Abstract
Dyskeratosis congenita (DC) is an inherited bone marrow failure syndrome caused by germline mutations in telomere biology genes. Patients have extremely short telomeres for their age and a complex phenotype including oral leukoplakia, abnormal skin pigmentation and dysplastic nails in addition to bone marrow failure, pulmonary fibrosis, stenosis of the esophagus, lacrimal ducts and urethra, developmental anomalies, and high risk of cancer.
We evaluated a patient with features of DC, mood dysregulation, diabetes, and lack of pubertal development. Family history was not available but genome-wide genotyping was consistent with consanguinity. Whole exome sequencing identified 82 variants of interest in 80 genes based on the following criteria: homozygous, <0.1% minor allele frequency in public and in-house databases, nonsynoymous, and predicted deleterious by multiple in silico prediction programs. Six genes were identified likely contributory to the clinical presentation.
The cause of DC is likely due to homozygous splice site variants in RTEL1, a known DC and telomere biology gene. A homozygous, missense variant in tryptophan hydroxylase 1 (TPH1) may be clinically important as this gene encodes the rate limiting step in serotonin biosynthesis, a biologic pathway connected with mood disorders. Four additional genes (SCN4A, LRP4, GDAP1L1, and SPTBN5) had rare, missense homozygous variants that we speculate may contribute to portions of the clinical phenotype.
This case illustrates the value of conducting detailed clinical and genomic evaluations on rare patients in order to identify new areas of research into the functional consequences of rare variants and their contribution to human disease.
Keywords: dyskeratosis congenita, mood dysregulation, RTEL1, TPH1, STPBN5, SCN4A, GDAP1L1, LRP4
INTRODUCTION
Dyskeratosis congenita (DC) is a cancer-prone inherited bone marrow failure syndrome and the prototypic telomere biology disorder (TBD)(Bertuch, 2016). The diagnostic triad consists of lacy reticular skin pigmentation, oral leukoplakia and dysplastic nails. Patients with DC are at a high risk of developing bone marrow failure with a cumulative incidence of at least 50% by the age of 40 years(Alter, Giri, Savage, & Rosenberg, 2009). Clinical features of DC vary and include pulmonary disease, liver disease, neurologic and developmental issues, gastrointestinal or genitourinary stenosis, and ophthalmologic problems. Patients with DC have exceedingly short telomeres, the nucleoprotein complex at chromosome ends essential for chromosomal stability, due to germline mutations in key telomere biology genes. Currently there are at least 13 known causative genes of DC and related TBDs namely DKC1, TINF2, TERT, TERC, NOP10, NHP2, CTC1, WRAP53, RTEL1, ACD, PARN, NAF1, and STN1, which cumulatively account for 70–80% of patients with DC(Bertuch, 2016; Simon et al., 2016; Stanley et al., 2016; Takai et al., 2016). Telomere length less than the first percentile for age, measured in leukocytes by flow cytometry with in situ hybridization (flow FISH) is diagnostic of DC(Alter et al., 2012).
This report details the clinical and genetic features of an individual who, in addition to DC had multiple other medical problems not likely directly related to DC including diabetes, lack of pubertal development, and mood dysregulation probably due to numerous homozygous genetic variants.
METHODS
Clinical characterization
Patient NCI-323-1 participated in an IRB-approved longitudinal cohort study at the National Cancer Institute (NCI) entitled “Etiologic Investigation of Cancer Susceptibility in Inherited Bone Marrow Failure Syndromes” (ClinicalTrials.gov Identifier: NCT00027274)(Alter et al., 2010). The patient’s legal guardian signed consent and the patient signed assent based upon his developmental abilities. Permission for the use of photos was also granted via signed consent from the legal guardian.
This study includes comprehensive family history and individual history questionnaires, detailed medical record review, and biospecimen collection. Detailed clinical evaluations were performed at the NIH Clinical Center per protocol. Telomere length was measured by flow cytometry with fluorescent in situ hybridization (flow FISH) in leukocytes as described(Alter et al., 2012).
Whole exome sequencing analyses
Whole exome sequencing (WES) on blood-derived DNA from the proband obtained at 16 years of age was performed at the NCI’s Cancer Genomics Research Laboratory, as previously described(B. J. Ballew et al., 2013). Exome enrichment was performed with NimbleGen’s SeqCap EZ Human Exome Library v3.0+UTR (Roche NimbleGen, Inc., Madison, WI, USA), targeting 96 Mb of exonic sequence and the flanking untranslated regions (UTR) on an Illumina HiSeq as previously described(B. Ballew et al., 2013; Mirabello et al., 2014). Annotation of each exome variant locus was performed using a custom software pipeline.
WES variants were identified as of interest if they met the following criteria: homozygous, nonsynonymous, had a minor allele frequency (MAF) <0.1% in the Exome Aggregation Consortium (ExAc) databases, and occurred <5 times in our in house database of 4,091 individuals. Variants of interest were validated to rule out false positive findings using an Ion 316 chip on the Ion PGM Sequencer (Life Technologies, Carlsbad, CA, USA).
Genotyping
Genome-wide single nucleotide polymorphisms (SNP) genotyping was conducted using the Illumina OmniExpress Beadchip at the NCI’s Cancer Genomics Research Laboratory. For SNP assays, sample completion rates, SNP call rates, and genotype concordance rates were calculated. Samples with completion rate less than 90% were excluded from CNV analysis.
Bioinformatics
The potential pathogenicity of variants was predicted using MetaSVM, a tool which is based on 10 component scores, SIFT, PolyPhen-2 HDIV, PolyPhen-2 HVAR, GERP++, MutationTaster, MutationAssessor, FATHMM, LRT, SiPhy, and PhyloP(Dong et al., 2015). Human Splicing Finder was used to predict potential splice site changes (Desmet et al., 2009). The database for Genotypes and Phenotypes (dbGaP) accession assigned to this study is phs001481.v1.p1.
RESULTS
Clinical presentation
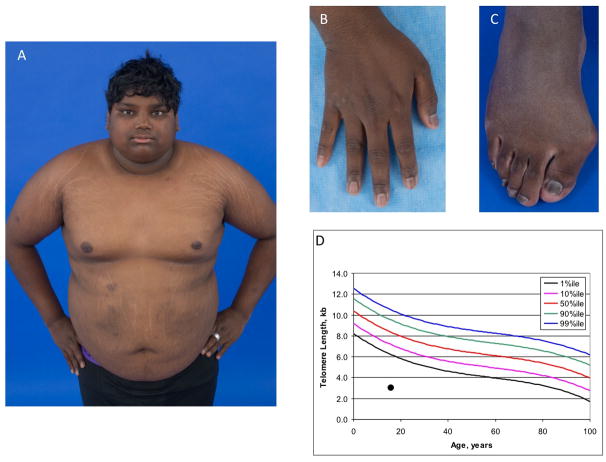
The proband, NCI-323-1, was a 21 year-old male of Indian ancestry evaluated for DC due to the presence of pancytopenia, developmental delay, and very short telomeres. His past medical history was significant for intrauterine growth restriction and a birth weight of 3 pounds 6 ounces at 38 weeks gestation. Microcephaly, undescended testes, and respiratory distress were reported after birth. He was cared for in a special care nursery due to poor feeding and infection. The family history is unknown as he was adopted at 6 months of age. Multiple respiratory infections, irritable airway, and failure to thrive were reported through at least 4 years of age. Developmental delay, microcephaly, short stature were noted in infancy and have persisted. He first spoke in full sentences at 5 years of age. He had non-specific gastrointestinal problems, including encopresis. Cytopenias were first noted at age 13 years during an evaluation for gastrointestinal symptoms (Table 1). Hypothyroidism was diagnosed at age 14 years. He was referred to a hematologist at age 16 years when he was found to have persistent thrombocytopenia and anemia while undergoing endocrine evaluation for short stature and delayed puberty. His bone marrow showed trilineage hypoplasia with cellularity of approximately 5%. Testing for Fanconi anemia was negative. Peripheral blood lymphocyte telomere lengths were very low at 3.1 kb (mean normal 16 year-old telomere length 8.3 kb, Figure 1). He was enrolled in the current study at that time and blood was drawn for exome sequencing. He was followed closely but not treated for the cytopenias. He also developed insulin dependent diabetes at 16 years of age.
Table 1. Laboratory findings at age 13, 16, and 21 years.
All values reflect fasting levels. Additional studies done and within normal limits at age 21 years included total iron, iron saturation, ferritin, total immunoglobulins, lymphocyte subsets, antibody titers to mumps, rubella, diphtheria, and tetanus, bilirubin, alpha fetoprotein, total protein, albumin.
| Age | Normal range/units | |||
|---|---|---|---|---|
| Laboratory study | 13 years | 16 years* | 21 years | |
| WBC | 4,300 | 4,100 | 4,710 | 4,200–9,000/uL |
| ANC | 1,247 | 1,568 | 1,484 | ≥1,500/uL |
| Hemoglobin | 10.9 | 8.7 | 11.4 | 13.7–17.5 gm/dL |
| MCV | 85.1 | 88.9 | 80.3 | 79–92.2 fL |
| Platelets | 43,000 | 25,000 | 38,000 | ≥150,000/uL |
| Fetal hemoglobin | 6.5 | 1.7 | 0–2% | |
| Erythropoietin | 233 | 2.6–18.5 mlU/mL | ||
| TSH | 1.1 | 3.67 | 0.4–4 mcIU/mL | |
| Glucose | 95 | 157 | 170 | 70–100 mg/dL |
| Insulin | 34.7 | 2.6–26.9 mcU/mL | ||
| AST | 21 | 42 | 0–41 U/L | |
| ALT | 28 | 48 | 0–40 U/L | |
| Testosterone | 15.6 | <20.0 | 262–1,593 ng/dL | |
| Bone marrow morphology | Hypocellular (5%); erythroid predominance & macrocytosis | Hypocellular (20%); erythroid predominance & occasional myeloid with hypogranularity | ||
| Bone marrow cytogenetics | +1,der(1;7)(q10;q10) [19]/46,XY[1] | |||
Abbreviations: WBC, white blood cells; ANC, absolute neutrophil count; MCV, mean corpuscular hemoglobin; TSH, thyroid stimulating hormone; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Blood was drawn for exome sequencing at 16 years of age, prior to the development of bone marrow cytogenetic abnormalities.
Figure 1. Clinical features of the proband.
A) body habitus, hypo- and hyper-pigmented patches as well as acanthosis nigricans and striae on abdomen, back, shoulders, and legs; B) ridged and peeling fingernails; C) thickened toenails, pes planus, and hallux valgus deformity; D) telomere length in total lymphocytes measured by flow cytometry with in situ hybridization
The proband had psychiatric manifestations from early childhood that included mood disorder with “constant meltdowns.” He was evaluated by a neurologist at age 4 years and treated with sertraline. Further child psychiatry evaluation at age 8 years for irritability and frequent crying episodes led to the diagnosis of bipolar disorder as per mother’s recollection. His mood stabilized with the addition of quetiapine fumarate to the sertraline regimen. Quetiapine fumarate was later switched to aripiprazole and paroxetine. Clonazepam was given, as needed, for severe agitation.
Physical exam at 21 years of age revealed short stature with height at 150.9 cm (<1st percentile, Z-score −3.5), weight 80.6 kg, and BMI of 35.4. Head circumference was 51.5 cm (1st percentile, Z-score −2.4). Relevant clinical and laboratory features are shown in Figure 1 and Table 1. He had a hyper-pigmented skin patch as well as acanthosis nigricans and striae on abdomen, back, shoulders, and legs. Toenails were thickened and rough. Fingernails were ridged and peeling. Pes planus and severe hallux valgus deformity were present. Oral exam revealed a darkly pigmented tongue with a 3 by 2 mm area of leukoplakia present. Delayed bone maturation was noted with a bone age of 17 years for a chronological age of 21 years. Pubertal development was Tanner stage 1 with very small testes (<1 cm) and micropenis associated with very low testosterone level. Brain and pituitary MRI were normal. Echocardiogram with microbubble study was normal. Abdominal ultrasound showed liver span of 14–15 cm with diffuse increased echogenicity compatible with steatosis. The spleen and kidneys were normal. Pulmonary function studies showed a moderately reduced carbon monoxide diffusion capacity at 45% with no desaturation after 6 minutes of walking. Psychiatric evaluation at the time found that his behavioral history with the background of intellectual disability was more consistent with severe mood dysregulation rather than bipolar disorder.
Unfortunately, at age 22 years, the patient developed severe pancytopenia and shortness of breath requiring hospitalization. He died two weeks later from progressive respiratory failure; no autopsy was performed.
Loss of heterozygosity and copy number variation
Illumina OmniExpress genotyping identified 940 regions of loss of heterozygosity (LOH) that were at least 500 kilobases (kb) in size and found on all chromosomes. There were 71 LOH regions greater than 1 megabases (mb) in size and of 40 were more than 3 mb. The extent of homozygosity was consistent with first degree relative consanguinity (Supplementary Table 1).
There were runs of homozygosity encompassing established neuropsychiatric risk copy number variants (CNVs) at 1q, 2p, 7q, 16p, 15q, and 17q(Stefansson et al., 2014). Notably, missense variants were present in two genes in these regions: rs375138650 in BCL9 at 1q21.2 and rs201093821 in CDR2 at 16p12.2. Both variants were predicted tolerated by meta-SVM but had CADD scores >20; the CADD score suggests they could be deleterious but functional studies are required.
Exome sequencing
There were 82 homozygous nonsynonymous variants in 80 genes with minor allele frequencies (MAF) less than 0.1% in population and in-house databases with high quality sequencing scores (details of filtering given in Methods) identified in the proband (Supplementary Table 2). A combination of literature searches and evaluation of the biological pathways for each of these genes led us to further evaluate six genes possibly related to the proband’s complex phenotype.
The cause of very short telomeres and DC clinical features, including nail abnormalities, oral leukoplakia, and bone marrow failure, in this patient is most likely due to the presence of homozygous variants in the regulator of telomere elongation helicase 1 (RTEL1) gene (chr20:62,321,005, c.2025+4A>C). This variant is predicted to be disease causing by Mutation Taster. Human Splicing Finder predicts this variant results in a broken donor site that could result in aberrant splicing (Supplementary Table 2).
The proband was homozygous for a missense variant in the tryptophan hydroxylase 1 (TPH1) gene (rs570648870, chr11:18,042,689, c.G1184A, p.R395H). Although previously undescribed as disease causing, all 10 pathogenicity predictors used by MetaSVM predicted this variant to be deleterious. TPH1 is the first and rate limiting step in serotonin biosynthesis as it required for the conversion of tryptophan to 5-OH-tryptophan which is then converted to serotonin (Waloen, Kleppe, Martinez, & Haavik, 2017).
Additional homozygous variants of potential but uncertain clinical interest were also identified. There were two homozygous variants in SPTBN5, p.R2141W and p.R1367G, (rs538566766 and rs530203497, respectively). Although MetaSVM predicts that these variants may be tolerated, it is not possible to predict the consequences of the presence of two rare homozygous variants in the same gene. SPTBN5 encodes spectrin beta-V, a protein with limited data but apparently important in the cytoskeleton structure and binding to microtubule-based motor proteins(Papal et al., 2013).
Homozygosity for a likely pathogenic variant, p.M150L (rs543502873) was identified in SCN4A which encodes the sodium channel voltage-gated, type IV, alpha subunit. Pathogenic germline variants in SCN4A have been identified as the cause of a group of related muscular disorders including hypokalemic periodic paralysis(Vicart et al., 1993).
A homozygous predicted damaging variant, p.R94Q, was present in the GDAP1L1 gene encoding ganglioside induced differentiation associated protein 1 like 1. This gene is similar in sequence to GDAP1, in which mutations cause Charcot-Marie-Tooth type 4A disease but it’s precise function is unknown(Bird, 1993). Finally, rs528376810 in LRP4 (p.E1691K) is also predicted to be damaging.
Germline mutations in LRP4, which encodes LDL receptor related protein 4, a member of the low-density lipoprotein receptor-related protein family, have been reported to cause autosomal recessive Cenani-Lenz syndrome, a disorder of limb and kidney malformations associated with altered Wnt and beta-catenin signaling(Li et al., 2010).
DISCUSSION
The patient reported here had multiple complex diseases, including DC, diabetes, mood dysregulation, and mildly dysmorphic features, requiring a multidisciplinary approach to diagnosis and medical management. Identification of the genetic etiology in this setting can lead to important insights into the underlying biology and lead to new avenues of research. Numerous likely-disease associated variants were identified that could contribute to the patient’s complex clinical phenotype.
The homozygous RTEL1 variant (chr20:62,321,005, c.2025+4A>C) is located in a region near other reported deleterious variants and is predicted to affect protein splicing(B. J. Ballew et al., 2013; Deng et al., 2013; Le Guen et al., 2013; Walne, Vulliamy, Kirwan, Plagnol, & Dokal, 2013). The c.2025+4A>C RTEL1 variant likely results in aberrant exon splicing and thus altered protein function. Therefore, it is highly likely that this variant is the cause of the proband’s very short telomeres and features of DC.
Several association studies suggest that common polymorphisms in TPH1 are associated with suicidal behavior, schizophrenia, and possibly bipolar disorder but data are inconsistent [reviewed in (Mirkovic et al., 2016)]. To date, rare, deleterious, missense variants in TPH1 have not been reported to be associated with human disease. However, given the key role of TPH1 in serotonin biosynthesis, the associations between common variants in TPH1 and similar clinical phenotypes, it is highly likely the homozygous TPH1 variant, p.R395H, contributed to mood dysregulation in this patient. Additionally, there were runs of homozygosity and rare homozygous missense variants in BCL9 and CDR2 present in chromosomal regions associated with neuropsychiatric disease.
It is not clear whether the other reported homozygous variants contributed significantly to the proband’s medical problems as many of the genes or variants with them have yet to be associated with disease. For example, SPTBN5 could possibly contribute to disease in this case because the presence of two different homozygous predicted likely deleterious variants would have significant functional consequences on the protein. Variants in SCN4A have been associated with hypokalemic periodic paralysis and related disorders. These clinical features were not present in our patient but the functional consequences of these variants are unknown.
GDAP1L1 encodes a protein similar to that encoded by a Charcot-Marie-Tooth disease gene type 4A, GDAP1(Bird, 1993). Our patient was able to ambulate without difficulty and had no neurosensory abnormalities which suggests that the GDAP1L1 variants may not contribute to that phenotype in this case.
This patient did have a homozygous variant in the LRP4 gene, a cause of Cenani-Lenz syndrome. However, he had a different homozygous missense variant and did not have the severe syndactyly synostosis or renal agenesis reported by Li et al(Li et al., 2010). It is notable that he had pes planus and severe hallux valgus deformity and remains conceivable that the LRP4 variants contribute to his phenotype.
In summary, this report illustrates the value of characterizing the clinical and genetic features of rare but potentially highly informative patients. The cause of DC is likely due to homozygous splice site variants in RTEL1 and his mood dysregulation disorder may be due to homozygous missense variant in TPH1. It is intriguing to speculate that the other variants described above could contribute to several aspects of his clinical phenotype. Future studies of patients with these or similar genetic variants have the potential to shed additional light into complex human diseases.
Supplementary Material
Acknowledgments
We thank the patient and his family for their valuable participation in our study. Lisa Leathwood, RN, Maureen Risch, RN, and Ann Carr, MS, CGC provided study support through contract HHSN261201100018C with Westat Inc (Rockville, MD). The NCI’s Division of Cancer Epidemiology and Genetics Exome Sequencing Working Group is acknowledged for providing in house control samples for variant comparisons. We also thank Dr. Francis McMahan, National Institute of Mental Health, for reviewing genomic regions of interest.
This work was supported by the intramural research program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute National Institutes of Health, and by the intramural research program of the National Institute of Mental Health, National Institutes of Health. The authors have no conflicts of interest to declare.
References
- Alter BP, Giri N, Savage SA, Peters JA, Loud JT, Leathwood L, … Rosenberg PS. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150(2):179–188. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113(26):6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97(3):353–359. doi: 10.3324/haematol.2011.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballew B, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, … Savage S. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Human Genetics. 2013;132(4):473–480. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballew BJ, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, … Savage SA. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Hum Genet. 2013;132(4):473–480. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuch AA. The molecular genetics of the telomere biology disorders. RNA Biol. 2016;13(8):696–706. doi: 10.1080/15476286.2015.1094596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird TD. In: Charcot-Marie-Tooth Neuropathy Type 4. Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews(R); Seattle (WA): 1993. [Google Scholar]
- Deng Z, Glousker G, Molczan A, Fox AJ, Lamm N, Dheekollu J, … Tzfati Y. Inherited mutations in the helicase RTEL1 cause telomere dysfunction and Hoyeraal-Hreidarsson syndrome. Proc Natl Acad Sci U S A. 2013;110(36):E3408–3416. doi: 10.1073/pnas.1300600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet FO, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37(9):e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Wei P, Jian X, Gibbs R, Boerwinkle E, Wang K, Liu X. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet. 2015;24(8):2125–2137. doi: 10.1093/hmg/ddu733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guen T, Jullien L, Touzot F, Schertzer M, Gaillard L, Perderiset M, … Revy P. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Hum Mol Genet. 2013;22(16):3239–3249. doi: 10.1093/hmg/ddt178. [DOI] [PubMed] [Google Scholar]
- Li Y, Pawlik B, Elcioglu N, Aglan M, Kayserili H, Yigit G, … Wollnik B. LRP4 mutations alter Wnt/beta-catenin signaling and cause limb and kidney malformations in Cenani-Lenz syndrome. Am J Hum Genet. 2010;86(5):696–706. doi: 10.1016/j.ajhg.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabello L, Macari ER, Jessop L, Ellis SR, Myers T, Giri N, … Savage SA. Whole-exome sequencing and functional studies identify RPS29 as a novel gene mutated in multicase Diamond-Blackfan anemia families. Blood. 2014;124(1):24–32. doi: 10.1182/blood-2013-11-540278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovic B, Laurent C, Podlipski MA, Frebourg T, Cohen D, Gerardin P. Genetic Association Studies of Suicidal Behavior: A Review of the Past 10 Years, Progress, Limitations, and Future Directions. Front Psychiatry. 2016;7:158. doi: 10.3389/fpsyt.2016.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papal S, Cortese M, Legendre K, Sorusch N, Dragavon J, Sahly I, … El-Amraoui A. The giant spectrin betaV couples the molecular motors to phototransduction and Usher syndrome type I proteins along their trafficking route. Hum Mol Genet. 2013;22(18):3773–3788. doi: 10.1093/hmg/ddt228. [DOI] [PubMed] [Google Scholar]
- Simon AJ, Lev A, Zhang Y, Weiss B, Rylova A, Eyal E, … Somech R. Mutations in STN1 cause Coats plus syndrome and are associated with genomic and telomere defects. J Exp Med. 2016;213(8):1429–1440. doi: 10.1084/jem.20151618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley SE, Gable DL, Wagner CL, Carlile TM, Hanumanthu VS, Podlevsky JD, … Armanios M. Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci Transl Med. 2016;8(351):351ra107. doi: 10.1126/scitranslmed.aaf7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnusdottir B, Morgen K, Arnarsdottir S, … Stefansson K. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505(7483):361–366. doi: 10.1038/nature12818. [DOI] [PubMed] [Google Scholar]
- Takai H, Jenkinson E, Kabir S, Babul-Hirji R, Najm-Tehrani N, Chitayat DA, … de Lange T. A POT1 mutation implicates defective telomere end fill-in and telomere truncations in Coats plus. Genes Dev. 2016;30(7):812–826. doi: 10.1101/gad.276873.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicart S, Sternberg D, Arzel-Hezode M, Franques J, Bendahhou S, Lory P, … Fontaine B. In: Hypokalemic Periodic Paralysis. Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews(R); Seattle (WA): 1993. [Google Scholar]
- Walne AJ, Vulliamy T, Kirwan M, Plagnol V, Dokal I. Constitutional Mutations in RTEL1 Cause Severe Dyskeratosis Congenita. Am J Hum Genet. 2013;92(3):448–453. doi: 10.1016/j.ajhg.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waloen K, Kleppe R, Martinez A, Haavik J. Tyrosine and tryptophan hydroxylases as therapeutic targets in human disease. Expert Opin Ther Targets. 2017;21(2):167–180. doi: 10.1080/14728222.2017.1272581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.