Abstract
Neonatal abstinence syndrome (NAS) is a condition affecting newborns that are exposed to an opioid in utero. In a randomized, controlled trial assessing the efficacy of buprenorphine and morphine in NAS, blood samples were analyzed from a subset of patients receiving buprenorphine along with NAS scores. The data were used to validate and adapt an existing model of buprenorphine in neonates and to identify relationships between buprenorphine or norbuprenorphine pharmacokinetics (PK) and efficacy or safety. The time to NAS stabilization was found to decrease with increasing buprenorphine exposure. This pharmacokinetic-pharmacodynamic (PK-PD) relationship was able to be quantified and adequately described with a mathematical model. The findings confirm a previous PK model of buprenorphine and extend the model to describe the PK of norbuprenorphine and to identify a novel PK-PD relationship of buprenorphine in NAS. This model will allow optimization of dosing strategies in future clinical trials.
Introduction
In utero exposure to xenobiotics which act on the central nervous system in some cases causes withdrawal symptoms in the post-natal period. While many exposures generate self-limited and mild manifestations, opioids can cause a severe and prolonged symptom complex. This neonatal abstinence syndrome, also known as the neonatal opioid withdrawal syndrome, results in signs including excessive crying, poor feeding, and disordered autonomic control.(1) The rate of NAS has increased from 1.2 per 1000 births in 2001 to 6 per 1000 births in 2013, with some states reporting rates as high as 33 cases per 1000 births.(2, 3) The continued upward vector of opioid use and morbidity since 2013 is geographically widespread and portends increasing rates of in utero opioid exposure.(4) All infants at risk of withdrawal are managed with non-pharmacologic therapies with the goals of managing symptoms, soothing irritability and facilitating normal weight gain patterns. However, up to 2/3 of infants require pharmacologic therapies to reach symptom control.(5) Opioids are the first line therapy for NAS.(6) Approximately 80% of US centers use morphine as primary therapy with the remainder using methadone.(7) Buprenorphine has been explored as a potential treatment in the neonatal abstinence syndrome.(8–10) While the exposure-response relationship for the efficacy and safety of buprenorphine in NAS remains largely undefined, in this study, we report the first pharmacokinetic (PK) and pharmacodynamic (PD) profile of buprenorphine in NAS from the Blinded Buprenorphine OR Neonatal morphine solution (BBORN) trial (ClinicalTrials.gov number NCT01452789).
Results
This analysis includes a total of 28 patients, 27 of which were exposed in utero primarily to methadone and 1 to buprenorphine. There were 265 observations of serum buprenorphine and norbuprenorphine; 44 samples of urine buprenorphine and buprenorphine glucuronide, urine norbuprenorphine and norbuprenorphine glucuronide; 4373 observations of NAS scores; and 7614 observations of respiratory rate. Further characteristics of the data are presented in Table 1.
Table 1.
Demographic Data
| Demographic Factors | Statistic | Value |
|---|---|---|
| N | Count | 28 |
| Female | Proportion | 39% |
| Birth Weight (kg) | Mean (SD) | 3.10 (0.43) |
| Age at Last Dose (days) | Mean (SD) | 21 (11.6) |
N=sample size, kg = kilograms, SD = standard deviation
Pharmacokinetic Models
First, we checked the concordance of the previously published model with the new serum PK data of buprenorphine. The model described the data with reasonable accuracy as displayed in Figure S1. The model had a mean squared error of 0.062 and a root mean squared error of 0.251.
Then, we removed the outliers and re-estimated the parameters using the same model structure of the older model and inter-subject variability (ISV). Certain parameters in the new model were fixed (ka, EMAX, BASE1) to the values published in the prior Ng model due to the sparse sampling and a lower range of patient ages compared to the older study. The lack of older patients may explain the relatively high values of relative standard error (RSE) on the volume parameters.
A 2-compartment model was sufficient to describe the serum buprenorphine PK data. This model utilized a combined additive and proportional intra-individual error structure. Generally, parameter values were similar to the original model with some slight alterations to the maturation functions. ISV was initially included on all parameters. However, removing ISV terms for the absorption constant (ka) and intercompartmental clearance (Q) did not result in a statistically significant increase in the objective function (p=1, likelihood ratio test). Thus, only ISV on total clearance (CL), central volume of distribution (V2), and peripheral volume of distribution (V3) were maintained in the final model. The parameter values are displayed in Table 2.
Table 2.
Parameter Estimates
| Parameters | Estimate | RSE% |
|---|---|---|
| Parent PK Model | ||
| Ka (hr−1) | 0.416 (FIX) | |
| CL (L/hr) | 203 | 12 |
| V2 (L) | 142 | 142 |
| Q (L/hr) | 1010 | 96 |
| V3 (L) | 6350 | 61 |
| KM (day) | 2.18 | 29 |
| SLP | 5 (FIX) | |
| EMAX | 0.477 (FIX) | |
| TF | 0.104 | 32 |
| KM1 (day) | 4.79 | 24 |
| SLP1 | 5 (FIX) | |
| BASE1 | 0.0268 (FIX) | |
| Additive Error | 0 | |
| Proportional Error | 0.58 | 6 |
| CL-ISV (%) | 49.9 | 17 |
| V2-ISV (%) | 363 | 53 |
| V3-ISV (%) | 74.1 | 12 |
| Metabolite PK Model | ||
| Fraction | 50% (FIX) | |
| V4 (L) | 2930 | 30 |
| CL40 (L/hr) | 187 | 12 |
| KM2 (day) | 6.99 | 17 |
| SLP2 | 5 (FIX) | |
| BASE2 | 0.479 | 23 |
| Additive Error | 0.010 | 48 |
| Proportional Error | 0.28 | 10 |
| V4-ISV (%) | 74.8 | 28 |
| CL40-ISV (%) | 50.9 | 13 |
| NAS PD Model | ||
| KNAS | 0.0724 | 32 |
| E2MAX | 1.25 | 58 |
| HILL | 1.36 | 55 |
| EC50 (ng/mL) | 0.509 | 78 |
| NASKM (day) | 0.0818 | 191 |
| NASHILL | 0.454 | 21 |
| NASMAX | 5.11 | 69 |
| DRUGK (1/day) | 0.56 | 28 |
| Additive Error | 2.32 | 3 |
| KNAS-ISV (%) | 0.0579 | 56 |
| NASKM-ISV (%) | 0.333 | 41 |
Ka = absorption rate constant, CL = clearance, V2 = volume of distribution of buprenorphine in the central compartment, V3 = volume of distribution of buprenorphine in the peripheral compartment, Q = intercompartmental clearance, KM/KM1= ontogeny maturation rate constants, SLP/SLP1/HILL = hill constant in sigmoidal model, EMAX = maximum hepatic function, TF = exponential growth factor, BASE1/2 = proportion of hepatic function at birth, ISV = inter-subject variability, V4 = apparent volume of distribution of norbuprenorphine, CL40 = apparent clearance of norbuprenorphine, KNAS = rate constant of NAS chnage, E2MAX = maximal pharmacodynamic effect of buprenorphine, EC50 = concentration producing half-maximal pharmacodynamics effect, NASKM, NASMAX = constant representing maximum rate of NAS worsening, NASHILL = constant describing slope of NAS course, DRUGK = rate constant representing the clearance of opioid transferred in utero, L=liters, hr=hours
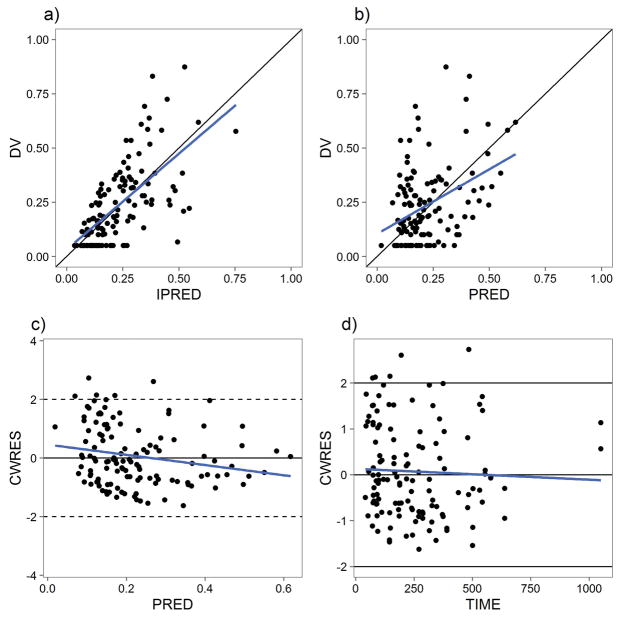
Diagnostic goodness-of-fit plots of the parent model are presented in Figure 1. We further evaluated the adapted parent model using predictive checks (PC) as shown in Figure S2. Figure S2A shows a visual predictive check (VPC). Figures S2B and S2C demonstrate the model’s performance in prediction of the peak and trough within a 95% population prediction interval. A prediction- and variability-corrected VPC (pvcVPC) is shown in Figure S3.
Figure 1.
Serum Buprenorphine PK Model Goodness-of-Fit Plots. A) Individual predictions (IPRED) vs observed values (DV). B) Population predictions (PRED) vs DV. C) Conditional weighted residuals (CWRES) vs PRED. D) CWRES vs TIME (hr).
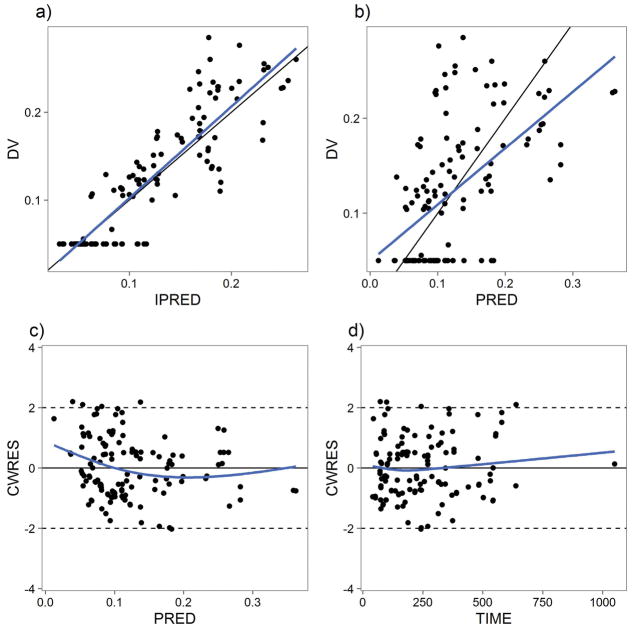
A 1-compartment model was sufficient to describe the serum metabolite, norbuprenorphine, concentration data. A 2-compartment model did not result in a significant decrease in the objective function (p =1, likelihood ratio test). Allometric scaling was used to relate weight to the volume of distribution and clearance parameters with exponents 1 and 0.75, respectively. ISV terms were used for both volume of distribution of norbuprenorphine (V4) and clearance of norbuprenorphine (CL40). Assuming that total mass balance was achieved in the urine samples obtained in patients, the fraction of buprenorphine converted to norbuprenorphine was fixed to 50%. Only one digit of significance was used to characterize the uncertainty inherent in the calculation based on the total molar ratios of buprenorphine, norbuprenorphine and glucuronides collected in the urine. A maturation function on CL40 was found to result in a statistically significant improvement in goodness of fit. The parameter values of the metabolite model are also shown in Table 2. The goodness-of-fit plot is shown in Figure 2. The VPC and pvcVPC are shown in Figures S4 and S5, respectively.
Figure 2.
Serum Norbuprenorphine PK Model Goodness-of-Fit Plots. A) IPRED vs DV. B) PRED vs DV. C) CWRES vs PRED. D) CWRES vs TIME(hr).
NAS PD Model
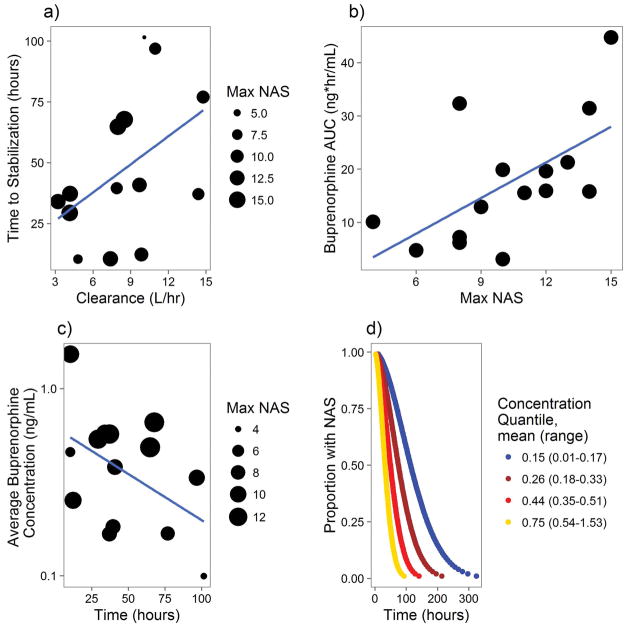
Next, the relationship between PK parameters and NAS disease course was analyzed. Patients who received phenobarbital or who breastfed from a mother taking buprenorphine were not included to avoid potential confounding. While buprenorphine and norbuprenorphine concentrations were highly correlated, buprenorphine was found to be a more significant driver of PD effects. The major source of variability between patients was the CL of buprenorphine. Neonates with a lower buprenorphine clearance had a shorter time to NAS stabilization (TNS) as shown in Figure 3A. Figure 3B demonstrates that the neonates with similar severity of NAS generally stabilized once they were exposed to a similar area under the curve (AUC) of buprenorphine. Furthermore, for any given group of NAS severity, neonates exposed to higher concentrations of buprenorphine tended to stabilize faster as shown in Figure 3C.
Figure 3.
Buprenorphine-NAS PK-PD Relationship. A) Relationship of Average Buprenorphine Clearance and Time to NAS Stabilization (TNS). The size of each dot represents each neonate’s maximum NAS Score. B) Relationship between Buprenorphine AUC and NAS Severity. Each neonate included is represented by a point, which represents the maximum NAS score and AUC of buprenorphine until that time. C) Relationship between TNS and Average Buprenorphine Concentration. Each neonate included is represented by a point, which represents the TNS and average concentration of buprenorphine until that time. D) NAS Survival Analysis. This graph represents the predicted stabilization of NAS over time for a theoretical neonate at one of the 4 quartiles of buprenorphine concentration and a max NAS score of 11.
There was a negative linear relationship between average concentration (Cave) and TNS, which provides more evidence that increasing the buprenorphine Cave can decrease TNS. This was further analyzed in a parametric survival regression model with the infants divided into quartiles of average concentration of buprenorphine from the first dose to stabilization with NAS severity included as a covariate. Cave was a statistically significant predictor of shorter TNS (p =0.002). Figure 3D shows the survival model prediction of TNS at each quartile of Cave given a peak average NAS severity of 11, which was close to the median in the study. Based on the analysis and the concentrations obtained in the study, average concentration of 0.8 ng/mL was chosen as a PK target for simulation. 0.8 ng/mL reflects the average concentration in the highest concentration quartile from the survival analysis rounded to 1 significant figure to reflect the uncertainty in the calculation.
A buprenorphine-NAS PK-PD model was built using the system of differential equations shown below:
where DNAS/DT represents the change in NAS score. KNAS is a first-order rate constant that represents the natural improvement of NAS with respect to time; WITHD represents the time course of onset of withdrawal as the opioid of abuse is removed from the neonate’s system with PNA (day) and DRUGK (1/day) that represent the neonate’s postnatal age and the rate of removal of the xenobiotic, respectively. NAST represents the increase in withdrawal as a function of time with NASMAX representing the severity of withdrawal while KMNAS contributes to the speed of increase of withdrawal symptoms.
E represents the effect of buprenorphine in the amelioration of NAS based on C2, the concentration of buprenorphine in the central compartment assuming rapid equilibration with the central nervous system. EC50 refers to the concentration producing half maximal response, and E2MAX refers to the theoretical maximum response. The effect of buprenorphine is described using a sigmoid Emax relationship.
In this disease model, ISV terms were used on KNAS and KMNAS, and an additive intra-individual error model was used. Figure 4 shows the goodness-of-fit plots for the NAS disease model. Figure S6 demonstrates the ability of the PD model to describe the disease course in a VPC and in a PC to predict observed TNS. Figure S7 shows the pvcVPC of the PK-PD model.
Figure 4.
Buprenorphine-NAS PK-PD Model Goodness-of-Fit Plots. A) IPRED vs DV. B) PRED vs DV. C) CWRES vs PRED. D) CWRES vs time.
Respiratory Rate Analysis
No enrolled subjects experienced clinical respiratory depression in the BBORN trial.(11) The relationship between PK parameters and respiratory rate was analyzed graphically and with a regression model. There was no relationship between observed concentration of buprenorphine and respiratory rate [data not shown]. Hysteresis plots and patient-level data were also analyzed but showed no relationship between buprenorphine or norbuprenorphine concentration and respiratory rate [data not shown].
Dose Selection
The survival analysis suggested a buprenorphine concentration target of 0.8 ng/mL and the respiratory rate analysis indicated that there was no major safety signal at the concentrations observed in this study. Based on the following calculation,
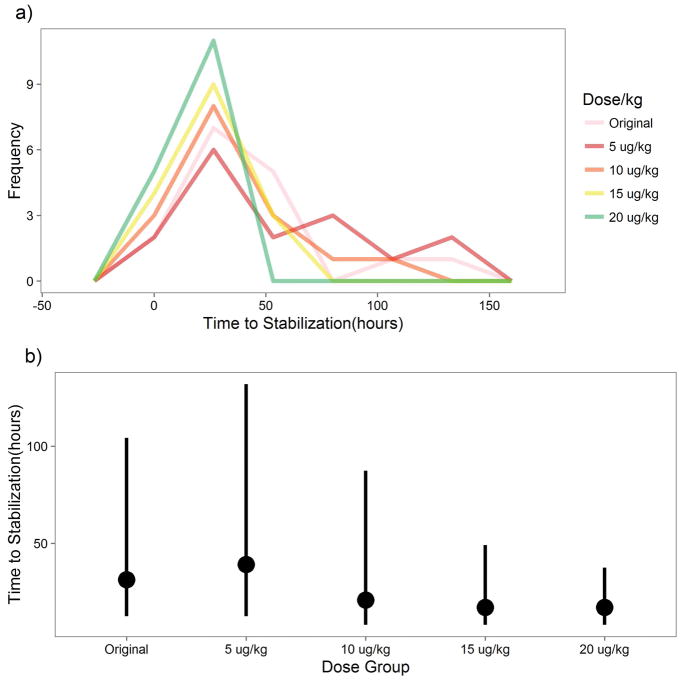
with the CL calculated based on the observed ontogeny at the specific dose time, a dose of 15 μg/kg q8 hour would reach the target concentration in most patients. Accordingly, doses from 5 μg/kg – 20 μg/kg q8 were analyzed for time to stabilization using the combined PK-PD model. The results are shown in Figure 5. Figure 5A shows the predicted TNS for each dose regimen. 15 and 20 μg/kg q8 appear to result in the fastest TNS as one might expect. However, Figure 5B further demonstrates that the medians of the higher doses are very similar indicating that even the 10 μg/kg q8 dose is sufficient for the majority of neonates but may need to be titrated upwards for neonates with higher clearance or more severe NAS.
Figure 5.
NAS Dose Simulation. A) This is a frequency polygon plot representing the prediction of when each neonate would have stabilized if given a different dose relative to the original based on simulations from the PK-PD model. B) This point range plot shows the median and 95% confidence interval of time to stabilization by dose based on the PK-PD simulation. Here, “Original” refers to the dosing performed in the study.
Discussion
In this analysis, we were able to build a PK-PD model for buprenorphine in infants with NAS. The diagnostic plots showed good agreement between the observed and predicted values of the data in the PK models of serum buprenorphine and norbuprenorphine. With respect to the buprenorphine-NAS PK-PD model, the NAS score data have high variability due to the inherently subjective nature of the NAS scoring system, and temporal variability of environmental cues such as maternal presence and feeding. However, there is good agreement between the trends in the observed and predicted values of NAS scores. The conditional weighted residuals (CWRES) plots reveal no systemic bias.
This is the first study to use population pharmacokinetic (PopPK) techniques to analyze the PK of norbuprenorphine in neonates and to categorize the PD of buprenorphine in NAS. This PK-PD model was well able to describe the variability in the observed concentrations of buprenorphine, norbuprenorphine, and the NAS scores. The model focused specifically on the PD endpoint of time to stabilization of NAS scores, and not on the exposure-response relationship during the weaning phase. It is unclear if a quicker time to symptom stabilization changes the speed with which doses can be reduced during the weaning phase.
The buprenorphine PK model was largely similar to the one previous PopPK model of the drug in infants.(12) The norbuprenorphine PK model was structured as a one-compartment model. However, it would appear that norbuprenorphine follows a two-compartment disposition that was unable to be elucidated in this sample due to sparse sampling. Although a steady-state equilibrium may not be able to be reached as the CL is changing rapidly in the neonatal population, we can consider that V4 is a pseudo-steady state volume (Vss) of norbuprenorphine, the combination of norbuprenorphine central and peripheral volume of distribution. At 3760 L, V4 (presumed Vss of norbuprenorphine) was smaller than the Vss of buprenorphine, 7600 L. This is physiologically plausible as buprenorphine is converted to norbuprenorphine by N-dealklyation, which results in a more polar compound that distributes to the tissues less. Furthermore, the clearance of norbuprenorphine was higher than buprenorphine and possessed a faster maturation function, which is also typical as the purpose of Phase I oxidation is generally to make xenobiotics more polar and therefore easier to eliminate renally through the urine.
While the disease model was not based on data from untreated patients, it was able to predict clinically relevant features such as the time to stabilization and the peak NAS score. The disease model suggests that neonatal abstinence syndrome of median severity (peak rolling average of 12 NAS scores equivalent to 11) without medical treatment lasts for approximately 12 days until stabilization, which is a reasonable estimate clinically for a subset of NAS patients. Furthermore, our results for the PD of buprenorphine are in concordance with a previously published PD model of buprenorphine in adult patients.(13) Our results have similar Hill coefficients: 1.21 in the Nasser study and 1.36 in the present study. The adult Nasser study identified an EC50 of 0.67 ng/mL, while the present pediatric study identified an EC50 of 0.509. This discrepancy may exist because the pediatric population was exposed to a lower range of concentrations, which may have prevented a full characterization of the PD relationship. Overall, this lends external validity to this study’s central PD relationship. This also suggests that the PD relationship in adults is similar to the relationship in pediatrics.
Although the study was not explicitly designed for dose selection, the PD model and the PK-PD analysis establish a PK target for neonatal abstinence syndrome stabilization. An AUC0-inf of 40 ng-hr/mL appeared to be required for NAS stabilization for moderately severe NAS, and higher concentrations produced shorter time to stabilization and control of symptoms. An initial dose of 15 μg/kg q8 reached the 0.8 ng/mL target within two days for a majority of patients and is shown to be an effective dose in the simulation. However, little is known about the proper titration strategy. In this sample of patients, the NAS stabilization was driven by exposure, which was affected by clearance. Neonates with lower clearances were treated adequately at lower doses of the drug. Thus, there was no data on slow-clearing neonates with higher doses.
While respiratory depression was not seen in neonates at the buprenorphine and norbuprenorphine exposures in this study, there is no data to suggest that higher doses in infants with relatively slow clearance would not cause respiratory depression in those patients. The use of a higher initial dose would also result in overtreatment in a fraction of infants who would not otherwise have required up-titration and thus could have weaned more quickly from a lower initial dose. Adult data has suggested control of withdrawal symptoms at a buprenorphine concentration of 0.7 ng/mL.(14) This value is similar to the survival analysis-derived target of 0.8 ng/mL in the current study. What is also striking is the high correlation between symptom control and buprenorphine concentration, suggesting drug exposure as the primary driver of response rather than other pharmacodynamically driven host factors.
Overall, buprenorphine presents a new therapeutic agent for NAS. In the BBORN trial, infants treated with buprenorphine had a median length of treatment of 15 days compared to 28 days with morphine. Our pharmacometric analysis further clarifies the PK of buprenorphine in neonates and establishes a strong PD relationship in the time to stabilization of NAS symptoms. This analysis can be used to design and eventually test a rational optimal treatment regimen using this agent. The analysis did not address optimization of weaning. However, the model allows for a rational approach to weaning that could include changing the frequency of dosing in the weaning phase. Potentially, a twice-daily or even once-daily dosing strategy to facilitate outpatient weaning could be used.
Methods
Study Protocol
The BBORN trial was a randomized, double blind, double dummy, clinical trial comparing sublingual buprenorphine to oral morphine for the treatment of the neonatal abstinence syndrome.(15) This study was performed at Thomas Jefferson University Hospital, Philadelphia, PA, USA. The clinical protocol and informed consent documents were approved by the university Institutional Review Board. Infants were eligible for participation in the study if they were ≥ 37 weeks of gestation, were exposed to opiates in utero and demonstrated signs and symptoms of NAS requiring treatment. Infants with exposure to benzodiazepines and those with major congenital malformations, intrauterine growth retardation, medical illness, concomitant use of CYP3A inhibitors, hypoglycemia, hyperbilirubinemia, or seizures were excluded from the study. Breast feeding infants were not eligible until the approval of a protocol amendment on October 17, 2013.
Consent was obtained from the parents of infants at risk for neonatal abstinence syndrome. All infants were monitored using the MOTHER NAS Scale, a modified Finnegan scoring instrument.(16) Infants were randomized to either buprenorphine or morphine if they had sum of 3 scores ≥24 or a single score ≥12. The neonates allocated to the buprenorphine group were treated with buprenorphine 5.3 μg/kg every eight hours. Doses were uptitrated by 25% for inadequate symptom control up to a maximum dose of 20 μg/kg. If symptoms were not controlled at this dose, a phenobarbital 20 mg/kg loading dose followed by daily 5 mg/kg was added. Once the infant was stabilized with an average score below 8 for at least 2 days, the dose was tapered at a rate of 10% per day until the dose was within 10% of the starting dose. At that point, the baby was discontinued from buprenorphine and monitored for at least 2 days before discharge.
Blood for PK analysis was drawn in all patients in the study using a sparse sampling regimen. At least one peak blood sample was drawn within 24 hours of initiation of therapy. Random samples were drawn either as a peak and trough surrounding a single dose or as a mid-dose interval timepoint. Samples were obtained by heel stick into lithium heparin pediatric tubes (BD microtainer, Ref # 365971). Blood was spun at 3000 RPM in a refrigerated centrifuge for 10 minutes. Plasma was collected, transferred to storage tubes and frozen at −70 C. Buprenorphine, norbuprenorphine and their urinary glucuronide concentrations were analyzed using previously described liquid chromatography/tandem mass spectrometry methods.(9, 12, 17) The limit of quantification was 0.1 ng/mL for buprenorphine, norbuprenorphine, and their respective glucuronides. When it did not impact clinical care, urine was collected from 4 hour bagged samples in the first and second weeks. Urine collection was subject to interruption or leakage, and some collections represented less than 4 hour collection time.
Pharmacokinetic Analysis
Data preparation, summary statistics and other statistical analysis were performed using R version 3.2.2. NONMEM 7.3, with the gfortran 4.9.2 compiler, was used for population pharmacokinetic and pharmacodynamic analyses. Exploratory analysis of output and diagnostic plots was accomplished using R and xpose4 version 4.6.0. The modeling workflow was handled using Pirana 2.9.2.(18)
The first-order conditional estimation (FOCE) method was used to estimate the fixed-effect and random-effect parameters. FOCE with interaction was used if the model utilized a proportional or combined residual error model. Unless otherwise noted, model goodness-of-fit was evaluated for appropriateness using diagnostic goodness-of-fit plots after successful parameter search, likelihood ratio tests and convergence diagnostics. A nested model evaluated by the likelihood ratio test was considered statistically significant if the objective function value decreased by more than 3.84 (p < 0.05). To avoid bias and to aid in model convergence, outliers were identified as observations that produced an absolute value of CWRES greater than 2.5 and were removed from the final model. Residual or IIV was modeled using an additive, proportional or combined error structures. ISV was assessed for all models using an exponential error structure.
Models were evaluated using internal validation techniques. A visual predictive check (VPC) was used to assess the ability of the model to predict the variability in observed concentrations.(19) The model was used to create 1000 replicate concentration datasets using Monte Carlo simulation. A prediction- and variability-corrected VPC (pvcVPC) was also used to attempt to correct for inter-subject differences.(20) These simulated datasets were then compared to the original observed data. In addition, predictive checks (PC) were used to ensure that the model accurately predicted clinically relevant features of the data. Values calculated from the simulated datasets were compared to values from the observed dataset.
PK Model Development
A population pharmacokinetic model was previously built based on observations of serum buprenorphine concentration in infants and adults.(12) This model featured a 2-compartment disposition model of buprenorphine with body-weight based allometric scaling on V2, V3, Q and CL. In addition, the model bridged PK between neonates and adults using maturation functions on CL and V3. This model was adapted to the current dataset by re-estimating all parameters given the new data with the allometric exponents fixed to 0.75 for Q and CL and to 1 for V2 and V3.
Once the parent buprenorphine model was fit to the data in the study, it was extended to a serum parent-metabolite PK model. The metabolite model was built using a sequential fit strategy; the individual predictions of the buprenorphine concentrations were used to drive the predicted concentration of norbuprenorphine. In addition, maturation functions on compartmental volumes of distribution and clearances were attempted to describe neonatal ontogeny.
PD Model Development
After the serum parent and metabolite models were deemed sufficient, the individual patient-level PK parameters for buprenorphine and norbuprenorphine were analyzed graphically for a relationship with TNS. For the purposes of standardization, NAS stabilization was considered to occur when the average of the previous 12 NAS scores decreased below 8. Trends were analyzed between TNS and PK parameters including CL, AUC, Cave, and maximum concentration (Cmax).
Using the insight from analyzing the relationship between summary buprenorphine PK parameters and TNS, a PD model was developed. The effective concentration for the NAS disease model was assumed to be equal to concentration of buprenorphine in V2, following the assumption that a rapid equilibrium exists between the blood and the brain.. Both buprenorphine and norbuprenorphine concentrations were considered as potential drivers of the PD effects separately. In adults, norbuprenorphine has limited nociceptive activity due to limited penetration into the CNS because it is a substrate of the efflux transporter P-glycoprotein (P-gp).(21) P-gp is not fully developed in neonates,(22) which may signify that norbuprenorphine contributes to pharmacodynamic activity.
Next, the data were analyzed to potentially identify a relationship between buprenorphine or norbuprenorphine concentration and respiratory rate. Similar to traditional opioids, the major expected adverse event in patients treated with buprenorphine is respiratory depression. In neonates, respiratory depression was considered to be a consistent respiratory rate of less than 30 breaths per minute, which was adapted as a lower bound of normal respiratory rate in infants from a systematic review.(11) This relationship was analyzed using concentration-response plots and hysteresis plots both in the full sample and on the patient level.
Dose Selection
The knowledge gained from the PK-PD model was used to select a dosing strategy. The NAS stabilization survival curve provides a PK target for simulation while the respiratory rate analysis gives an upper limit representing potential toxicity. For the purpose of this analysis, the conditional estimates of PK and PD parameters were used in the simulation. This allowed the exploration of the question of how the neonates would have responded differently with different treatment regimens, which in turn can be used to suggest new dosing strategies for future study.
Supplementary Material
Figure S1. Validation of Previous Serum Buprenorphine PK Model
Figure S2. Serum Buprenorphine PK Model Predictive Checks
Figure S3. Serum Buprenorphine PK Model pvcVPC
Figure S4. Serum Norbuprenorphine PK Model VPC
Figure S5. Serum Norbuprenorphine PK Model pvcVPC
Figure S6. Serum Buprenorphine-NAS PK-PD Model Predictive Checks
Figure S7. Buprenorphine-NAS PK-PD Model pvcVPC
Table S1. Urine Pharmacokinetic Data
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The pharmacokinetics (PK) of buprenorphine in infants with neonatal abstinence syndrome (NAS) has been reported on previously. It is known to have beneficial effects in NAS.
WHAT QUESTION DID THE STUDY ADDRESS?
This study addressed the PK of buprenorphine and its metabolite norbuprenorphine and related the PK profile to the pharmacodynamics (PD).
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study furthers our understanding of buprenorphine PK in neonates with a special focus on maturation of clearance. It also presents a novel disease model for NAS to link buprenorphine PK to therapeutic effect.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study adds supportive evidence for the efficacy of buprenorphine in NAS by establishing an exposure-response relationship for therapeutic success. Awareness of this relationship may be essential in the further exploration of the dose. The novel disease model identified in this study may also be useful for other medications in NAS.
Acknowledgments
Funding Information
Jason Moore was supported by NIH grant T32GM008562. The study was supported by the National Institute for Drug Abuse R01 DA029076.
Footnotes
Conflict of Interest Statement
Dr. Kraft is an unpaid consultant to Chiesi. The authors have no other conflicts of interest to disclose.
Author Contributions
J.M. and W.K. wrote the manuscript; W.K., M.E., and S.A. performed the research; J.M., M.G., and C.N. analyzed the data; D.M. and W.F. contributed new reagents/analytical tools.
References
- 1.Hudak ML, Tan RC. Neonatal drug withdrawal. Pediatrics. 2012;129:e540–60. doi: 10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- 2.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307:1934–40. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 3.Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD. Incidence of Neonatal Abstinence Syndrome - 28 States, 1999–2013. MMWR Morb Mortal Wkly Rep. 2016;65:799–802. doi: 10.15585/mmwr.mm6531a2. [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell JK, Gladden RM, Seth P. Trends in Deaths Involving Heroin and Synthetic Opioids Excluding Methadone, and Law Enforcement Drug Product Reports, by Census Region - United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:897–903. doi: 10.15585/mmwr.mm6634a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolia VN, et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med. 2015;372:2118–26. doi: 10.1056/NEJMsa1500439. [DOI] [PubMed] [Google Scholar]
- 6.Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2010:CD002059. doi: 10.1002/14651858.CD002059.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Patrick SW, Kaplan HC, Passarella M, Davis MM, Lorch SA. Variation in treatment of neonatal abstinence syndrome in US children’s hospitals, 2004–2011. J Perinatol. 2014;34:867–72. doi: 10.1038/jp.2014.114. [DOI] [PubMed] [Google Scholar]
- 8.Kraft WK, Dysart K, Greenspan JS, Gibson E, Kaltenbach K, Ehrlich ME. Revised dose schema of sublingual buprenorphine in the treatment of the neonatal opioid abstinence syndrome. Addiction. 2011;106:574–80. doi: 10.1111/j.1360-0443.2010.03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraft WK, et al. Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial. Pediatrics. 2008;122:e601–7. doi: 10.1542/peds.2008-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall ES, et al. A Cohort Comparison of Buprenorphine versus Methadone Treatment for Neonatal Abstinence Syndrome. J Pediatr. 2016;170:39–44e1. doi: 10.1016/j.jpeds.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Fleming S, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377:1011–8. doi: 10.1016/S0140-6736(10)62226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng CM, et al. Population Pharmacokinetic Model of Sublingual Buprenorphine in Neonatal Abstinence Syndrome. Pharmacotherapy. 2015;35:670–80. doi: 10.1002/phar.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laffont CM, Gomeni R, Heidbreder C, Jones JP, 3rd, Nasser AF. Population Pharmacokinetic Modeling After Repeated Administrations of RBP-6000, a New, Subcutaneously Injectable, Long-Acting, Sustained-Release Formulation of Buprenorphine, for the Treatment of Opioid Use Disorder. J Clin Pharmacol. 2016;56:806–15. doi: 10.1002/jcph.665. [DOI] [PubMed] [Google Scholar]
- 14.Kuhlman JJ, Jr, Levine B, Johnson RE, Fudala PJ, Cone EJ. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction. 1998;93:549–59. doi: 10.1046/j.1360-0443.1998.93454910.x. [DOI] [PubMed] [Google Scholar]
- 15.Kraft WK, et al. Buprenorphine for the Treatment of the Neonatal Abstinence Syndrome. N Engl J Med. 2017;376:2341–8. doi: 10.1056/NEJMoa1614835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaltenbach K, Jones HE. Neonatal Abstinence Syndrome: Presentation and Treatment Considerations. J Addict Med. 2016;10:217–23. doi: 10.1097/ADM.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 17.Huang W, Moody DE, McCance-Katz EF. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-electrospray ionization-tandem mass spectrometry. Ther Drug Monit. 2006;28:245–51. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- 18.Keizer RJ, Karlsson MO, Hooker A. Modeling and Simulation Workbench for NONMEM: Tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol. 2013;2:e50. doi: 10.1038/psp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn. 2001;28:171–92. doi: 10.1023/a:1011555016423. [DOI] [PubMed] [Google Scholar]
- 20.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown SM, Campbell SD, Crafford A, Regina KJ, Holtzman MJ, Kharasch ED. P-glycoprotein is a major determinant of norbuprenorphine brain exposure and antinociception. J Pharmacol Exp Ther. 2012;343:53–61. doi: 10.1124/jpet.112.193433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam J, et al. The ontogeny of P-glycoprotein in the developing human blood-brain barrier: implication for opioid toxicity in neonates. Pediatr Res. 2015;78:417–21. doi: 10.1038/pr.2015.119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Validation of Previous Serum Buprenorphine PK Model
Figure S2. Serum Buprenorphine PK Model Predictive Checks
Figure S3. Serum Buprenorphine PK Model pvcVPC
Figure S4. Serum Norbuprenorphine PK Model VPC
Figure S5. Serum Norbuprenorphine PK Model pvcVPC
Figure S6. Serum Buprenorphine-NAS PK-PD Model Predictive Checks
Figure S7. Buprenorphine-NAS PK-PD Model pvcVPC
Table S1. Urine Pharmacokinetic Data