Abstract
Deficiencies of galactosylceramidase and glucocerebrosidase result in the accumulation of galactosylsphingosine (GalSph) and glucosylsphingosine (GluSph) in Krabbe and Gaucher diseases, respectively. GalSph and GluSph are useful biomarkers for both diagnosis and monitoring of treatment effects. We have developed and validated a sensitive, accurate, high throughput assay for simultaneous determination of the concentration of GalSph and GluSph in mouse serum. GalSph and GluSph and their deuterated internal standards were extracted by protein precipitation in quantitative recoveries, baseline separated by hydrophilic interaction chromatography, and detected by positive-ion electrospray mass spectrometry in multiple reaction monitoring mode. Total run time was 7 minutes. The lower limits of quantification were 0.2 ng/ml for both GalSph and GluSph. Sample stability, assay precision and accuracy, and method robustness were demonstrated. This method has been successfully applied to measurement of these lipid biomarkers in a natural history study in twitcher (Krabbe) mice.
Keywords: Galactosylsphingosine, Glucosylsphingosine, Hydrophilic interaction chromatography-tandem mass spectrometry, Krabbe disease, Gaucher disease
1. INTRODUCTION
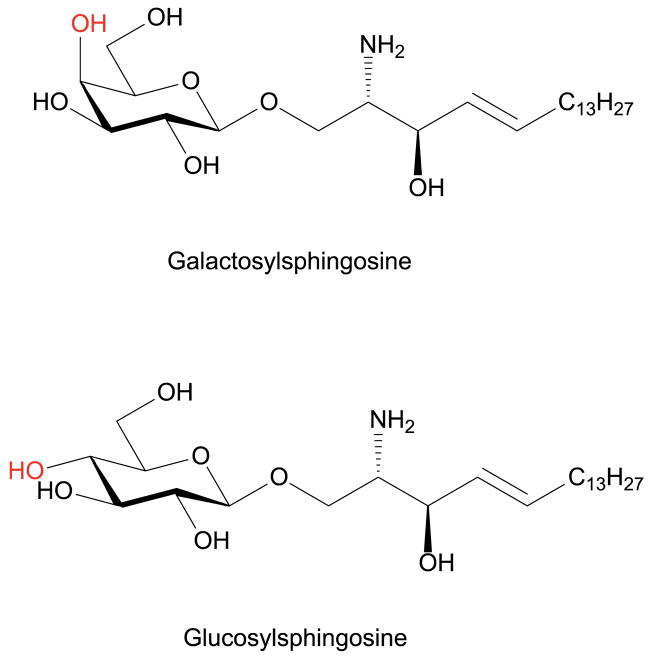
GalSph (psychosine) and GluSph are lyso-glycosphingolipids in which 1-hydroxyl of sphingosine is conjugated with galactose and glucose, respectively (Figure 1). GalSph is a highly cytotoxic lipid and accumulates in Krabbe disease, a fatal neurodegenerative disorder, caused by deficiency of the lysosomal enzyme galactosylceramidase (GALC) (Igisu and Suzuki 1984b, Suzuki and Suzuki 1970, Svennerholm, et al. 1980). GalSph has been proposed to trigger apoptosis of oligodendrocytes and Schwann cells by disrupting lipid rafts in myelinating glia (Hawkins-Salsbury, et al. 2013, White, et al. 2009, Zaka and Wenger 2004), leading to severe demyelination, neurodegeneration and the clinical signs of Krabbe disease (Husain, et al. 2004, Kohlschutter 2013, Miyatake and Suzuki 1972, Pastores 2009). Accumulated GalSph has been suggested as a biomarker for disease progression (Bradbury, et al. 2016, Igisu and Suzuki 1984b, Turgeon, et al. 2015, Whitfield, et al. 2001, Zanfini, et al. 2013, Zhu, et al. 2012). Moderate increase of GluSph was also found in plasma from Niemann-Pick C patients (Welford, et al. 2014), and more strikingly in Gaucher disease resulting from glucocerebrosidase deficiency (Brady, et al. 1966, Dekker, et al. 2011, Murugesan, et al. 2016, Orvisky, et al. 2002, Rolfs, et al. 2013). GluSph has been used for primary diagnosis and long-term monitoring of the efficacy of therapy in Gaucher disease (Dekker, van Dussen, Hollak, et al. 2011, Murugesan, Chuang, Liu, et al. 2016, Rolfs, Giese, Grittner, et al. 2013). Although GalSph and GluSph have been shown to be neurotoxic, the exact mechanisms of their toxicities are not well understood.
Figure 1.
Structures of GalSph and GluSph. The 4-hydroxyl groups that distinguishes GalSph and GluSph are highlighted in red.
A limitation to use of GalSph and GluSph as disease biomarkers has been the difficulty in simultaneous separation and quantification of these isomeric lyso-glycosphingolipids in biological samples. Various methods have previously been employed to analyze GalSph and GluSph, including silica thin layer chromatography (TLC) (Bodennec, et al. 2003a, Bodennec, et al. 2003b, Ichioka, et al. 1987, Li, et al. 2011, Motta, et al. 2016) or high performance liquid chromatography (HPLC) after derivatization (Igisu and Suzuki 1984a, Nozawa, et al. 1992, Orvisky, Park, LaMarca, et al. 2002), electrospray ionization-tandem mass spectrometry (ESI-MS/MS) (Jiang, et al. 2009, Whitfield, Sharp, Taylor, et al. 2001), reversed phased liquid chromatography-tandem mass spectrometry (RPLC-MS/MS) (Dekker, van Dussen, Hollak, et al. 2011, Fuller, et al. 2015, Galbiati, et al. 2007, Mirzaian, et al. 2015, Murugesan, Chuang, Liu, et al. 2016, Rolfs, Giese, Grittner, et al. 2013, Zanfini, Dreassi, Berardi, et al. 2013), and hydrophilic interaction chromatography-tandem mass spectrometry (HILIC-MS/MS) (Chuang, et al. 2013, Marshall, et al. 2016, Sun, et al. 2013, Turgeon, Orsini, Sanders, et al. 2015, Welford, Garzotti, Marques Lourenco, et al. 2014). Most methods involve tedious and elaborate Bligh-Dyer extraction or solid phase extraction. Only normal phase stationary phase chromatography such as silica TLC (Bodennec, Trajkovic-Bodennec and Futerman 2003b) and HILIC (Chuang, Pacheco, Zhang, et al. 2013, Nozawa, Iwamoto, Tokoro, et al. 1992, Turgeon, Orsini, Sanders, et al. 2015, Welford, Garzotti, Marques Lourenco, et al. 2014) can separate GalSph and GluSph as well as their derivatives. However, both methods have significant drawbacks, as the silica TLC methods require use of radiolabeled reagents and the HILIC-MS/MS methods require long run times due to long equilibration time of HILIC. Here we report an improved HILIC-MS/MS method for simultaneous determination of GalSph and GluSph in mouse serum, which offers easy sample preparation, robustness, and short analysis times. The method has been validated and shown to be sensitive, precise, accurate and reproducible. The performance of this assay was further examined using serum samples obtained from a natural history study of twitcher mice.
2. EXPERIMENTAL
2.1 Chemicals and reagents
GalSph, GluSph, d5-GalSph and d5-GluSph were obtained from Avanti Polar Lipids (Alabaster, AL). 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) was obtained from VWR (West Chester, PA). Formic acid, ammonium formate, and sodium hydroxide were obtained from Sigma–Aldrich (St. Louis, MO). All HPLC solvents (methanol, and acetonitrile) were HPLC grade and were purchased from EMD Chemicals (Gibbstown, NJ). Milli-Q ultrapure water was prepared with a Milli-Q Integral Water Purification System (Billerica, MA). Pooled control mouse serum and six lots of individual mouse sera were purchased from BioChemed Services (Winchester, VA).
2.2 Experimental animals
Twitcher and wild type mice were used in this study. The Institutional Animal Care and Use Committee at Washington University School of Medicine approved all animal protocols. Heterozygous twitcher (GALC+/−) mice on a C57BL/6 background were obtained from the Jackson Laboratory (Bar Harbor, ME), and heterozygote matings were used to generate the homozygous twitcher (GALC−/−) mice used in this study. Genotypes were determined on day one post birth polymerase chain reaction as previously described (Lin, et al. 2005, Sakai, et al. 1996). Twitcher and wild type mice were housed under standard conditions with ad libitum access to food and water and were maintained on a 12 hours light/dark cycle. Sera were collected from mice at postnatal day 6, 13, 23, and 36 (n=3 animals/age group).
2.3 Stock solution preparation
All the stock solutions (1 mg/mL) were prepared in methanol. A working solution containing GalSph (10 μg/mL) and GluSph (10 μg/mL) was prepared by the dilution of the stock solution with methanol. The internal standard working solution (1/1 ng/mL of d5-GalSph/d5-GluSph) was prepared in acetonitrile.
2.4 Standard curves
Because of the endogenous presence of GalSph and GluSph in mouse serum, 2% CHAPS aqueous solution was used to prepare the calibration standards. Calibration curves were prepared by spiking the GalSph and GluSph working solution into 2% CHAPS solution, and preparing serial dilutions that yielded eight calibration standards (0.2/0.2, 0.4/0.4, 1/1, 5/5, 10/10, 20/20, 50/50, 100/100 ng/mL of GalSph/GluSph). 2% CHAPS solution served as blank. The same calibration standards in mouse serum were also prepared and used to assess responsiveness in different matrixes, which was evaluated by parallelism between standard curves prepared in biological matrix (mouse serum) and surrogate matrix (2% CHAPS solution).
2.5 Quality control samples
The pooled mouse serum was analyzed to establish the mean concentration of endogenous GalSph and GluSph by the HILIC-MS/MS method. The lower limit (LLQC), low (LQC), middle (MQC), high (HQC), dilution (DQC) quality control samples (endogenous level + 0/0 ng/mL, endogenous level + 1/1 ng/mL, endogenous level + 40/40 ng/mL, endogenous level + 80/80 ng/mL, and endogenous level + 160/160 ng/mL) were prepared. The lower limit of quantification (LLOQ) sample (0.2/0.2 ng/mL of GalSph/GluSph) was prepared in 2% CHAPS solution. The GalSph and GluSph in DQC sampler were higher than the upper limit of quantification (ULOQ: 100/100 ng/mL of GalSph/GluSph). The DQC sample was diluted 1:4 with 2% CHAPS solution, prior to extraction.
2.6 Sample preparation
Standards, QCs, blank or study samples (50 μL) were aliquotted into 2 mL polypropylene tubes. To each tube was added 10 μL of 0.5 N NaOH aqueous solution, and the mixture was vortexed for 15 seconds. The internal standard working solution (1 mL) was added except that acetonitrile (1 mL) was used for a blank. The samples were vortexed for approximately 3 minutes and then centrifuged at 10,000 rpm for 10 minutes. The supernatants were transferred to 1.2 mL glass inserts (VWR, West Chester, PA) containing 10 μL of 10% formic acid in acetonitrile (v/v) in 96 well plates.
2.7 LC-MS/MS analysis
LC–MS/MS analysis was conducted on a Shimadzu (Columbia, MD) Prominence HPLC system coupled with an Applied Biosystems/MDS Sciex (Ontario, Canada) 4000QTRAP mass spectrometer using multiple reaction monitoring (MRM). The HPLC system consists of Prominence HPLC system with a CBM-20A system controller, 2 LC-20AD pumps, a SIL-20ACHT autosampler, and a DGU-20A5R degasser.
The chromatography was performed at ambient temperature using Ascentis® Express HILIC (4.6 × 50 mm, 2.7 μm, Supelco, Bellefonte, PA) protected with a HILIC Securityguard™ column (4 × 3.0 mm, Phenomenex, Torrance, CA). The compartment of the autosampler was set at 4 °C. Mobile phase A (0.1% formic acid and 1 mM ammonium formate in water) and mobile phase B (0.1% formic acid and 1 mM ammonium formate in acetonitrile-water (95:5)) were operated with a gradient elution as follows: 0 – 0.2 min 100 - 95% B, 0.2 – 3.5 min 95% B, 3.5 – 4.0 min 95 - 10% B, 4.0 – 5.0 min 10% B, 5.0 – 5.1 min 10 – 100% B, and 5.1 – 7.0 min 100% B at a flow rate of 1.5 ml/min. The HPLC flow was diverted to waste except for 2–4 min to mass spectrometer. The injection volume was 100 μL. The ESI source temperature was 600 °C; the ESI needle was 5000 V; the declustering potential was 76 V; both the entrance potential and the collision cell exit potential was 10 V. The collision and curtain gas were set at medium and 20, respectively. Both desolvation gas and nebulizing gas were set at 45. For MRM, the collision energies for mass transitions of m/z 462.3 to 282.3 (quantifier for GalSph and GluSph), m/z 462.3 to 264.3 (qualifier for GalSph and GluSph) and m/z 467.3 to 287.3 (d5-GalSph and d5-GluSph, internal standards) were 31, 26, and 31 V, respectively. The dwell time was set at 50 ms for each mass transition. Data were acquired and analyzed by Analyst software (version 1.5.2). Calibration curves were constructed by plotting the corresponding peak area ratios of analyte/internal standard versus the corresponding analyte concentrations using weighted (1/x2) least squares regression analysis.
2.8 Linearity, precision and accuracy
The linearity response of analytes was assessed over their respective calibration range from three batches of analytical runs. The precision and accuracy of the assay were determined for each analyte at LLOQ, LLQC, LQC, MQC and HQC concentration levels in mouse serum over the three batch runs. The DQC was used to assess the dilution integration. These QC concentrations included the known fortified levels added to the mouse serum plus the endogenous concentration of analytes. For each QC concentration, analysis was performed in six replicates on each day except for DQCs for which three replicates were prepared in the first batch. Accuracy and precision are denoted by percent relative error (%RE) and percent coefficient of variance (%CV), respectively. The accuracy and precision were required to be within ± 15% RE of the nominal concentration and ≤15% CV, respectively, for LLQC, LQC, MQC, HQC, and DQC samples. The accuracy and precision were required to be within ± 20% RE of the nominal concentration and ≤20% CV for LLOQ samples in the intra-batch and inter-batch assays (US Department of Health and Human Services 2001).
2.9 Sample stability
For GalSph and GluSph, long-term storage, freeze/thaw stabilities, and stabilities on the bench-top and in the autosampler were determined at the LQC and HQC concentration levels (n = 3). Long-term storage stability of analyte in mouse serum was tested up to 162 days upon storage at −80 °C, respectively. Bench-top stability was evaluated from mouse serum that was kept on lab bench at room temperature for 18 hours before sample extraction. Freeze/thaw stability was tested by freezing the samples overnight, followed by thawing to room temperature the next day. This process was repeated three times. In the autosampler, stability at 4 °C was tested over 20 days by injecting the first batch of the validation samples. Stock solution stability was established by quantification of samples from dilution of two stock solutions that have been stored at −20 °C for 162 days and at room temperature on the bench for 65 hours, respectively, to the final solution (100 ng/mL in 95% acetonitrile). A fresh standard curve was established each time.
2.10 Analysis of mouse serum samples
Samples consisted of calibration standards in duplicate, a blank, a blank with internal standard, QC standards (LQC, MQC and HQC), and unknown study samples were analyzed. The standard curve covered the expected unknown sample concentration range, and samples that exceeded the highest standard could be diluted and re-assayed. In the dilution sample re-assay, a diluted QC in triplicate would be also included in the analytical run. The results of the QC samples provided the basis of accepting or rejecting the run according to FDA guidelines (US Department of Health and Human Services 2001).
2.11 Statistics
Results are expressed as mean ± SD. For group comparisons, and the statistical significance of differences in mean values was determined by a two-tailed Student’s t test. A p value of 0.05 or less was considered significant.
3. RESULTS
3.1 LC–MS/MS method development
In our method, we applied minimal cleanup of the serum samples using only a simple protein precipitation procedure in order to achieve high throughput. d5-GalSph and d5-GluSph spiked serum was used to optimize the extraction. Direct protein precipitation with acetonitrile or methanol yielded ~85% recovery. The remaining 15% of d5-GalSph and d5-GluSph were lost to the precipitated proteins likely via ion-ion interaction. To interrupt ion-ion interaction between GalSph/GluSph with proteins, and further improve recovery, protein precipitations under acidic and basic conditions were evaluated. Alkalinization of serum with sodium hydroxide to pH 13 followed by protein precipitation offered quantitative recovery of GalSph and GluSph. The extract was neutralized with formic acid to prevent damage of HILIC column stationary phase. Drying the extract with nitrogen flow at 50 °C followed by reconstitution in 95% acetonitrile led to loss of GalSph and GluSph. The recoveries of GalSph and GluSph were 75% and 79% from serum after extracts were dried and reconstituted, respectively, and 74% and 75% from 2% CHAPS, respectively. The GalSph and GluSph were probably lost via non-specific binding to negatively charged surface of glass insert (Ji, et al. 2010). To avoid drying, acetonitrile was selected as precipitation solvent since the extract can be directly injected to HILIC column.
GalSph and GluSph are two distinct compounds and differ only in configuration of 4-hydroxyl group of sugar moiety (Figure 1). HILIC is necessary to separate these two isomers as interactions with HILIC stationary phase are dominated by the polar sugar moiety. In previously published methods, long columns were used to achieve separation of GalSph and GluSph, and long equilibration time after column cleanup with low organic mobile phase was necessary for consistent retention time, leading to long run time. Although isocratic elution can avoid long equilibration time, we observed retention shift and loss of resolution when even a small batch of biological samples (about 20 samples) were injected to Ascentis Express HILIC (2 × 250 mm, 5 μ) column without thorough pre-column cleanup.
We screened columns in an attempt to find a short HILIC column for achieving chromatographic resolution of GalSph and GluSph so that short equilibration time could be used. The screening results of Atlantis HILIC Silica, Zorbax RX-SIL Silica, Ascentis Express HILIC, Cortecs HILIC, XBridge BEH Amide, Luna HILIC, Luna Silica, Luna NH2, Halo HILIC, ACE Excel 2 SIL, ZIC-HILIC, Hypersil Gold HILIC showed that Hypersil Gold HILIC provided best resolution. However, its performance quickly degraded after injection of a few biological samples. Ascentis Express HILIC (4.6 × 50 mm, 2.7 μ) also offered sufficient resolution as well as stable performance, and, therefore, was selected to support further method development.
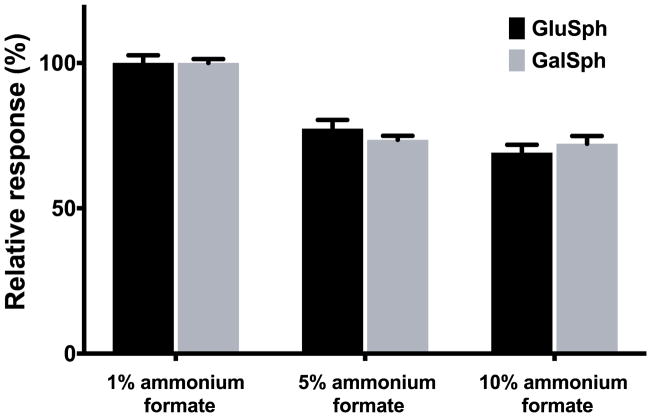
GalSph and GluSph exhibited significant peak tailing as a result of ion-ion interaction of protonated amino group in the basic analytes with silanol of silica. As a strategy to improve peak shape, we used a mobile-phase modifier with a higher ionic strength. The combination of formic acid and ammonium formate was found to improve peak shape and increase sample load tolerance. However, ammonium formate led to signal suppression in electrospray ionization (Figure 2), which required optimization of the ammonium formate concentration. The addition of only 1 mM of ammonium formate to mobile phases with 0.1% formic acid maintained good shapes for GalSph and GluSph with minimal loss of sensitivities.
Figure 2.
Effect of ammonium formate concentrations in mobile phases on the mass spectrometric responses of GalSph and GluSph. The data are presented as mean percentage + SD normalized to peak areas of GalSph and GluSph in mobile phases with 1 mM ammonium formate (n = 3).
The chromatographic throughput and robustness were improved in this study. To reduce the run time we used high flow (1.5 mL/min) to achieve fast elution and equilibration taking advantage of low back-pressure on HILIC owing to the high organic nature of the mobile phase and the high permeability of the column. After GalSph and GluSph were eluted, we used a high aqueous component mobile phase (90% mobile phase A) to wash the column for 1 min in order to prevent the accumulation of polar endogenous components on column. As a result, a large number of samples could be injected in one batch, and column performance did not deteriorate, even after injection of more than 2000 extracted biological samples.
The fragmentation patterns of GalSph and GluSph in collision-induced dissociation are nearly indistinguishable, and have been studied previously (Dekker, van Dussen, Hollak, et al. 2011, Jiang, Yang and Han 2009). The two most abundant product ions at m/z 282.3 and 264.3 resulted from the neutral loss of a galactose and a galactose plus a water molecule, respectively. The multiple reaction monitoring (MRM) transition m/z 462.3→282.3 showed the higher sensitivity than m/z 462.3 → 264.3 and therefore was chosen as quantifier for GalSph and GluSph. The MRM transition m/z 462.3 → 264.3 was used as qualifier. Similarly, the MRM transition m/z 467.3 → 287.3 was chosen for monitoring of d5-GalSph and d5-GluSph (internal standards).
3.2 Selection of surrogate matrix for standard curves
Surrogate analyte and surrogate matrix are two standard approaches for analyzing endogenous compounds (Jones, et al. 2012). For both approaches, it is essential to establish parallelism between surrogate curve and an authentic standard curve in the native matrix. For this study, we used the surrogate matrix approach. To establish parallelism between surrogate and authentic matrix standard curves, several challenges needed to be overcome due to differences in matrix components between the surrogate and native matrices, which may affect compound characteristics such as matrix effect, recovery, stability or adsorption. Thus, the selection of a surrogate matrix is critical and should generally meet three criteria: 1) the matrix is analyte free; 2) the matrix shows no adsorption loss and decomposition of analyte; and 3) there is similar recovery of analyte in both surrogate and authentic matrices. Based on these criteria, we chose 2% CHAPS solution as the surrogate matrix for this assay.
The standard curves prepared in mouse serum were parallel to those prepared in 2% CHAPS solution, and the differences in slopes of the standard curves in surrogate and authentic matrixes were 5.9% and 2.2% for GalSph and GluSph, respectively. These results suggested that the same responsiveness of GalSph and GluSph in different matrices was observed, and calibration curves prepared in surrogate matrix were suitable for analysis of serum samples.
3.3 Validation
3.3.1 Extraction efficiency and matrix effects
To evaluate the recoveries of the GalSph and GluSph from mouse serum and surrogate standard curve matrix (2% CHAPS), signals of d5-GluSph and d5-GalSph from pre-extraction spiked samples were compared to those of post-extraction spiked samples. The recoveries of GalSph and GluSph from mouse serum were 90% and 98%, respectively, and from 2% CHAPS 93% and 96%, respectively. The matrix factors (Viswanathan, et al. 2007) for GalSph and GluSph were assessed by comparing the peak response of the d5-GalSph and d5-GluSph from post-extraction spiked samples to equivalent pure compound solutions in 95% acetonitrile. Matrix factors were 0.98 and 1.07 in mouse serum, and 0.99 and 1.01 in 2% CHAPS for GalSph and GluSph, respectively, suggesting that there are no significant matrix effects for GalSph and GluSph in these matrices.
3.3.2 Selectivity
To ascertain the method selectivity, blank (2% CHAPS solution) with and without internal standard and six individual wide type and twitcher mouse sera were analyzed. As shown in Figure 3a, no interfering peaks to analyte and internal standard from blanks were observed. There are no interfering peaks to analyte from blank with internal standard (Figure 3b). In the highest calibrator (ULOQ, 100 ng/mL) without internal standards, there are no interfering peaks to internal standard (data not shown).
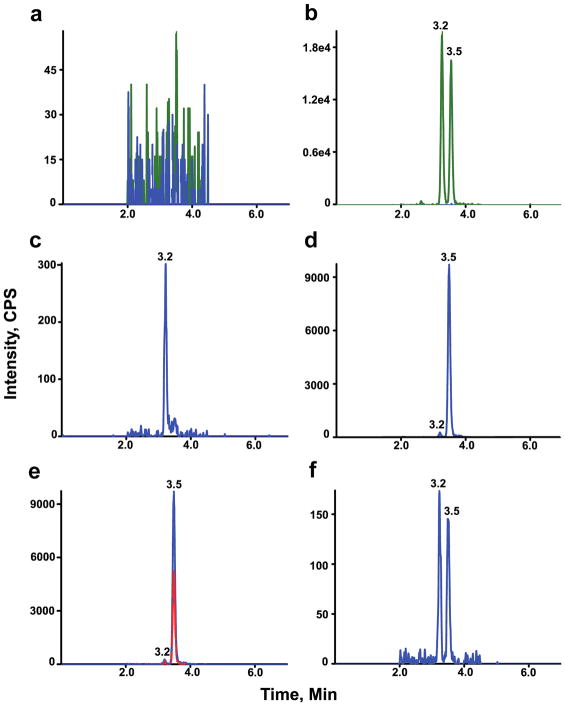
Figure 3.
HILIC-MS/MS chromatograms for GalSph and GluSph. Chromatograms of analytes and internal standards in blank (a), analytes and internal standards in blank with internal standard (b), analytes in wild type mouse serum (c), analytes in twitcher mouse serum (d), analytes in twitcher mouse serum in 2 MRM transitions (e), and analytes in LLOQ (f). Retention times of GalSph and GluSph are 3.5 and 3.2 min, respectively. The analytes (m/z 462→282) are shown in blue and internal standards (m/z 467→287) in green. The qualifier MRM transition m/z 462→264 for GalSph and GluSph is shown in red (e).
Wild type mouse sera used in the validation contained low levels of GluSph and nearly undetectable levels of GalSph (Figure 3c), whereas in twitcher mouse sera both GluSph and GalSph were present (Figure 3d). There were no interfering peaks from the mouse sera at the retention time and in the MRM channel of the internal standards. The identities of GluSph and GalSph were confirmed by the qualifier (Figure 3e).
3.3.3 Sensitivity
The LLOQ for GalSph and GluSph was prepared in 2% CHAPS solution at 0.2 and 0.2 ng/mL, respectively. The LLOQ samples were processed and analyzed with a calibration curve and QC samples (Table 1). The intra-run accuracy levels were −4.7 – 8.7% RE and 0.3 – 7.8% RE for GalSph and GluSph, respectively. The intra-run precisions at LLOQ level were 1.7 – 5.6% CV and 1.8 – 8.9% CV for GalSph and GluSph, respectively. The inter-run accuracies were 1.9% RE and 4.7% RE for GalSph and GluSph, respectively. The inter-run precisions were 7.1% CV and 6.4% CV for GalSph and GluSph, respectively. A typical MRM chromatogram at the LLOQ concentration is shown in Figure 1f.
Table 1.
Accuracy and precision of QC samples
| Analyte | GalSph | GluSph | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analytical run number | QC Level | LLOQ | LLQC | LQC | MQC | HQC | DQC | LLOQ | LLQC | LQC | MQC | HQC | DQC |
| Nominal concentration | 0.2 | 0 | 1 | 40 | 80 | 160 | 0.2 | 0.2 | 1.2 | 40.2 | 80.2 | 160 | |
| 1 | Intra-run mean | 0.203 | 0 | 1.05 | 40.2 | 81.4 | 179 | 0.201 | 0.197 | 1.19 | 39.7 | 80.2 | 170 |
| Intra-run SD | 0.0113 | 0 | 0.0228 | 0.848 | 1.51 | 2.65 | 0.0179 | 0.0168 | 0.0549 | 1.43 | 1.32 | 1.53 | |
| Intra-run %CV | 5.6 | 0 | 2.2 | 2.1 | 1.9 | 1.5 | 8.9 | 8.5 | 4.6 | 3.6 | 1.7 | 0.9 | |
| Intra-run %RE | 1.7 | 0 | 5 | 0.4 | 1.8 | 11.9 | 0.3 | −1.6 | −0.7 | −1.4 | −0.06 | 6.5 | |
| n | 6 | 6 | 6 | 6 | 6 | 3 | 6 | 6 | 6 | 6 | 6 | 3 | |
| 2 | Intra-run mean | 0.217 | 0 | 1.04 | 38.8 | 78.8 | 0.208 | 0.199 | 1.22 | 38.2 | 76.3 | ||
| Intra-run SD | 0.0062 | 0 | 0.046 | 0.991 | 0.987 | 0.0114 | 0.0086 | 0.0476 | 0.404 | 1.20 | |||
| Intra-run %CV | 2.9 | 0 | 4.4 | 2.6 | 1.3 | 5.4 | 4.3 | 3.9 | 1.1 | 1.6 | |||
| Intra-run %RE | 8.7 | 0 | 4.1 | −3.0 | −1.5 | 5.8 | −0.4 | 1.3 | −4.9 | −4.9 | |||
| n | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |||
| 3 | Intra-run mean | 0.191 | 0 | 1.04 | 40.1 | 79.0 | 0.216 | 0.216 | 1.22 | 39.0 | 75.9 | ||
| Intra-run SD | 0.0107 | 0 | 0.0497 | 1.02 | 2.17 | 0.0039 | 0.0096 | 0.0354 | 0.838 | 1.86 | |||
| Intra-run %CV | 5.6 | 0 | 4.8 | 2.6 | 2.8 | 1.8 | 4.5 | 2.9 | 2.2 | 2.5 | |||
| Intra-run %RE | −4.7 | 0 | 3.5 | 0.2 | −1.3 | 7.8 | 7.8 | 1.8 | −2.9 | −5.4 | |||
| n | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |||
| Inter-run mean | 0.204 | 0 | 1.04 | 39.7 | 79.7 | 0.209 | 0.202 | 1.21 | 39.0 | 77.4 | |||
| Inter-run SD | 0.0144 | 0 | 0.0393 | 1.11 | 1.97 | 0.0134 | 0.0153 | 0.0458 | 1.10 | 2.42 | |||
| Inter-run %CV | 7.1 | 0 | 3.8 | 2.8 | 2.5 | 6.4 | 7.6 | 3.8 | 2.8 | 3.1 | |||
| Inter-run %RE | 1.9 | 0 | 4.2 | −0.8 | −0.3 | 4.7 | 1.0 | 0.8 | −3.0 | −3.4 | |||
| n | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | |||
3.3.4 Accuracy and precision
The accuracy and precision of the method were assessed by analyzing QC samples along with a calibration curve on three different days (Table 1). The calibration curve consisted of eight standards of different concentrations, each in duplicate, ranging from 0.2 to 100 ng/mL. Excellent results were obtained for the calibration curves, as the deviations of the back-calculated concentrations from their nominal values were within 15% for all the calibration standards in the three days of validation. All the QC samples were prepared in pooled mouse serum, and the endogenous level of GalSph and GluSph determined by mean of multiple replicates (n=12). The pooled mouse serum serves as LLQC, in which the signal to noise ratio of endogenous GalSph was less than 3 and assigned as 0 ng/mL. The endogenous levels were used to calculate the nominal concentrations of the spiked QC (LQC, MQC, HQC, and DQC). The DQC were diluted 5 times with 2% CHAPS solutions before extraction, and followed the procedure for other samples. The results of the QC samples in the three validation runs and dilution integration demonstrated acceptable accuracy and precision based on the preset validation criteria of 15% RE and ±15% CV.
3.3.5 Stability
Stability of the GalSph and GluSph in the mouse serum was evaluated under a variety of conditions to establish length of storage and sample processing conditions. The bench-top stability study showed that the GalSph and GluSph were stable in mouse serum for 18 hours at room temperature. The stability of GalSph and GluSph was determined to be acceptable in mouse serum following three freeze-thaw cycles. For processed samples (autosampler stability), the GalSph and GluSph were stable for 20 days at 4 °C. The GalSph and GluSph were determined to be stable for 162 days at −80 °C in mouse serum.
The GluSph and GalSph in standard curve matrix were stable for 65 hours at room temperature and for 162 days at −80 °C. In stock solution, GluSph and GalSph were stable for 18 hours at room temperature and for 162 days at −20 °C.
3.3.6 Carryover and run size evaluation
To evaluate carryover, a blank sample was immediately injected following the highest standard (100 ng/mL GalSph and GluSph). No carryover was observed in the regions of interest. The assay was validated to successfully analyze batch size of 228 samples and all the QC samples met accuracy and precision requirements.
3.4 Application of assay for natural history studies in Krabbe mouse model
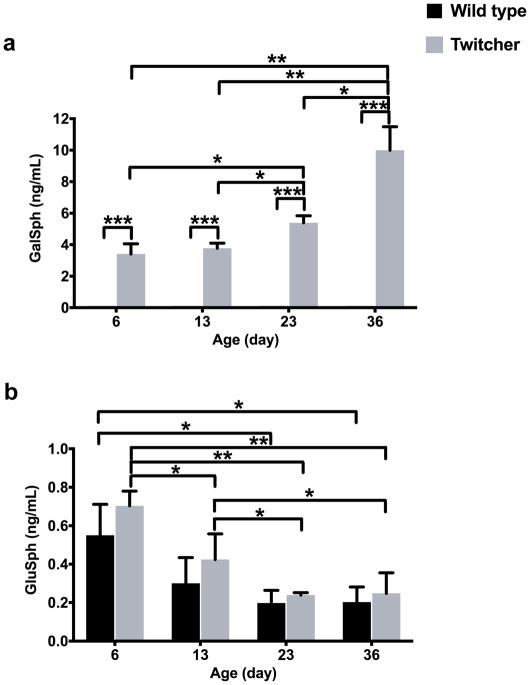
The method was used to analyze GalSph and GluSph in serum samples obtained from twitcher and wild type mice in natural history studies (Figure 2). The serum samples were collected from mice at age of 6, 13, 23, and 36 days (3 animals/age group). The GalSph in serum of the wild type at all ages was below the LLOQ of 0.2 ng/mL, while it was clearly present in the sera from the twitcher mice at all ages (Figure 2a). The GalSph level in serum of the affected mice was not significantly changed in pre-symptomatic stage (6 vs. 13 days); however, it was increased significantly with age and the progression of the disease in post-symptomatic stage (6 vs. 23, 23 vs. 36 days). GluSph was detectable in all the sera from twitcher and wild type mice (Figure 2b). There were no significant differences in GluSph between age-matched twitcher and wild type mice. In twitcher and wild type mice, GluSph was the highest at postnatal day 6 and decreased after 23 days age.
4 DISCUSSION
Glycosphingoid bases (lyso-glycosphingolipids) such as GalSph and GluSph are markedly elevated in Krabbe and Gaucher diseases, respectively. Both markers in blood have been proposed as biomarkers to assist clinicians in decision-making on initiation and optimizing individual dosing of therapy (Bradbury, Bagel, Jiang, et al. 2016, Carter, et al. 2016, Dekker, van Dussen, Hollak, et al. 2011, Murugesan, Chuang, Liu, et al. 2016, Rolfs, Giese, Grittner, et al. 2013, Turgeon, Orsini, Sanders, et al. 2015). Mouse models for Krabbe and Gaucher diseases have been used to develop new therapies, and GalSph and GluSph levels were used to monitor the therapeutic efficacies (Cabrera-Salazar, et al. 2010, Cabrera-Salazar, et al. 2012, Dahl, et al. 2015, Li and Sands 2014, Lin, Fantz, Levy, et al. 2005, Marshall, Sun, Bangari, et al. 2016, Mikulka and Sands 2016, Qin, et al. 2012, Reddy, et al. 2011, Sun, et al. 2011). A significant reduction in GalSph or GluSph in the treated mouse models has been considered a reliable metric for monitoring the beneficial effects of newly developing therapies. A method for accurate quantification of GalSph and GluSph with easy sample preparation, short analysis times, good sensitivity and specificity is needed.
To date, accurate quantification of GalSph and GluSph has been achieved only with HILIC-MS/MS methods, in which two isomers are separated. The major limitations of current methods are the long run time, tedious sample preparation, and lack of robustness, which limited their utilities in the treatment development for Krabbe and Gaucher diseases, where GalSph and GluSph are used for assessment of treatment efficacies. Here we have developed a robust mass spectrometric detection method for sensitive and high throughput quantification of GalSph and GluSph. The HILIC condition was optimized to reach 7 min total run time per sample with baseline separation of GalSph and GluSph. The isotope labeled internal standards were used and allowed compensation for fluctuations in extraction, ionization efficiency, and mass spectrometric performance. Since the isotope-labeled standards are chemically identical to the analytes of interest, the elution pattern from LC as well as the ionization efficiency in the mass spectrometer are identical to the analytes. The 2% CHAPS was used as surrogate matrix for standard curve as no analyte-free mouse serum is available. It is important to reach similar recoveries from biological and surrogate matrices. The approximate15% loss of GalSph and GluSph that were bound to precipitated proteins during neutral protein precipitation of serum samples cannot be compensated by their internal standards that were dissolved in precipitation solvent, while the recoveries of GalSph and GluSph from 2% CHAPS were quantitative, leading to underestimates of these two compounds using standard curves in 2% CHAPS. The quantitative extraction recoveries for GalSph and GluSph from mouse serum and surrogate matrices were achieved under basic extraction condition to permit dilute-and-shoot, and thus significantly improved throughput. Assay performance was robust as up to 228 samples could be injected consecutively without any degradation in the quality of the chromatography, analyte detection, accuracy, and precision. The method also showed excellent sensitivity, accuracy, and precision. Compared to previously reported methods, our method delivers reliable data at higher throughput.
We used our method to analyze sera of wild type and twitcher mice at different ages. The affected twitcher mice show no symptoms until postnatal day 15 and are indistinguishable from their heterozygous and wild type littermates (Duchen, et al. 1980, Taniike and Suzuki 1994). However, the GalSph is significantly elevated in twitcher serum compared to wild type even at postnatal day 6. The pre-symptomatic elevation of serum GalSph in neonatal twitcher mouse is in agreement with the finding that GalSph measurement can be used as the primary newborn screening marker for asymptomatic early infantile Krabbe disease (Turgeon, Orsini, Sanders, et al. 2015). Our GalSph data from post-symptomatic mice also confirm the previous report that in twitcher serum GalSph rapidly increases after clinical signs appear and directly relates to the progression of the disease (Zanfini, Dreassi, Berardi, et al. 2013, Zhu, Lopez-Rosas, Qiu, et al. 2012). Although serum GluSph shows no significant difference between age-matched twitcher and wild type mice, the biomarker levels are lower in older as compared with younger mice from day 3 to 36. A paper reported that the plasma GluSph level in 12 weeks old wile type mice was 0.5 ng/mL (Ferraz, et al. 2016), which was 2.5-fold of that in 36 days old mice. A more thorough natural history study is needed to obtain a complete profile of GluSph in serum over age.
In conclusion, we have developed a robust and high throughput HILIC-MS/MS method for the accurate quantification of GalSph and GluSph in mouse serum, which are biomarkers to monitor the treatment efficacy for Krabbe and Gaucher diseases, respectively. This method could be readily extended to quantification of GalSph and GluSph in human sera, and could prove useful for monitoring of these biomarkers in clinical studies in the context of development of new therapies for these disorders.
Figure 4.
GalSph (a) and GluSph (b) in serum samples obtained from wide type (n = 3) and twitcher (n = 3) mice at different ages, expressed as mean values ± SD. The GalSph levels in all the wild type samples are below LLOQ and assigned as half LLOQ (0.1 ng/mL) for purposes of plotting and statistical analysis. *: p < 0.05; **: p < 0.01; ***: p < 0.0001.
Acknowledgments
This work was supported by NIH grant to M.S (R01 NS084861) and was performed in the Washington University Metabolomics Facility (NIH P30 DK020579 and P30 DK056341).
Abbreviations
- CHAPS
3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate
- %CV
percent coefficient of variance
- DQC
dilution quality control
- ESI
electrospray ionization
- ESI-MS/MS
electrospray ionization-tandem mass spectrometry
- GALC
galactosylceramidase
- GalSph
galactosylsphingosine
- GluSph
glucosylsphingosine
- HILIC
hydrophilic interaction chromatography
- HILIC-MS/MS
hydrophilic interaction chromatography-tandem mass spectrometry
- HPLC
high performance liquid chromatography
- HQC
high quality control
- LC
liquid chromatography
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LLOQ
lower limit of quantification
- LLQC
lower limit quality control
- LQC
low quality control
- MQC
middle quality control
- MS
mass spectrometry
- MRM
multiple reaction monitoring
- %RE
percent relative error
- RPLC-MS/MS
reversed phased liquid chromatography-tandem mass spectrometry
- SD
standard deviation
- TLC
thin layer chromatography
- ULOQ
upper limit of quantification
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
References
- Bodennec J, Pelled D, Futerman AH. Aminopropyl solid phase extraction and 2 D TLC of neutral glycosphingolipids and neutral lysoglycosphingolipids. J Lipid Res. 2003a;44:218–26. doi: 10.1194/jlr.d200026-jlr200. [DOI] [PubMed] [Google Scholar]
- Bodennec J, Trajkovic-Bodennec S, Futerman AH. Simultaneous quantification of lyso-neutral glycosphingolipids and neutral glycosphingolipids by N-acetylation with [3H]acetic anhydride. J Lipid Res. 2003b;44:1413–9. doi: 10.1194/jlr.D300010-JLR200. [DOI] [PubMed] [Google Scholar]
- Bradbury AM, Bagel JH, Jiang X, Swain GP, Prociuk ML, Fitzgerald CA, O’Donnell PA, Braund KG, Ory DS, Vite CH. Clinical, electrophysiological, and biochemical markers of peripheral and central nervous system disease in canine globoid cell leukodystrophy (Krabbe’s disease) J Neurosci Res. 2016;94:1007–17. doi: 10.1002/jnr.23838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO, Kanfer JN, Bradley RM, Shapiro D. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher’s disease. J Clin Invest. 1966;45:1112–5. doi: 10.1172/JCI105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Salazar MA, Bercury SD, Ziegler RJ, Marshall J, Hodges BL, Chuang WL, Pacheco J, Li L, Cheng SH, Scheule RK. Intracerebroventricular delivery of glucocerebrosidase reduces substrates and increases lifespan in a mouse model of neuronopathic Gaucher disease. Exp Neurol. 2010;225:436–44. doi: 10.1016/j.expneurol.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Cabrera-Salazar MA, Deriso M, Bercury SD, Li L, Lydon JT, Weber W, Pande N, Cromwell MA, Copeland D, Leonard J, Cheng SH, Scheule RK. Systemic delivery of a glucosylceramide synthase inhibitor reduces CNS substrates and increases lifespan in a mouse model of type 2 Gaucher disease. PLoS One. 2012;7:e43310. doi: 10.1371/journal.pone.0043310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RL, Wrabetz L, Jalal K, Orsini JJ, Barczykowski AL, Matern D, Langan TJ. Can psychosine and galactocerebrosidase activity predict early-infantile Krabbe’s disease presymptomatically? J Neurosci Res. 2016;94:1084–93. doi: 10.1002/jnr.23793. [DOI] [PubMed] [Google Scholar]
- Chuang WL, Pacheco J, Zhang XK, Martin MM, Biski CK, Keutzer JM, Wenger DA, Caggana M, Orsini JJ., Jr Determination of psychosine concentration in dried blood spots from newborns that were identified via newborn screening to be at risk for Krabbe disease. Clin Chim Acta. 2013;419:73–6. doi: 10.1016/j.cca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Dahl M, Doyle A, Olsson K, Mansson JE, Marques AR, Mirzaian M, Aerts JM, Ehinger M, Rothe M, Modlich U, Schambach A, Karlsson S. Lentiviral gene therapy using cellular promoters cures type 1 Gaucher disease in mice. Mol Ther. 2015;23:835–44. doi: 10.1038/mt.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker N, van Dussen L, Hollak CE, Overkleeft H, Scheij S, Ghauharali K, van Breemen MJ, Ferraz MJ, Groener JE, Maas M, Wijburg FA, Speijer D, Tylki-Szymanska A, Mistry PK, Boot RG, Aerts JM. Elevated plasma glucosylsphingosine in Gaucher disease: relation to phenotype, storage cell markers, and therapeutic response. Blood. 2011;118:e118–27. doi: 10.1182/blood-2011-05-352971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen LW, Eicher EM, Jacobs JM, Scaravilli F, Teixeira F. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain. 1980;103:695–710. doi: 10.1093/brain/103.3.695. [DOI] [PubMed] [Google Scholar]
- Ferraz MJ, Marques AR, Gaspar P, Mirzaian M, van Roomen C, Ottenhoff R, Alfonso P, Irun P, Giraldo P, Wisse P, Sa Miranda C, Overkleeft HS, Aerts JM. Lyso-glycosphingolipid abnormalities in different murine models of lysosomal storage disorders. Mol Genet Metab. 2016;117:186–93. doi: 10.1016/j.ymgme.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Fuller M, Szer J, Stark S, Fletcher JM. Rapid, single-phase extraction of glucosylsphingosine from plasma: A universal screening and monitoring tool. Clin Chim Acta. 2015;450:6–10. doi: 10.1016/j.cca.2015.07.026. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Basso V, Cantuti L, Givogri MI, Lopez-Rosas A, Perez N, Vasu C, Cao H, van Breemen R, Mondino A, Bongarzone ER. Autonomic denervation of lymphoid organs leads to epigenetic immune atrophy in a mouse model of Krabbe disease. J Neurosci. 2007;27:13730–8. doi: 10.1523/JNEUROSCI.3379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins-Salsbury JA, Parameswar AR, Jiang X, Schlesinger PH, Bongarzone E, Ory DS, Demchenko AV, Sands MS. Psychosine, the cytotoxic sphingolipid that accumulates in globoid cell leukodystrophy, alters membrane architecture. J Lipid Res. 2013;54:3303–11. doi: 10.1194/jlr.M039610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain AM, Altuwaijri M, Aldosari M. Krabbe disease: neurophysiologic studies and MRI correlations. Neurology. 2004;63:617–20. doi: 10.1212/01.wnl.0000134651.38196.f8. [DOI] [PubMed] [Google Scholar]
- Ichioka T, Kishimoto Y, Yeager AM. Simultaneous determination of psychosine and cerebrosides. Anal Biochem. 1987;166:178–82. doi: 10.1016/0003-2697(87)90560-4. [DOI] [PubMed] [Google Scholar]
- Igisu H, Suzuki K. Analysis of galactosylsphingosine (psychosine) in the brain. J Lipid Res. 1984a;25:1000–6. [PubMed] [Google Scholar]
- Igisu H, Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984b;224:753–5. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- Ji AJ, Jiang Z, Livson Y, Davis JA, Chu JX, Weng N. Challenges in urine bioanalytical assays: overcoming nonspecific binding. Bioanalysis. 2010;2:1573–86. doi: 10.4155/bio.10.114. [DOI] [PubMed] [Google Scholar]
- Jiang X, Yang K, Han X. Direct quantitation of psychosine from alkaline-treated lipid extracts with a semi-synthetic internal standard. J Lipid Res. 2009;50:162–72. doi: 10.1194/jlr.D800036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BR, Schultz GA, Eckstein JA, Ackermann BL. Surrogate matrix and surrogate analyte approaches for definitive quantitation of endogenous biomolecules. Bioanalysis. 2012;4:2343–56. doi: 10.4155/bio.12.200. [DOI] [PubMed] [Google Scholar]
- Kohlschutter A. Lysosomal leukodystrophies: Krabbe disease and metachromatic leukodystrophy. Handb Clin Neurol. 2013;113:1611–8. doi: 10.1016/B978-0-444-59565-2.00029-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Sands MS. Experimental therapies in the murine model of globoid cell leukodystrophy. Pediatr Neurol. 2014;51:600–6. doi: 10.1016/j.pediatrneurol.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YT, Li SC, Buck WR, Haskins ME, Wu SW, Khoo KH, Sidransky E, Bunnell BA. Selective extraction and effective separation of galactosylsphingosine (psychosine) and glucosylsphingosine from other glycosphingolipids in pathological tissue samples. Neurochem Res. 2011;36:1612–22. doi: 10.1007/s11064-010-0348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Fantz CR, Levy B, Rafi MA, Vogler C, Wenger DA, Sands MS. AAV2/5 vector expressing galactocerebrosidase ameliorates CNS disease in the murine model of globoid-cell leukodystrophy more efficiently than AAV2. Mol Ther. 2005;12:422–30. doi: 10.1016/j.ymthe.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Marshall J, Sun Y, Bangari DS, Budman E, Park H, Nietupski JB, Allaire A, Cromwell MA, Wang B, Grabowski GA, Leonard JP, Cheng SH. CNS-accessible Inhibitor of Glucosylceramide Synthase for Substrate Reduction Therapy of Neuronopathic Gaucher Disease. Mol Ther. 2016;24:1019–29. doi: 10.1038/mt.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulka CR, Sands MS. Treatment for Krabbe’s disease: Finding the combination. J Neurosci Res. 2016;94:1126–37. doi: 10.1002/jnr.23822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaian M, Wisse P, Ferraz MJ, Gold H, Donker-Koopman WE, Verhoek M, Overkleeft HS, Boot RG, Kramer G, Dekker N, Aerts JM. Mass spectrometric quantification of glucosylsphingosine in plasma and urine of type 1 Gaucher patients using an isotope standard. Blood Cells Mol Dis. 2015;54:307–14. doi: 10.1016/j.bcmd.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Miyatake T, Suzuki K. Globoid cell leukodystrophy: additional deficiency of psychosine galactosidase. Biochem Biophys Res Commun. 1972;48:539–43. doi: 10.1016/0006-291x(72)90381-6. [DOI] [PubMed] [Google Scholar]
- Motta M, Tatti M, Furlan F, Celato A, Di Fruscio G, Polo G, Manara R, Nigro V, Tartaglia M, Burlina A, Salvioli R. Clinical, biochemical and molecular characterization of prosaposin deficiency. Clin Genet. 2016;90:220–9. doi: 10.1111/cge.12753. [DOI] [PubMed] [Google Scholar]
- Murugesan V, Chuang WL, Liu J, Lischuk A, Kacena K, Lin H, Pastores GM, Yang R, Keutzer J, Zhang K, Mistry PK. Glucosylsphingosine is a key biomarker of Gaucher disease. Am J Hematol. 2016;91:1082–1089. doi: 10.1002/ajh.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa M, Iwamoto T, Tokoro T, Eto Y. Novel procedure for measuring psychosine derivatives by an HPLC method. J Neurochem. 1992;59:607–9. doi: 10.1111/j.1471-4159.1992.tb09412.x. [DOI] [PubMed] [Google Scholar]
- Orvisky E, Park JK, LaMarca ME, Ginns EI, Martin BM, Tayebi N, Sidransky E. Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: correlation with phenotype and genotype. Mol Genet Metab. 2002;76:262–70. doi: 10.1016/s1096-7192(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Pastores GM. Krabbe disease: an overview. Int J Clin Pharmacol Ther. 2009;47(Suppl 1):S75–81. doi: 10.5414/cpp47075. [DOI] [PubMed] [Google Scholar]
- Qin EY, Hawkins-Salsbury JA, Jiang X, Reddy AS, Farber NB, Ory DS, Sands MS. Bone marrow transplantation increases efficacy of central nervous system-directed enzyme replacement therapy in the murine model of globoid cell leukodystrophy. Mol Genet Metab. 2012;107:186–96. doi: 10.1016/j.ymgme.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AS, Kim JH, Hawkins-Salsbury JA, Macauley SL, Tracy ET, Vogler CA, Han X, Song SK, Wozniak DF, Fowler SC, Klein RS, Sands MS. Bone marrow transplantation augments the effect of brain- and spinal cord-directed adeno-associated virus 2/5 gene therapy by altering inflammation in the murine model of globoid-cell leukodystrophy. J Neurosci. 2011;31:9945–57. doi: 10.1523/JNEUROSCI.1802-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs A, Giese AK, Grittner U, Mascher D, Elstein D, Zimran A, Bottcher T, Lukas J, Hubner R, Golnitz U, Rohle A, Dudesek A, Meyer W, Wittstock M, Mascher H. Glucosylsphingosine is a highly sensitive and specific biomarker for primary diagnostic and follow-up monitoring in Gaucher disease in a non-Jewish, Caucasian cohort of Gaucher disease patients. PLoS One. 2013;8:e79732. doi: 10.1371/journal.pone.0079732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Inui K, Tatsumi N, Fukushima H, Nishigaki T, Taniike M, Nishimoto J, Tsukamoto H, Yanagihara I, Ozono K, Okada S. Molecular cloning and expression of cDNA for murine galactocerebrosidase and mutation analysis of the twitcher mouse, a model of Krabbe’s disease. J Neurochem. 1996;66:1118–24. doi: 10.1046/j.1471-4159.1996.66031118.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ran H, Liou B, Quinn B, Zamzow M, Zhang W, Bielawski J, Kitatani K, Setchell KD, Hannun YA, Grabowski GA. Isofagomine in vivo effects in a neuronopathic Gaucher disease mouse. PLoS One. 2011;6:e19037. doi: 10.1371/journal.pone.0019037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zamzow M, Ran H, Zhang W, Quinn B, Barnes S, Witte DP, Setchell KD, Williams MT, Vorhees CV, Grabowski GA. Tissue-specific effects of saposin A and saposin B on glycosphingolipid degradation in mutant mice. Hum Mol Genet. 2013;22:2435–50. doi: 10.1093/hmg/ddt096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Suzuki Y. Globoid cell leucodystrophy (Krabbe’s disease): deficiency of galactocerebroside beta-galactosidase. Proc Natl Acad Sci U S A. 1970;66:302–9. doi: 10.1073/pnas.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L, Vanier MT, Mansson JE. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J Lipid Res. 1980;21:53–64. [PubMed] [Google Scholar]
- Taniike M, Suzuki K. Spacio-temporal progression of demyelination in twitcher mouse: with clinico-pathological correlation. Acta Neuropathol. 1994;88:228–36. doi: 10.1007/BF00293398. [DOI] [PubMed] [Google Scholar]
- Turgeon CT, Orsini JJ, Sanders KA, Magera MJ, Langan TJ, Escolar ML, Duffner P, Oglesbee D, Gavrilov D, Tortorelli S, Rinaldo P, Raymond K, Matern D. Measurement of psychosine in dried blood spots--a possible improvement to newborn screening programs for Krabbe disease. J Inherit Metab Dis. 2015;38:923–9. doi: 10.1007/s10545-015-9822-z. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services FaDA, Center for Drug Evaluation and Research and Center for Veterinary Medicine. Guidance for Industry: Bioanalytical Method Validations. 2001. [Google Scholar]
- Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, Shah VP, Skelly JP, Swann PG, Weiner R. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm Res. 2007;24:1962–73. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]
- Welford RW, Garzotti M, Marques Lourenco C, Mengel E, Marquardt T, Reunert J, Amraoui Y, Kolb SA, Morand O, Groenen P. Plasma lysosphingomyelin demonstrates great potential as a diagnostic biomarker for Niemann-Pick disease type C in a retrospective study. PLoS One. 2014;9:e114669. doi: 10.1371/journal.pone.0114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AB, Givogri MI, Lopez-Rosas A, Cao H, van Breemen R, Thinakaran G, Bongarzone ER. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J Neurosci. 2009;29:6068–77. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield PD, Sharp PC, Taylor R, Meikle P. Quantification of galactosylsphingosine in the twitcher mouse using electrospray ionization-tandem mass spectrometry. J Lipid Res. 2001;42:2092–5. [PubMed] [Google Scholar]
- Zaka M, Wenger DA. Psychosine-induced apoptosis in a mouse oligodendrocyte progenitor cell line is mediated by caspase activation. Neurosci Lett. 2004;358:205–9. doi: 10.1016/j.neulet.2003.12.126. [DOI] [PubMed] [Google Scholar]
- Zanfini A, Dreassi E, Berardi A, Governini L, Corbini G, Costantino-Ceccarini E, Balestri P, Luddi A. Quantification of psychosine in the serum of twitcher mouse by LC-ESI-tandem-MS analysis. J Pharm Biomed Anal. 2013;80:44–9. doi: 10.1016/j.jpba.2013.02.039. [DOI] [PubMed] [Google Scholar]
- Zhu H, Lopez-Rosas A, Qiu X, Van Breemen RB, Bongarzone ER. Detection of the neurotoxin psychosine in samples of peripheral blood: application in diagnostics and follow-up of Krabbe disease. Arch Pathol Lab Med. 2012;136:709–10. doi: 10.5858/arpa.2011-0667-LE. [DOI] [PMC free article] [PubMed] [Google Scholar]