To the Editor
Bronchiolitis is the most common lower respiratory infection in infants, and the leading cause of infant hospitalizations in the U.S. (approximately 130,000 hospitalizations annually).1 Prior research has linked vitamin D status to bronchiolitis pathobiology. Indeed, 25-hydroxyvitamin D (25OHD) undergoes enzymatic conversions to its biologically-active form, 1,25-dihydroxyvitamin D (1,25OH2D), in immune cells (e.g., macrophages, dendritic cells and T cells), and plays a pleiotropic regulatory role on both innate and adaptive immune systems.2
Epidemiological investigations have reported the associations of lower blood 25OHD levels at birth or early infancy with an increased risk and severity of acute respiratory infections (ARIs), including bronchiolitis.2 Similarly, a 2017 meta-analysis of individual participant data from 25 trials demonstrated that vitamin D supplementation is protective against ARIs in subjects with low baseline 25OHD concentrations.3 While these earlier reports have evaluated circulating 25OHD levels in relation to disease processes,2,3 it remains unclear how vitamin D interacts with the numerous downstream functional molecules that can influence clinical outcomes. Metabolomics, a high-throughput technology, addresses this knowledge gap through comprehensively characterizing small-molecule metabolites that are a function of the infant’s genetic make-up and environmental factors,4 such as vitamin D status.
In the current study, by testing the serum metabolome from a nested subset of a multicenter bronchiolitis cohort study, we investigated the relationships between serum 25OHD levels, metabolome, and disease severity. More specifically, we tested the hypothesis that 25OHD-related serum metabolites are associated with the risk of positive pressure ventilation (PPV) use among infants with bronchiolitis.
This is an analysis of the data from a multicenter prospective cohort study of infants with severe bronchiolitis (i.e., bronchiolitis requiring hospitalization). Details of the study design, setting, subjects, data measurement, and analysis may be found in the Online Supplement (Supplemental Methods). In brief, the 35th Multicenter Airway Research Collaboration (MARC-35) cohort study5 enrolled 1,016 infants (age <1 year) hospitalized with bronchiolitis at 17 sites across 14 U.S. states (Table E1) during the 2011–2014 winter seasons. Bronchiolitis was defined using the American Academy of Pediatrics guidelines – acute respiratory illness with some combination of rhinitis, cough, tachypnea, wheezing, crackles, and retractions.6 We excluded patients who were transferred to a participating hospital >24 hours after the original hospital admission, those who were consented >24 hours after admission, or those with known heart-lung disease, immunodeficiency, immunosuppression, or gestational age <32 weeks. The institutional review board of each participating hospital approved the study. We obtained written informed consent from the infant’s parent or guardian.
In addition to the collection of phenotypic data via structured interview and chart review, blood specimens were collected within 24 hours of hospitalization using a standardized protocol.5 Serum total 25OHD levels were quantified by immunoassays; bioavailable and free 25OHD levels were computed using previously-validated formulas. Serum metabolites (metabolome) were profiled using ultra-high performance liquid chromatography-tandem mass spectrometry by Metabolon (Durham, NC, USA). The details of the tests and quality control measures may be found in Supplemental Methods. The clinical outcome of interest was the use of PPV – defined as use of continuous positive airway pressure and/or intubation during hospitalization.5
For the present analysis, we selected 140 infants who had sufficient volume of serum specimens and performed serum metabolomic profiling. First, to derive a subset of serum metabolites associated with the circulating 25OHD (three components – total, bioavailable, and free) levels, we constructed sparse partial least squares (sPLS) regression models with cross-validation and LASSO regularization that minimize potential overfitting. The sPLS method has advances that it handles correlated variables (e.g., metabolites) and that it can also simultaneously model multiple response variables (total, bioavailable, and free 25OHD levels in the current study). To determine the association between each of these selected serum metabolites and the risk of PPV use, we next constructed multivariable logistic regression and random-effects models adjusting for age, sex, history of premature birth, feeding status, weight at presentation, in-hospital corticosteroid use, detected virus, and potential patient clustering within the hospitals. P-values were adjusted for multiple testing with the Benjamini-Hochberg false discovery rate (FDR) method. All analyses were conducted in R version 3.4.
The analytic (n=140) and non-analytic (n=876) cohorts were not significantly different for most patient characteristics, including the serum 25OHD levels (P>0.05; Table E2), except the analytic cohort had a lower proportion of respiratory syncytial virus infection and higher proportion of rhinovirus infection (both P<0.05). Of 140 infants in the analytic cohort, the median age was 3 months (interquartile range [IQR] 1–6 months), 62% were male, and 39% were non-Hispanic white. The median level of serum total 25OHD was 24.8 ng/ml (IQR 15.9–32.8 ng/ml). Its level was significantly correlated with that of bioavailable 25OHD (r=0.73; P<0.001) and free 25OHD (r=0.66; P<0.001; Figure E1). Most patient characteristics did not differ significantly by total 25OHD status (Table 1), while infants with lower total 25OH levels were younger, less likely to have a previous breathing problem, and had a lower weight at presentation (all P<0.05). Additionally, these infants had a higher likelihood of PPV use and longer hospital length-of-stay (both P<0.05) than those with higher total 25OHD levels.
Table 1.
Patient Characteristics of Infants Hospitalized for Bronchiolitis According to Serum Total 25-hydroxyvitamin D Level
| Variables | Total 25OHD <24.8 ng/ml | Total 25OHD ≥24.8 ng/ml | P-value |
|---|---|---|---|
| n=70 | n=70 | ||
| Characteristics | |||
| Age (mo), median, (IQR) | 1.9 (0.9–3.5) | 5.0 (2.6–8.0) | <0.001 |
| Male sex | 45 (64.3) | 42 (60.0) | 0.73 |
| Race/ethnicity | 0.56 | ||
| Non-Hispanic white | 25 (35.7) | 29 (41.4) | |
| Non-Hispanic black | 13 (18.6) | 16 (22.9) | |
| Hispanic | 29 (41.4) | 24 (34.3) | |
| Other | 3 (4.3) | 1 (1.4) | |
| Parental history of asthma | 23 (32.9) | 21 (30.4) | 0.90 |
| Maternal smoking during pregnancy | 8 (11.4) | 9 (13.0) | 0.98 |
| C-section delivery | 23 (32.9) | 29 (42.0) | 0.35 |
| Prematurity (32–37 weeks) | 17 (24.3) | 17 (24.3) | 0.99 |
| Previous breathing problems before the index hospitalization* | 11 (15.7) | 23 (32.9) | 0.03 |
| History of eczema | 8 (11.4) | 12 (17.1) | 0.47 |
| Mostly breastfed for the first 3 months of age | 40 (59.7) | 26 (41.3) | 0.054 |
| Smoke exposure at home | 4 (5.7) | 11 (15.7) | 0.10 |
| Clinical presentation | |||
| Weight at presentation (kg), median (IQR) | 5.1 (4.0–6.5) | 7.2 (5.5–8.4) | <0.001 |
| Respiratory rate at presentation (per minute), median (IQR) | 46 (40–60) | 52 (44–62) | 0.03 |
| Oxygen saturation at presentation | 0.32 | ||
| <90% | 12 (16.2) | 7 (8.7) | |
| 90%–93% | 10 (14.7) | 16 (23.2) | |
| ≥94% | 47 (68.1) | 47 (68.1) | |
| Received corticosteroids during pre- hospitalization visit | 8 (11.4) | 8 (11.4) | 0.99 |
| Virology | |||
| Sole RSV infection | 35 (50.0) | 28 (40.0) | 0.31 |
| Sole rhinovirus infection | 12 (17.1) | 14 (20.0) | 0.83 |
| RSV + rhinovirus coinfection | 5 (7.1) | 7 (10.0) | 0.76 |
| Others | 18 (25.7) | 21 (30.0) | 0.57 |
| Laboratory data | |||
| Serum bioavailable 25OHD (ng/ml), median (IQR) | 2.6 (1.7–3.7) | 5.2 (4.0–8.1) | <0.001 |
| Serum free 25OHD (pg/ml), median (IQR) | 8.4 (5.4–12.2) | 15.5 (10.9–23.8) | <0.001 |
| Serum vitamin D-binding protein (μg/ml), median (IQR) | 134.6 (85.1–170.6) | 161.3 (103.5–223.1) | 0.03 |
| Serum LL-37 (ng/ml), median (IQR) | 36.0 (28.0–55.5) | 48.0 (28.5–65.5) | 0.23 |
| Food sensitization† | 10 (14.3) | 16 (22.9) | 0.28 |
| Aeroallergen sensitization† | 0 (0) | 2 (2.9) | 0.48 |
| Hospital course | |||
| Positive pressure ventilation‡ | 25 (35.7) | 13 (18.6) | 0.04 |
| Admission to intensive care unit | 39 (55.7) | 31 (44.3) | 0.24 |
| Hospital length-of-stay ≥3 days | 39 (55.7) | 33 (47.1) | 0.40 |
| Hospital length-of-stay (day), median (IQR) | 3 (2–8) | 2 (2–5) | 0.04 |
Data are no. (%) of infants unless otherwise indicated.
Abbreviations: IQR, interquartile range; RSV, respiratory syncytial virus; 25OHD, 25-hydroxyvitamin D
Defined as a child having cough that wakes him/her at night and/or causes emesis, or when the child has wheezing or shortness of breath without cough
Defined by having one or more positive values for allergen-specific IgE
Defined as continuous positive airway pressure and/or intubation during inpatient stay, regardless of location, during the index hospitalization
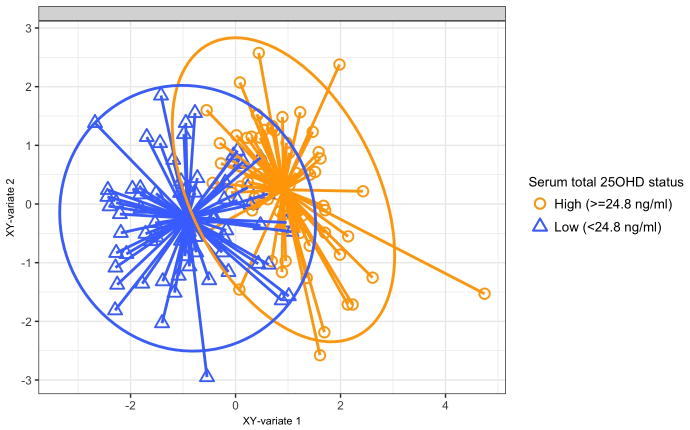
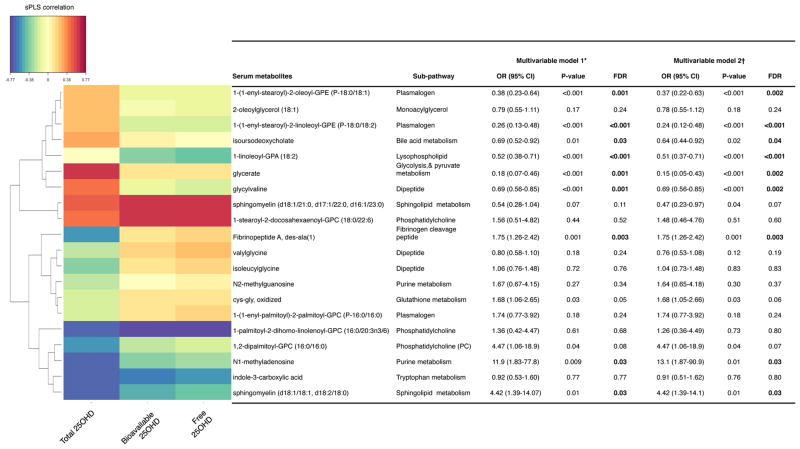
Metabolomics testing of serum specimens detected a total of 707 named metabolites from 96 sub-pathways in 8 super-pathways. Based on the sPLS ordination, the overall metabolome profiles clustered by total 25OHD status (Figures 1 and E2). By computing their loading scores with the sPLS model, we identified a set of top 20 serum metabolites that were most strongly associated with the serum 25OHD (total, bioavailable, and free) levels (Figures 2 and E3). These metabolites were products of predominantly lipid metabolism (11 metabolites), followed by amino acid/peptide, nucleotide, and carbohydrate metabolism. In the multivariable logistic regression models adjusting for potential confounders and the random-effects models accounting for site-effects, 9 of these 20 metabolites were significantly associated with the risk of PPV use (all FDR<0.05). Specifically, among the metabolites that were correlated with lower 25OHD levels, 3 metabolites – fibrinopeptide A, N1-methyladenosine, and sphingomyelin (d18:1/18:1, d18:2/18:0) – were associated with a significantly higher risk of PPV use (all FDR<0.05; Figure 2). By contrast, among the metabolites that were correlated with higher 25OHD levels, 6 metabolites – 1-(1-enyl-stearoyl)-2-oleoyl-glycerophosphorylethanolamine (GPE), 1-(1-enyl-stearoyl)-2-linoleoyl-GPE, isoursodeoxycholate, 1-linoleoyl-glycerophosphate (GPA), glycerate, and glycylvaline – were associated with a significantly lower risk of PPV use (all FDR<0.05). The discrimination ability of the 20 selected metabolites on the risk of PPV use was high, with an area-under-the receiver operating characteristic curve of 0.92 (Figure E4).
Figure 1. Sparse partial least squares analysis score plot of serum metabolome according to total 25-hydroxyvitamin D levels.
Each dot represents the global serum metabolomic profile of a single infant, by plotting the component scores in the smaller subspace spanned by latent variables of sparse partial least squares (sPLS) model. The scatter plot indicates the similarities and dissimilarities in the global metabolome profile between infants, grouped by serum total 25OHD status (defined by the median values). The eclipses are 95% confidence intervals. The arrows start from the centroid of each group and end for each patient belonging to each group.
Figure 2. Correlations between serum 25-hydroxyvitamin D (25OHD) and selected serum metabolites, and their associations with the risk of positive pressure ventilation use among infants hospitalized with bronchiolitis.
The heatmap (left) visualizes the correlations between the serum 25OHD (total, bioavailable, and free) levels and 20 selected metabolites that were examined using hierarchical clustering with an average linkage algorithm. The table (right) shows the adjusted associations between each of selected serum metabolites with the risk of PPV use. Bold results are statistically significant with FDR of <0.05. OR are calculated per each incremental increase in scaled level.
Abbreviations: 25OHD, 25-hydroxyvitamin D; CI, confidence interval; FDR, false discovery rate; GPA, glycerophosphate; GPC, glycerophosphocholine; GPE, glycerophosphorylethanolamine; OR, odds ratio; sPLS, partial least squares.
* Logistic regression models adjusting for patients’ age, sex, history of premature birth, feeding status, weight at presentation, corticosteroid use, and detected virus.
† Random-effects models adjusting for the covariates above and accounting for potential patient clustering within the hospitals.
Based on the data from a prospective multicenter cohort of infants hospitalized for bronchiolitis, serum 25OHD levels were associated with differences in the serum metabolome profile – a metabolomic signature. Specifically, from the 707 named metabolites detected, we identified a set of 20 metabolites (predominantly lipids) that were associated with circulating 25OHD (total, bioavailable, and free) levels. Among these, metabolites correlated with lower 25OHD (e.g., sphingolipids) were associated with a significantly higher risk of PPV use. In contrast, metabolites correlated with higher 25OHD (e.g., plasmalogen pathway metabolites [GPE and GPA]) were associated with a significantly lower risk of PPV use. This is the first investigation that has examined the interrelationships between circulating 25OHD, metabolome, and clinical outcomes among infants with bronchiolitis.
Consistent with our observations, earlier reports of several disease conditions, including wheezing in young children, have found that the circulating total 25OHD concentrations were associated with specific blood and urine metabolomic profiles, particularly phospholipids.7,8 For instance, a recent nested case-control study of 84 children with recurrent wheeze (or asthma) and 161 controls reported that, at age 3 years, the sphingolipid and glycerophospholipid (including GPE) metabolism pathways are associated with both serum 25OHD levels and clinical outcomes.7 The literature has also demonstrated that 1,25OH2D influences the metabolism of sphingolipids by regulating multiple components (e.g., the expression of sphingosine 1-phosphate [S1P] phosphatase, S1P receptors, sphingomyelinase).9 Sphingolipids are not only building blocks of the cell membrane bilayer but also serve as signaling molecules with important roles in immune response and the pathobiology of inflammatory diseases (e.g., asthma, autoimmune diseases).9 Emerging evidence has also shown that an elevated level of sphingolipids in the upper airway is associated with higher severity in infants with bronchiolitis.5
The current study also demonstrated that glycerophospholipids, particularly plasmalogen pathway metabolites (e.g., GPE and GPA), were associated with both higher circulating 25OHD levels and lower risk of PPV use. Consistent with this finding, a cohort study of 392 Finnish adults demonstrated the relationships between serum 25OHD levels and these lipids in the plasmalogen pathway.8 Plasmalogen is not only a major structural component of cell membrane and pulmonary surfactant, but also plays biological roles (e.g., generation of inflammatory mediators and protection against oxidation of surfactant lipids).10 The literature has also demonstrated that levels of plasmalogen are insufficient in premature infants with bronchopulmonary dysplasia and smokers with COPD.10 Our study corroborates these earlier reports, and extends them by demonstrating the interrelations between circulating 25OHD, metabolome, and disease severity in infants with bronchiolitis.
We acknowledge several potential limitations to the study. First, 25OHD and metabolites were measured early in the hospital course of critical illness at a single time-point and from a single bio-fluid (i.e., serum). Second, free and bioavailable 25OHD levels were not directly measured but computed using previously-validated formulas. Additionally, between-assay variability of total 25OHD measurement is possible because of the lack of standardization. Third, the current study did not have comparative metabolic information from a “healthy control” group of 25OHD-sufficient infants. Nonetheless, the study aim was not to evaluate the associations between 25OHD, metabolome, and the development of bronchiolitis, but to examine their contributions to acute severity among infants hospitalized for bronchiolitis. Fourth, we were unable to fully account for reverse causation and potential confounding, such as differences in intensive care resources. Yet, we statistically accounted for potential clustering of patients at the hospital-level. Fifth, while we performed cross-validations and LASSO regularization to limit potential over-fitting, validation in a separate population is required to confirm our inferences. The findings are, however, consistent with recent publications on related topics.7,8 Finally, while the study population consisted of a racially/ethnically- and geographically-diverse sample, all were hospitalized for bronchiolitis and, as such, the findings may not be generalizable to children with mild-to-moderate bronchiolitis. Still, our inference remain directly relevant to 130,000 hospitalized US children each year.1
In sum, based on the multicenter data of 140 infants hospitalized for bronchiolitis, we found that serum 25OHD levels were associated with a set of serum metabolites – a metabolomic signature. Among these, the metabolites correlated with lower 25OHD levels (e.g., sphingomyelin) were associated with a significantly higher risk of PPV use while those correlated with higher 25OHD levels (e.g., plasmalogen pathway metabolites) were associated with a lower risk. Although causality remains to be elucidated, prior research and our observations collectively suggest that circulating 25OHD, through affecting metabolites associated with inflammatory processes, may influence bronchiolitis severity. Our findings advance research into the complex interrelations between the environmental factors (e.g., vitamin D), host immunity, and the pathobiology of bronchiolitis in infants.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by grants UG3 OD-023253, U01 AI-087881, R21 HL-129909, and R01AI-134940 from the National Institutes of Health (Bethesda, MD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank the MARC-35 study hospitals and research personnel for their ongoing dedication to bronchiolitis and asthma research (Table E1 in the Online Supplement). We also thank Janice A. Espinola, MPH, and Ashley F. Sullivan, MS, MPH (Massachusetts General Hospital, Boston, MA), as well as Alkis Togias, MD (National Institute of Allergy and Infectious Diseases) for their contributions to the study.
Footnotes
Conflict of Interest: The authors have no financial relationships relevant to this article to disclose.
Author Contributions: KH, CJS, and CAC conceived the study. JCC, JMM, and CT contributed to data collection and management. CAC obtained research funding and supervised the conduct of the study. CJS contributed to the metabolomics processing and testing. KH analyzed the data, and drafted the manuscript. All authors contributed substantially to its revision. KH takes responsibility for the paper as a whole.
References
- 1.Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA., Jr Trends in bronchiolitis hospitalizations in the United States, 2000–2009. Pediatrics. 2013;132(1):28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camargo CA., Jr . Vitamin D, acute respiratory infections, and asthma/COPD. In: Feldman D, Pike W, Bouillon R, Giovannucci E, Goltzman D, Hewison M, editors. Vitamin D. 4. Cambridge, Massachusetts: Elsevier Academic Press; 2018. [Google Scholar]
- 3.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly RS, Dahlin A, McGeachie MJ, et al. Asthma Metabolomics and the Potential for Integrative Omics in Research and the Clinic. Chest. 2017;151(2):262–277. doi: 10.1016/j.chest.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart CJ, Mansbach JM, Wong MC, et al. Associations of nasopharyngeal metabolome and microbiome with severity among infants with bronchiolitis: A multi-omic analysis. Am J Respir Crit Care Med. 2017;196(7):882–891. doi: 10.1164/rccm.201701-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 7.Kelly RS, Litonjua AA, Weiss S, Lasky-Su J. Metabolomics as a novel method to explore the relationship between asthma and vitamin D. Am J Respir Crit Care Med. 2017;195:A4980. [Google Scholar]
- 8.Nelson SM, Panagiotou OA, Anic GM, et al. Metabolomics analysis of serum 25-hydroxy-vitamin D in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study. Int J Epidemiol. 2016;45(5):1458–1468. doi: 10.1093/ije/dyw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Gil M, Pierucci F, Vestri A, Meacci E. Crosstalk between sphingolipids and vitamin D3: potential role in the nervous system. Br J Pharmacol. 2017;174(8):605–627. doi: 10.1111/bph.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822(9):1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.