Abstract
Background
Eosinophils in the nasal mucosa are an elemental feature of allergic rhinitis.
Objective
Our objective was to explore eosinophilic inflammation and its impact on respiratory virus infection at the nasal mucosa.
Methods
Inflammation in the nasal mucosae of mice was evaluated in response to repetitive stimulation with strict intranasal volumes of a filtrate of Alternaria alternata. Mice were then challenged with influenza virus.
Results
Repetitive stimulation with A. alternata resulted in eosinophil recruitment to the nasal passages in association with elevated levels of IL-5, IL-13, and eotaxin-1; eosinophil recruitment was diminished in eotaxin-1−/− mice, and abolished in Rag1−/− mice. A. alternata also resulted in elevated levels of nasal-wash IgA in both wild-type and eosinophil-deficient ΔdblGATA mice. Interestingly, A. alternata-treated mice responded to an influenza virus infection with profound weight loss and mortality compared to mice that received diluent alone (0% vs. 100% survival, ***p < 0.001); the lethal response was blunted when A. alternata was heat-inactivated. Minimal differences in virus titer were detected, and eosinophils present in the nasal passages at the time of virus inoculation provided no protection against the lethal sequelae. Interestingly, nasal-wash fluids from mice treated with A. alternata included more neutrophils and higher levels of proinflammatory mediators in response to virus challenge, among these, IL-6, a biomarker for disease severity in human influenza.
Conclusions and Clinical Relevance
Repetitive administration of A. alternata resulted in inflammation of the nasal mucosae and unanticipated morbidity and mortality in response to subsequent challenge with influenza virus. Interestingly, and in contrast to findings in the lower airways, eosinophils recruited to the nasal passages provided no protection against lethal infection. As increased susceptibility to influenza virus among individuals with rhinitis has been the subject of several clinical reports, this model may be used for further exploration of these observations.
Keywords: eosinophil, respiratory virus, inflammation, cytokine
Introduction
Eosinophils are tissue leukocytes that have complex and multi-factorial roles in promoting health and disease [1, 2]. While relatively few eosinophils are found in the respiratory tract at homeostasis [3], eosinophils can be mobilized in large numbers in response to allergic stimuli, notably via the coordinate actions of Th2 cytokines and chemoattractants [4]. Until recently, eosinophils recruited to tissues were perceived as uniformly destructive and cytotoxic. However, current research has led to a new appreciation of eosinophils as having more sophisticated, immunomodulatory properties, and the capacity to promote tissue regeneration and repair [5].
Several lines of evidence have suggested a role for eosinophils in modulating respiratory virus infection [6, 7]. Among these, severe infection with respiratory syncytial virus (RSV), the most common infection among infants and children worldwide, is accompanied by eosinophil recruitment to the lower airways [8]. While epidemiologic studies have defined a relationship between severe RSV infection and recurrent wheezing during childhood, the numerous factors contributing to this observation remain to be fully elucidated [9]. Likewise, infection with rhinovirus (RV) has been identified as a prominent factor contributing to asthma exacerbations [10]. Beale and colleagues [11] have pointed to RV-mediated induction of IL-25, a cytokine with the capacity to augment eosinophilic inflammation, as a critical element promoting this response, although Hong and colleagues [12] suggest that RV-induced exacerbations are mediated primarily by neutrophils.
Particularly intriguing are the reports that consider eosinophils as mediators of antiviral host defense. For example, Adamko and colleagues [13] reported that ovalbumin-sensitized guinea pigs displayed IL5- and eosinophil-dependent antiviral activity against mouse parainfluenza virus. Phipps and colleagues [14] found that RSV virions were cleared more rapidly from the lungs of eosinophil-enriched IL5tg mice, and that this response was dependent on MyD88-signaling. Percopo and colleagues [15] reported that eosinophils in lung tissue were activated by and underwent degranulation in response to acute infection with pneumonia virus of mice (PVM), and likewise that eosinophils provided crucial protection against the lethal sequelae of this infection. Most recently, Samarasinghe and colleagues [16] found that eosinophils themselves were susceptible to infection with influenza virus (H1N1), and that adoptive transfer of eosinophils from the lungs of allergic mice into the lungs of influenza virus-infected mice resulted in a significant reduction in lung virus titer.
All the aforementioned studies focused on eosinophils and viruses interacting with one another in the lower airways. In this study, our intent was to examine eosinophil-mediated responses to virus infection in the upper airways. The nasal epithelium is a critical interface and an important portal of entry for influenza virus [17, 18].
In order to explore the interactions between eosinophils and virus infection in the upper airways, we subjected mice to a regimen of repetitive inhalation of strict-intranasal volumes (as defined experimentally by Southam and colleagues [19]) of a filtrate of the aeroallergen, Alternaria alternata. Fungal allergens have been featured previously in mouse models of allergic rhinitis [20, 21]; this strategy, which utilizes intranasal challenges only without systemic sensitization, is based directly on the allergic rhinitis model developed by McCusker and colleagues [22] who documented eosinophil recruitment to the nasal mucosae of BALB/c mice, also in response to strict intranasal inoculation with increasing concentrations of ovalbumin. We have featured A. alternata in our studies, as it is a prominent environmental allergen, prevalent in soil and as well as within homes with abundant moisture or insect infestation [23]. From a human clinical perspective, repetitive exposure to A. alternata is among the major risk factors for developing allergic manifestations, including rhinitis [24].
Here, we report that repetitive intranasal stimulation with A. alternata results in eosinophil recruitment to the upper airways and, surprisingly, unanticipated morbidity and mortality in response to a minimal inoculum of influenza virus, the latter associated with neutrophil recruitment and local over-production of proinflammatory cytokines, including IL-6 and CCL-2. Unexpectedly, and in contrast to earlier findings focused on eosinophils in the lower airways [13 – 16] eosinophils in the nasal passages had no measurable impact against lethal respiratory virus infection.
Methods
Mice
Wild-type C57BL/6 and BALB/c mice (6 – 10 weeks old) were from Charles River Laboratories, Frederick, MD. Rag1−/− mice (C57BL/6) are maintained by the NIAID/Taconic consortium. Eotaxin-1−/− (BALB/c) and ΔdblGATA (C57BL/6) mice are maintained at NIAID in the14BS vivarium. As gender plays a role in the inflammatory responses to influenza infection in C57BL/6 mice [25], only female, 6 – 8 week-old wild-type and ΔdblGATA (C57BL/6) mice were utilized for influenza virus infection studies. The National Institute of Allergy and Infectious Diseases Division of Intramural Research Animal Care and Use Committee, as part of the National Institutes of Health Intramural Research Program, approved all the experimental procedures as per protocol LAD 8E.
Allergen challenge
Mice under isoflurane anesthesia were inoculated intranasally with a reconstituted filtrate of A. alternata (Greer Allergy Immunotherapy; 10 mg/mL in Hanks’ buffered saline solution, 50 μg/mouse delivered in a strict intranasal dose of 2.5 μL per nare [19] on days 0, 2, 4 (week 1), 7, 9, 11 (week 2), and 14, 16, and 18 (week 3) or diluent alone at each time point as shown in Fig 1A. In some experiments, mice were inoculated as above with A. alternata that was heated to 95°C in a thermocycler for 10 min and stored at −80°C prior to inoculation. At time points indicated (days 7, 14, or 21, at week 1, 2, and 3, respectively), mice were sacrificed and subjected to nasal wash with a total per mouse of 0.8 mL phosphate buffered saline (PBS) with 0.1% bovine serum albumin (BSA; [26]) without protease-digestion of the submucosa. Typical recovery was 0.5 to 0.6 mL per mouse. Given the limited volume of nasal wash fluid obtained, all assays were not performed on each sample.
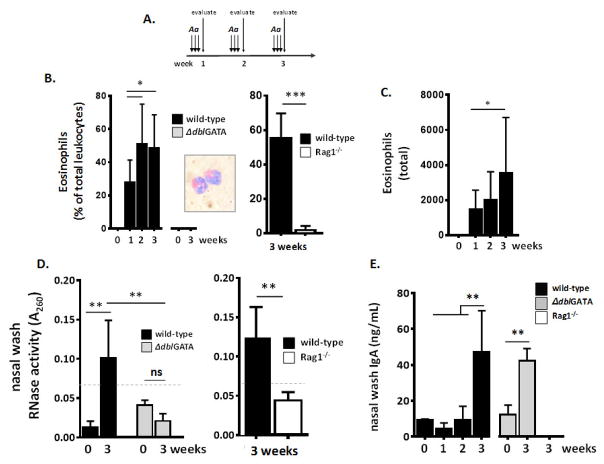
Fig. 1. Repetitive administration of the aeroallergen, Alternaria alternata, to the nasal passages results in eosinophilic inflammation.
A. Strategy for repetitive challenge with A. alternata antigens (filtrate; 10 mg/mL) administered at strict intranasal volumes (2.5 μL per nare [19]) over a three (3) week period as described in the Methods Section. B. Eosinophils (percent of total leukocytes) are prominent in nasal wash fluid of wild-type but not eosinophil-deficient ΔdblGATA mice or lymphocyte-deficient Rag1−/− mice in response to A. alternata administered as in Fig. 1A; inset, eosinophils from nasal wash fluid of wild-type mice stained with modified Giemsa. C. Eosinophils (total in 0.6 mL nasal wash fluid) at weeks 0, 1, 2 and 3 as in Fig. 1A. D. RNase activity, a measure of eosinophil degranulation, detected in nasal wash fluid of wild-type, but not ΔdblGATA or Rag1−/− mice; dotted line, limit of background activity. E. IgA detected in nasal wash fluid of wild-type and ΔdblGATA mice, but not Rag1−/− mice; n = 3 – 8 mice per time point, ***p < 0.005, **p < 0.01, *p < 0.05, ns, no statistical significance, 1-way ANOVA or Mann-Whitney U-test.
Protease activity
Protease activities of active and heat-inactivated extracts of A. alternata were assessed using the Fluoro Protease Assay kit (G-Biosciences), an assay that uses fluorescein isothiocyanate (FITC)-labeled casein as a generic protease substrate. Samples were diluted in 1x Fluoro™ Assay Buffer and added to wells of a 96-well fluorometer-compatible titer plate. FITC-conjugated casein assay substrate was added to the wells and incubated at room temperature for 2 hours. Fluorescence intensity was determined using a FilterMax F5 multi-mode microplate reader at an excitation wavelength of 485 and an emission wavelength of 530nm. Buffer without protease was used as a blank for background subtraction.
Influenza virus infection
Influenza A/HK/1/68 (gift from J. Keicher, Symmune Therapeutics, Raleigh, NC) was provided to us as egg-passaged stock, and was passaged three times in wild-type specific pathogen-free mice in our high-barrier facility prior to utilization in in vivo experiments. This was done to avoid inoculating foreign (egg) antigens during the experimental trials. Influenza virus stocks were maintained at −80°C as clarified lung homogenates in phosphate buffered saline (PBS) at 3 × 107 TCID50 units/mL. In experimental studies, allergen or diluent-challenged mice were inoculated under isoflurane anesthesia with influenza virus at varying dilutions (30, 150, or 500 TCID50 units/mouse). Virus was administered intranasally in a total volume of 2.5 μL per nare (5 μL per mouse) and evaluated by serial weights and survival. Using this volume of inoculum, we are unable to obtain reliable MLD50 data in naïve C57BL/6 recipient mice, even using 10-times more virus (i.e., 5000 TCID50 units/mouse). In other experiments, mice were sacrificed on day 6 after virus inoculation for evaluations as below.
Evaluation of cells and cytokines in nasal wash fluid
Cytospins were prepared (100 μL per slide) and stained with modified Giemsa (Diff-Quik, ThermoScientific); total leukocytes and leukocyte differential, including percent eosinophils, neutrophils, and macrophages were determined by visual inspection and scoring of minimum of 100 cells per mouse. Cytokine levels in nasal wash fluid were evaluated by DuoSet ELISA assays (R&D Systems). In experiments with virus-infected mice, cytokines were first evaluated by Proteome profiler cytokine array kit (ARY006; R&D Systems) as per manufacturer’s instructions (1 mL nasal wash fluid per filter; 0.2 mL per mouse combined from 5 mice per condition). Cytokines of specific interest were re-evaluated by DuoSet assay.
Histology
The heads of A. alternata-challenged mice were excised and fixed in cold 10% buffered formalin. After fixation, the tissue was decalcified, paraffin embedded, and sectioned from anterior to posterior, and stained with hematoxylin and eosin by Histoserv, Inc. (Germantown, MD). Reference points for sections were as described by Mery and colleagues [27]. The scale bars in the images were estimated based on the known diameter of a mouse eosinophil at 10 μm [28].
RNase assay
Enzymatic activity in 50 μL samples of cell-free nasal wash fluid was determined as previously described [29].
Nasal wash IgA
Total IgA in cell-free nasal wash samples was determined by ELISA as per manufacturer’s instructions (Abcam).
Tissue Culture Infectious Dose (TCID)50 assay
This assay was performed as previously described to titrate pneumoviruses [30] with some specific changes as follows: Briefly, Madin-Darby Canine Kidney (MDCK) cells were seeded at 0.8 × 105 per well in 250 μL growth medium (DMEM high glucose with 7.5% fetal bovine serum (FBS), 4 mM glutamine and penicillin/stretptomycin) in a 48 well plate. At t = 24 hrs, wells were rinsed with serum free-medium (as above, without FBS) and serial dilutions of virus were added in infection medium (growth medium without FBS with 1 μg/mL TPCK trypsin). Virus-free control wells are included. After 2 hrs virus adsorption at 34°C in CO2 incubator, virus-containing medium was removed and fresh infection medium was added. Plates were incubated at 34°C in CO2 incubator and cytopathic effect (CPE) is monitored for 1 week. TCID50 titer per mL of virus stock, per mL of bronchoalveolar lavage fluid or per mg lung tissue homogenate (the latter determined by BCA assay (Pierce)) were calculated by the method of Reed and Muench as described.
Virus titration by qPCR
Virus was evaluated in nasal wash fluid and in whole lung tissue using a qPCR assay that targets the Influenza A/HK/1/68 matrix (M1) protein (Genbank Accession no. AF348188) via a method analogous to that described by our laboratory for evaluating pneumonia virus of mice (PVM; [31, 32]). Briefly, RNA was prepared from mouse lung tissue that had been immersed and stored in RNAlater (Ambion, Austin, TX). Isolated RNA (RNAzol, Tel-test, Friendswood, TX) was treated with DNase I to remove genomic DNA contaminants. Reverse transcription was performed using a first-strand cDNA synthesis kit (Roche) with random primers; a no reverse transcriptase control was included. The qPCR reactions were amplified in triplicate, with the ABI 2x TaqMan reagent, primer-probe mixes, and cDNA or plasmid standard in a 25-μl final volume (Applied Biosystems). Thermal cycling parameters for the ABI7500 absolute quantitation program (Applied Biosystems) include 50°C for 2 min, 95°C for 10 min, and 40 amplification cycles alternating 95°C for 15 sec and 60°C for 1 min. Custom design primer-probes include primer 1, 5′-AAG ACC AAT CCT GTC ACC -3′; primer 2, 5′-CAA AGC GTC TAC GCT GCA GTC C-3′; probe 6FAM-TTT GTG TTC ACG CTC ACC GTG CC-TAMRA). A 1002 bp PCR amplicon of the M1 protein (bp 1 to 1002, GenBank ID CY112250.1) was used to generate a standard curve for absolute quantification. Experimental triplicate data points were interpolated to linear standard curves over the concentration ranges indicated. A sample calculation from data generated by this method for PVM is shown in Supplemental Figure 1 of reference 32. The data from BAL fluid are presented as copies/mL. The data from lung tissue are normalized to absolute copies of GAPDH. This value is generated using commercially available mouse GAPDH primer-probes (Applied Biosystems catalog no. 4308313); values obtained are interpolated to a standard curve generated using a mouse GAPDH plasmid in pCMV pSport 6 (American Type Culture Collection cat no. 10539385), also as previously described [31, 32].
Statistical analysis
All quantitative findings were from two or more replicate datasets; specific number of mice per group in individual experiments are as indicated in the Figure Legends. Data were analyzed via appropriate algorithms within GraphPad PRISM or http://www.socscistatistics.com/tests/mannwhitney, also as indicated in the Figure Legends.
Results
Repetitive administration of A. alternata to the nasal passages results in eosinophilic inflammation
The strategy for repetitive challenge with A. alternata antigens administered in strict intranasal volumes (2.5 μL per nare [19]) over three weeks is shown in Fig. 1A. No cells were detected in nasal wash at week 0. Eosinophils were initially detected in nasal wash fluid after one week (27 ± 14% of total leukocytes) and reached peak values of 51 ± 24% and 49 ± 20% at 2 and 3 weeks, respectively, as shown in Fig. 1B. Eosinophils remained detectable in nasal wash fluid at 6 and 8 weeks without further provocation (data not shown). No eosinophils were detected in the nasal wash fluid of eosinophil-deficient ΔdblGATA mice, and few eosinophils were detected in the absence of lymphocytes (Rag1−/− mice). Eosinophils detected in nasal wash are of typical morphology with standard staining properties [Fig. 1B, inset]. Total eosinophils detected in nasal wash fluid at weeks 0 through 3 is shown in Fig. 1C. Ribonuclease activity, a measure of eosinophil activation and degranulation [33, 34], was detected above background levels in nasal wash fluid from wild-type but not ΔdblGATA or Rag1−/− mice [Fig. 1D]. Secretory IgA was detected in nasal wash fluid of both wild-type and ΔdblGATA mice after 3 weeks of repetitive stimulation, but not in nasal wash fluid from lymphocyte deficient Rag1−/− mice ([Fig. 1E]; see Discussion).
Eotaxin-1 (CCL-11) promotes eosinophil recruitment to nasal passages
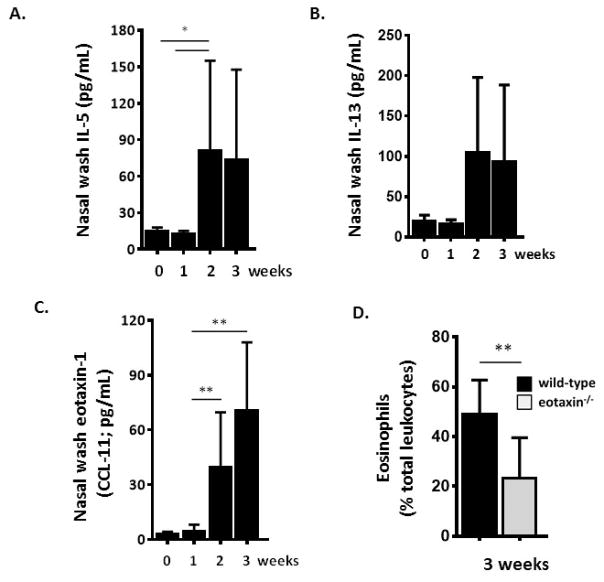
CCL-11 is a major eosinophil chemoattractant and typically pairs with IL-5 to activate and recruit eosinophils in response to allergic provocation [35]. Repetitive stimulation with a filtrate of A. alternata results in elevated levels of IL-5 [Fig. 2A] and IL-13 [Fig. 2B] in nasal wash fluid. Eotaxin-1 was detected at 39 ± 10 and 70 ± 12 pg/mL at weeks 2 and 3, respectively, at levels 13-fold and 24-fold over baseline [Fig. 2C]. Administration of A. alternata to eotaxin-1 gene-deleted mice resulted in 56% fewer eosinophils in nasal wash fluid after three weeks [Fig. 2D]
Fig. 2. Eotaxin-1 (CCL-11) promotes eosinophil recruitment to nasal passages.
Detection of immunoreactive A. Interleukin-5 (IL-5) B. IL-13 and C. Eotaxin-1 (CCL-11) in nasal wash fluid after 1, 2 and 3 weeks of repetitive challenge with A. alternata as in Fig. 1A. D. Eosinophils (percent of total leukocytes) detected in the airways are diminished in eotaxin-1 gene-deleted mice; n = 3 – 5 mice per time point, **p < 0.01, *p < 0.05; 1-way ANOVA or Mann-Whitney U-test.
Histology of the nasal passages of mice subjected to repetitive challenge with A. alternata antigens
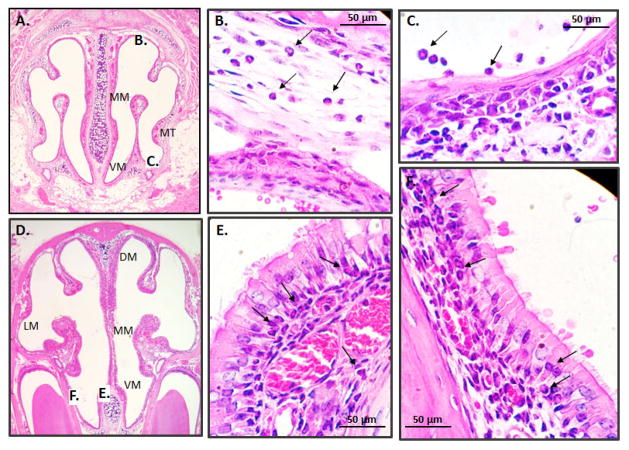
Shown in Fig. 3 are coronal sections of the nasal passages of wild-type mice subjected to the full protocol shown in Fig. 1A. Histology provides additional evidence for eosinophil recruitment in response to A. alternata and documents their distribution in distinct submucosal compartments. For example, eosinophils are prominent not only within the lumen, but within the mucosal tissue underlying the squamous epithelial cells at the medial meatus, at the base of the columnar epithelial cells lining the ventral meatus, as shown.
Fig. 3. Histology of the nasal passages of mice subjected to repetitive challenge with A. alternata antigens.
A. Anterior cross section featuring the medial meatus (MM), ventral meatus (VM), and medial turbinate (MT) with areas marked B. and C. featured in enlargements on the panels to follow. B. Eosinophils (examples at arrows) are prominent in the mucosal tissue underlying the squamous epithelium and C. within the lumen of the nasal passage. D. Posterior cross section including the dorsal meatus (DM) and lateral meatus (LM); areas marked E. and F are featured in enlargements in panels to follow. E. Eosinophils are detected at the base of the ciliated columnar respiratory epithelial cells and F. within the mucosal tissue. Original magnification 4X; panels A and D and 40X; panels B, C, E and F).
Eosinophilic inflammation in the upper airways does not protect against influenza virus infection
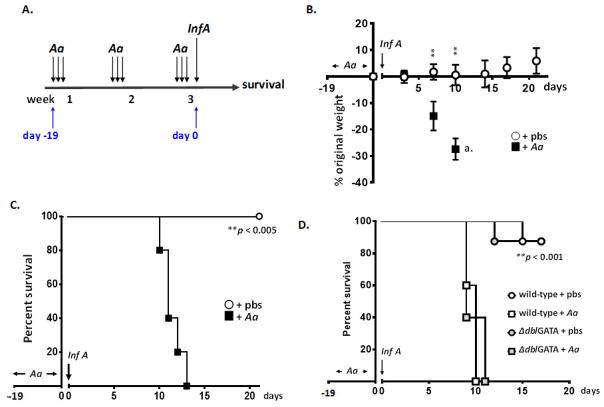
Wild-type female C57BL/6 mice subjected to repetitive stimulation with A. alternata or diluent control as per Fig. 1A were inoculated with Influenza A/HK/1/68 (also in strict intranasal volumes, 2.5 μL/nare, total 5 μL/mouse) on day 0 as shown in Fig. 4A. Mice treated with diluent (pbs) prior to influenza virus (30 TCID50 units) responded with minimal weight loss only, observed in some mice at days 10 and 14 after inoculation. In contrast, the identical inoculum led to rapid weight loss [Fig. 4B] and ultimately mortality [Fig. 4C] among mice that had been treated with A. alternata. Interestingly, the presence of eosinophils had no impact on this response. Eosinophils have been characterized mediators of antiviral host defense in the lower airways [13 – 16], and have characterized activity against the influenza A H1N1 [16] and H3N2 in the lower airways [Suppl. Fig. 1]. However, eosinophil-sufficient wild-type and eosinophil-deficient ΔdblGATA, both subjected to repetitive administration of A. alternata, succumbed to influenza infection by day 11 [Fig. 4D].
Fig. 4. Eosinophilic inflammation in the upper airways does not protect against influenza virus infection.
A. Strategy for influenza virus (Inf A) infection and evaluation. Inf A was administered to mice previously challenged with A. alternata or diluent control (days −19 through days −1) as previously indicated in Fig. 1A. B. Weight loss (% original weight determined for each mouse) in response to influenza virus infection. Mice were challenged with A. alternata or diluent control and inoculated on day 0 with 30 TCID50 units Inf A in 5 μL (2.5 μL per nare) as in Fig. 4A; n = 5 mice per group, **p < 0.001 at time points indicated (Student’s t-test). C. Survival of influenza virus-infected mice (30 TCID50 units in 5 μL on day 0 as in B.) that had been subjected to repetitive challenge with A. alternata or diluent control; n = 5 mice per group, **p < 0.005 Log-rank test. D. Survival of wild-type and eosinophil-deficient ΔdblGATA mice subjected to repetitive challenge with A. alternata or diluent control (pbs) prior to infection with Inf A (500 TCID50 units in 5 μL); n = 4 – 5 mice per group, **p < 0.001 Log-rank test.
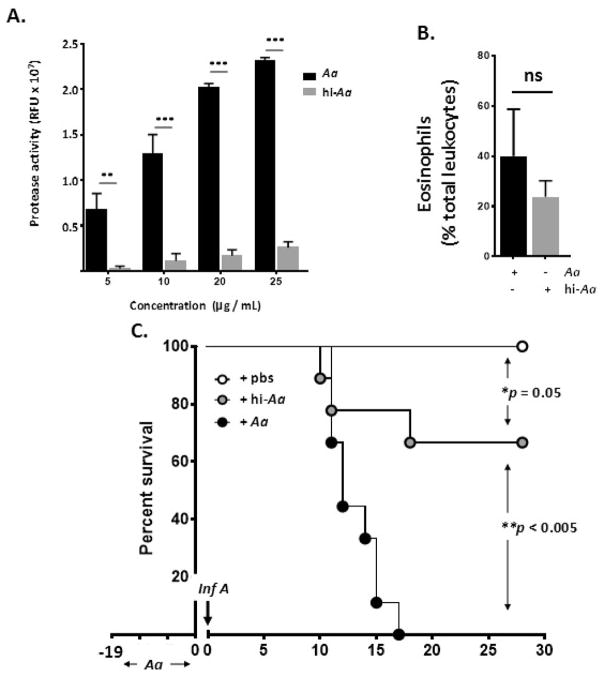
Heat-inactivation destroys A. alternata protease activity and blunts lethal response to influenza virus
A. alternata elicits inflammation in the lower respiratory tract in part via the actions of its serine proteases, which ultimately result in induction of proinflammatory cytokines and leukocyte recruitment; results from cell culture and ex vivo studies suggest that the mechanism may (or may not) involve targeting of protease activated receptor-2 (PAR2) [36 – 38]. The A. alternata filtrate in our studies cleaved a fluorescent-tagged casein substrate in a dose-dependent fashion, and that heat-inactivation (95°C for 10 minutes) reduced proteolytic activity to background levels [Fig. 5A]. Eosinophilic inflammation was maintained in response to administration of heat-inactivated A. alternata [Fig. 5B], but the lethal response to influenza virus (150 TCID50 units in 5 μL) was reduced significantly [Fig. 5C]. These results, which suggest that protease activity amplifies pathology in response to influenza infection, are intriguing, given recent findings that, by contrast, implicate PAR2 activation as a protective mechanism in a similar setting [39]; this is considered further in the Discussion.
Fig. 5. Heat-inactivation of A. alternata blunts the lethal response to influenza virus.
A. Filtrate of A. alternata heated to 95°C for 10 minutes retains little to no residual protease activity using casein as a substrate; ***p < 0.001. B. Heat-inactivated filtrate of A. alternata (hi-Aa) administered to upper airways as in Fig. 1A promotes eosinophil recruitment to the upper airways as does the active filtrate (Aa), n = 5 mice per group; ns, no significant difference, Mann-Whitney U-test. C. Survival of wild-type mice challenged with A. alternata (Aa), hi-Aa or diluent control as in Fig. 4A followed by Inf A (150 TCID50 units in 5 μL); n = 9 – 10 mice per group, *p = 0.05, **p < 0.005, Log-rank.
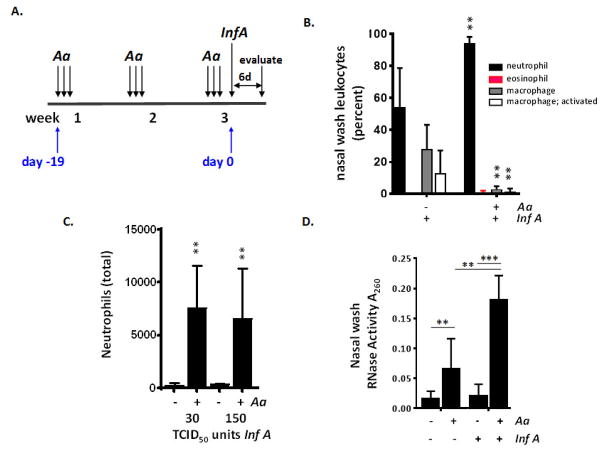
Repetitive stimulation with A. alternata followed by influenza virus infection results in a significant increase in neutrophil recruitment
Wild-type female C57BL/6 mice subjected to repetitive stimulation with A. alternata or diluent control were inoculated with influenza virus on day 0 as in Fig. 4A and evaluated on day 6 [Fig. 6A]. As shown in Fig. 6B, influenza virus (150 TCID50 units in 5 μL) resulted in neutrophil recruitment to the upper airways of both diluent (pbs) and A. alternata-treated mice. The percent neutrophils detected was significantly higher in A. alternata treated mice, reaching 93 ± 4.1% vs. 58 ± 19% of the total leukocytes in A. alternata vs. pbs-treated mice, respectively (**p < 0.01). Total neutrophils in nasal wash fluid from influenza-infected A. alternata-treated mice were 18 to 30-fold higher than that detected from influenza-infected mice treated with pbs alone [Fig. 6C]. Although eosinophils were a small fraction of leukocyte differential detected at day 6 [Fig. 6B], influenza virus infection was associated with an increase in eosinophil degranulation [Fig. 6D], a finding consistent with our earlier studies on eosinophils in the lung during pneumonia virus of mice infection [15] and influenza-mediated activation of eosinophils reported by Samarasinghe and colleagues [16].
Fig. 6. Influenza virus infection of mice challenged with A. alternata results in differential recruitment of proinflammatory neutrophils.
A. Strategy for influenza virus (Inf A) infection and evaluation. Influenza virus was administered to mice previously challenged with A. alternata or diluent control as in Fig. 4A and evaluated on day 6. B. Neutrophils (% total leukocytes) are more prominent in nasal wash samples from wild-type mice challenged with A. alternata prior to influenza infection (150 TCID50 units in 5 μL) than those from mice challenged with diluent control; n = 5 mice per group, **p < 0.01, Mann-Whitney U-test. C. Total neutrophils in nasal wash samples from mice infected with 30 or 150 TCID50 units influenza A; n = 5 mice per group, **p < 0.01, Mann Whitney U-test. D. RNase activity detected in nasal wash fluid in response to influenza infection (500 TCID50 units); n = 5 mice per group, **p < 0.01; ***p < 0.005., 1-way ANOVA, Student’s t-test.
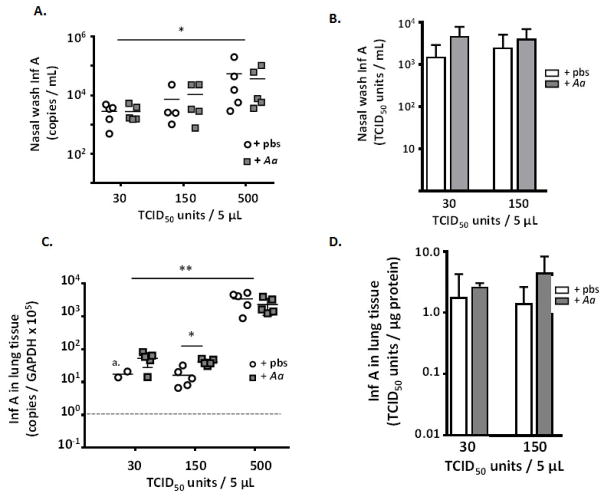
Pre-treatment with A. alternata has a minimal impact on virus burden
Despite significant differential survival, titration of virus by qPCR and tissue culture methods showed few to no differences when comparing nasal wash fluids or lung tissue from influenza-virus infected mice that had been treated with A. alternata to those that had been treated with diluent control [Fig. 7]. While higher virus titers were recovered from both A. alternata- and diluent-treated mice that were inoculated with 500 TCID50 units vs. those inoculated with 150 or 30 TCID50 units, *p < 0.05, **p < 0.01 [Fig. 7A and Fig. 7C], differences between recoveries at a single inoculating dose were non-existent or minimal (well under 1 log; Fig. 7C) and not reproduced by TCID50-analysis [Fig 7D].
Fig. 7. Eosinophilic inflammation in the upper airways has no impact on virus recovery.
No significant differences were observed in comparisons of mice treated with repetitive administration of A. alternata vs. pbs diluent control, evaluated at day 6 of infection with influenza virus (inoculation of 30, 150 or 500 TCID50 in 5 μL volume). In BAL fluid, A. copies per mL or B. TCID50 units/mL; in lung tissue C. copies per GAPDH x 105 or TCID50 units/μg lung protein or D. TCID50 units per μg protein; a. three points below detectable limits at dotted line; n = 4 – 5 mice per point, *p < 0.05; **p < 0.01.
Rhinitis followed by virus infection results in an increase in critical pro-inflammatory cytokines
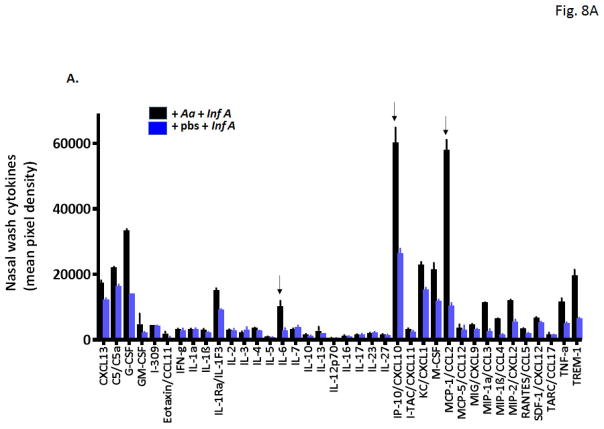
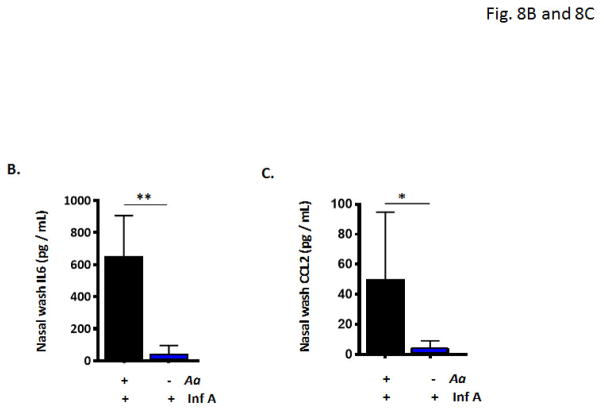
Nasal wash fluids from mice subjected to repetitive administration of A. alternata or diluent control followed by infection with influenza virus [as in Fig. 6A] were evaluated by cytokine profiling. As shown in Fig. 8A, nasal wash fluids from A. alternata treated mice displayed higher levels of nearly all 40 proinflammatory mediators evaluated. Among the most prominent are CCL-2 (5.5-fold higher levels in nasal wash fluids from mice subjected to A. alternata vs. diluent prior to influenza virus infection), MIP-1α (4.1-fold higher), MIP-1β (3.7-fold higher), IL-6 (3.6-fold higher), MIP-2 (2.2-fold higher) and CXCL-10 (2.3-fold higher). Direct evaluation of nasal wash fluids by ELISA confirmed significant increases in IL-6 [Fig. 8B] and CCL-2 [Fig. 8C], both cytokines implicated in pathogenic responses in acute influenza infection [40].
Fig. 8. Eosinophilic inflammation in the nasal passages followed by influenza virus infection results in differential release of critical proinflammatory cytokines.
A. Cytokine profiling reveals differential release of numerous proinflammatory cytokines in the upper airways, including elevated levels of (a) IL-6, (b) CXCL10, and (c) CCL2, cytokines that have been previously associated with poor outcomes in acute respiratory virus infection (at arrows). Direct evaluation confirmed differential detection of B. IL-6 and C. CCL2 in nasal wash fluid of Aa-treated wild-type mice, when compared to diluent-treated, Inf A-infected mice; n = 5 mice per group, **p < 0.01, *p < 0.05.
Discussion
In this study, our primary goal was to examine eosinophil-mediated responses to influenza virus infection in the upper airways. Toward this end, we have adapted a strategy of repetitive dosing with a filtrate of the saprophytic fungus, A. alternata, used in earlier studies to elicit allergic lung disease [41 – 43]. Here, we show that repetitive stimulation with this filtrate in volumes that are limited to the nasal cavity [19, 22] results in prominent eosinophilic inflammation within the nasal mucosa. Eosinophil recruitment is absent in Rag1−/− mice, suggesting a critical role for Th2 lymphocytes [44]. Eosinophil recruitment is also associated with cytokines IL-5 and IL-13, and is diminished, but not eliminated, in mice devoid of the chemoattractant, eotaxin-1. By week 3 of this protocol, profound elevations of nasal wash fluid IgA were detected in both wild-type and eosinophil-deficient ΔdblGATA mice. This is notable in light of recent observations that highlight interactions between eosinophils and B cells, including release of crucial eosinophil-derived cytokines that promote survival of antibody producing cells in the bone marrow and gastrointestinal tract [45]. Our data suggest that eosinophils are dispensable for support of B cells and IgA production in the upper respiratory tract in response to repetitive stimulation with A. alternata. These observations may be explained by the fact that IgA production in this setting results from allergic provocation, an activity that might be distinguished from eosinophil-mediated homeostatic support of B cell function [3, 45].
Before returning to the issue of eosinophils, we were surprised to find that wild-type mice subjected to repetitive stimulation with A. alternata responded to an otherwise survivable inoculum of influenza with significant morbidity and mortality (see Fig. 4B and 4C). This response is blunted in mice subjected to repetitive treatment with heat-inactivated A. alternata (see Fig. 5C), in which fungal proteases have been inactivated (Fig. 5A). Serine proteases from A. alternata induce lung inflammation in mice, observations which have been linked to activation of protease-activated receptor-2 (PAR2) on respiratory epithelial cells [36, 37]. In unrelated work, Feld and colleagues [39] have reported that PAR2 activation is a protective mechanism in influenza infection, in studies that focus on infected neutrophils. Further study of A. alternata, its serine proteases, and its interactions with influenza virus infection may provide further insight into this issue.
While virus infection is a critical feature of the morbidity and mortality observed in this study, the enhanced response to influenza virus was not related to a significant increase in virus burden among mice treated with A. alternata. Virus titers were not significantly different from one another when comparing responses from A. alternata-treated vs. control diluent-treated mice (see Fig. 7). By contrast, virus infection in the A. alternata-treated mice was accompanied by a more prominent local proinflammatory response, including up to 30-fold more neutrophils (see Fig. 6C) and >100-times more IL-6 (see Fig. 8B). IL-6 is among the proinflammatory cytokines that have been associated with severe disease and poor outcomes in influenza-infected patients [46, 47], although mouse model studies of acute influenza virus infection do not support a unique focus on IL6 as a negative modulator of disease [48, 49]. These findings are consistent with and extend the current appreciation of host inflammatory responses as providing major contributions to the outcome of acute respiratory virus infection [50].
Returning the focus to eosinophils in the respiratory tract, we and others have shown previously that eosinophils recruited to the lungs in response to cytokines or allergens reduced the negative sequelae of acute respiratory virus infections in vivo [13 – 15 see also Suppl. Fig. 1]; recently, Samarasinghe and colleagues [16] found that eosinophils isolated from an allergen-sensitized and challenged mouse adoptively transferred into a wild-type mouse had a distinct impact on weight loss secondary to infection with Influenza A H1N1. However, in contrast to what has been observed in the lower airways, activated eosinophils in the nasal passages have no apparent impact on the lethal sequelae of acute influenza virus infection. The mechanisms underlying this distinction remain unclear, and await further characterization of the eosinophils and the inflammatory milieu.
In summary, we report that repetitive intranasal stimulation with a filtrate of A. alternata results in eosinophil recruitment to the nasal mucosa, and, surprisingly, significant morbidity and mortality in response to influenza virus in association with local over-production of proinflammatory cytokines, including IL6. Interestingly, and in contrast to our findings in the lower airways, eosinophils recruited to the nasal passages in response to A. alternata have no measurable impact against lethal respiratory virus infection. To the best of our knowledge, this is the first rhinitis model in which this unique and powerful response to influenza virus has been described. There are several reports that examine susceptibility to influenza A among individuals with allergic rhinitis [51 – 53]; a model such as this might be useful for further exploration of these clinical observations.
Supplementary Material
A. Strategy for influenza A virus (Inf A) infection of A. alternata-challenged mice. Inf A was administered on day 0 via intranasal inoculation (50 μL, 1500 TCID50 units/mouse) to mice that had received A. alternata (500 μg/50 μL inoculum on days indicated or diluent only) on days −7, − 4 and −1 as indicated. B. Percent original weight per mouse. One death in the diluent only group, on day 15; n = 5 mice per group, *p < 0.05, Student’s t-test.
Acknowledgments
This work was supported by NIAID Division of Intramural Research (ZIA-AI000941 and ZIA-AI000943 to HFR and ZIA-AI000939 to KMD)
Footnotes
Author Contributions
MM performed most of the experimental work, analyzed data, and reviewed the manuscript.
JLR provided experimental studies for the revised manuscript, analyzed data, and reviewed the manuscript.
CMP assisted MM on experimental studies and analysis and reviewed the manuscript.
KMD provided critical assistance to JLR and reviewed the manuscript.
HFR analyzed data and wrote the first and subsequent drafts of the manuscript
References
- 1.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 2013. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuta GT, Atkins FD, Lee NA, Lee JJ. Changing roles of eosinophils in health and disease. Ann Allergy Asthma Immunol. 2014;113:3–8. doi: 10.1016/j.anai.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.95. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster PS, Maltby S, Rosenberg HF, Tay HL, Hogan SP, Collison AM, Yang M, Kaiko GE, Hansbro PM, Kumar RK, Mattes J. Modeling Th2 responses and airway inflammation to understand fundamental mechanisms regulating the pathogenesis of asthma. Immunol Rev. 2017;278:20–40. doi: 10.1111/imr.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563–75. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar RK, Foster PS, Rosenberg HF. Respiratory viral infection, epithelial cytokines, and innate lymphoid cells in asthma exacerbations. J Leukoc Biol. 2014;96:391–6. doi: 10.1189/jlb.3RI0314-129R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg HF, Dyer KD, Domachowske JB. Respiratory viruses and eosinophils: exploring the connections. Antiviral Res. 2009;83:1–9. doi: 10.1016/j.antiviral.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison AM, Bonville CA, Rosenberg HF, Domachowske JB. Respiratory syncytial virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am J Respir Crit Care Med. 1999;159:1918–24. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- 9.Rossi GA, Colin AA. Respiratory syncytial virus-host interaction in the pathogenesis of bronchiolitis and its impact on respiratory morbidity in later life. Pediatr Allergy Immunol. 2017;28:320–331. doi: 10.1111/pai.12716. [DOI] [PubMed] [Google Scholar]
- 10.Steinke JW, Borish L. Immune responses in rhinovirus-induced asthma exacerbations. Curr Allergy Asthma Rep. 2016;16:78. doi: 10.1007/s11882-016-0661-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beale J, Jayaraman A, Jackson DJ, Macintyre JDR, Edwards MR, Walton RP, Zhu J, Man Ching Y, Shamji B, Edwards M, Westwick J, Cousins DJ, Yi Hwang Y, McKenzie A, Johnston SL, Bartlett NW. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci Transl Med. 2014;6:256ra134. doi: 10.1126/scitranslmed.3009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong JY, Chung Y, Steenrod J, Chen Q, Lei J, Comstock AT, Goldsmith AM, Bentley JK, Sajjan US, Hershenson MB. Macrophage activation state determines the response to rhinovirus infection in a mouse model of allergic asthma. Respir Res. 2014;15:63. doi: 10.1186/1465-9921-15-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999;190:1465–78. doi: 10.1084/jem.190.10.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–86. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 15.Percopo CM, Dyer KD, Ochkur SI, Luo JL, Fischer ER, Lee JJ, Lee NA, Domachowske JB, Rosenberg HF. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood. 2014;123:743–52. doi: 10.1182/blood-2013-05-502443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samarasinghe AE, Melo RC, Duan S, LeMessurier KS, Liedmann S, Surman SL, Lee JJ, Hurwitz JL, Thomas PG, McCullers JA. Eosinophils promote antiviral immunity in mice infected with Influenza A virus. J Immunol. 2017;198:3214–26. doi: 10.4049/jimmunol.1600787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebisawa IT, Kitamoto O. Persistence of viral antigen in the nasal epithelium in complicated influenza. Am Rev Respir Dis. 1968;99:275–278. doi: 10.1164/arrd.1969.99.2.275. [DOI] [PubMed] [Google Scholar]
- 18.Baccam P, Beauchemin C, Macken CA, Hayden FG, Perelson AS. Kinetics of Influenza A virus infection in humans. J Virol. 2006;80:7590–7599. doi: 10.1128/JVI.01623-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Southam DS, Dolovich M, O’Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L833–9. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 20.Cho SH, Oh Sy, Zhu Z, Lee J, Lane AP. Spontaneous eosinophilic nasal inflammation in a genetically-mutant mouse: comparative study with an allergic inflammation model. PLoS One. 2012;7:e35114. doi: 10.1371/journal.pone.0035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JH, Yi JS, Gong CH, Jang YJ. Development of Aspergillus protease with ovalbumin-induced allergic chronic rhinosinusitis model in the mouse. Am J Rhinol Allergy. 2014;28:465–70. doi: 10.2500/ajra.2014.28.4100. [DOI] [PubMed] [Google Scholar]
- 22.McCusker C, Chicoine M, Hamid Q, Mazer B. Site-specific sensitization in a murine model of allergic rhinitis: role of the upper airways in lower airways disease. J Allergy Clin Immunol. 2002;110:891–8. doi: 10.1067/mai.2002.130048. [DOI] [PubMed] [Google Scholar]
- 23.Salo PM, Arbes SJ, Jr, Crockett PW, Thorne PS, Cohn RD, Zeldin DC. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol. 2008;121:678–84. doi: 10.1016/j.jaci.2007.12.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kustrzeba-Wojcicka I, Siwak E, Terlecki G, Wolanczyk-Medrala A, Medrala W. Alternaria alternata and its allergens: a comprehensive review 2014. Clin Rev Allerg Immunol. 2014;47:354–65. doi: 10.1007/s12016-014-8447-6. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo ME, Hodgson A, Robinson DP, Kaplan JB, Pekosz A, Klein SL. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine. 2011;29:9246–55. doi: 10.1016/j.vaccine.2011.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puchta A, Verschoor CP, Thurn T, Bowdish DM. Characterization of inflammatory responses during intranasal colonization with Streptococcus pneumoniae. J Vis Exp. 2014;83:e50490. doi: 10.3791/50490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mery S, Gross EA, Joyner DR, Godo M, Morgan KT. Nasal diagrams: a tool for recording the distribution of nasal lesions in rats and mice. Toxicol Pathol. 1994;22:353–72. doi: 10.1177/019262339402200402. [DOI] [PubMed] [Google Scholar]
- 28.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, Luo H, Zellner KR, Protheroe CA, Willetts L, Lesuer WE, Colbert DC, Hlemers RA, Lacy P, Moqbel R, Lee NA. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130:572–84. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg HF, Domachowske JB. Assays for detection of RNase A superfamily ribonucleases. Methods Mol Biol. 2001;160:355–62. doi: 10.1385/1-59259-233-3:355. [DOI] [PubMed] [Google Scholar]
- 30.Percopo CM, Dubovi EJ, Renshaw RW, Dyer KD, Domachowske JB, Rosenberg HF. Canine pneumovirus replicates in mouse lung tissue and elicits inflammatory pathology. Virology. 2011;416:26–31. doi: 10.1016/j.virol.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Percopo CM, Dyer KD, Karpe KA, Domachowske JB, Rosenberg HF. Eosinophils and respiratory virus infection: a dual-standard curve qRT-PCR-based method for determining virus recovery from mouse lung tissue. Methods Mol Biol. 2014;1178:257–66. doi: 10.1007/978-1-4939-1016-8_22. [DOI] [PubMed] [Google Scholar]
- 32.Gabryzewski SJ, Bachar O, Dyer KD, Percopo CM, Killoran KE, Domachowske JB, Rosenberg HF. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J Immunol. 2011;186:1151–61. doi: 10.4049/jimmunol.1001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Dyer KD, Rosenberg HF. Evolution of the rodent eosinophil-associated RNase gene family by rapid gene sorting and positive selection. Proc Natl Acad Sci USA. 2000;97:4701–6. doi: 10.1073/pnas.080071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shamri R, Melo RC, Young KM, Bivas-Benita M, Xenakis JJ, Spencer LA, Weller PF. CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules. FASEB J. 2012;26:2084–93. doi: 10.1096/fj.11-200246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster PS, Mould AW, Yang M, Mackenzie J, Mattes J, Hogan SP, Mahalingam S, McKenzie AN, Rothenberg ME, Young IG, Matthaei KI, Webb DC. Elemental signals regulating eosinophil accumulation in the lung. Immunol Rev. 2001;179:173–81. doi: 10.1034/j.1600-065x.2001.790117.x. [DOI] [PubMed] [Google Scholar]
- 36.Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, Daines MO. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am J Physiol Lung Cell Mol Physiol. 2011;300:L605–14. doi: 10.1152/ajplung.00359.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33 mediates asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–92. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Grady SM, Patil N, Melkamu T, Maniak PJ, Lancto C, Kita H. ATP release and Ca2+ signaling by human bronchial epithelial cells following Alternaria aeroallergen exposure. J Physiol. 2013;591:4595–609. doi: 10.1113/jphysiol.2013.254649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feld M, Shpacovitch V, Ehrhardt C, Fastrich M, Goerge T, Ludwig S, Steinhoff M. Proteinase-activated receptor-2 agonist activates anti-influenza mechanisms and modulates interferon-gamma-induced antiviral pathways in human neutrophils. Biomed Res Int. 2013;2013:2013, 879080. doi: 10.1155/2013/879080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo XJ, Thomas PG. New fronts emerge in the influenza cytokine storm. Semin Immunopathol. 2017;39:541–550. doi: 10.1007/s00281-017-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass-induced lung inflammation. Int Arch Allergy Immunol. 2014;163:92–115. doi: 10.1159/000356341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabriel MF, Potigo I, Tomaz CT, Martinez Alternaria alternata allergens: markers of exposure, phylogeny and risk of fungi-induced respiratory allergy. Environ International. 2016;89–90:71–80. doi: 10.1016/j.envint.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Shinkai Y, Young F, Alt FW. Probing immune functions in RAG-deficient mice. Curr Opin Immunol. 1994;6:313–9. doi: 10.1016/0952-7915(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 45.Berek C. Eosinophils: important players in humoral immunity. Clin Exp Immunol. 2015;183:57–64. doi: 10.1111/cei.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001;64:262–8. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 47.Oshansky CM, Gartland AJ, Wong SS, Jeevan T, Wang D, Roddam PL, Caniza MA, Hertz T, Devincenzo JP, Webby RJ, Thomas PG. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am J Respir Crit Care Med. 2014;189:449–62. doi: 10.1164/rccm.201309-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dienz O, Rug JG, Eaton SM, Lanthier PA, Burg E, Drew A, Bunn J, Suratt BT, Haynes L, Rincon M. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5:258–66. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lauder SN, Jones E, Smart K, Bloom A, Williams AS, Hindley JP, Ondondo B, Taylor PR, Clement M, Fielding C, Godkin AJ, Jones SA, Gallimore AM. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur J Immunol. 2013;43:2613–25. doi: 10.1002/eji.201243018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newton AH, Cardani A, Braciale TJ. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol. 2016;38:471–82. doi: 10.1007/s00281-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santillan Salas CF, Mehra S, Pardo Crespo MR, Juhn YU. Atopic conditions other than asthma and risk of the 2009 novel H1N1 infection in children: a case control study. Allergy Asthma Proc. 2013;34:459–66. doi: 10.2500/aap.2013.34.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fireman P. Virus-provoked rhinitis in patients who have allergies. Allergy Asthma Proc. 2002;23:99–102. [PubMed] [Google Scholar]
- 53.Gentile DA, Doyle WJ, Fireman P, Skoner DP. Effect of experimental influenza A infection on systemic immune and inflammatory parameters in allergy and non-allergic adult subjects. Ann Allergy Asthma Immunol. 2001;87:496–5000. doi: 10.1016/S1081-1206(10)62263-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Strategy for influenza A virus (Inf A) infection of A. alternata-challenged mice. Inf A was administered on day 0 via intranasal inoculation (50 μL, 1500 TCID50 units/mouse) to mice that had received A. alternata (500 μg/50 μL inoculum on days indicated or diluent only) on days −7, − 4 and −1 as indicated. B. Percent original weight per mouse. One death in the diluent only group, on day 15; n = 5 mice per group, *p < 0.05, Student’s t-test.