Abstract
Hematopoietic stem and progenitor cells (HSPC) are necessary for life-long blood production and replenishment of the hematopoietic system during stress. We recently reported that nuclear factor I/X (Nfix) promotes HSPC survival post-transplant. Here, we report that ectopic expression of Nfix in primary mouse HSPCs extends their ex vivo culture from about 20 days to 40 days. HSPCs overexpressing Nfix display hypersensitivity to supportive cytokines and reduced apoptosis when subjected to cytokine deprivation relative to controls. Ectopic Nfix resulted in elevated levels of c-Mpl transcripts and cell surface protein on primary murine HSPCs as well as increased phosphorylation of STAT5, which is known to be activated down-stream of c-MPL. Blocking c-MPL signaling by removal of thrombopoietin or addition of a c-MPL neutralizing antibody negated the anti-apoptotic effect of Nfix overexpression on cultured HSPCs. Further, NFIX was capable of binding to and transcriptionally activating a proximal c-Mpl promoter fragment. In sum, these data suggest that NFIX-mediated up-regulation of c-Mpl transcription can protect primitive hematopoietic cells from stress ex vivo.
Keywords: Hematopoietic Stem Cells, Ectopic Gene Expression, Apoptosis, Cell Survival, NFIX, NFI Transcription Factors, Thrombopoietin, c-Mpl
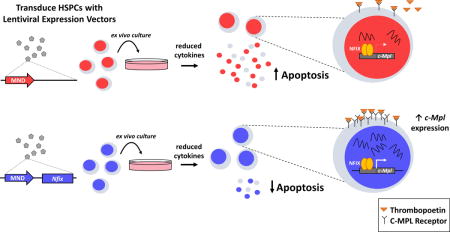
Graphical abstract
Hematopoietic stem and progenitor cells (HSPC) are necessary for lifelong blood production and replenishment of the hematopoietic system during stress. Here, we show that nuclear factor I/X (Nfix) is capable of protecting HSPC from stress-induced apoptosis during ex vivo culture. This protection relies on proper thrombopoietin/c-MPL signaling, as NFIX directly regulates c-Mpl expression.
Introduction
Hematopoietic stem and progenitor cells (HSPCs) are necessary for replenishing the blood system during native hematopoiesis and times of stress, such as during a hematopoietic stem cell transplant (HSCT), which is employed routinely in the clinic to treat hematologic disease. Transplant-induced stress exerted on HSPCs has been well documented, resulting in reduced stem cell pools and decreased self-renewal ability [1-3], but regulation of their ability to overcome this stress and successfully replenish hematopoiesis is not well understood. Cell-extrinsic and cell-intrinsic regulators of HSCT have been implicated in HSPC self-renewal and mobilization and homing [4-6]. Better understanding the mechanisms that allow HSPC engraftment post-transplant will facilitate efforts to improve transplantation protocols and clinical outcomes.
Recently, our lab completed a functional screen that identified 17 novel regulators of murine HSCT, including the nuclear factor I (NFI) family member, Nfix [7]. NFI family members function as transcriptional activators and repressors [8,9]. Although Nfix−/− mice display no overt hematopoietic phenotypes during native hematopoiesis, shRNA-mediated knock-down or genetic deletion of Nfix in HSPCs results in a profound loss of competitive in vivo repopulating potential, a loss of niche retention post-transplant and increased apoptosis [10]. NFIX and NFIA, a related family member, have also been implicated in regulating hematopoietic lineage fate decisions, with ectopic expression of NFIA or Nfix promoting HSPC commitment to erythropoiesis or myelopoiesis and depletion promoting granulopoiesis or lymphopoiesis, respectively [11,12].
Although Nfix is clearly required by HSPCs during HSCT, little is known about how NFIX regulates HSPCs at the molecular and cellular level. Here we report that Nfix can promote ex vivo growth, cytokine hypersensitivity, and survival of primitive hematopoietic populations ex vivo. We further demonstrate that these effects are in part mediated via up-regulation of the thrombopoietin (TPO) receptor, c-Mpl, thus revealing NFIX as a novel transcriptional regulator of c-Mpl and illuminating one molecular pathway targeted by NFIX in HSPC.
Materials and Methods
Complete materials and methods can be found in the Supplemental Data.
Results and Discussion
We have previously shown that Nfix is critical to HSPC survival post-transplantation [10]. To further interrogate the role of Nfix in HSPC biology, we ectopically expressed Nfix in Lineage−Sca-1+c-Kit+ (LSK) cells cultured under serum-free conditions (Supporting Information Fig. S1A). During culture, cells were assessed for growth rate, retention of vector+ (NFIX+) cells, and persistence of an LSK phenotype (Fig. 1A). Nfix was over-expressed 20-fold in NFIX+ cells, while other NFI genes remained unperturbed (Fig. 1B). Remarkably, ectopic Nfix expression prolonged hematopoietic cell cultures two-fold, allowing cells to persist up to 40 days ex vivo (Fig. 1C). However, the relative growth of control and NFIX+ cultures did not significantly diverge until control cells began to display culture exhaustion (p = 0.036) (Fig. 1C). During this extended time, a steady selection for NFIX+ cells was apparent (Fig. 1D). These data suggest that Nfix can promote the extended cell culture of hematopoietic progenitors.
Figure 1. Nfix induces longevity in HSPC ex vivo culture.
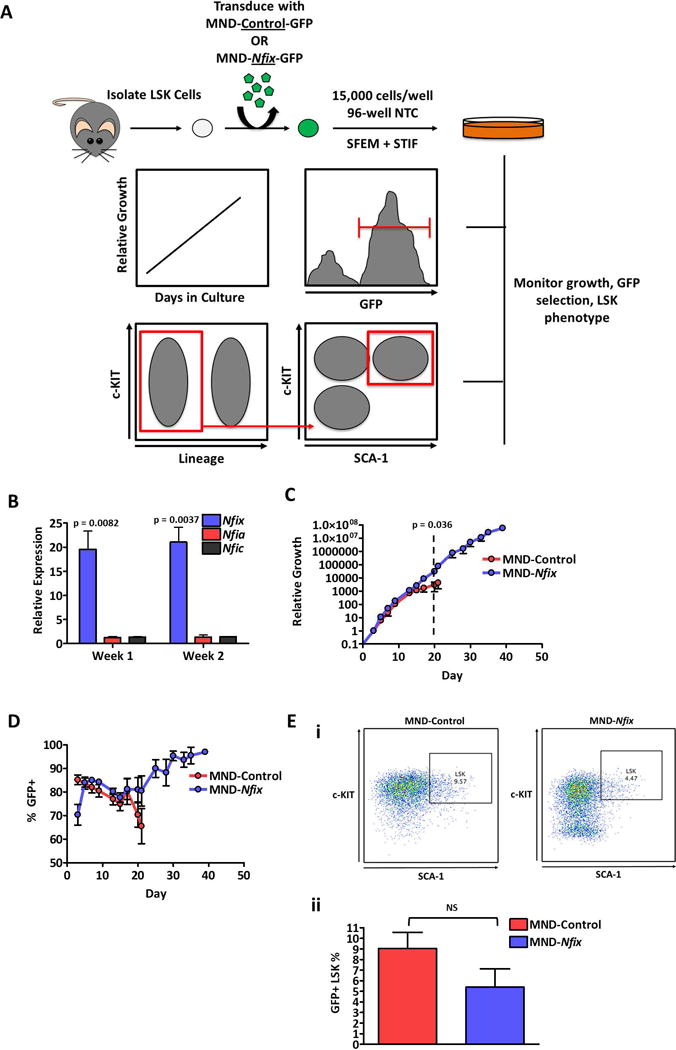
(A) Experimental schematic. 48 hours post-transduction, LSK cells transduced with MND-Control or MND-Nfix were re-plated in 96-well non-tissue culture treated plates in serum-free expansion medium (SFEM) supplemented with mSCF, mTPO, mIGF-2, and hFGF-a (STIF). Every 48-72 hours of culture, cells were counted, assessed for GFP+ cells, and passaged 1:4. Cells were also periodically assessed for LSK immuno-phenotype. (B) Relative expression of NFI-family genes in NFIX+ cells compared to control cells, quantified by qRT-PCR (n = 3). Tbp was used as a housekeeping gene. (C) Relative growth of control and NFIX+ cells during ex vivo culture (n = 4). Dotted line indicates the divergence in relative growth between control and NFIX+ cells. (D) GFP percentage of control and NFIX+ cells during ex vivo culture, assessed by flow cytometry (n = 4). (E) Percentage of control and NFIX+ cells with an LSK immuno-phenotype at day seven of ex vivo culture, depicted as a (i) representative dot plot and (ii) bar plot (n = 6). All values represent mean ± standard deviation. NS denotes not significant.
By seven days of culture, the majority of cells in both control and NFIX+ cultures had lost the LSK cell surface phenotype (Fig. 1E), with immunophenotypic LSK cells being almost completely lost from culture by day 14 (Supporting Information Fig. S1B, S1C). However, Nfix overexpression appeared to accelerate the loss of this phenotype, evident by the appearance of a Sca-1−c-Kit− population in NFIX+ cells and reduced overall levels of cell surface c-Kit, relative to control (Fig. 1Ei, 1Eii, Supporting Information Fig. S1D). These data suggest that Nfix might promote LSK cell differentiation during ex vivo culture. At day seven of culture, control and NFIX+ cells displayed a similar blast-like morphology, with NFIX+ cells retaining this morphology through day 30 of culture (Supporting Information Fig. S2). However, LSK cells overexpressing Nfix displayed a loss of in vivo competitive hematopoietic repopulating potential, a myeloid bias in peripheral blood production and a loss of colony-forming unit (CFU) potential compared to control cells by seven days of culture, with a significant loss in CFU potential by 21 days in culture (p = 0.023) (Supporting Information Fig. S3A–D and S4). A majority of expanded control and NFIX+ cells were negative for all major lineage markers (excepting CD8) and expressed c-Kit and CD71, which is a marker of proliferating progenitors (Supporting Information Fig. S5A-B). High CD71 expression can also be indicative of erythroid progenitors, and while NFIX+ cells show a significantly higher percentage of a CD71hi population compared to controls (p = 0.017), this population represents only a small portion (15-25%) of cells throughout the entirety of the culture (Supporting Information Fig. S5C). Together, these data suggest that Nfix promotes differentiation of LSK cells towards a heterogeneous immature progenitor population that ultimately lacks CFU potential, suggesting arrested differentiation potential.
As Nfix-deficient HSPCs display elevated apoptosis post-transplant [10], we next tested if ectopic Nfix protects primitive hematopoietic cells from apoptosis during ex vivo culture. Towards this, NFIX+ HSPCs were cultured under normal or reduced cytokine conditions and monitored for growth rate, NFIX+ cell selection, cell cycle, and apoptosis (Fig. 2A). Control cells cultured in reduced cytokines displayed a significantly lower growth rate by day 13 (p = 0.048) and an attenuated culture lifespan relative to cells maintained at normal cytokine levels (Fig. 2B). Remarkably, reduced cytokine levels had no effect on the extended culture of NFIX+ cells (Fig. 2B). NFIX+ cells cultured under reduced cytokines were selected for at a significantly accelerated rate compared to NFIX+ cells cultured under normal cytokine levels (p = 0.041) (Fig. 2C). There were no significant differences in cell cycle status between control and NFIX+ cells regardless of cytokine levels (Fig. 2D), suggesting that the reduced growth rate of cytokine-deprived control cells was not due to a reduction in cycling. However, control cells displayed a significant increase in apoptosis (p = 0.032) when cultured in reduced cytokines (Fig. 2E). In contrast, the apoptotic status of NFIX+ cells was unaffected by reduced cytokines (Fig. 2E), even in immunophenotypic HSPCs (Supporting Information Fig. S6). These data reveal that Nfix promotes primitive hematopoietic cell survival ex vivo.
Figure 2. HSPCs overexpressing Nfix can withstand cytokine deprivation and display reduced apoptosis during ex vivo culture.
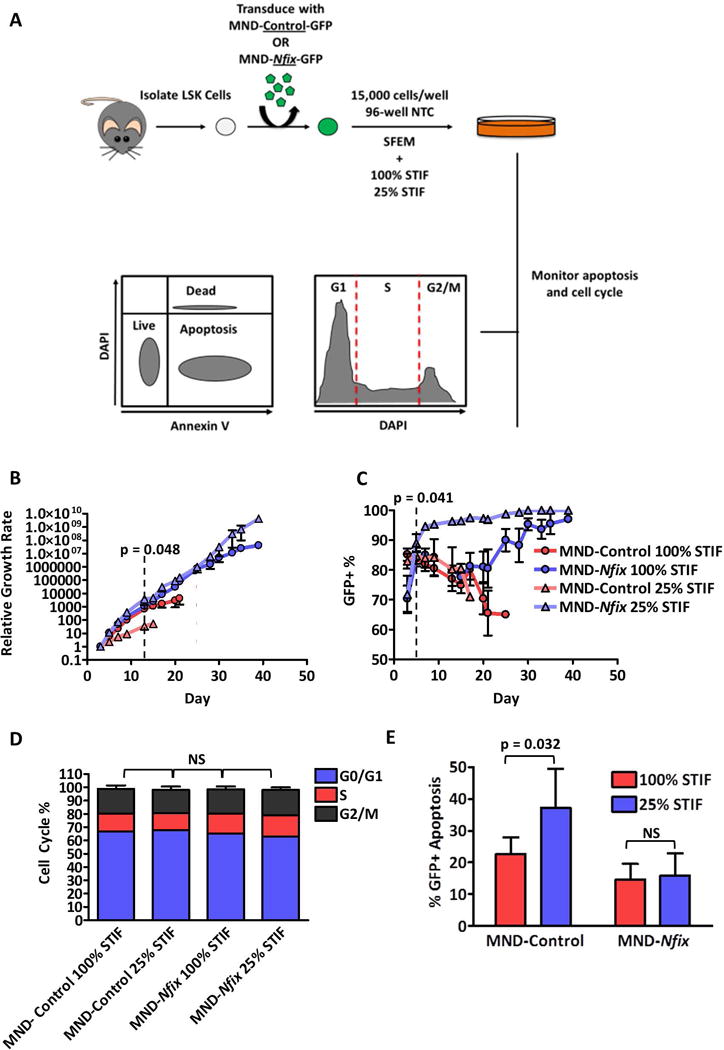
(A) Experimental schematic. 48 hours post-transduction, LSK cells transduced with MND-Control or MND-Nfix were re-plated in 96-well non-tissue culture treated plates in serum-free expansion medium (SFEM) supplemented with either normal (100%) or reduced (25%) levels of STIF cytokines. Every 48-72 hours of culture, cells were counted, assessed for GFP+ cells, and passaged 1:4. Cells were also assessed by flow cytometry for apoptosis via Annexin V and cell cycle via DAPI at day seven of culture. (B) Relative growth of control and NFIX+ cells during ex vivo culture (n = 4). Dotted line indicates the divergence in relative growth between control 100% and control 25% cells. (C) GFP percentage of control and NFIX+ cells during ex vivo culture, assessed by flow cytometry (n = 4). Dotted line indicates significant selection of NFIX+ cells under 25% cytokines. (D) Cell cycle analysis of GFP+ control or NFIX+ cells at day seven of ex vivo culture (n = 3). (E) Percentage of GFP+ apoptotic cells within control or NFIX+ cell cultures at day seven of ex vivo culture (n = 6). All values represent mean ± standard deviation. NS denotes not significant.
We previously observed by global gene expression analyses [10] that Nfix knockdown in HSPC reduced expression of multiple genes implicated in HSPC survival and maintenance including c-Mpl, a known regulator of HSC maintenance in the bone marrow niche that has been shown to affect apoptosis via multiple downstream signaling cascades [13-16]. c-MPL is the receptor for TPO, which is added as a supplement to our ex vivo serum-free cultures of HSPCs. To further explore possible regulation of c-Mpl levels by NFIX, we assessed the expression of c-Mpl in NFIX+ cells after seven days of ex vivo culture by qRT-PCR and flow cytometry (Fig. 3A, 3B). We found that c-Mpl transcripts increased two-fold in NFIX+ cells (p = 0.028) (Fig. 3A). We also observed a two-fold increase in c-MPL cell surface antigen on NFIX+ cells relative to control via flow cytometry (p = 0.042) (Fig. 3Bi, 3Bii). Also, from the number of additional HSPC genes previously observed to be perturbed by loss of Nfix [10], Erg was significantly upregulated (p = 0.022) (Supporting Information Fig. S7). TPO/c-MPL signaling is classically involved in megakaryopoiesis and platelet production [17-19]. Thus, as expected, NFIX+ cells also displayed a substantial increase in the cell-surface antigen CD41 (Fig. 3C), a known marker of megakaryocytes [20]. Since our data suggested that Nfix was driving HSPC towards an immature progenitor population (Supporting Information Fig. S2-S5), we further interrogated our cultures for CFU-Megs. NFIX+ cells appeared to generate more CFU-Megs than control cells after seven days of culture (p = 0.052), but the absolute frequency of CFU-Megs in NFIX+ cultures was minute (0.016), revealing that megakaryocytic progenitors with colony forming potential are rare in NFIX+ cultures (Supporting Information Fig. S8A-B). This is consistent with the observed low percentage of immunophenotypic megakaryocyte progenitors (c-Kit+Sca-1−CD127−CD9+CD32/CD16loCD41+) (Supporting Information Fig. S8C) [21]. Indeed, by day 30 almost no CFU-Megs were present in NFIX+ cultures (Supporting Information Fig. S8A).
Figure 3. NFIX up-regulates c-Mpl expression and downstream signaling in HSPC during ex vivo culture.
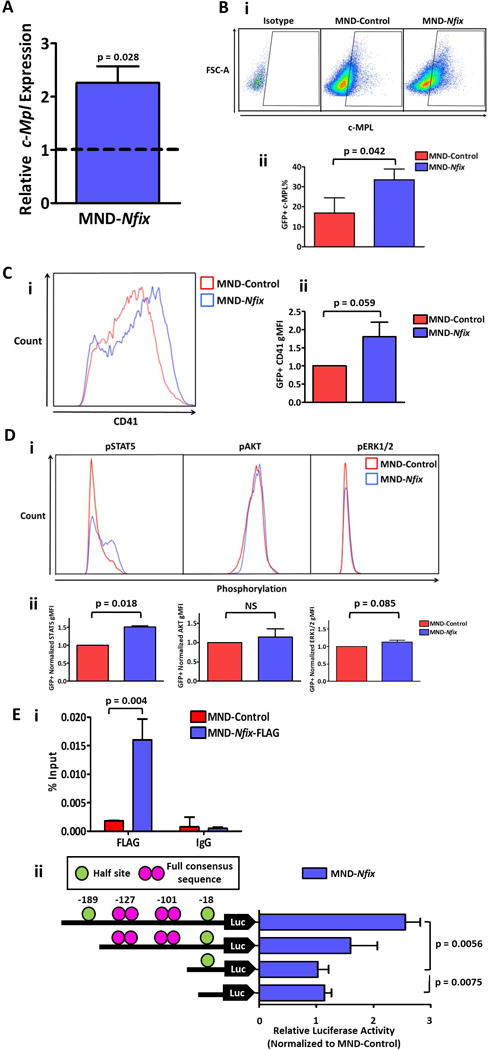
(A) Expression of (i) c-Mpl in LSK cells transduced with MND-Nfix relative to controls at day seven of culture, quantified by qRT-PCR (n = 3). Tbp was used as a housekeeping gene. (B) Percentage of c-MPL+ cells at four days of ex vivo culture for control and NFIX+ HSPCs, depicted as (i) representative dot plots and (ii) bar plots (n = 3). (C) Relative level of CD41 cell surface expression in NFIX+ cells compared to controls after four days of ex vivo culture, measured by flow cytometry as gMFI, depicted as (i) representative fluorescence histogram and (ii) bar plot (n = 3). (D) Relative levels of STAT5, AKT, and ERK1/2 phosphorylation in NFIX+ cells compared to controls after four days of ex vivo culture, measured by flow cytometry as geometric mean fluorescence intensity (gMFI). Depicted as (i) representative fluorescence histograms and (ii) bar plots (n = 3). (E) (i) Quantitative ChIP analysis of c-Mpl proximal promoter in HPC5 cells. Data are presented as a percentage of total input chromatin (n = 3). (ii) Left, Schematic representation of the c-Mpl promoter with half and full NFI consensus sites cloned into luciferase reporter backbone pGL4.14. Right, Results showing luciferase activity normalized to Renilla luminescence and relative to MND-control samples (n = 3-5). Values represent mean ± standard error. NS denotes not significant.
TPO/c-MPL can activate JAK/STAT, PI3K/AKT, and MAPK/ERK downstream signaling pathways [22]. To determine if NFIX-mediated up-regulation of c-Mpl also increased TPO/c-MPL signaling, we examined the phosphorylation status of STAT5, AKT, and ERK1/2 via flow cytometry. NFIX+ cells displayed significant enhancement of STAT5 phosphorylation compared to control cells (p = 0.018), while AKT trended towards enhanced phosphorylation after prolonged TPO treatment (Fig. 3D, Supporting Information Fig. 9A). Also, NFIX+ cells showed no difference in phosphorylation of ERK1/2 compared to control cells (Fig. 3D, Supporting Information Fig. 9A). This suggests that the anti-apoptotic effects displayed by NFIX+ HSPC may be mediated through the STAT5 signaling pathway. Indeed, expression of Bcl-XL, an anti-apoptotic factor induced by STAT5 [23], was also significantly upregulated in NFIX+ cells by two weeks of culture compared to controls (p = 0.0038) (Supporting Information Fig. S9B). In sum, these data reveal that up-regulation of Nfix induces both c-Mpl expression and signaling downstream of c-MPL in primitive hematopoietic cells.
Examination of the c-Mpl locus revealed palindromic NFI binding sites within the c-Mpl promoter (Figure 3Eii). Promoter analysis by TRANSFAC revealed full NFI consensus sites 101 and 127 nucleotides upstream of the c-Mpl transcription start site (TTS, +1) (Figure 3Eii). NFI members are known to bind both full and half NFI consensus sites [24]. Two half sites were identified 18 and 189 nucleotides upstream of the c-Mpl TSS (Figure 3Eii). To assess NFIX transcriptional activity against these putative NFI binding sites in the c-Mpl proximal promoter, a c-Mpl 243 bp genomic fragment 5′ of the c-Mpl promoter containing the four identified putative NFI binding sites was sub-cloned into the pGL4.14 promoterless luciferase vector. Transient transfection of this vector into K562 cells yielded nearly three-fold higher levels of promoter activity when co-transfected with MND-NFIX relative to co-transfection with MND-Control (Figure 3Eii). This enriched activity was diminished when the half NFI site (−189) furthest from the TSS was removed and was significantly reduced by the additional removal of the two full NFI sites (−127 and −101) (p = 0.0056) (Figure 3Eii). Further, chromatin immunoprecipitation (ChIP) was used to show direct NFIX binding to the c-Mpl proximal promoter in the HPC5 bone marrow derived cell line. Primers were designed and validated to amplify the promoter region containing two full NFI consensus sites. In Figure 3Ei, a near 9-fold enrichment is observed in samples where a FLAG-tagged NFIX is present compared to controls. These data suggest that NFIX may directly activate c-Mpl promoter activity in a hematopoietic cell line.
To determine if the anti-apoptotic effects of ectopic Nfix in primitive hematopoietic cells depends on enhanced TPO/c-MPL signaling, we cultured NFIX+ HSPC in reduced cytokines while also either removing TPO or blocking ligand binding to c-MPL via a neutralizing antibody (AMM2) [13] for 72 hours. Although removal of TPO and neutralization of c-MPL led to reduced cell expansion in both control and NFIX+ cultures, NFIX+ cultures were significantly more sensitive to the loss of c-MPL stimulation after TPO removal or the addition of AMM2 (p = 0.0021 and 0.033, respectively) (Fig. 4A). The selection for NFIX+ cells under reduced cytokines was also lost when TPO/c-MPL signaling was blocked by TPO removal or the addition of AMM2 (p = 0.0054 and 0.0019, respectively) (Fig. 4B), suggesting an enhanced reliance on TPO/c-MPL signaling for expansion of NFIX+ cells. NFIX+ cells display an accelerated loss of the LSK immuno-phenotype (Fig. 1E), possibly due to enhanced differentiation towards a downstream progenitor (Supporting Information Fig. S3-S5). This loss of immuno-phenotype was mostly due to down-regulation of c-Kit cell surface expression (Fig. 1Ei). When NFIX+ cells were cultured in the absence of TPO or the presence of AMM2, c-Kit was no longer rapidly down-regulated relative to control (Fig. 4C). Finally, while apoptosis was relatively unaffected by a loss of c-MPL signaling in control cells, NFIX+ cells displayed a significant increase in apoptosis after TPO removal (p = 0.045) or addition of AMM2 (p = 0.0098) (Fig. 4D). These data reveal that NFIX-mediated up-regulation of c-MPL, and subsequent downstream signaling, functionally contributes to Nfix-induced protection from apoptosis and accelerated differentiation in primitive hematopoietic cells ex vivo.
Figure 4. The anti-apoptotic effect of Nfix in HSPC depends on c-MPL signaling.
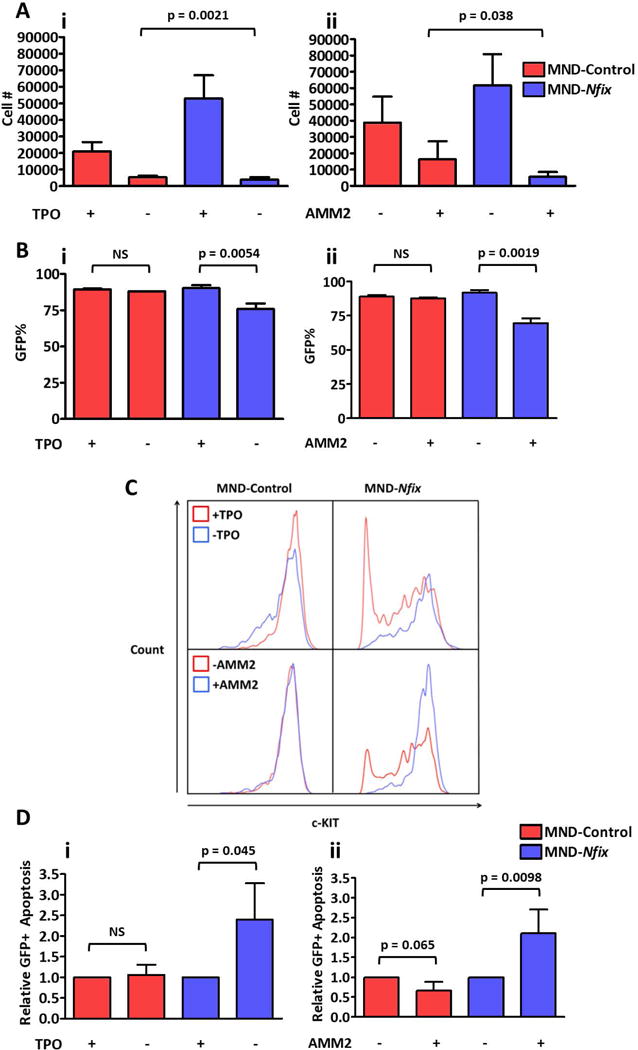
(A) Relative number of cells in control and NFIX+ cultures (i) with or without TPO and (ii) with or without AMM2 72 hours after replating (TPO, n = 3; AMM2, n = 6). Bar indicates significant difference in the extent of cell loss after 72 hours of culture between control and NFIX+ cells. (B) Percentage of GFP+ cells in control and NFIX+ cultures (i) with or without TPO and (ii) with or without AMM2 72 hours after replating (TPO, n = 3; AMM2, n = 6). (C) Representative fluorescence histograms of GFP+ control and NFIX+ cultures to illustrate shift in c-KIT intensity 72 hours after removal of mTPO or addition of AMM2 (TPO, n = 3; AMM2, n = 6). (D) Relative levels of apoptosis in GFP+ compartment of control and NFIX+ cultures (i) with or without TPO and (ii) with or without AMM2 72 hours after replating (TPO, n = 3; AMM2, n = 6). All values represent mean ± standard deviation. NS denotes not significant.
Conclusion
In this study we have utilized ex vivo culture of HSPCs to further interrogate the molecular regulation of HSPCs by Nfix, which is required for their in vivo repopulation potential [10]. Primitive hematopoietic cells overexpressing Nfix persist in culture significantly longer than control cells, even when severely deprived of cytokines. We show that this persistence is due to enhanced survival that is mediated, in part, by up-regulation of the TPO receptor, c-Mpl, and correlates with our previous finding that loss of Nfix is detrimental to HSPC survival post-transplant [10]. Nfix appears to promote differentiation of cultured LSK cells towards a heterogeneous mixture of immature progenitors that lack transplantation and CFU potential (Supporting Information Fig. S2-S5, S8), likely indicative of a differentiation block. It is also possible that Nfix expression selects for a cell in these cultures that depends on c-MPL signaling for survival. However, the enhanced survival of NFIX+ cells can also be observed in immunophenotypic HSPCs (Supporting Information Fig. S6), demonstrating that this phenomenon is not confined to a particular population.
We further demonstrate that NFIX may function as a transcriptional regulator of c-Mpl. Indeed, NFIX was capable of activating a promoter containing multiple NFI consensus binding sites located upstream of the c-Mpl promoter. We also show NFIX-FLAG directly associated with the proximal promoter. NFIX may also regulate downstream effectors of the TPO/c-MPL signaling pathway, as Stat5a is significantly upregulated in NFIX+ cells compared to controls (p = 0.0012, Supporting Information Fig. 9C). However, this effect may be indirect as there are no NFI consensus binding sites proximal to the Stat5a promoter (data not shown). NFI proteins favor binding in the proximal promoter region.
c-Mpl is a well-known regulator of HSPC function, as it is required for the maintenance of adult quiescent HSCs and protection from DNA-damage induced apoptosis in vivo [13-15]. Given that Nfix is required for HSPC survival during transplant hematopoiesis [10], our data further implicate Nfix as a novel regulator of this important HSPC regulatory axis. Further work will be required to determine if Nfix-mediated regulation of HSPC responsiveness to TPO contributes to loss of HSPC survival and niche retention following transplant in vivo.
Supplementary Material
Supplemental Figure 1. NFIX overexpression promotes accelerated differentiation of LSK cells during ex vivo culture. (A) FACS plots depicting the sorting schematic for freshly isolated LSK cells. (B) LSK immunophenotype of control and NFIX+ cells at day 0, 7, and 14 of culture depicted a representative dot plot from three independent experiments. (C) LSK immunophenotype of control and NFIX+ cells at day 14 of culture depicted as bar plot (n = 3). (D) One-way FACS histogram depicting a reduction in c-KIT+ cells among the lineage negative population of control and NFIX+ cells after seven days of ex vivo culture. All values represent mean ± standard deviation. NS denotes not significant.
Supplemental Figure 2. HSPCs overexpressing Nfix display an immature blast-like morphology similar to control cells. Romanowsky stain of fresh bone marrow (BM) LSK cells, culture day seven (D7) GFP+ MND-Control, D7 GFP+ NFIX+ cells, and day thirty (D30) GFP+ NFIX+ cells. Representative images from two independent experiments are shown. Scale bars represent 50μm.
Supplemental Figure 3. HSPCs overexpressing Nfix fail to repopulate the bone marrow of irradiated recipients and display a myeloid bias in lineage distribution. (A) Schematic displaying competitive transplantation assay to assess hematopoietic repopulation potential of HSPC. CD45.2 “test” LSK cells were harvested from bone marrow and transduced with either MND-control or MND-Nfix lentiviral vectors. CD45.1 “competitor” LSK cells were mock transduced. 24 hours post-transduction, 5000 test and 5000 competitor cells were harvested and transplanted into irradiated recipients. (B) Percentage of CD45.2 “test” cells in the peripheral blood of transplanted recipients over a 16 week period. (C) Percentage of GFP+ cells within CD45.2 “test” cells in the peripheral blood of transplanted recipients over a 16 week period. (D) Percentage of T-, B-, and myeloid cells within CD45.2 “test” cells in the peripheral blood of transplanted recipients over a 16 week period.
Supplemental Figure 4. HSPC overexpressing Nfix display reduced CFU potential. (A) Frequency of colony-forming units among GFP+ control and NFIX+ cells cultured for seven days ex vivo (n = 3). (B) Frequency of colony-forming units among GFP+ control and NFIX+ cells cultured for 21 days ex vivo (n = 3). The frequency of colony forming units refers to the number of colonies scored divided by the total number of cells plated in methylcelluose. All values represent mean ± standard deviation. NS denotes not significant.
Supplemental Figure 5. Nfix-overexpressing cells display no major lineage markers and an immature progenitor immuno-phenotype. (A) Percentage of lineage+ cells among GFP+ control and NFIX+ cells as one (n = 3), three (n = 2), and four (n = 3) weeks in ex vivo culture. (B) Percentage of c-Kit+ CD71+ cells among GFP+ control and NFIX+ cells at various time-points during ex vivo culture. (C) Representative FACS plot depicting the percentage of CD71hi cells in GFP+ control and NFIX+ cultures at day seven and day 30 of ex vivo culture. All values represent mean ± standard deviation. Note: all comparisons in (A) are not significant.
Supplemental Figure 6. LSK cells overexpressing Nfix display reduced apoptosis under cytokine deprivation during ex vivo culture. Percentage of GFP+ apoptotic cells within control or NFIX+ LSK cells at day seven of ex vivo culture (n = 3). All values represent mean ± standard deviation. NS denotes not significant.
Supplemental Figure 7. Nfix overexpression affects the expression of other known regulators of HSPC biology. Relative expression of several regulators of HSPC biology in NFIX+ cells compared to control cells known to be down-regulated upon shRNA-induced Nfix knockdown [10] at day seven of ex vivo culture (n = 3). All values represent mean ± standard deviation. NS denotes not significant.
Supplemental Figure 8. HSPCs overexpressing Nfix are not enriched for megakaryocyte progenitors or CFU-Megs. (A) Frequency of GFP+ CFU-Megs from control day seven, NFIX+ day seven, and NFIX+ day 30 ex vivo cells (n = 2). (B) Representative images of CFU-Megs from control day seven, NFIX+ day seven, and NFIX+ day 30 ex vivo cells (n = 2). (C) Percentage of megakaryocyte progenitors (c-Kit+Sca-1−CD127−CD9+CD32/CD16loCD41+) among GFP+ control and GFP+ NFIX+ cells (n = 4). All values represent mean ± standard deviation. Scale bars represent 50μm. MND-Control D7 = 100× magnification; MND-Nfix D7 = 100× magnification; MND-Nfix D30 = 200× magnification.
Supplemental Figure 9. NFIX+ cells display enhanced TPO/c-MPL signaling sensitivity to mTPO exposure. (A) Relative phosphorylation status of STAT5, AKT, and ERK1/2 in NFIX+ cells during a time-course of mTPO exposure following cytokine starvation, as measured by phosphoflow (STAT5, ERK1/2: n = 4; AKT: n = 3). gMFI: Geometric mean fluorescence intensity. (B) Relative expression of Bcl-xL in NFIX+ cells compared to control cells at different time points during ex vivo culture, quantified by qRT-PCR (n = 3). Tbp was used as a housekeeping gene. (C) Relative expression of Stat5a and Stat5b in NFIX+ cells compared to control cells at day seven of ex vivo culture, quantified by qRT-PCR (n = 6). Tbp was used as a housekeeping gene. All values represent mean ± standard deviation. NS denotes not significant.
Acknowledgments
We thank Sandy Schwemberger, Stacie Woolard, Deanna Langfitt, Liusheng He, and Richard Ashmun of St. Jude Children’s Research Hospital Flow Cytometry Core for FACS support. We also thank the St. Jude Children’s Research Hospital Veterinary Pathology Core and Microscopy Core for cellular morphology support. Finally, we thank the McKinney-Freeman laboratory for manuscript commentary.
Funded by: National Institute of Diabetes and Digestive and Kidney Disease at the National Institutes of Health. Grant number R01DK104028; the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children’s Research Hospital (Memphis, TN); The Hartwell Foundation
Footnotes
Trent Hall: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing
Megan Walker: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing
Miguel Ganuza: conception and design, collection and/or assembly of data, data analysis and interpretation
Per Holmfeldt: conception and design, collection and/or assembly of data, data analysis and interpretation
Marie Bordas: conception and design, collection and/or assembly of data, data analysis and interpretation
Guolian Kang: data analysis and interpretation
Wenjian Bi: data analysis and interpretation
Lance E. Palmer: data analysis and interpretation
David Finkelstein: data analysis and interpretation
Shannon McKinney-Freeman: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript
References
- 1.Harrison DE, Stone M, Astle CM. Effects of transplantation on the primitive immunohematopoietic stem cell. J Exp Med. 1990;172(2):431–437. doi: 10.1084/jem.172.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selleri C, Maciejewski J, De Rosa G, et al. Long-lasting decrease of marrow and circulating long-term culture initiating cells after allogeneic bone marrow transplant. Bone Marrow Transplant. 1999;23(10):1029–1037. doi: 10.1038/sj.bmt.1701759. [DOI] [PubMed] [Google Scholar]
- 3.Mauch P, Rosenblatt M, Hellman S. Permanent loss in stem cell self renewal capacity following stress to the marrow. Blood. 1988;72(4):1193–1196. [PubMed] [Google Scholar]
- 4.Goncalves Kevin A, Silberstein L, Li S, et al. Angiogenin promotes hematopoietic regeneration by dichotomously regulating quiescence of stem and progenitor cells. Cell. 2016;166(4):894–906. doi: 10.1016/j.cell.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H, Yuan Y, Shen H, et al. Hematopoietic stem cell exhaustion impacted by p18INK4C and p21Cip1/Waf1 in opposite manners. Blood. 2006;107(3):1200–1206. doi: 10.1182/blood-2005-02-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marquez-Curtis LA, Turner AR, Sridharan S, et al. The ins and outs of hematopoietic stem cells: studies to improve transplantation outcomes. Stem Cell Rev. 2011;7(3):590–607. doi: 10.1007/s12015-010-9212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmfeldt P, Ganuza M, Marathe H, et al. Functional screen identifies regulators of murine hematopoietic stem cell repopulation. J Exp Med. 2016;213(3):433–449. doi: 10.1084/jem.20150806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pjanic M, Pjanic P, Schmid C, et al. Nuclear factor I revealed as family of promoter binding transcription activators. BMC Genomics. 2011;12(1):1–10. doi: 10.1186/1471-2164-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gronostajski RM. Roles of the NFI/CTF gene family in transcription and development. Gene. 2000;249(1–2):31–45. doi: 10.1016/s0378-1119(00)00140-2. [DOI] [PubMed] [Google Scholar]
- 10.Holmfeldt P, Pardieck J, Saulsberry AC, et al. Nfix is a novel regulator of murine hematopoietic stem and progenitor cell survival. Blood. 2013;122(17):2987–2996. doi: 10.1182/blood-2013-04-493973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starnes LM, Sorrentino A, Pelosi E, et al. NFI-A directs the fate of hematopoietic progenitors to the erythroid or granulocytic lineage and controls β-globin and G-CSF receptor expression. Blood. 2009;114(9):1753–1763. doi: 10.1182/blood-2008-12-196196. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor C, Campos J, Osinski JM, et al. Nfix expression critically modulates early B lymphopoiesis and myelopoiesis. PLoS One. 2015;10(3):e0120102. doi: 10.1371/journal.pone.0120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshihara H, Arai F, Hosokawa K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1(6):685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Qian H, Buza-Vidas N, Hyland CD, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1(6):671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Pestina TI, Cleveland JL, Yang C, et al. Mpl ligand prevents lethal myelosuppression by inhibiting p53-dependent apoptosis. Blood. 2001;98(7):2084–2090. doi: 10.1182/blood.v98.7.2084. [DOI] [PubMed] [Google Scholar]
- 16.Chou F-S, Griesinger A, Wunderlich M, et al. The thrombopoietin/MPL/Bcl-xL pathway is essential for survival and self-renewal in human preleukemia induced by AML1-ETO. Blood. 2012;120(4):709–719. doi: 10.1182/blood-2012-01-403212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartley TD, Bogenberger J, Hunt P, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994;77(7):1117–1124. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 18.de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 19.Kaushansky K, Broudy VC, Lin N, et al. Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development. Proc Natl Acad Sci U S A. 1995;92(8):3234–3238. doi: 10.1073/pnas.92.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips D, Charo I, Parise L, et al. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988;71(4):831–843. [PubMed] [Google Scholar]
- 21.Nakorn TN, Miyamoto T, Weissman IL. Characterization of mouse clonogenic megakaryocyte progenitors. Proc Natl Acad Sci U S A. 2003;100(1):205–210. doi: 10.1073/pnas.262655099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Graaf CA, Metcalf D. Thrombopoietin and hematopoietic stem cells. Cell Cycle. 2011;10(10):1582–1589. doi: 10.4161/cc.10.10.15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Socolovsky M, Fallon AEJ, Wang S, et al. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-XL induction. Cell. 1999;98(2):181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 24.Bachurski CJ, Kelly SE, Glasser SW, Currier TA. Nuclear Factor I Family Members Regulate the Transcription of Surfactant Protein-C. Journal of Biological Chemistry. 1997;272(52):32759–32766. doi: 10.1074/jbc.272.52.32759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. NFIX overexpression promotes accelerated differentiation of LSK cells during ex vivo culture. (A) FACS plots depicting the sorting schematic for freshly isolated LSK cells. (B) LSK immunophenotype of control and NFIX+ cells at day 0, 7, and 14 of culture depicted a representative dot plot from three independent experiments. (C) LSK immunophenotype of control and NFIX+ cells at day 14 of culture depicted as bar plot (n = 3). (D) One-way FACS histogram depicting a reduction in c-KIT+ cells among the lineage negative population of control and NFIX+ cells after seven days of ex vivo culture. All values represent mean ± standard deviation. NS denotes not significant.
Supplemental Figure 2. HSPCs overexpressing Nfix display an immature blast-like morphology similar to control cells. Romanowsky stain of fresh bone marrow (BM) LSK cells, culture day seven (D7) GFP+ MND-Control, D7 GFP+ NFIX+ cells, and day thirty (D30) GFP+ NFIX+ cells. Representative images from two independent experiments are shown. Scale bars represent 50μm.
Supplemental Figure 3. HSPCs overexpressing Nfix fail to repopulate the bone marrow of irradiated recipients and display a myeloid bias in lineage distribution. (A) Schematic displaying competitive transplantation assay to assess hematopoietic repopulation potential of HSPC. CD45.2 “test” LSK cells were harvested from bone marrow and transduced with either MND-control or MND-Nfix lentiviral vectors. CD45.1 “competitor” LSK cells were mock transduced. 24 hours post-transduction, 5000 test and 5000 competitor cells were harvested and transplanted into irradiated recipients. (B) Percentage of CD45.2 “test” cells in the peripheral blood of transplanted recipients over a 16 week period. (C) Percentage of GFP+ cells within CD45.2 “test” cells in the peripheral blood of transplanted recipients over a 16 week period. (D) Percentage of T-, B-, and myeloid cells within CD45.2 “test” cells in the peripheral blood of transplanted recipients over a 16 week period.
Supplemental Figure 4. HSPC overexpressing Nfix display reduced CFU potential. (A) Frequency of colony-forming units among GFP+ control and NFIX+ cells cultured for seven days ex vivo (n = 3). (B) Frequency of colony-forming units among GFP+ control and NFIX+ cells cultured for 21 days ex vivo (n = 3). The frequency of colony forming units refers to the number of colonies scored divided by the total number of cells plated in methylcelluose. All values represent mean ± standard deviation. NS denotes not significant.
Supplemental Figure 5. Nfix-overexpressing cells display no major lineage markers and an immature progenitor immuno-phenotype. (A) Percentage of lineage+ cells among GFP+ control and NFIX+ cells as one (n = 3), three (n = 2), and four (n = 3) weeks in ex vivo culture. (B) Percentage of c-Kit+ CD71+ cells among GFP+ control and NFIX+ cells at various time-points during ex vivo culture. (C) Representative FACS plot depicting the percentage of CD71hi cells in GFP+ control and NFIX+ cultures at day seven and day 30 of ex vivo culture. All values represent mean ± standard deviation. Note: all comparisons in (A) are not significant.
Supplemental Figure 6. LSK cells overexpressing Nfix display reduced apoptosis under cytokine deprivation during ex vivo culture. Percentage of GFP+ apoptotic cells within control or NFIX+ LSK cells at day seven of ex vivo culture (n = 3). All values represent mean ± standard deviation. NS denotes not significant.
Supplemental Figure 7. Nfix overexpression affects the expression of other known regulators of HSPC biology. Relative expression of several regulators of HSPC biology in NFIX+ cells compared to control cells known to be down-regulated upon shRNA-induced Nfix knockdown [10] at day seven of ex vivo culture (n = 3). All values represent mean ± standard deviation. NS denotes not significant.
Supplemental Figure 8. HSPCs overexpressing Nfix are not enriched for megakaryocyte progenitors or CFU-Megs. (A) Frequency of GFP+ CFU-Megs from control day seven, NFIX+ day seven, and NFIX+ day 30 ex vivo cells (n = 2). (B) Representative images of CFU-Megs from control day seven, NFIX+ day seven, and NFIX+ day 30 ex vivo cells (n = 2). (C) Percentage of megakaryocyte progenitors (c-Kit+Sca-1−CD127−CD9+CD32/CD16loCD41+) among GFP+ control and GFP+ NFIX+ cells (n = 4). All values represent mean ± standard deviation. Scale bars represent 50μm. MND-Control D7 = 100× magnification; MND-Nfix D7 = 100× magnification; MND-Nfix D30 = 200× magnification.
Supplemental Figure 9. NFIX+ cells display enhanced TPO/c-MPL signaling sensitivity to mTPO exposure. (A) Relative phosphorylation status of STAT5, AKT, and ERK1/2 in NFIX+ cells during a time-course of mTPO exposure following cytokine starvation, as measured by phosphoflow (STAT5, ERK1/2: n = 4; AKT: n = 3). gMFI: Geometric mean fluorescence intensity. (B) Relative expression of Bcl-xL in NFIX+ cells compared to control cells at different time points during ex vivo culture, quantified by qRT-PCR (n = 3). Tbp was used as a housekeeping gene. (C) Relative expression of Stat5a and Stat5b in NFIX+ cells compared to control cells at day seven of ex vivo culture, quantified by qRT-PCR (n = 6). Tbp was used as a housekeeping gene. All values represent mean ± standard deviation. NS denotes not significant.


