present study, we characterized the clinical, endoscopic, histopathological, and immunophenotypic features of a large cohort of patients with Ipi-AC, and compared these features to patients with ulcerative colitis (UC) and healthy controls.
Methods
Patient Population
We examined the clinical and histological features of patients with new-onset Ipi-AC, UC, and normal controls (Ctrl) at the Johns Hopkins Hospital. Cases were identified by searching the Johns Hopkins Pathology Data Systems (PDS) for diagnostic biopsies of the colon and ileum performed during the period of January 2010 to September 2015. Patients with a new diagnosis of Ipi-AC or UC were included in our study if they had at least one biopsy of the left colon with sufficient tissue for histopathological analysis. Biopsies of the right colon and ileum were also analyzed if available. The UC group was composed of patients undergoing an initial diagnostic biopsy before the initiation of treatment. Ipi-AC biopsies were selected regardless of treatment history. Control biopsies were obtained from patients with diarrhea who underwent a colonoscopy and had normal biopsies. All patients with other gastrointestinal pathologies were excluded from our study. Stool cultures and CMV staining were used to exclude infectious colitis when clinically indicated. Demographic information, clinical history, and endoscopic reports were reviewed for all cases. The Johns Hopkins Institutional Review Board (IRB) approved this study.
Histopathology
All available biopsies of the left colon, right colon and ileum were stained with hematoxylin and eosin and histopathological features of the biopsies were scored. Two pathologists (MKP and RAA), blinded to the clinical history of the patients, reviewed the slides and a third pathologist (LV) adjudicated occasional discrepancies. Slides were scored for the presence or absence of ulceration, cryptitis, crypt abscesses, basal plasmacytosis, and distortion of crypt architecture in the left colon, right colon and ileum. We defined these histological features as present if they were seen in any of the biopsy sites including the left colon, right colon or ileum. Crypt distortion was further characterized as mild, moderate, or severe based on the following criteria: 1) mild crypt distortion was defined as minimal crypt disarray and shortening with mild basal plasmacytosis, 2) moderate crypt distortion was defined as aberrantly shaped crypts (branching, growing sideways) with moderate basal plasmacytosis and rare glandular dropout, and 3) marked crypt distortion was defined as extensive areas of glandular dropout with aberrantly shaped crypts, marked basal plasmacytosis, and villiform surface configuration. Cryptitis, quantified as the number of crypts infiltrated by neutrophils, and crypt abscesses were quantified per 10 high power field (HPF). Apoptotic cells within the crypt were quantified in 10 HPF at 40x magnification with a field diameter of 0.35 mm.
Immunohistochemistry
All slides from each area of the GI tract were stained in batch for CD4, CD8, CD20, FOXP3, and CD138 in a central reference laboratory at the Johns Hopkins Hospital according to standard automated protocols. Briefly, biopsy samples were fixed in formalin and embedded in paraffin, and 5 µm sections were cut and mounted on glass slides. Deparaffinization and rehydration were performed, followed by antigen retrieval and antibody staining as previously described [29]. The slides were digitally scanned at 20× objective equivalent (0.49 microns/pixel) using an Aperio Scanscope (Leica Biosystems, Wetzlar, Germany). The density of CD4, CD8, CD20, FOXP3, and CD138− positive cells was measured as the number of cells / surface area analyzed using a digital image analysis software platform (HALO Image Analysis, Corrales, NM) as previously described [30]. The total number of labeled cells per unit area in the left colon, right colon and ileum, and the ratio of FOXP3/CD8 and FOXP3/CD4 cells for each respective biopsy site within each group were reported.
Statistics
The frequency and severity of the histopathological and immunohistochemistry features in the Ipi-AC group were compared with those in the UC and control groups. Categorical variables were compared using the chi-squared test, while continuous variables were described as means with standard deviations and compared using Student’s t-test. Statistical analysis was performed using JMP Version 9 (SAS Institute Inc., Cary, NC). All tests were two-sided and a p-value of <0.05 was used to define statistical significance.
Results
Study population
Histopathological and immunohistochemical features of 22 cases of Ipi-AC, 12 cases of treatment-naïve UC, and 5 Ctrls with diarrhea but normal biopsies were evaluated. Patients with Ipi-AC were older than patients with UC and Ctrls (62 ± 11.7 years for Ipi-AC vs 42 ± 17.8 for UC, p<0.05). Patients in the Ipi-AC group had received ipilimumab for various malignancies, including metastatic melanoma (n=15), metastatic pancreatic adenocarcinoma (n=4), metastatic lung carcinoma (n=2), and metastatic renal cell carcinoma (n=1). Seven of the 22 Ipi-AC patients received concurrent anti-PD-1 therapy (nivolumab, n=6; pembrolizumab, n=1). Half of the patients in the Ipi-AC group (n=11) received empiric steroids prior to biopsy, and one of these 11 patients received infliximab prior to biopsy. The median duration of ipilimumab therapy prior to the onset of colitis in our study group was 46 days (range 14 – 396 days), and the median number of doses of ipilimumab prior to the onset of colitis was 2 (range 1–10).
Watery and bloody diarrhea were observed in both the Ipi-AC and UC groups. The most common clinical symptom in the Ipi-AC group was watery diarrhea (n=21, 95%), whereas the most common symptom in the UC group was hematochezia (n=9, 75%). On endoscopic exam, the mucosa in the Ipi-AC group appeared edematous and erythematous in 8 patients (36%), ulcerated in 7 (32%), and normal in 5 (21%). The most common endoscopic finding in the UC group was an erythematous, friable and ulcerated mucosa in the left-sided colon in 9 patients (75%). The endoscopic examination was normal for all patients in the Ctrl group (see table 1).
Table 1.
Demographic, clinical characteristics, endoscopic findings, and histopathologic findings of ipilimumab-associated colitis (Ipi-AC, n=22), ulcerative colitis (UC, n=12), and normal controls (Ctrl, n=5). The presence of mucosal ulcerations, cryptitis, crypt abscesses, basal plasmacytosis, and crypt distortion is depicted as the number and percentage of patients with the histopathologic feature in at least one biopsy site. Crypt distortion was further subclassified as mild, moderate or severe depending on the severity of crypt irregularities. Cryptitis, crypt abscesses, and apoptotic bodies were quantified per 10 high power field (HPF) in the left colon.
| Ipi-AC (n=22) | UC (n=12) | Ctrl (n=5) | ||
|---|---|---|---|---|
|
| ||||
| Age (years) | 62 ± 11.7 | 42 ± 17.8* | 49 ± 16.6# | |
|
| ||||
| Sex (% female) | 7 (32%) | 8 (67%) | 3 (60%) | |
|
| ||||
| Most common clinical symptom | Watery diarrhea (n=21, 95%) | Hematochezia (n=9, 75%) | Watery diarrhea (100%) | |
|
| ||||
| Most common endoscopic findings | Edematous and erythematous mucosa (n=8, 36%) | Erythematous, friable and ulcerated mucosa (n=9, 75%) | Normal (n=5, 100%) | |
|
| ||||
| Sites biopsied [N (%)] | ||||
| left colon | 22 (100%) | 12 (100%) | 5 (100%) | |
| Right colon | 11 (50%) | 7 (58%) | 2 (40%) | |
| Ileum | 6 (27%) | 3 (25%) | 3 (60%) | |
|
| ||||
| Presence of mucosal ulceration [N (%)] | 10 (45%) | 7 (58%) | 0 (0%)# | |
|
| ||||
| Cryptitis | ||||
| Presence [N (%)] | 16 (73%) | 10 (83%) | 0 (0%)## | |
| Quantitative (#/10 HPF) | 3.6 ± 5.3 | 11.6 ± 6.3*** | 0 ± 0## | |
|
| ||||
| Crypt Abscesses | ||||
| Presence [N (%)] | 7 (32%) | 8 (67%)* | 0 (0%) | |
| Quantitative (#/10 HPF) | 1.8 ± 3.8 | 1.8 ± 2.4 | 0 ± 0 | |
|
| ||||
| Presence of basal plasmacytosis [N (%)] | 3 (14%) | 11 (92%)*** | 0 (0%) | |
|
| ||||
| Crypt distortion [N (%)] | ||||
| Presence (any) | 5 (23%) | 9 (75%)** | 0 (0%) | |
| Mild | 4 (18%) | 3 (25%) | 0 (0%) | |
| Moderate | 1 (5%) | 4 (33%) | 0 (0%) | |
| Severe | 0 (0%) | 2 (17%) | 0 (0%) | |
|
| ||||
| Apoptotic Bodies (per 10 HPF) | 16.6 ± 15.6 | 7.3 ± 4.7* | 0.8 ± 0.4### | |
Data are depicted as mean ± SD. Comparisons were made between Ipi-AC and UC as well as Ipi-AC and Ctrls using chi-squared analysis for categorical variables and the Student’s t-test for continuous variables.
For Ipi-AC vs. UC: * p<0.05, ** p<0.01, *** p<0.001.
For Ipi-AC vs. Ctrl: # p<0.05, # p<0.01, ## p<0.001
Histopathological Features of Ipi-AC, UC, and normal controls
The histopathological features of Ipi-AC, UC, and controls are summarized in Table 1. All cases included biopsies of the left colon, whereas only a subset of cases included biopsies of the right colon or ileum. A total of 71 biopsies were reviewed for the 39 patients. The average number of biopsies per subject and the frequency of right colon and ileum biopsies were similar across the three groups.
Both the Ipi-AC and UC groups had lymphoplasmacytic expansion of the lamina propria. The most frequently observed histopathological features in the Ipi-AC group were cryptitis (n=16, 73%) and mucosal ulcerations (n=10, 45%), both of which were present in a similar percent of patients as UC (n=10, 83% with cryptitis; n=7, 58% with ulcerations; p>0.05) (Table 1 and Figure 2a). Crypt abscesses were also common in Ipi-AC (n=7, 32%), but were more likely to be present in UC (n=8, 67%, p=0.049) (Table 1). Compared to UC, fewer patients in the Ipi-AC group had basal plasmacytosis (n=3, 14% for Ipi-AC vs n=11, 92% for UC, p<0.0001) and crypt distortion (n=5, 23% for Ipi-AC vs n=9, 75% for UC, p=0.003) (Table 1 and Figure 2b–c). Crypt distortion was not only more frequently observed in UC, but it was more severe when present; only one Ipi-AC patient (5%) had moderate crypt distortion, whereas 50% of the UC group had moderate or severe crypt distortion. These histopathologic findings were observed in the left colon, right colon and ileum of the Ipi-AC group, whereas the ileum of the UC group was spared in the three biopsies that were analyzed. None of these histopathological features were observed in any biopsy site from the normal control group.
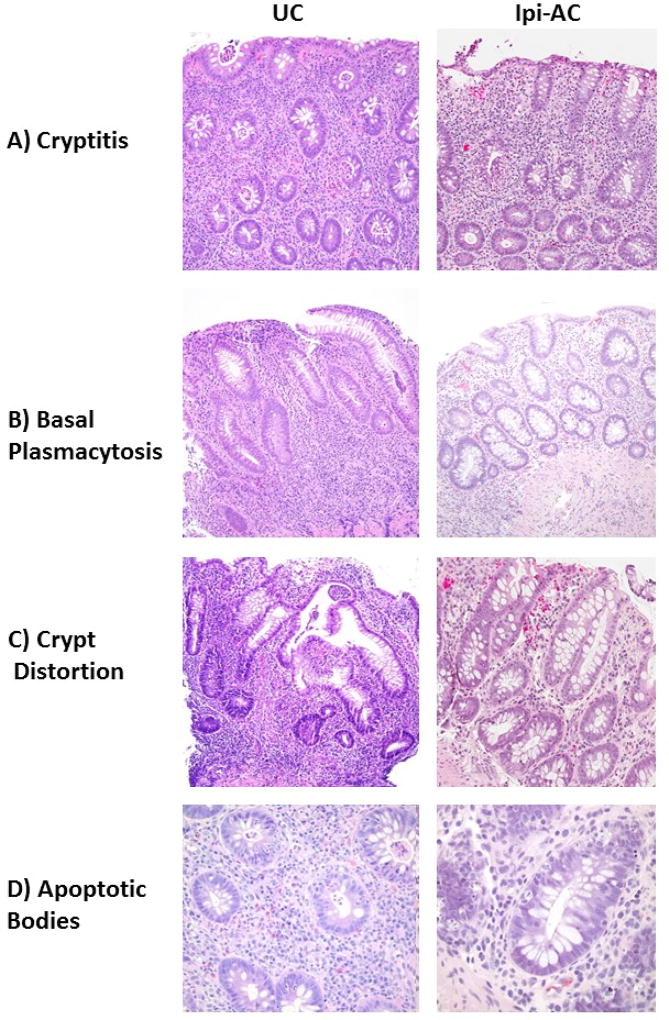
Figure 2.
Representative images of histopathologic features in ulcerative colitis (UC) and ipilimumab-associated colitis (Ipi-AC). A) Ipi-AC had more focal cryptitis compared to the diffuse cryptitis pattern seen in UC. B) UC had prominent basal plasmacytosis compared to Ipi-AC. C) UC had severe architectural distortion of crypts, compared to Ipi-AC which had focal and mild crypt distortion. D) Ipi-AC had more apoptotic bodies within the crypts compared to UC.
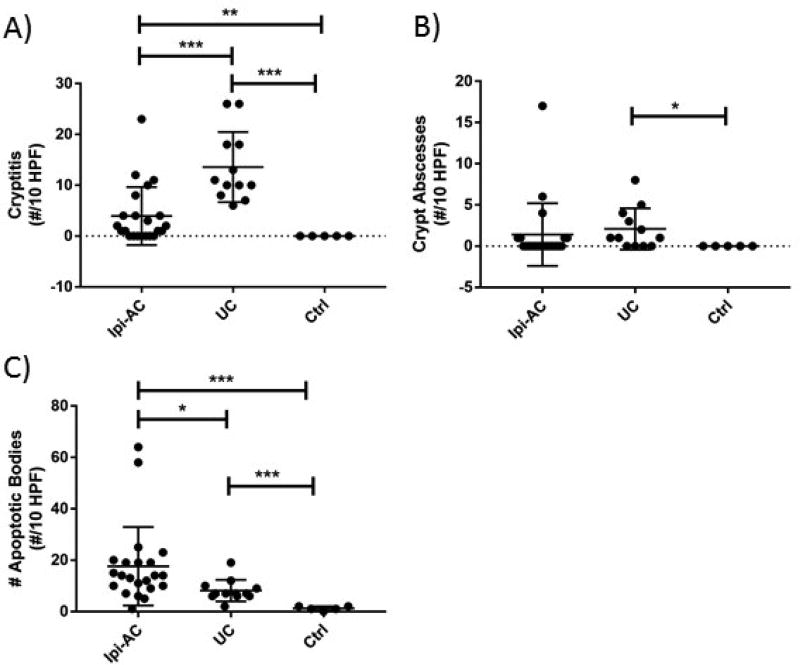
The severity of histopathologic features including cryptitis, crypt abscesses, and apoptotic bodies was evaluated quantitatively in the Ipi-AC group and compared to UC and controls. Comparisons were made for only the left colon because UC is known to spare other parts of the GI tract. Although a similar proportion of subjects with Ipi-AC and UC had cryptitis observed in at least one biopsy site, the severity of cryptitis in the Ipi-AC group was less than the UC group as assessed by the number of crypts infiltrated by neutrophils (4.0 ± 5.7 for Ipi-AC vs 13.6 ± 6.9 for UC, p=0.0006) (Figure 1a). Crypt abscesses were less likely to be present in Ipi-AC compared to UC, but there was no difference in the number of crypt abscesses when they were quantified in both groups (1.4 ± 3.8 for Ipi-AC vs 2.1 ± 2.5 for UC, p>0.05) (Figure 1b). The Ipi-AC group had more apoptotic bodies in the left colon compared to UC (17.6 ± 15.3 for Ipi-AC vs 8.2 ± 4.2 for UC, p=0.011) (Figure 1c). Apoptotic bodies were predominately located at the base of the crypts (Figure 2d). As compared to controls, Ipi-AC had more severe cryptitis and more crypt abscesses and apoptotic bodies (p<0.05 for cryptitis and apoptotic bodies, p=0.096 for crypt abscesses).
Figure 1.
The severity of cryptitis and crypt abscesses and the number of apoptotic bodies were quantified in the left colon of ipilimumab-associated colitis (Ipi-AC), ulcerative colitis (UC), and normal controls (Ctrl). (A) There was less cryptitis, quantified as the number of crypts with intraepithelial neutrophils, in the Ipi-AC group compared to UC (p=0.0006). (B) There was no difference in quantitative crypt abscesses between Ipi-AC and UC (p>0.05). (C) The Ipi-AC group had more apoptotic bodies compared to the UC group (p=0.011) and controls (p<0.0001). Unpaired Student’s t-test was used for all analyses. * p< 0.05, ** p<0.01, *** p<0.001
Quantitative Immunophenotyping of Mucosal Lymphocytes in the GI tract from Ipi-AC Compared to UC and Controls
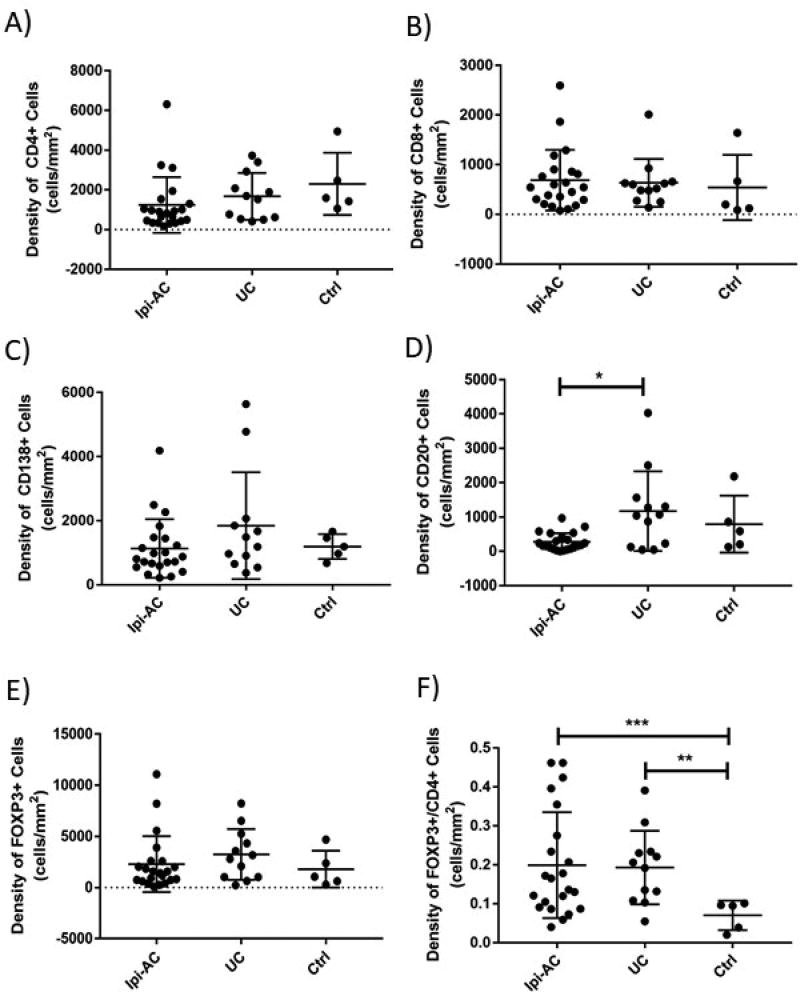
We performed digital image analysis on immunohistochemically stained slides to determine the density of CD4, CD8, CD20, CD138, and FOXP3-positive lymphocytes in Ipi-AC and compared expression of these markers to UC and controls. We did not find a significant difference in the density of CD4, CD8, CD20, CD138, or FOXP3-positive cells in the left colon of Ipi-AC and controls (Figure 3a–e). There was no difference in the FOXP3/CD8 ratio (p>0.05) (data not shown). The FOXP3/CD4 ratio in the left colon was significantly higher in the Ipi-AC group compared to controls (0.20 ± 0.14 vs 0.07 ± 0.04, p=0.0008) (Figure 3f), and this difference was also observed in the right colon and ileum (data not shown).
Figure 3.
Immunophenotypic features of the left colon of ipilimumab-associated colitis (Ipi-AC), ulcerative colitis (UC), and normal controls (Ctrl). The density of cells that stained positive for CD4, CD8, CD20, CD138, and FOXP3 was assessed using immunohistochemistry. There was no difference in the density of (A) CD4, (B) CD8, or (C) CD138-positive cells between any of the groups (p>0.05). (D) The Ipi-AC group had less CD20-positive cells in the left colon compared to UC (p=0.022). There was no difference in the density of CD20-positive cells between the Ipi-AC and Ctrl groups (p>0.05). (E) The total density of FOXP3-positive cells was similar among all groups (p>0.05). (F) There was no difference in the FOXP3/CD4 ratio between Ipi-AC and UC (p>0.05), but the Ipi-AC group had an increased FOXP3/CD4 ratio compared to Ctrls (p=0.0008). Unpaired Student’s t-test was used for all analyses. * p< 0.05, ** p<0.01, *** p<0.001
We also compared the immunologic features of mucosal lymphocytes from Ipi-AC and UC. As compared to UC, the left colon of Ipi-AC had a significantly lower density of CD20-positive cells (275.8 ± 253.3 cells/mm2 for Ipi-AC vs 1173.3 ± 1158.2 cells/mm2 for UC, p=0.022) (Figure 3d). There was no difference in the density of CD4, CD8, CD138 or FOXP3-expressing cells or the ratio of FOXP3/CD8 or FOXP3/CD4 in the left colon between Ipi-AC and UC (p>0.05 for all comparisons) (Figure 3a–c, e–f).
To determine if the histopathological or immunohistochemical features noted above were affected by treatment with steroids, we compared Ipi-AC patients treated with steroids prior to biopsy (Ipi-AC-S, n=11) with treatment-naïve patients (Ipi-AC, n=11). The only histopathological or immunophenotypic marker that was different between the Ipi-AC and Ipi-AC-S groups was the density of CD138-positive lymphocytes (733.9 ± 631.6 cells/mm2 for Ipi-AC vs 1531.1 ± 1003.5 cells/mm2 for Ipi-AC-S, p=0.040). When Ipi-AC-S patients were excluded from analysis, the density of CD138-positive lymphocytes was lower in Ipi-AC compared to UC (733.9 ± 631.6 cells/mm2 for Ipi-AC vs 1843.3 ± 1667.0 cells/mm2, p=0.050).
Discussion
We described the endoscopic, histopathologic, and immunophenotypic features in GI mucosa from patients with Ipi-AC, and compared these features to patients with treatment-naïve UC and normal controls. Cryptitis, mucosal ulcerations, and crypt abscesses were common in Ipi-AC, consistent with the prior observations of Verschuren and colleagues [22]. Compared to this previously described cohort of 25 patients with Ipi-AC, patients in our study had a modestly lower prevalence of ulcerations, cryptitis, crypt abscesses, and crypt irregularities. Some of these differences may be attributed to the lower number of biopsies obtained per patient in our cohort, which may have decreased the chance of detecting histological abnormalities. The cohort published by Verschuren and colleagues may have had more severe disease because the patients were treatment-naïve at the time of biopsy, whereas half of the Ipi-AC patients in our cohort had received empiric immunosuppression prior to biopsy. However, we did not find any significant differences in histopathological features between Ipi-AC patients who received steroids and those who were treatment-naïve.
The histopathologic features of Ipi-AC were similar to UC, but there were several notable differences. The distribution of pathology was more evenly distributed throughout the GI tract in Ipi-AC, whereas UC disproportionately affected the distal colon. Patients with UC generally had more severe histopathologic abnormalities with increased cryptitis, crypt abscesses, crypt distortion and basal plasmacytosis compared to Ipi-AC, whereas Ipi-AC had significantly more apoptotic bodies. The presence and severity of crypt distortion and basal plasmacytosis were particularly prominent distinguishing features between Ipi-AC and UC. A prior study also demonstrated that sera from patients with Ipi-AC and classic IBD have distinct patterns of antibody titers to microbial flora [31]. These findings support the idea that Ipi-AC is a distinct histopathologic entity compared to UC.
Prior studies of Ipi-AC are conflicting on the effect of ipilimumab on Tregs in the GI mucosa, with some studies describing an increase in Tregs in the GI mucosa and others reporting no significant change [26–28]. Although we did not find an increase in the number of cells expressing the Treg marker FOXP3, we did observe an increase in the FOXP3/CD4 cell ratio as compared to control subjects. The increased FOXP3/CD4 ratio may represent a shift toward increased Treg cells as a compensatory mechanism to suppress the inflammatory process in the GI tract. This result was surprising given the clinical observation in other settings that ipilimumab therapy may deplete Treg cells through its effects on CTLA4 expressed on Tregs. It is possible that despite an increase in FOXP3/CD4 ratio, ipilimumab may cause functional changes in Tregs similar to what has been observed in murine models of Ipi-AC [4], or that ipilimumab may selectively deplete a subset of Tregs normally responsible for the prevention of colitis.
We also noted an increase in B-lineage cells as assessed by CD20 staining and more basal plasmacytosis in the UC group compared to Ipi-AC. This observation is consistent with prior pathophysiologic studies of IBD [32] and supports the notion that humoral immunity is more important in the pathogenesis of UC than Ipi-AC, which is predominately a T-cell driven disease. This may also explain why abatacept, a recombinant fusion protein comprising the extracellular domain of human CTLA-4 designed to selectively inhibit T-cell activation, recently failed in a large clinical trial of IBD [33].
Strengths of this study include a robust clinical cohort of well-characterized subjects with Ipi-AC, UC, and controls with the use of highly specific cell labeling and quantitative analyses to detect differences in the histology and immunophenotypic features of these groups. Relative weaknesses of this investigation include the potential for selection biases affecting the disease severity of the Ipi-AC, UC, and control cohorts, as patients with mild colitis may not have pursued a diagnostic colonoscopy. It is also possible that the Ipi-AC group may have had a shorter duration of colitis prior to biopsy because they were more closely monitored, which may have resulted in less severe histopathologic features. We also did not adjust our statistical analyses for multiple comparisons. In conclusion, Ipi-AC has many overlapping features with ulcerative colitis but is a distinct pathologic entity with notable clinical and histopathological differences. Our results have implications for the diagnosis of Ipi-AC and provide insights into the pathophysiology of iAEs from ipilimumab therapy.
Acknowledgments
This work was supported by the NCI SPORE in Gastrointestinal Cancers P50 CA062924-22 (RA), The Johns Hopkins Bloomberg Kimmel Institute for Cancer Immunotherapy (JT, EJ, and RA), the Viragh Foundation, NIH RO1 FD004819 (DL), NIH Training Grant T32AR048522 (BA), and NIH Center Core Grant (P30CA006973).
Footnotes
Disclosures: There are no potential conflicts of interest relevant to the manuscript.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 4.Read S, Greenwald R, Izcue A, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–83. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 6.Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reuben JM, Lee B-N, Li C, et al. Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma. Cancer. 2006;106:2437–44. doi: 10.1002/cncr.21854. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (Anti-CTLA4 Antibody) Causes Regression of Metastatic Renal Cell Cancer Associated With Enteritis and Hypophysitis. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS, Kähler KC, Hauschild A. Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 12.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–16. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–50. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375:1749–55. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in Patients With Cancer After Antibody Blockade of Cytotoxic T-Lymphocyte-Associated Antigen 4. J Clin Oncol. 2006;24:2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell KA, Kluger H, Sznol M, Hartman DJ. Ipilimumab-induced perforating colitis. J Clin Gastroenterol. 2013;47:781–5. doi: 10.1097/MCG.0b013e31828f1d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minor DR, Chin K, Kashani-Sabet M. Infliximab in the Treatment of Anti-CTLA4 Antibody (Ipilimumab) Induced Immune-Related Colitis. Cancer Biother Radiopharm. 2009;24:321–5. doi: 10.1089/cbr.2008.0607. [DOI] [PubMed] [Google Scholar]
- 20.Jain A, Lipson EJ, Sharfman WH, Brant SR, Lazarev MG. Colonic ulcerations may predict steroid-refractory course in patients with ipilimumab-mediated enterocolitis. World J Gastroenterol. 2017;23:2023. doi: 10.3748/wjg.v23.i11.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rastogi P, Sultan M, Charabaty AJ, Atkins MB, Mattar MC. Ipilimumab associated colitis: an IpiColitis case series at MedStar Georgetown University Hospital. World J Gastroenterol. 2015;21:4373–8. doi: 10.3748/wjg.v21.i14.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verschuren EC, van den Eertwegh AJ, Wonders J, et al. Clinical, Endoscopic, and Histologic Characteristics of Ipilimumab-Associated Colitis. Clin Gastroenterol Hepatol. 2016;14:836–42. doi: 10.1016/j.cgh.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–54. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arriola E, Wheater M, Lopez MA, Thomas G, Ottensmeier C. Evaluation of immune infiltration in the colonic mucosa of patients with ipilimumab-related colitis. Oncoimmunology. 2016;5:e1209615. doi: 10.1080/2162402X.2016.1209615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oble DA, Mino-Kenudson M, Goldsmith J, et al. Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32:1130–7. doi: 10.1097/PAS.0b013e31817150e3. [DOI] [PubMed] [Google Scholar]
- 28.Lord JD, Hackman RC, Moklebust A, et al. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Dig Dis Sci. 2010;55:1396–405. doi: 10.1007/s10620-009-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794–801. doi: 10.1136/gutjnl-2015-310839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010;10:11. [PMC free article] [PubMed] [Google Scholar]
- 32.Scott BB, Goodall A, Stephenson P, Jenkins D. Rectal mucosal plasma cells in inflammatory bowel disease. Gut. 1983;24:519–24. doi: 10.1136/gut.24.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandborn WJ, Colombel J, Sands BE, et al. Abatacept for Crohn’s Disease and Ulcerative Colitis. Gastroenterology. 2012;143:62–69.e4. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]