Abstract
The hippocampus may play a role in categorization because of the need to differentiate stimulus categories (pattern separation) and to recognize category membership of stimuli from partial information (pattern completion). We hypothesized that the hippocampus would be more crucial for categorization of low-density (few relevant features) stimuli--due to the higher demand on pattern separation and pattern completion--than for categorization of high-density (many relevant features) stimuli. Using a touchscreen apparatus, rats were trained to categorize multiple abstract stimuli into two different categories. Each stimulus was a pentagonal configuration of five visual features; some of the visual features were relevant for defining the category whereas others were irrelevant. Two groups of rats were trained with either a high (dense, n = 8) or low (sparse, n = 8) number of category-relevant features. Upon reaching criterion discrimination (≥ 75% correct, on 2 consecutive days), bilateral cannulas were implanted in the dorsal hippocampus. The rats were then given either vehicle or muscimol infusions into the hippocampus just prior to various testing sessions. They were tested with: the previously trained stimuli (trained), novel stimuli involving new irrelevant features (novel), stimuli involving relocated features (relocation), and a single relevant feature (singleton). In training, the dense group reached criterion faster than the sparse group, indicating that the sparse task was more difficult than the dense task. In testing, accuracy of both groups was equally high for trained and novel stimuli. However, both groups showed impaired accuracy in the relocation and singleton conditions, with a greater deficit in the sparse group. The testing data indicate that rats encode both the relevant features and the spatial locations of the features. Hippocampal inactivation impaired visual categorization regardless of the density of the category-relevant features for the trained, novel, relocation, and singleton stimuli. Hippocampus-mediated pattern completion and pattern separation mechanisms may be necessary for visual categorization involving overlapping irrelevant features.
Keywords: Categorization, generalization, discrimination, reversible inactivation, touchscreen, learning
1 | INTRODUCTION
Categorization is fundamental for organisms’ adaptively responding to stimuli and events. Perceptual or similarity-based categorization has been operationally defined as responding equivalently to members of the same stimulus class, responding differently to members of different stimulus classes, and transferring appropriate responding to novel members of these classes. An extensive literature exists on the empirical and theoretical analysis of perceptual categorization in humans and other species (Ashby & Valentin, 2017; Kruschke, 1992; Love, Medin, & Gureckis, 2004; Sloutsky, 2010; Smith et al., 2012). Rodents have only recently been used in visual categorization research, but several studies have found that rats can discriminate complex visual stimuli and learn perceptual categories (Brooks et al., 2013; Furtak, Ahmed, & Burwell, 2012; Vermaercke, Cop, Willems, D’Hooge, & Op de Beeck, 2014; Vinken, Vermaercke, & Op de Beeck, 2014; Wasserman, Castro, & Freeman, 2012; Zoccolan, 2015). For example, a study from our group found that rats learned to categorize photographs of flowers, chairs, cars, and humans (Brooks et al., 2013). These perceptual categorization studies in animals demonstrate robust category learning and transfer to novel stimuli, consistent with human perceptual categorization.
Neural mechanisms of perceptual categorization have been incorporated into a computational theory called COVIS (Ashby & Valentin, 2017). According to COVIS, there are two neural systems for category learning that map onto declarative and procedural (nondeclarative) memory. The declarative memory system is posited to be necessary for category learning based on a single rule or ruled-based (RB) learning, whereas category learning that requires the integration of two or more dimensions or information integration (II) learning is mediated by the procedural memory system. COVIS hypothesizes that RB category learning depends on the prefrontal cortex, hippocampus, and the anterior caudate nucleus; II learning, on the other hand, requires corticostriatal circuitry.
Evidence supporting a role for the hippocampus in RB category learning comes primarily from studies of humans with medial temporal lobe (MTL) amnesia and from studies involving functional imaging. Humans with MTL amnesia can learn RB categories with a simple rule and II categories, but they have difficulty when the category task is more complicated, where identification of the rule may exceed the capacity of working memory (Ashby & Valentin, 2017). Humans show differential activation of the hippocampus during RB category learning and in the caudate during II category learning (Nomura et al., 2007). Hippocampal activation is also seen in humans performing II tasks when conscious recall is engaged (Seger, Dennison, Lopez-Paniagua, Peterson, & Roark, 2011).
The hippocampal activation observed in human imaging studies is consistent with the activity of single CA1 and CA3 neurons in monkeys performing a delayed matching-to-sample task (Hampson, Pons, Stanford, & Deadwyler, 2004), which can be characterized as a RB task. Hippocampal neurons showed incidental category responding to perceptually similar stimuli used in the task. When probe trials were given with altered versions of the stimuli, individual monkeys differed in the altered stimuli to which they responded and so too did hippocampal neurons. The findings of the human imaging and monkey neurophysiology studies support the hypothesis of COVIS that the hippocampus plays a role in perceptual categorization with visual stimuli, especially under conditions that require explicit rule learning.
The discrimination and generalization mechanisms involved in categorization can be related to pattern separation and pattern completion mechanisms within the hippocampus (Rolls, 2016). Discrimination in category learning requires the development of distinct representations, which could be facilitated by pattern separation, especially when the exemplars of categories have overlapping features. Generalization to novel exemplars usually necessitates the retrieval of category-defining information based on partial input. Moreover, the ability to use pattern completion in categorization tasks obviates the need to remember each individual stimulus.
Numerous studies indicate that pattern separation is initially mediated by the dentate gyrus (DG) (Rolls, 2016). Selective lesions or blockade of neurogenesis in the DG produce impairments in tasks requiring discrimination between relatively similar stimuli or events (Gilbert, Kesner, & Lee, 2001; Hunsaker & Kesner, 2008; Hunsaker, Rosenberg, & Kesner, 2008; Morris, Churchwell, Kesner, & Gilbert, 2012). Pattern completion requires the CA3 region of the hippocampus, with lesions impairing memory based on partial input during retrieval (Gold & Kesner, 2005; Hunsaker & Kesner, 2008; Hunsaker et al., 2008). Neurophsyiological recordings from the DG show distinctive coding and sensitivity to changes in perceptual input, whereas neurons in CA3 are relatively insensitive to changes in sensory input and to degraded input from the DG for spatial representations (Guzowski, Knierim, & Moser, 2004; Leutgeb, Leutgeb, Moser, & Moser, 2007; Leutgeb et al., 2005; Neunuebel & Knierim, 2014). These findings suggest that both category learning and category generalization should depend on the hippocampus (Rolls, 2016). However, selective lesions or reversible inactivations have not yet been deployed to demonstrate a causal role of hippocampal processing in visual categorization.
The current study examined the role of the hippocampus in visual categorization by rats using reversible inactivation with muscimol. Rats were trained in a touchscreen apparatus to discriminate two categories of stimuli with five features that were manipulated to alter category structure and to test the nature of the category representation. A prior study in human categorization reported that category structure may determine which mechanisms are used to learn categories (Kloos & Sloutsky, 2008): Different learning mechanisms were engaged depending on whether the category structure contained many (i.e., dense) or few (i.e., sparse) category relevant features. Here, we examined whether category density played a role in determining whether the hippocampus is crucial for categorization by rodents.
Different groups of rats were therefore trained with either dense or sparse categories. The dense categories had 3 category-relevant features and 2 irrelevant features. The sparse categories had 1 category-relevant feature and 4 irrelevant features. Our hypothesis was that the hippocampus would be more crucial for categorization with sparse stimuli because there are fewer relevant features to help distinguish categories, placing a heavier load on pattern separation. Moreover, fewer category-relevant features should place a higher load on pattern completion as well, when rats are presented with novel test stimuli. After the rats reached a discrimination criterion, several test sessions were given with muscimol or vehicle infusions into the dorsal hippocampus. Test trials included novel exemplars of the categories, rearranged stimuli where some of the relevant and irrelevant features changed spatial locations, and presentations of a single relevant feature in a novel spatial location within the stimulus area. The findings that we report indicate that the dorsal hippocampus is crucial for categorization of both dense and sparse categories.
2 | MATERIALS AND METHODS
2.1 | Animals
Subjects were 27 male Long-Evans rats (300 – 350 g); 16 rats were randomly assigned to either the dense or the sparse groups (n = 8 per group) and the remaining 11 rats were trained and tested with a control discrimination task. All rats were individually housed in standard rodent cages on a 12 hr light/dark cycle. All training and testing sessions were conducted during the light phase. Rats were food-restricted to a minimum of 85% free-feeding weight and water was available ad libitum. The Institutional Animal Care and Use Committee at the University of Iowa approved all procedures.
2.2 | Categorization task
2.2.1 | Behavioral Apparatus and Visual Stimuli
The training chamber was 36 (w) × 41 (d) × 36 (h) cm in size. Inside the chamber, a metal mesh grid (34 [w] × 39 [d] × 5 [h] cm in size) was placed on the floor. An aluminum food tray (6.5 [w] × 13 [d] × 4.5 [h] cm in size) was located on the wall opposite the touchscreen. A rotary pellet feeder (Med Associates Inc., Georgia, VT, model ENV-203IR) delivered a 45-mg food pellet into the food tray. A small house light was above the food tray and was illuminated throughout the behavioral sessions. Both feeder and house light were controlled by a relay controller (Model RS-232; National Control Devices, Osceola, MO). An infrared touchscreen panel (15-in., EloTouch Systems, Fremont, CA) was positioned in front of an LCD flat-screen monitor (Model 1550V, NEC, Melville, NY). The touchscreen panel, visual stimuli on the LCD monitor, and relay controller were controlled by custom-written Matlab codes (Matworks, Natick, MA) with PsychToolbox extensions (Brainard, 1997; Pelli, 1997) for the Apple iMac (Model iMac 10.1, Apple, Cupertino, CA). White noise was played during all of the training/testing sessions to mask extraneous noise.
All category stimuli and a white reward stimulus (RGB: 255 255 255) were 238 × 238 pixels in size. The trial-initiating star stimulus (white star on a black background) was 150 × 150 pixels in size. All visual stimuli appeared on a uniform gray background (RGB: 200 200 200) of 1024 × 768 pixel resolution.
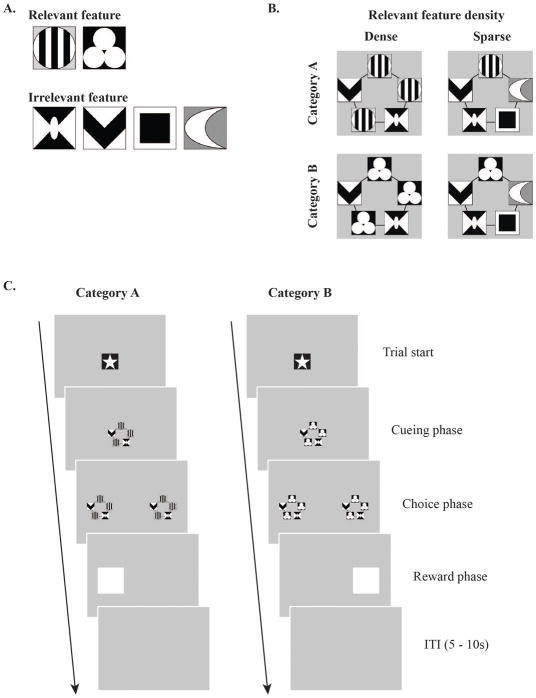
Each category stimulus was composed of five visual feature holders (VFHs). The five VFHs formed a pentagon shape on top of the 238 × 238 pixel square space. In each VFH, either category-relevant or category-irrelevant features appeared, depending on the training and testing conditions. In terms of category density, the category feature appeared in either three (dense) or one (sparse) of the five VFHs. In the dense condition, the top, top-right, and bottom-left VFHs were used for category-relevant feature presentations. In the sparse condition, only the top VFH was used for the category-relevant feature presentation. A grating of vertical black and white stripes and a triangular arrangement of 3 white circles were used as the category-relevant features (Fig. 1A). Other visually distinctive geometrical patterns were presented as category-irrelevant features; they were presented with the same probability in both categories. The combinations of category-relevant and category-irrelevant features resulted in a total of 12 exemplars in each category, in both category density groups (Fig. 1A and Supplementary Fig. 1A). The frequency of all visual features’ appearance during the experiment was controlled and counterbalanced within subjects as well as between subjects.
Figure 1. Visual category stimuli, task design, and sample trial sequence.
A. Visual features were used as either category-relevant or category-irrelevant features. Category-relevant features defined each category, whereas category-irrelevant features appeared in both sets of exemplars (12 for each category). Across category-feature density conditions (dense or sparse), 3 or 1 category-relevant feature(s) appeared with 2 or 4 category-irrelevant features, respectively. Occurrences of the category-irrelevant features were controlled and counterbalanced. B. Each trial began with the white star in the center of the screen (Trial start). After a single touch, a category cue stimulus appeared on the screen and the rat had to touch it three times (cueing phase) to initiate the choice phase. Touch coordinates on the cue category stimuli were recorded for later analyses. During the choice phase, the category cue stimulus appeared on both left and right sides of the screen and the rat was required to touch one of them for food reward depending on which category cue was given (e.g., left for category A and right for category B, counterbalanced across the rats). A reward was delivered to the food tray on correct trials and a penalty ITI (10 ~ 15 s) was given on error trials (Reward/Penalty phase). Before the rat reached the learning criterion for testing, corrections were given for every error trial in which the trial repeated from the cueing phase until the rat selected the correct choice. After a 3 s ITI, the next trial started.
2.2.2 | Handling/Shaping
Specific handling and shaping procedures were described in a previous publication (Kim, Wasserman, Castro, & Freeman, 2016). Briefly, all rats were individually handled daily under food restriction after a 1-week acclimation period. The rats were then encouraged to forage for rodent food pellets (45-mg; MLab Rodent Tablet 45 mg, TestDiet Inc., IN) and the session repeated daily until all the pellets (n = 20) were consumed within 10 minutes, on 2 consecutive days (7 – 10 days).
After handling, each rat was placed into a training chamber (Kim et al., 2016; see below for details about the apparatus and visual stimuli) for shaping. Each shaping trial started with the presentation of a star stimulus in the center of the screen. By touching the star stimulus, a white reward stimulus box appeared on either the left or right side of the screen. Touching the reward stimulus delivered a single pellet into the food tray. A total of 60 trials were given daily until all of the trials were finished in 25 min, on 2 consecutive days (7 – 14 days).
2.2.3 | Visual Category Training
Each training session included 72 trials (category [2] × exemplar [12] × block [3]). Every trial was initiated by touching the star stimulus which appeared in the center of the screen. Rats were thus able to initiate each trial in a self-paced manner. By touching the star stimulus, one category exemplar was presented in the center of the screen as a category cue for the given trial (cueing-phase). Rats were trained to touch the category cue three times because the multiple-touch requirement on the cue stimulus forces the rat to explore the visual stimuli and allows for tracking the location of responses within the stimulus prior to the choice phase (Kim et al., 2016). After three touches on the cue, the category exemplar appeared on both the left and right sides of the screen (choice-phase), separated by a large gap (390 pixels). Rats had to choose one of those stimuli, depending on which category cue was given (e.g., left for category A and right for category B, counterbalanced across the rats). If the correct choice stimulus was selected, then a white reward box appeared on the correct side. By touching the reward box, rats could retrieve a food reward from the food tray. The next trial was presented after an ITI of 3 s. If rats made an incorrect choice, then a longer ITI was given (10 ~ 15 s) and the same trial was repeated from the cueing-phase until they made the correct choice. Correction trials were not included in the calculation of accuracy.
This training procedure continued until rats reached the learning criterion (≥ 75% in both category conditions on 2 consecutive days). After rats reached the learning criterion, training trials continued without correction; every error trial proceeded to the next trial without food reward (Fig. 1B). Once rats reached the same criterion, now without correction, bilateral cannulae were implanted in the dorsal hippocampus. After 1 week of recovery, training resumed and continued until the rats again reached the same learning criterion. The rats were then given testing sessions with muscimol or vehicle infusions on different testing days.
2.2.4 | Visual Category Testing
Rats were shown the category stimuli presented during training along with probe trials that presented novel stimuli, relocated stimuli, and singleton stimuli. Testing sessions were given 30 min following phosphate-buffered saline (PBS) or muscimol infusion into the dorsal hippocampus. During Testing Session 1, 12 trials were presented with novel category stimuli (novel), in which the category-irrelevant features were replaced with novel irrelevant features that had not been presented during training (Supplementary Fig. 1B). The remaining 60 trials involved presentations of the trained stimuli. Testing Session 1 represented a standard test for determining whether the rats learned a category or were solving the task by simply remembering each training stimulus.
During Testing Session 2, 12 trials were presented with relocated category features (relocation). Both category-relevant and category-irrelevant features were relocated. Specifically, either two out of three (dense group) or one (sparse group) category-relevant feature(s) appeared at the locations in which irrelevant features had been presented (Supplementary Fig. 1C). The relocation condition examined whether the spatial configuration of features was encoded for the category stimuli. Thus, if the relative spatial locations of the relevant or irrelevant features were encoded, then the rat’s accuracy should drop during trials with relocated stimuli. In addition, another 8 trials were assigned for the singleton condition, in which only a single category-relevant feature was presented in the center of the 238 × 238 pixel square space (singleton) (Supplementary Fig. 1C). The singleton condition examined whether the current category stimuli were discriminated by simple rules such as “vertical lines – go left.” If the rats relied on a simple rule, then performance would be intact because no distractor was presented in the singleton condition. On the other hand, if the rats used more complex information such as associating the relevant feature with its spatial location, then performance would be impaired. As in Testing Session 1, there were 60 trials with trained category stimuli. Testing Session 1 was repeated twice per infusion type (i.e., PBS or muscimol), so a total of four sessions of data was available for analysis. Testing Session 2 was repeated four times per infusion type because the relocation condition needed more trials for proper counterbalancing. On all testing trials—novel, relocation, and singleton—rats received pellet reinforcement no matter which choice response they made.
2.2.5 | Simple Visual Discrimination Task (Control Task)
Because the categorization task involved mapping categories onto spatial locations (left vs. right), it is possible that the hippocampus is necessary for performing the task, regardless of whether categories are learned or not. To confirm that any effects of dorsal hippocampal inactivation were specific to the category discrimination, a control group of rats was tested with a control task in which a single stimulus was mapped to each location (left vs. right). The category stimuli were replaced by grey (RGB: 171 189 189) and black (RGB: 6 8 8) rectangles (238 × 238 pixel2). All other procedures were exactly same as in the visual categorization task.
2.2.6 | Touch Recording and Analyses
The details of the touch recording procedures were described in a previous publication (Kim et al., 2016). Briefly, an infrared touchscreen (1024 × 769 pixels resolution; Elo Touch Solutions Inc., Menlo Park, CA) in front of the LCD monitor recorded all touches during the session. The initial breakage of the infrared field was regarded as a touch. Rats were allowed to touch any location on the touchscreen, but only touches within the specific areas of interest (i.e., the areas in which the visual stimuli appeared) counted for transitions between trial phases and were stored for the later use.
Touch coordinates were analyzed in each category condition, across training and testing sessions. The coordinates of the three touches during the cueing-phase were analyzed for anticipatory touches toward the upcoming rewarded response location (left vs. right). The average x-coordinates of touches on the cue stimulus for categories A and B were computed. Then the average values of each category condition were subtracted by the average x-coordinates from all trials. The difference between the values from each category was defined as touch separation. The purpose of this measure was to examine whether the touches in each category deviated from the center and whether the touches moved toward the upcoming reward locations as rats learned the visual categorization task; if so, then we would have an additional measure of category discrimination, at the very beginning of the trial, before the required category choice had to be made.
Touch patterns were further analyzed using a support vector machine (SVM) classifier. Touch patterns in each category were used to train an SVM classifier across the training sessions (Kernel type = Gaussian Radial Basis Function). Then, SVM performance on classifying touches into categories was evaluated with leave-one-out cross-validation (Stone, 1974).
2.3 Surgery
Two guide cannulae (26G, 5 mm long; Plastics One, Roanoke, VA) coupled with stylets (1-mm protrusion from the tip of the guide cannula) were bilaterally implanted targeting the dorsal hippocampus. Under isoflurane (1 – 4%) anesthesia, the skull was drilled for cannula insertion (surgery coordinates: APbregma – 3.8 mm, ML ± 2.5) and the cannulae were positioned DV – 3.2 mm below skull surface. The cannulae were secured to the skull by bone cement (Zimmer, Warsaw, IN) and micro screws. The rats were given post-operative analgesics for 48 hr and recovered for at least 7 d before re-training.
2.4 Drug Infusion Procedure
The general infusion procedure was described in a previous publication (Kim et al., 2016). The rats were briefly anesthetized with isoflurane and the stylet was replaced by the infusion needle (30G; 1-mm protrusion from the tip of the guide cannula). The infusion needle was connected to a 10-μl of gas-tight syringe (Hamilton, Reno, NV) via polyethylene tubing (PE50; 110–120 cm). The injection was controlled by a micro-infusion pump (Harvard Apparatus, Holliston, MA). A quantity of 0.3 μl of either PBS (pH 7.4) or muscimol (2.0 mM, pH 7.4) was infused per injection site over 2 min, at a rate of 8.0 μl/h. All test sessions started 30 min after the infusion and a 1-d break was given after each infusion session to minimize residual drug effects and tolerance.
2.5 | Histology
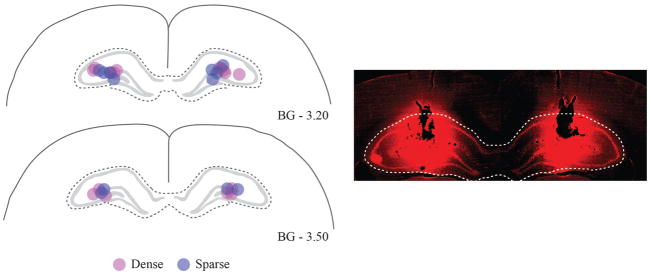
Cannula positions were histologically verified after the rats finished all behavioral tests. The rats were deeply anesthetized with sodium pentobarbital and then perfused with ~300 ml PBS and ~300 ml 10% formalin. Brains were stored in 10% formalin and 30% sucrose at 4˚C for 2 – 3 days before sectioning. Coronal sections (50 μm) of the brain were collected using a sliding microtome (Thermo Fisher Scientific, Waltham, MA) for later thionin staining (Sigma-Aldrich, St. Louis, MO). The diffusion range of the drug was also histologically verified using fluorescent muscimol (BODIOY TMR-X Muscimol; Molecular Probes, Eugene, OR). The same amount (0.3 μl per injection site; 2.0 mM) was infused 30 min before the perfusion. The unstained sections were examined under a red fluorescent filter. It is important to note that the molecular weight of fluorescent muscimol is heavier than the muscimol used for test sessions (Allen et al., 2008), so the diffusion range may be underestimated in Figure 4.
Figure 4. Verification of cannula tips and estimation of muscimol diffusion in dorsal hippocampus.
A. Position of cannula tips from each category-feature density group were marked in target loci. B. Diffusion of muscimol infusions in the dorsal hippocampus was estimated with fluorescent muscimol. The dotted white line indicates the anatomical boundary of the dorsal hippocampus. Magenta and blue dots represent dense and sparse group, respectively.
2.6 | Statistical Analyses
Choice accuracy, touch separation, and SVM classifier performance were the main dependent variables of the current study. Univariate and repeated-measures analysis of variance (ANOVA) and Pearson’s bivariate correlation analysis were used. The alpha level of statistical significance was set to 0.05. Post-hoc pairwise comparisons with Bonferroni corrections were conducted if there were significant main effects or interactions.
3 | RESULTS
3.1 | Success rate and learning rate for visual category learning
Rats were trained on the visual categorization task until they passed the learning criterion (≥ 75% in both category conditions on 2 consecutive days without correction trials). Most rats in both groups were able to learn the current visual categorization task. However, although all 8 of the rats successfully reached criterion in the dense group, 6 out of 8 rats passed the criterion in the sparse group. It took 24.38 d on average for the dense group (range = 12 – 36 d) and 40.83 d (range = 22 – 74 d) for learners in the sparse group to reach the criterion. An independent-samples t-test statistically confirmed the acquisition rate difference between the groups (t(12) = 17.87, p < 0.01). The results indicate that category-feature density was a significant factor for the success rate.
3.2 | Visual category learning and touch pattern segregation by category
In addition to accuracy across training sessions, we recorded the rats’ touch patterns on the category cue stimuli. A previous study showed that rats trained in this type of discrimination task begin to touch the side of the cue stimulus that would be correct during the upcoming choice phase (Kim et al., 2016). Touch location is therefore a useful index of prospective coding of the response location associated with reward.
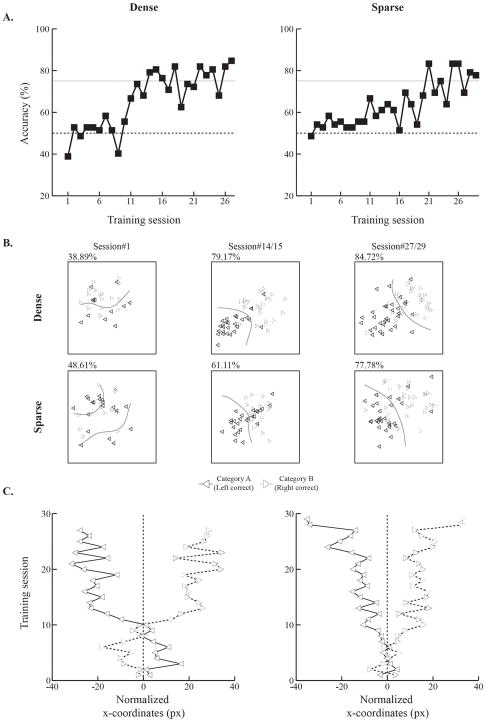
Figure 2 shows both accuracy (Fig. 2A) and touch location scores (Figs. 2B and 2C) for one rat in each group displaying similar numbers of training sessions to reach criterion. During the very first training session, touches from each category condition were intermixed and it was hard to separate them visually. We trained an SVM classifier to plot the boundary (i.e., hyperplane) on touches by category condition, but the segregation was still not accurate. However, as training progressed, touches proved to be more visually separable. The same was true for the SVM classifier. When rats reached the learning criterion, clear separation of touches by category condition was evident (Fig. 2B).
Figure 2. Visual categorization learning in individual rats from the dense and sparse groups.
A. Accuracy increased in rats from both category-feature density groups as training proceeded. Rats with similar numbers of training sessions to reach criterion were selected for comparison in this figure. Accuracy deviated from the chance level (dotted lines) after 1 or 2 weeks of training for rats in both groups. Later, however, rats in the dense group reached the learning criterion (solid lines) earlier than the sparse rats B. Touch patterns on the cue category stimuli across training. Rats were trained to touch the cue category stimuli 3 times to move on to the next phases in the trial sequence. The trial-averaged coordinates of touch patterns were plotted by category condition (left-arrow for Category A and right-arrow for Category B) across the very first, middle, and last sessions. Touch patterns were also used to train an SVM classifier across training. Solid curved lines represent hyperplanes generated by the SVM classifier. The numbers on top of each pattern denote accuracy during the specified training sessions. Touches for Category A became more separable from Category B as learning proceeded in both rats. The hyperplanes enhanced the clarity of these separations. C. Touch coordinates for Categories A and B diverged across training. The visual patterns from B were quantified and plotted across the training sessions. During initial learning, touches were occasionally mapped in opposite directions or gathered around the chance level (vertical dotted lines). As rats learned the task, however, the x-coordinates from each category progressively diverged.
We quantified touch separation by computing the normalized x-coordinates of the touches of these rats per training session. The mean x-coordinates for each trial from both category conditions were subtracted from the mean x-coordinates on all trials. Then, the normalized x-coordinates for the two categories were plotted across training sessions (Fig. 2C). During the initial training sessions, in which accuracy was at around chance level (Fig. 2A), touches for each category were scattered evenly across the cue stimulus. As accuracy increased, however, touches by category became increasingly separable and this separation continued until the end of training.
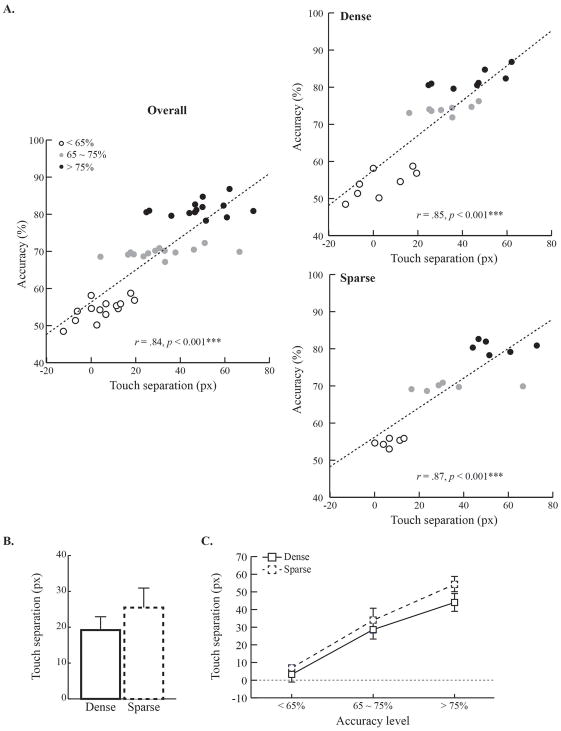
We examined whether the same patterns were observable at the population level (Fig. 3). Touch separation between category conditions and accuracy were the variables for the scatterplots. If the data at the individual level still held at the population level, then there should be a statistically significant relationship between touch separation and accuracy. In both groups, there were strong linear relationships between touch separation and accuracy (rs > 0.84, ps < 0.001); the higher the accuracy, the greater the touch separation. The results indicate that touch separation was a solid behavioral index of category learning in the current paradigm; the touch separation by category also indicates that rats developed clear anticipatory responding toward the upcoming reward location (Fig. 2C).
Figure 3. Relationship between touch separation and visual categorization learning.
A. Bivariate correlation between the degree of touch separation and accuracy in both category-feature density groups. Both groups showed a highly significant correlation between touch separation and choice accuracy. B. The degree of touch separation in each group. C. The changes of touch separation across accuracy levels and by group. Touch separation increased as accuracy reached higher levels. Error bars indicate the standard error.
3.3 | Touch separation on cue stimuli by category-feature density
We examined whether the amount of the touch separation on the cue stimulus differed between groups because the degree of the touch separation may reflect differences in the difficulty of the discrimination. The results showed that the touch separation in the dense group was about 19.20 pixels and about 25.49 pixels in the sparse group. Nevertheless, an independent-samples t-test showed that the degree of touch separation did not differ between groups (t(12) < 1, n.s.)(Fig. 3B).
Next, we examined how touch separation emerged across training in both dense and sparse groups. The training data sets were divided into three accuracy levels (< 65%, between 65% and 75%, and >75%). Touch separation was calculated at each accuracy level and plotted by group. As shown in Figure 3, in both groups, there was more touch separation as choice accuracy increased; however, no group differences were observed. Statistical tests confirmed this conclusion by showing a significant main effect of accuracy level (F(2, 24) = 63.99, p < 0.001). However, neither the main effect of group (F(1, 12) = 1.36, p = 0.27) nor the interaction between group and accuracy level were significant (F(2, 24) < 1, n.s.). Subsequent post-hoc comparisons confirmed that touch separation significantly increased across accuracy levels (ps < 0.001) similarly in both groups (Fig. 3C).
Recall that the number of training sessions differed between groups, because the sparse group learned more slowly than the dense group. We therefore conducted additional analyses to determine whether the group difference in touch separation could be accounted for by the difference in the number of training sessions. Correlations between touch separation and the number of training sessions were not significant for the dense group, the sparse group, or when the two groups were combined. These analyses indicate that the group difference in touch separation cannot be accounted for by differences in the number of training sessions.
3.4 | Category prediction across touches 1–3 on cue stimuli
We examined whether the touch divergence on the cue stimuli increased from Touch 1 (T1) to Touch 3 (T3). Touch separation for each touch on the cue stimulus was computed. The results showed that T1 was not separable by category for either group. From the T2 to T3, however, touches became more and more separable. Again, the separation was similar across category density groups. Statistical tests confirmed these observations by showing a main effect of touch order (F(2, 24) = 53.64, p < 0.001). Neither group (F(1, 12) = 1.08, p = 0.32) nor the interaction of the two factors (F(2, 24) = 1.379, p = 0.27) was statistically significant. Post-hoc comparisons showed that T2 and T3 involved greater spatial separation than T1 in both groups (ps < 0.001) (Supplementary Fig. 2A).
We also calculated an SVM classifier performance on single touches. If the category information from touches were homogeneously distributed, then the classifier would perform similarly with any touch in partitioning the touches into two categories. The results showed that the SVM classifier performed better with later touches. The statistical tests revealed a reliable main effect of touch order (F(2, 24) = 79.03, p < 0.001). Both group and the interaction between touch order and group were not statistically significant (Fs < 1, n.s.). Post-hoc tests showed that the SVM classifier performed better with the later touches (ps < 0.01). (Supplementary Fig. 2B).
We also examined the relationship between SVM classifier performance and accuracy across touches. The results showed that classifier performance with all three touches was strongly correlated with accuracy in both groups (rs > 0.7, ps < 0.001). However, when the classifier was trained and tested with the first touch only, the correlation was not significant in either group (rs < 0.1, ps > 0.67). From the second to the third touch, the correlation became statistically significant (rs > 0.45, ps < 0.05) (Supplementary Fig. 3). The overall results indicate that the degree of touch separation reflected category learning. However, these touches on the cue stimuli were quantitatively as well as qualitatively different from each other; the later touches were more strongly anticipatory of the location that would later be associated with reward than the earlier ones (see also Kim et al., 2016).
3.5 | Cannula placements and drug diffusion
Once rats reached the criterion (n = 8/8 for the dense group and n = 6/8 for the sparse group), cannulae were implanted bilaterally into the dorsal hippocampus. The placement of cannula tips into the target areas is illustrated in Figure 4. The cannula tip locations were found between 3.2 to 3.5 mm posterior to bregma, 2.2 to 2.5 mm lateral to midline, and 3.1 to 3.3 mm ventral to the skull surface. We verified that all infusion cannula tips were placed within the target loci. In addition, the fluorescent images showed that the drug infusions were well localized within the dorsal hippocampus.
3.6 | Dorsal hippocampal inactivation effects on visual categorization and generalization to novel exemplars
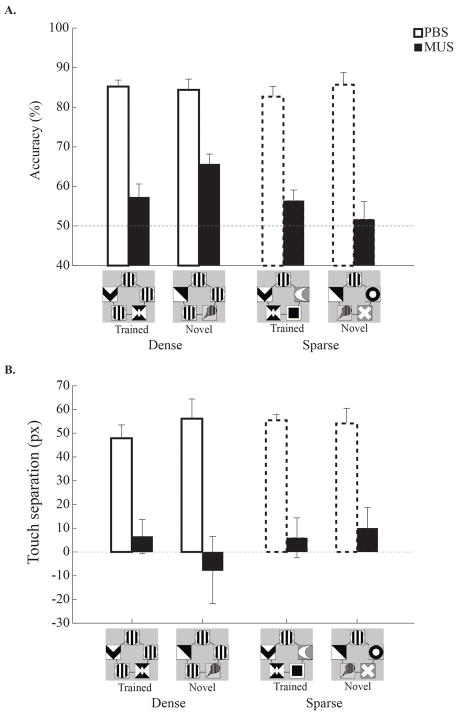
At least one full week following surgery, rats were re-trained until they again reached the learning criterion. Testing sessions then started with either PBS or muscimol infusions into the dorsal hippocampus. First, rats in both category-feature density groups were tested with the trained categorization stimuli, as well as with novel category stimuli, to assess their generalization performance. For the novel category stimuli, previous category-irrelevant features were replaced by new category-irrelevant features (Supplementary Fig. 1B). Both the retrieval of training and generalization capabilities were assessed for each rat with and without dorsal hippocampal inactivation. As shown in Fig. 5A, rats successfully retrieved trained category stimuli, and they also generalized their learning to novel category stimuli in both groups when the dorsal hippocampus was not inactivated. With hippocampal inactivation, however, both training and generalization performance were severely impaired. A 3-way ANOVA with drug [PBS and muscimol], group [dense and sparse], and test condition [trained and novel] as factors was conducted to verify these observations. There was a significant main effect of drug (F(1, 12) = 131.64, p < 0.001). All of the other factors and interactions were not statistically significant (Fs < 4.11, ps > 0.07). The results indicate that intact dorsal hippocampal function is critical for retrieving the previously learned category information and for generalizing this learning to new visual category stimuli.
Figure 5. Hippocampal dependency of retention and generalization in visual categorization.
A and B. Accuracy and touch separation during retention testing for the trained category stimuli as well as during generalization to novel stimuli in Testing Session 1. Both category-feature density groups were tested following either PBS or muscimol infusions into the dorsal hippocampus. With PBS infusions, performance was intact in both trained and novel conditions, regardless of category-feature density. However, both measures were significantly impaired in both groups during muscimol inactivation. Error bars indicate the standard error.
The degree of touch separation for retrieval and generalization was additionally examined across drug conditions. When PBS was infused into the dorsal hippocampus, touch separation in both trained and novel conditions was as high as the pre-infusion training level. However, once the dorsal hippocampus was inactivated, the degree of touch separation dropped to chance level, regardless of the test condition (Fig. 5B). A 3-way ANOVA revealed a main effect of drug (F(1, 12) = 43.16, p < 0.001). The main effects of group and test condition and the interaction were not statistically significant (Fs < 2.69, ps > 0.13). Overall, results from Testing Session 1 showed that rats could generalize their discriminative performance to exemplars in which category-irrelevant features were replaced with novel features, regardless of their having being trained with dense or sparse categories. However, once the dorsal hippocampus was inactivated, rats in both groups showed impaired performance with both training and generalization exemplars. These results indicate that visual categorization is critically dependent on the dorsal hippocampus.
3.7 | Category-feature density and generalization to relocated and singleton category features
Testing Session 1 showed that rats robustly generalized discriminative responding when category-irrelevant features were changed. In Testing Session 2, we further tested generalization when both category-relevant and category-irrelevant features were relocated within the stimulus space (relocation; Supplementary Fig. 1C). The positions of two category-relevant features were relocated with category-irrelevant features in the dense group; the one category-relevant feature was placed in one of the remaining four locations in the sparse group. Relocation stimuli were used to determine whether the spatial configuration of the relevant or irrelevant stimulus features had been encoded during categorization of the training stimuli. We also examined whether rats solved the current task as a visual cueing or categorization task by presenting only one category-relevant feature in the center of the screen (singleton; Supplementary Fig. 1C). If rats performed the task by simply paying attention to the category-relevant feature, then performance in the singleton condition should be equal to or higher than the trained condition because there were no distractors. However, if rats’ categorization behavior was based on the configuration of features, then their performance should be impaired in the singleton condition.
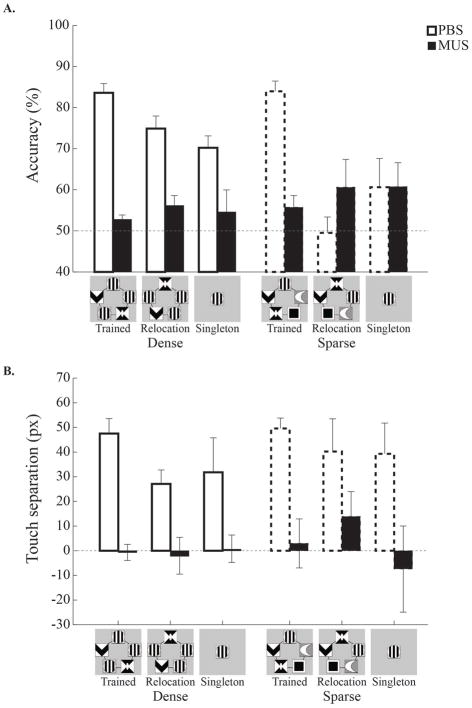
Accuracy in the testing conditions was plotted in both category-feature density groups across infusion sessions (Fig. 6A). With vehicle infusions, rats in both groups performed well with the training stimuli (> 80% accuracy). In the relocation condition, however, accuracy was impaired in both groups, but to different degrees; the impairment was mild in the dense group, but severe in the sparse group. Both groups were equivalently impaired during singleton trials. During inactivation of the dorsal hippocampus, rats in both groups were similarly impaired in all testing conditions; accuracy dropped to near chance level (< 60%). These results again indicate that the current visual categorization task is critically dependent on the dorsal hippocampus.
Figure 6. Hippocampal inactivation effects on testing with relocated and singleton category features.
A and B. Performance on trained, relocated, and singleton trials in Testing Session 2. Muscimol infusions again impaired performance during all three testing conditions. With PBS infusions, the dense group showed slightly impaired performance when the category-relevant features were relocated or when a single relevant feature appeared. The sparse group, by contrast, showed a substantial drop in accuracy during relocation and singleton trials. Muscimol infusions into the dorsal hippocampus resulted in chance performance in all testing conditions. Note that the sparse group had a floor effect for relocation and singleton trials, precluding assessment of hippocampal inactivation in these groups. Error bars indicate the standard error.
A 3-way ANOVA (drug × testing condition × group) found a main effect of drug (F(1, 12) = 12.42, p < 0.01). The impairment with the relocation and singleton stimuli compared to the trained stimuli in both groups was indicated by a main effect of testing condition (F(2, 24) = 5.75, p < 0.01). In addition, the smaller impairment in the testing conditions for the dense group than the sparse group during PBS infusions was supported by a 3-way interaction (F(2, 24) = 3.70, p < 0.05). Post-hoc pairwise comparisons showed that, with PBS infusions, the dense group performed better with the relocation stimuli than the sparse group (p < 0.001). None of the other comparisons between the groups was statistically significant (ps > 0.21). Within the dense group with PBS infusions, accuracy for the trained stimuli was similar to accuracy for the relocation stimuli (p = 0.09), whereas the difference with the singleton stimuli was significant (p < 0.05). The comparison between the relocation and singleton stimuli was not significant (p = 0.95). Within the sparse group, accuracy for the trained stimuli was significantly higher than accuracy for both the relocation and singleton stimuli (p < 0.001). Accuracy for the relocation stimuli was not statistically different from accuracy for the singleton stimuli (p = 0.16).
The touch separation results also showed a severe impairment due to dorsal hippocampal inactivation (Fig. 6B). In both category-feature density groups, a high degree of touch separation following PBS infusions decreased to chance level with dorsal hippocampal inactivation. Also, in both groups, there was a trend for touch separation to decrease from the trained to the relocation and the singleton stimuli. A 3-way ANOVA disclosed that only the main effect of the drug was statistically significant (F(1, 11) = 19.33, p < 0.01). All of the other main effects (testing condition, F(2, 22) = 1.08, p = 0.36; group, F(1, 11) < 1, n.s.) and the interaction (Fs < 1, n.s.) failed to attain statistical significance.
These results reveal the crucial role of the hippocampus in the current visual categorization task. Both accuracy and anticipatory touches toward the upcoming goal location were severely impaired when the dorsal hippocampus was inactivated. The results also reveal that category-feature density modulated the generalization to the relocated category stimuli; the dense group generalized better than the sparse group when the category-relevant features were switched with category-irrelevant features. Lastly, the impairment in the relocation and singleton conditions in both groups indicates that rats categorized the current visual stimuli based on the configuration of features and their spatial locations.
A limitation of the singleton test trials is that a category-relevant feature was both moved to a novel location and stripped of the other features, making it impossible to determine which change had the greater effect on accuracy. We have subsequently changed the singleton test trials in ongoing experiments to position the singleton feature in the training locations of the relevant or irrelevant features. The results of these ongoing experiments show a substantial decrement in rats’ accuracy regardless of the spatial location of the singleton feature. Thus, the most parsimonious interpretation of the testing results from the current study is that accuracy dropped during presentations of the singleton stimulus primarily because the other features were removed.
3.8 | Dorsal hippocampal inactivation and non-category visual discrimination
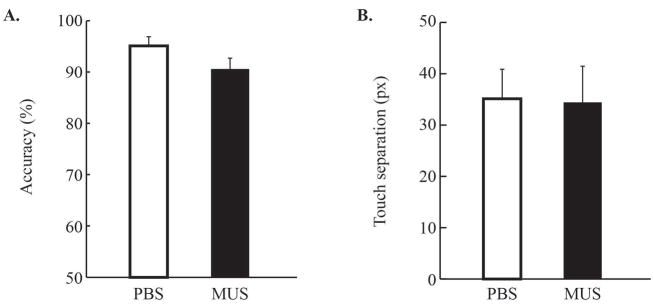
To demonstrate that the dorsal hippocampus was specifically involved in the categorization process, other rats (n = 11) were trained and tested with a simple visual discrimination task. All experimental procedures were the same as in the visual categorization task, except that the category stimuli were replaced with dark and bright patches. Once rats reached criterion (M = 6.73 d; range: 5 – 10 d), they were tested under either PBS or muscimol infusions into the dorsal hippocampus on different testing days. The results showed that both accuracy and touch separation remained high and similar between infusion conditions. Accuracy was higher than 90% and touch separation was higher than 30 px in both conditions, with and without the drug (Fig. 7). Paired-samples t-test confirmed these observations by showing no significant difference (ts > 2.06, ps > 0.06). Although the slight drop in accuracy was nearly significant, the magnitude of the decrease was substantially lower than with the category stimuli. These results suggest that the impairment in the visual categorization task following muscimol infusion was not due to a general impairment in visual discrimination or in mapping stimuli to spatial locations (i.e., left vs. right).
Figure 7. No reliable effects of dorsal hippocampus inactivation on a visual discrimination task.
A. When category stimuli were replaced with bright and dark patches that did not vary across trials, accuracy was very high (over about 90%) and was minimally affected by dorsal hippocampal inactivation. B. Touch separation was not affected either by the hippocampal inactivation. Error bars indicate the standard error.
4 | DISCUSSION
Rats trained with dense (3/5 relevant features) or sparse (1/5 relevant features) categories learned their respective discrimination tasks and generalized their behavior to novel exemplars, indicating successful categorization, although learning the sparse categories was more difficult. Learning was evident in both choice accuracy and touch separation on the cue stimulus (Figs. 2–3). Touch separation reflected the development of anticipatory touches on the side of the cue stimulus proximal to the upcoming location associated with reward. During testing trials with relocated features (spatial configuration of relevant and irrelevant features switched) and the singleton stimulus (a single relevant feature), rats in the dense category group showed a modest decrements in accuracy, whereas accuracy in the sparse category group dropped to chance performance. Inactivation of the dorsal hippocampus severely impaired categorization with the training stimuli and novel exemplars in both groups (Fig. 5). Accuracy for relocated stimuli and singleton stimuli was also impaired in the dense category group (Fig. 6). Hippocampal inactivation did not significantly impair accuracy in a discrimination control task (Fig. 7).
4.1 | Touch location on the category cue stimuli predicts later choice behavior
The current visual categorization task required rats to touch the cue stimulus three times before making a left or right choice to receive food reward. This procedure facilitates learning of visual tasks in rats by forcing them to focus attention on the category stimulus (Brooks et al., 2013; Kim et al., 2016). Early in learning, touches on the cue category stimuli were distributed randomly across the stimulus. However, as accuracy increased, touches on the cue stimulus moved toward the correct location (Fig. 2B & 2C). These anticipatory touches were highly correlated with later choice accuracy (Fig. 3). However, the three touches were not equal in terms of category anticipation. The very first touch was rarely category predictive. Touches 2 and 3 showed far greater separation as well as higher SVM performance (Supplementary Figs. 1–2). The fact that rats increasingly touched lateral areas of the central cue stimulus as they learned the task suggests that retrieval of the category membership of the cue stimulus was reflected in touch patterns. Touches on the cue stimulus that are proximal to the location associated with upcoming reward also suggests prospective coding of the expected location associated with reward.
4.2 | Decrements in accuracy and touch separation during relocation and singleton trials suggest arbitrary feature-location mapping within categories
The drop in accuracy during relocation and singleton test trials indicates that the rats did not learn the task by applying the simplest possible rules, e.g., ‘go left when the vertical lines are presented.’ Instead, the rats developed a feature-location mapping for the relevant features. This type of arbitrary association could be mediated by the CA3 autoassociative network (Rolls, 2016). The autoassociative network could automatically encode the relative location of the relevant feature(s), even though such spatial information is not necessary for solving the task. These arbitrary feature-location associations seemed to be particularly crucial for rats trained with sparse stimuli. Rats in this group showed chance level accuracy on relocation and singleton test trials. The feature-location association may be particularly crucial for the sparse group because only one feature is relevant. Movement from its expected location therefore produced a larger change in the stimulus than in the dense group, where there is greater redundancy in the relevant features as they appear in three locations (not just one location) during training. Additional behavioral analysis is necessary to probe differences in the nature of category representations as a function of differences in category structure.
4.3 | Hippocampal inactivation impaired categorization, not general task performance
The categorization task used in this study and in a previous study (Brooks et al., 2013) required the rats to map category membership onto a spatial location on the touchscreen (left or right). It is well documented that the hippocampus plays a crucial role in spatial memory (Buzsaki & Moser, 2013; O’Keefe & Dostrovsky, 1971; O’Keefe, Nadel, Keightley, & Kill, 1975). It is possible, therefore, that the impairment in our categorization task with hippocampal inactivation could be caused by a deficit in the ability to perform the stimulus-location mapping necessary for solving the task. However, rats given hippocampal inactivation were not significantly impaired on a discrimination task that required single stimulus-location mapping, indicating that the impairment during testing may be related to difficulty in some key aspect of categorization.
A limitation of the control discrimination task is that the stimuli were less complex than the stimuli in the categorization tasks, raising the possibility that the impairment in categorization following hippocampal inactivation was caused by a deficit in discriminating complex stimuli. It is unlikely, however, that stimulus complexity alone fully explains the effects of hippocampal inactivation on the current categorization tasks. The sparse task is more complex and difficult than the dense task, and yet both tasks were severely impaired by hippocampal inactivation. If stimulus complexity or task difficulty was the primary factor we would expect a less severe deficit in the dense category group for the training stimuli, but this did not occur, in two rounds of testing.
4.4 | Hippocampal inactivation may impair categorization by affecting pattern completion and pattern separation
Hippocampal inactivation impaired accuracy and touch separation for both the dense and sparse groups in all of the test conditions (training stimuli, novel exemplars, relocated stimuli, and singleton stimuli), even though the dense group showed a seemingly more robust representation during testing with relocated and singleton test stimuli. Hippocampal inactivation may have produced this global impairment in categorization by blocking pattern completion or pattern separation. A pattern separation deficit might explain why the rats were impaired when tested with both the training stimuli and the novel stimuli during hippocampal inactivation. That is, the presence of irrelevant features that were by definition identical, may have taxed the pattern separation needed to differentiate the categories.
A pattern completion deficit might explain why the rats were impaired during novel, relocation, and singleton trials during hippocampal inactivation. In addition to presenting a challenge for pattern separation, novel stimulus presentations may have taxed pattern completion by presenting category stimuli with 2 or 4 features that had never before been seen. The relocation trials involved moving relevant features to new locations, which would have made pattern completion more difficult, as the relevant features had been mapped to particular locations on the stimulus. On singleton trials, all category information is stripped away except for the relevant feature, thereby placing a load on pattern completion. The current results suggest that hippocampal pattern completion and separation might play crucial roles in visual categorization. However, a precise assessment of the role of hippocampal pattern separation and completion in rat visual categorization will require testing a variety of conditions specifically designed to measure these different processes.
4.5 | Hippocampal inactivation effects on categorization: memory or perception?
Although we have interpreted our findings in terms of memory mechanisms, it is important to note that the deficits following hippocampal inactivation in the current study could also be affected by impaired perception. Numerous studies support the hypothesis that the hippocampus is crucial for high-level visual perception (Baxter, 2009; Kent, Hvoslef-Eide, Saksida, & Bussey, 2016; Lee, Yeung, & Barense, 2012; Murray, Bussey, & Saksida, 2007; Zeidman & Maguire, 2016). Similar representations might be necessary for categorizing the stimuli in the current tasks because the category features are associated with particular spatial locations. The visual stimuli presented to humans and monkeys in studies of hippocampal perception mechanisms are more complex than the stimuli used in the current study, but the rat visual system might use similar perceptual mechanisms for less complex stimuli.
5 | Conclusions
The current study documents a crucial role for the dorsal hippocampus in rat visual categorization. Previous neurophysiological and neuroimaging studies have demonstrated correlates of categorization, but the current study is the first to demonstrate the necessity of the dorsal hippocampus in visual categorization. Our findings also indicate that the hippocampus is crucial for visual categorization with both dense and sparse category structures (Kloos & Sloutsky, 2008). We hypothesize that hippocampus-mediated pattern separation and pattern completion are both necessary for visual categorization by orthogonalizing perceptual categories with overlapping stimulus features and by retrieving category membership for stimuli with altered features, respectively. Future studies will test this hypothesis by manipulating category stimulus features to specifically tax pattern separation and pattern completion both with and without CA3 inactivation. Our visual categorization task might also be useful for examining the nature of hippocampal ensemble representations of categories with various degrees of overlapping features.
Supplementary Material
Only the stimuli from Category A are illustrated to avoid redundancy. A. Exemplars from Category A in the dense and sparse groups. B. Novel exemplars tested for Category A in the dense and sparse groups. C. Relocation stimuli tested for Category A in the dense and sparse groups. D. Singleton stimulus from Category A used for testing in the dense and sparse groups.
A. Touch separation was plotted for each of three touches as a function of category-feature density group. The very first touch (T1) on the cue category stimuli was not separable by category in either of the groups. Later touches (T2 and T3) were better separable and both groups showed significant touch separation. B. SVM classifier’s accuracy was higher to later touches than to earlier ones for both category-feature density groups. Error bars indicate the standard error.
Each row shows the results from each category-feature density group (A. dense; B. sparse). In both groups, accuracy was highly correlated with SVM performance when all of the touches were used to train the classifier. When touches in a sequence were parsed individually to train the classifier, accuracy was correlated with the second (T2) and third (T3) touches, but not with the first (T1) touch. These results indicate that later touches were more informative in anticipating the upcoming reward location.
Acknowledgments
Grant sponsor: NICHD; Grant number: P01HD080679.
We thank Lilian McKenzie for shaping and training the rats, and Vladimir Sloutsky for helpful discussions. Support for this work was provided by the National Institute of Child Health and Human Development grant P01HD080679.
References
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. J Neurosci Methods. 2008;171(1):30–38. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Valentin VV. Multiple Systems of Perceptual Category Learning: Theory and Cognitive Tests. In: Henri Cohen CL, editor. Handbook of Categorization in Cognitive Science. Vol. 2. Amsterdam, Netherlands: Elsevier; 2017. pp. 157–188. [Google Scholar]
- Baxter MG. Involvement of medial temporal lobe structures in memory and perception. Neuron. 2009;61(5):667–677. doi: 10.1016/j.neuron.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Brooks DI, Ng KH, Buss EW, Marshall AT, Freeman JH, Wasserman EA. Categorization of photographic images by rats using shape-based image dimensions. Journal of experimental psychology Animal behavior processes. 2013;39(1):85. doi: 10.1037/a0030404. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16(2):130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Ahmed OJ, Burwell RD. Single neuron activity and theta modulation in postrhinal cortex during visual object discrimination. Neuron. 2012;76(5):976–988. doi: 10.1016/j.neuron.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11(6):626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15(6):808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44(4):581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Pons TP, Stanford TR, Deadwyler SA. Categorization in the monkey hippocampus: a possible mechanism for encoding information into memory. Proc Natl Acad Sci U S A. 2004;101(9):3184–3189. doi: 10.1073/pnas.0400162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18(9):955–964. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18(10):1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- Kent BA, Hvoslef-Eide M, Saksida LM, Bussey TJ. The representational-hierarchical view of pattern separation: Not just hippocampus, not just space, not just memory? Neurobiol Learn Mem. 2016;129:99–106. doi: 10.1016/j.nlm.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Kim J, Wasserman EA, Castro L, Freeman JH. Anterior cingulate cortex inactivation impairs rodent visual selective attention and prospective memory. Behav Neurosci. 2016;130(1):75–90. doi: 10.1037/bne0000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos H, Sloutsky VM. What’s behind different kinds of kinds: effects of statistical density on learning and representation of categories. J Exp Psychol Gen. 2008;137(1):52–72. doi: 10.1037/0096-3445.137.1.52. [DOI] [PubMed] [Google Scholar]
- Kruschke JK. ALCOVE: an exemplar-based connectionist model of category learning. Psychol Rev. 1992;99(1):22–44. doi: 10.1037/0033-295x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Lee AC, Yeung LK, Barense MD. The hippocampus and visual perception. Front Hum Neurosci. 2012;6:91. doi: 10.3389/fnhum.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, … Moser EI. Progressive transformation of hippocampal neuronal representations in “morphed” environments. Neuron. 2005;48(2):345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Love BC, Medin DL, Gureckis TM. SUSTAIN: a network model of category learning. Psychol Rev. 2004;111(2):309–332. doi: 10.1037/0033-295X.111.2.309. [DOI] [PubMed] [Google Scholar]
- Morris AM, Churchwell JC, Kesner RP, Gilbert PE. Selective lesions of the dentate gyrus produce disruptions in place learning for adjacent spatial locations. Neurobiol Learn Mem. 2012;97(3):326–331. doi: 10.1016/j.nlm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81(2):416–427. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura EM, Maddox WT, Filoteo JV, Ing AD, Gitelman DR, Parrish TB, … Reber PJ. Neural correlates of rule-based and information-integration visual category learning. Cereb Cortex. 2007;17(1):37–43. doi: 10.1093/cercor/bhj122. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L, Keightley S, Kill D. Fornix lesions selectively abolish place learning in the rat. Exp Neurol. 1975;48(1):152–166. doi: 10.1016/0014-4886(75)90230-7. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Rolls ET. Pattern separation, completion, and categorisation in the hippocampus and neocortex. Neurobiol Learn Mem. 2016;129:4–28. doi: 10.1016/j.nlm.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Seger CA, Dennison CS, Lopez-Paniagua D, Peterson EJ, Roark AA. Dissociating hippocampal and basal ganglia contributions to category learning using stimulus novelty and subjective judgments. Neuroimage. 2011;55(4):1739–1753. doi: 10.1016/j.neuroimage.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloutsky VM. From Perceptual Categories to Concepts: What Develops? Cogn Sci. 2010;34(7):1244–1286. doi: 10.1111/j.1551-6709.2010.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Berg ME, Cook RG, Murphy MS, Crossley MJ, Boomer J, … Grace RC. Implicit and explicit categorization: a tale of four species. Neurosci Biobehav Rev. 2012;36(10):2355–2369. doi: 10.1016/j.neubiorev.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M. Cross-Validatory Choice and Assessment of Statistical Predictions. Journal of the Royal Statistical Society Series B (Methodological) 1974;36(2):111–147. [Google Scholar]
- Vermaercke B, Cop E, Willems S, D’Hooge R, Op de Beeck HP. More complex brains are not always better: rats outperform humans in implicit category-based generalization by implementing a similarity-based strategy. Psychon Bull Rev. 2014;21(4):1080–1086. doi: 10.3758/s13423-013-0579-9. [DOI] [PubMed] [Google Scholar]
- Vinken K, Vermaercke B, Op de Beeck HP. Visual categorization of natural movies by rats. J Neurosci. 2014;34(32):10645–10658. doi: 10.1523/JNEUROSCI.3663-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman EA, Castro L, Freeman JH. Same-different categorization in rats. Learning & memory (Cold Spring Harbor, NY) 2012;19(4):142. doi: 10.1101/lm.025437.111. [DOI] [PubMed] [Google Scholar]
- Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016;17(3):173–182. doi: 10.1038/nrn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolan D. Invariant visual object recognition and shape processing in rats. Behav Brain Res. 2015;285:10–33. doi: 10.1016/j.bbr.2014.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Only the stimuli from Category A are illustrated to avoid redundancy. A. Exemplars from Category A in the dense and sparse groups. B. Novel exemplars tested for Category A in the dense and sparse groups. C. Relocation stimuli tested for Category A in the dense and sparse groups. D. Singleton stimulus from Category A used for testing in the dense and sparse groups.
A. Touch separation was plotted for each of three touches as a function of category-feature density group. The very first touch (T1) on the cue category stimuli was not separable by category in either of the groups. Later touches (T2 and T3) were better separable and both groups showed significant touch separation. B. SVM classifier’s accuracy was higher to later touches than to earlier ones for both category-feature density groups. Error bars indicate the standard error.
Each row shows the results from each category-feature density group (A. dense; B. sparse). In both groups, accuracy was highly correlated with SVM performance when all of the touches were used to train the classifier. When touches in a sequence were parsed individually to train the classifier, accuracy was correlated with the second (T2) and third (T3) touches, but not with the first (T1) touch. These results indicate that later touches were more informative in anticipating the upcoming reward location.